User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
HCV continuum critical to providing better care in urban areas
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
FROM HEPATOLOGY
Key clinical point: Education regarding appropriate hepatitis testing, referral, support, and care is critical to mitigating loss of patients at each stage of the hepatitis C virus treatment continuum.
Major finding: Across an HCV care continuum with approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) were estimated to have HCV. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
Data source: Retrospective population-based study.
Disclosures: Authors reported no financial conflicts of interest.
Measures predict outcomes of chronic HCV with compensated cirrhosis
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Readily available clinical measures can be used to reliably predict long-term outcome in patients with chronic HCV infections and well-compensated advanced liver disease, Dr. Adriaan J van der Meer, of Erasmus University Medical Center, Rotterdam, The Netherlands, and his colleagues report.
The researchers devised risk scores for mortality and for cirrhosis-related complications from a cohort of 405 patients, 100 of whom died during about 8 years of follow up. They then applied the model to 296 patients, 59 of whom died during 6 years of follow up. Independent predictive factors included age, male sex, platelet count, and aspartate aminotransferase/alanine aminotransferase ratio, the researcher said in an article published in the January issue of Gut ( Gut 2015;64:322-331).
Click here to read the article in Gut: http://gut.bmj.com/content/64/2/322.abstract
Sofosbuvir and ribavirin effective in transplant patients with compensated recurrent HCV
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Patients who develop HCV infections after liver transplant may respond to a 24-week course of sofosbuvir and ribavirin, Dr. Michael Charlton, of the Mayo Clinic in Rochester, Minn., and his colleagues reported.
The researchers enrolled and treated 40 liver transplant patients with compensated recurrent HCV infection of any genotype; 83% had HCV genotype 1, 40% had cirrhosis (based on biopsy), and 88% had been previously treated with interferon. All patients received 24 weeks of sofosbuvir 400 mg daily and ribavirin starting at 400 mg daily, which was adjusted according to creatinine clearance and hemoglobin values, the researchers said in the January 2015 issue of Gastroenterology.
After 12 weeks, 28 of 40 had a sustained virologic response (70%; 90% confidence interval: 56%−82%). Relapse accounted for all cases of virologic failure. No patients had detectable viral resistance during or after treatment.
Click here to read the study: http://www.ncbi.nlm.nih.gov/pubmed/25304641
Sofosbuvir and ribavirin prevent HCV recurrence after liver transplantation
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Sofosbuvir and ribavirin given before liver transplantation prevented most cases of post-transplant HCV recurrence, according to Dr. Michael P. Curry, of Beth Israel Deaconess Medical Center, Boston, and his colleagues.
Up to 48 weeks of sofosbuvir (400 mg) and ribavirin were given to hepatocellular carcinoma patients on organ transplant waitlists. The patients had HCV of any genotype and cirrhosis (Child–Turcotte–Pugh score of 7 or less). The primary end point of the study (ClinicalTrials.gov: NCT01559844) was the proportion of 43 patients who had HCV-RNA levels of less than 25 IU/ml at transplant and at 12 weeks after transplant.
Of the 43 patients, 30 (70%) had a post-transplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died, the researchers reported in the January issue of Gastroenterology.
Click here to read the entire article: http://www.gastrojournal.org/article/S0016-5085%2814%2901145-7/fulltext
Aetna customers to receive discount on Gilead’s hepatitis C treatment
Gilead Sciences Inc. and Aetna Inc. have announced a partnership wherein the drug company will offer Aetna’s approximately 20 million health plan members a discounted rate on its hepatitis C drugs, Sovaldi (sofosbuvir) and Harvoni (ledipasvir and sofosbuvir).
The amount of the discount has not yet been announced, although it should be significantly less than the current rate: Sovaldi costs $84,000 for a 12-week course of treatment, and Harvoni costs $94,500 for 12 weeks.
For more information, go to www.reuters.com.
Gilead Sciences Inc. and Aetna Inc. have announced a partnership wherein the drug company will offer Aetna’s approximately 20 million health plan members a discounted rate on its hepatitis C drugs, Sovaldi (sofosbuvir) and Harvoni (ledipasvir and sofosbuvir).
The amount of the discount has not yet been announced, although it should be significantly less than the current rate: Sovaldi costs $84,000 for a 12-week course of treatment, and Harvoni costs $94,500 for 12 weeks.
For more information, go to www.reuters.com.
