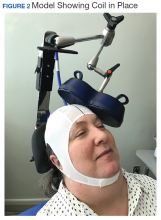
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
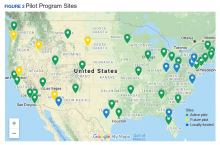
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
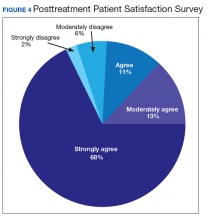
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.