User login
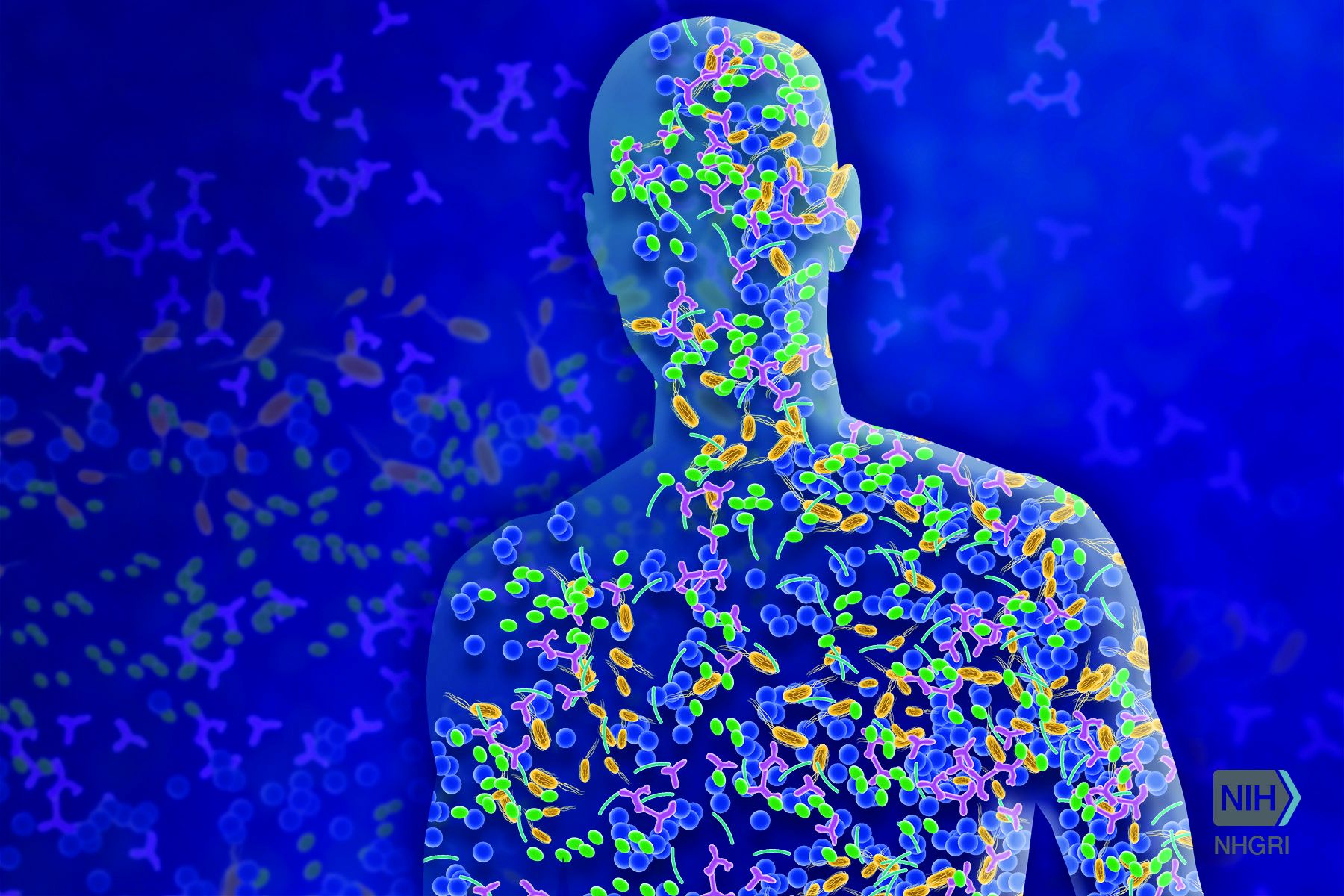
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.