Gilead Sciences Inc. and Aetna Inc. have announced a partnership wherein the drug company will offer Aetna’s approximately 20 million health plan members a discounted rate on its hepatitis C drugs, Sovaldi (sofosbuvir) and Harvoni (ledipasvir and sofosbuvir).
The amount of the discount has not yet been announced, although it should be significantly less than the current rate: Sovaldi costs $84,000 for a 12-week course of treatment, and Harvoni costs $94,500 for 12 weeks.
For more information, go to www.reuters.com.
Analysis: Push for expanded hepatitis C screening appears premature
The recent advent of new treatments for hepatitis C prompted organizations including the Centers for Disease Control and Prevention, the U.S. Preventive Services Task Force, and the World Health Organization to recommend expanded hepatitis C screening, but such screening may be premature, according to a subject analysis.
Too much uncertainty exists regarding the validity of surrogate markers for treatment efficacy that were used in trials, and evidence regarding clinical outcomes and screening strategies is lacking, according to Dr. Ronald L. Koretz of the University of California, Los Angeles, and his colleagues, who evaluated the current understanding of the incidence and natural course of hepatitis C infection, treatment efficacy, and potential harms of treatment for their analysis.
The best available data suggest that 80%-85% of patients with chronic hepatitis C will die of nonhepatic causes; thus screening could lead to unnecessary treatment. This is important, as safety data for newer drugs are limited, and the existing data suggest a small but concerning rate of serious adverse events associated with the use of some treatments and treatment regimens; the risk-benefit profile of treatment cannot be adequately evaluated because of the lack of data regarding treatment benefits, the investigators reported online Jan. 13 in the British Medical Journal ([doi:10.10036/bmj.g7809]).
Clinical trials to determine the outcomes of treatment in screen-detected patients, as well as the long-term hazards of treatment, are needed, they said, noting that currently available trials included small numbers of patients and/or only short-term follow-up. Until data from such trials are available, physicians should not be pressured to enforce recommended screening strategies “out of enthusiasm for new treatments that have not yet been shown to cause long-term clinical improvement,” they concluded.
Dr. Koretz is a member of the editorial board of the Cochrane Hepato-Biliary Group. The authors reported having no other financial conflicts.
The authors question the merits of hepatitis C (HCV) screening despite the endorsement of the Centers for Disease Control and Prevention, U.S. Preventive Services Task Force, and World Health Organization. They suggest HCV does not lead to sufficient mortality, treatment may cause harm, sustained virologic response (SVR) does not represent a ‘cure’ and question if treating HCV truly reduces long term morbidity/mortality. While it is true the majority of patients with HCV will not develop cirrhosis or death attributable to their liver disease, the authors downplayed the morbidity and mortality related to HCV and the significant cost to the health care system as well (Ann. Intern. Med. 2012;156:271; Hepatology 2013;57:2164]. The authors also downplayed the importance of sustained viral response (SVR), suggesting that it does not represent a cure, yet neglect to mention several studies demonstrating late recurrence in only 1%-3% of patients who have achieved SVR. Furthermore, the authors describe the “harms of treatment,” yet they largely reference therapies that are no longer used and make minimal comment on the clear safety and efficacy of the current interferon-free regimens. Additionally, they report that while clearing HCV may reduce the risk of decompensated cirrhosis and hepatocellular carcinoma, it does not completely eliminate these risks and therefore treatment may not be of value. This brings us back to the motivation of the CDC, USPSTF, and WHO to recommend screening for HCV – identifying asymptomatic infection to allow for administration of safe, effective antiviral therapies before the development of cirrhosis and all of its complications.
Dr. Sean Koppe is director of hepatology, University of Illinois Hospital & Health Sciences System. He has no conflicts of interest.
The authors question the merits of hepatitis C (HCV) screening despite the endorsement of the Centers for Disease Control and Prevention, U.S. Preventive Services Task Force, and World Health Organization. They suggest HCV does not lead to sufficient mortality, treatment may cause harm, sustained virologic response (SVR) does not represent a ‘cure’ and question if treating HCV truly reduces long term morbidity/mortality. While it is true the majority of patients with HCV will not develop cirrhosis or death attributable to their liver disease, the authors downplayed the morbidity and mortality related to HCV and the significant cost to the health care system as well (Ann. Intern. Med. 2012;156:271; Hepatology 2013;57:2164]. The authors also downplayed the importance of sustained viral response (SVR), suggesting that it does not represent a cure, yet neglect to mention several studies demonstrating late recurrence in only 1%-3% of patients who have achieved SVR. Furthermore, the authors describe the “harms of treatment,” yet they largely reference therapies that are no longer used and make minimal comment on the clear safety and efficacy of the current interferon-free regimens. Additionally, they report that while clearing HCV may reduce the risk of decompensated cirrhosis and hepatocellular carcinoma, it does not completely eliminate these risks and therefore treatment may not be of value. This brings us back to the motivation of the CDC, USPSTF, and WHO to recommend screening for HCV – identifying asymptomatic infection to allow for administration of safe, effective antiviral therapies before the development of cirrhosis and all of its complications.
