Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions. 13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
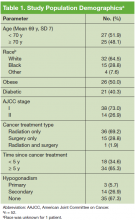
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The mean ADAM score was 4.7 (a value > 3 indicates a positive screen for hypogonad symptoms). Based on laboratory results, 32.6% of patients had hypogonadism; 26.9% of the patients had secondary hypogonadism, and 6% had primary hypogonadism. Based on the ADAM questionnaire, the most common symptoms were erectile dysfunction, low libido, and low physical performance (Table 2).The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.