User login
A Clinical Program to Implement Repetitive Transcranial Magnetic Stimulation for Depression in the Department of Veterans Affairs
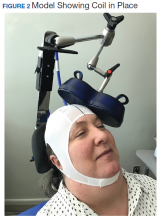
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
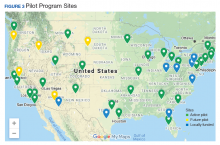
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
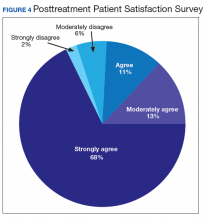
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury (FULL)
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
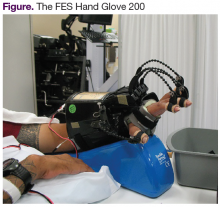
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
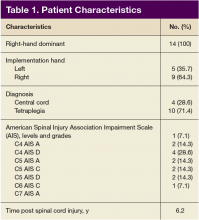
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
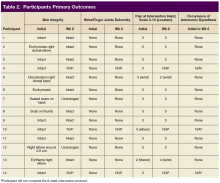
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
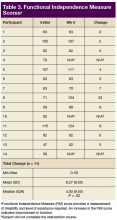
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.