Dr. Sean Koppe is director of hepatology, University of Illinois Hospital & Health Sciences System. He has no conflicts of interest.
The authors question the merits of hepatitis C (HCV) screening despite the endorsement of the Centers for Disease Control and Prevention, U.S. Preventive Services Task Force, and World Health Organization. They suggest HCV does not lead to sufficient mortality, treatment may cause harm, sustained virologic response (SVR) does not represent a ‘cure’ and question if treating HCV truly reduces long term morbidity/mortality. While it is true the majority of patients with HCV will not develop cirrhosis or death attributable to their liver disease, the authors downplayed the morbidity and mortality related to HCV and the significant cost to the health care system as well (Ann. Intern. Med. 2012;156:271; Hepatology 2013;57:2164]. The authors also downplayed the importance of sustained viral response (SVR), suggesting that it does not represent a cure, yet neglect to mention several studies demonstrating late recurrence in only 1%-3% of patients who have achieved SVR. Furthermore, the authors describe the “harms of treatment,” yet they largely reference therapies that are no longer used and make minimal comment on the clear safety and efficacy of the current interferon-free regimens. Additionally, they report that while clearing HCV may reduce the risk of decompensated cirrhosis and hepatocellular carcinoma, it does not completely eliminate these risks and therefore treatment may not be of value. This brings us back to the motivation of the CDC, USPSTF, and WHO to recommend screening for HCV – identifying asymptomatic infection to allow for administration of safe, effective antiviral therapies before the development of cirrhosis and all of its complications.
Dr. Sean Koppe is director of hepatology, University of Illinois Hospital & Health Sciences System. He has no conflicts of interest.
The recent advent of new treatments for hepatitis C prompted organizations including the Centers for Disease Control and Prevention, the U.S. Preventive Services Task Force, and the World Health Organization to recommend expanded hepatitis C screening, but such screening may be premature, according to a subject analysis.
Too much uncertainty exists regarding the validity of surrogate markers for treatment efficacy that were used in trials, and evidence regarding clinical outcomes and screening strategies is lacking, according to Dr. Ronald L. Koretz of the University of California, Los Angeles, and his colleagues, who evaluated the current understanding of the incidence and natural course of hepatitis C infection, treatment efficacy, and potential harms of treatment for their analysis.
The best available data suggest that 80%-85% of patients with chronic hepatitis C will die of nonhepatic causes; thus screening could lead to unnecessary treatment. This is important, as safety data for newer drugs are limited, and the existing data suggest a small but concerning rate of serious adverse events associated with the use of some treatments and treatment regimens; the risk-benefit profile of treatment cannot be adequately evaluated because of the lack of data regarding treatment benefits, the investigators reported online Jan. 13 in the British Medical Journal ([doi:10.10036/bmj.g7809]).
Clinical trials to determine the outcomes of treatment in screen-detected patients, as well as the long-term hazards of treatment, are needed, they said, noting that currently available trials included small numbers of patients and/or only short-term follow-up. Until data from such trials are available, physicians should not be pressured to enforce recommended screening strategies “out of enthusiasm for new treatments that have not yet been shown to cause long-term clinical improvement,” they concluded.
Dr. Koretz is a member of the editorial board of the Cochrane Hepato-Biliary Group. The authors reported having no other financial conflicts.
The recent advent of new treatments for hepatitis C prompted organizations including the Centers for Disease Control and Prevention, the U.S. Preventive Services Task Force, and the World Health Organization to recommend expanded hepatitis C screening, but such screening may be premature, according to a subject analysis.
Too much uncertainty exists regarding the validity of surrogate markers for treatment efficacy that were used in trials, and evidence regarding clinical outcomes and screening strategies is lacking, according to Dr. Ronald L. Koretz of the University of California, Los Angeles, and his colleagues, who evaluated the current understanding of the incidence and natural course of hepatitis C infection, treatment efficacy, and potential harms of treatment for their analysis.
The best available data suggest that 80%-85% of patients with chronic hepatitis C will die of nonhepatic causes; thus screening could lead to unnecessary treatment. This is important, as safety data for newer drugs are limited, and the existing data suggest a small but concerning rate of serious adverse events associated with the use of some treatments and treatment regimens; the risk-benefit profile of treatment cannot be adequately evaluated because of the lack of data regarding treatment benefits, the investigators reported online Jan. 13 in the British Medical Journal ([doi:10.10036/bmj.g7809]).
Clinical trials to determine the outcomes of treatment in screen-detected patients, as well as the long-term hazards of treatment, are needed, they said, noting that currently available trials included small numbers of patients and/or only short-term follow-up. Until data from such trials are available, physicians should not be pressured to enforce recommended screening strategies “out of enthusiasm for new treatments that have not yet been shown to cause long-term clinical improvement,” they concluded.
Dr. Koretz is a member of the editorial board of the Cochrane Hepato-Biliary Group. The authors reported having no other financial conflicts.
Key clinical point: Evidence to support expanded hepatitis C screening is lacking.
Major finding: An estimated 80%-85% of patients with chronic hepatitis C will die from nonhepatic causes.
Data source: An analysis of existing evidence.
Disclosures: Dr. Koretz is a member of the editorial board of the Cochrane Hepato-Biliary Group. The authors reported having no other relevant financial disclosures.
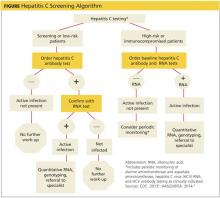
HCV Screening Decision Tool
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
HCV SCREENING DECISION TOOL
The initial screening tool for HCV infection is an HCV antibody test. A positive anti-HCV antibody result can signify either current or resolved infection, followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present. Screening should be offered to all individuals falling within one or more of the following at-risk categories or behaviors.1-4
HIGH-RISK INDIVIDUALS HAVE/ARE…
• Been born between 1945 and 1965, regardless of other risk factors; should be screened one time
• Currently or formerly used injection drugs, including injecting only once many years ago; current injection drug users should be screened annually
• Received clotting factor concentrates made before 1987 (before more advanced methods for manufacturing those products were developed)
• Received blood transfusions or solid organ transplants before July 1992 (before better testing of blood donors became available)
• Born to HCV-positive mothers (If diagnosis is required for children younger than 18 months, use the HCV ribonucleic acid (RNA) test at 1 to 2 months.)
• Ever received long-term hemodialysis
• Human immunodeficiency virus (HIV) infection
• Known exposures to HCV
• Health care workers after needle stick injuries involving HCV-positive blood
• Recipients of blood or organs from donors who tested positive for HCV
• HIV-positive men who have sex with men; should be screened annually
• Signs and symptoms of liver disease (elevated transaminase levels)
LOWER-RISK INDIVIDUALS HAVE...
• Heterosexual intercourse with an HCV-infected person or multiple sexual partners
• Shared personal items that may contain blood, such as razors and toothbrushes
• Other invasive health care procedures, such as injections
• Cosmetic procedures, such as tattoos and piercings, where infection control is substandard
• Used intranasal drugs, cocaine, or marijuana
The following HCV screening algorithm, which includes the points at which referrals to specialists are indicated, is useful for determining the right test to use for a specific risk group.
Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease.
Read Sturm D, Gurevitz SL, Davidson D, Fritchley A, Wagaman A. Chronic hepatitis C infection: Bane of baby boomers. 2014;24(11):24-32 to earn 1 hour AAPA Category 1 CME credit, expires November 31, 2015.
URL: http://www.clinicianreviews.com/articles/cecme-activities/article/chronic-hepatitis-c-infection-bane-of-baby-boomers/73b211cce27d01b93463584dd2c44083.html
REFERENCES
4. [1.] The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed January 7, 2015.
8. [2.] CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. [3.] Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. [4.] American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed January 7, 2015.
Sofosbuvir and ribavirin critical to preventing posttransplantation HCV recurrence
Sofosbuvir and ribavirin treatments should be administered to patients with hepatitis C virus who undergo liver transplantations in order to significantly decrease the risks of posttransplant HCV recurrence, according to two new studies published in the January issue of Gastroenterology (10.1053/j.gastro.2014.09.023 and 10.1053/j.gastro.2014.10.001).
“In clinical trials, administration of sofosbuvir with ribavirin was associated with rapid decreases of HCV RNA to undetectable levels in patients with HCV genotype 1, 2, 3, 4, and 6 infections,” wrote lead author Dr. Michael P. Curry of the Beth Israel Deaconess Medical Center in Boston, and his coauthors on the first of these two studies. “In more than 3,000 patients treated to date, sofosbuvir has been shown to be safe, viral breakthrough during treatment has been rare (and associated with nonadherence), and few drug interactions have been observed.”
In a phase II, open-label study, Dr. Curry and his coinvestigators enrolled 61 patients with HCV of any genotype, and cirrhosis with a Child-Turcotte-Pugh score no greater than 7, who were all wait-listed to receive liver transplantations. Subjects received up to 48 weeks of treatment with 400 mg of sofosbuvir, and a separate dose of ribavirin prior to liver transplantation, while 43 patients received transplantations alone. The primary outcome sought by investigators was HCV-RNA levels less than 25 IU/mL at 12 weeks after transplantation among patients that had this level prior to the operation.
The investigators found that 43 subjects had the desired HCV-RNA levels; of that population, 49% had a posttransplantation virologic response, with the most frequent side effects reported by subjects being fatigue (38%), headache (23%), and anemia (21%). Of the 43 applicable subjects, 30 (70% of the population) had a posttransplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died.
“This study provides proof of concept that virologic suppression without interferon significantly can reduce the rate of recurrent HCV after liver transplantation,” the study says, adding that the results “compare favorably with those observed in other trials of pretransplantation antiviral therapy.”
In the second study, the authors ascertained that combination therapy consisting of sofosbuvir and ribavirin for 24 weeks is effective at preventing hepatitis C virus recurrence in patients who undergo liver transplantations.
“Recurrent HCV infection is the most common cause of mortality and graft loss following transplantation, and up to 30% of patients with recurrent infection develop cirrhosis within 5 years,” wrote the study’s authors, led by Dr. Michael Charlton of the Mayo Clinic in Rochester, Minn.
Using a prospective, multicenter, open-label pilot study, investigators enrolled and treated 40 patients with a 24-week regimen of 400 mg sofosbuvir and ribavirin starting at 400 mg, which was subsequently adjusted per patient based on individual creatinine clearance and hemoglobin levels. Subjects were 78% male and 85% white, with 83% having HCV genotype 1, 40% having cirrhosis, and 88% having been previously treated with interferon. The primary outcome investigators looked for was “sustained virologic response 12 weeks after treatment (SVR12).”
Data showed that SVR12 was achieved by 28 of the 40 subjects that received treatment, or 70%. The most commonly reported adverse effects were fatigue (30%), diarrhea (28%), headache (25%), and anemia (20%). No patients exhibited detectable viral resistance during or after treatment, and although two patients terminated their treatment because of adverse events, investigators reported no deaths, graft losses, or episodes of rejection.
“In contrast,” Dr. Charlton and his coauthors noted, “interferon-based treatments have been associated with posttreatment immunological dysfunction (particularly plasma cell hepatitis) and even hepatic decompensation in LT [liver transplant] recipients.”
The authors of the first study disclosed that Dr. Curry has received grants from and been affiliated with Gilead, which was a sponsor of the study. The authors of the second study reported no relevant financial disclosures.
Sofosbuvir and ribavirin treatments should be administered to patients with hepatitis C virus who undergo liver transplantations in order to significantly decrease the risks of posttransplant HCV recurrence, according to two new studies published in the January issue of Gastroenterology (10.1053/j.gastro.2014.09.023 and 10.1053/j.gastro.2014.10.001).
“In clinical trials, administration of sofosbuvir with ribavirin was associated with rapid decreases of HCV RNA to undetectable levels in patients with HCV genotype 1, 2, 3, 4, and 6 infections,” wrote lead author Dr. Michael P. Curry of the Beth Israel Deaconess Medical Center in Boston, and his coauthors on the first of these two studies. “In more than 3,000 patients treated to date, sofosbuvir has been shown to be safe, viral breakthrough during treatment has been rare (and associated with nonadherence), and few drug interactions have been observed.”
In a phase II, open-label study, Dr. Curry and his coinvestigators enrolled 61 patients with HCV of any genotype, and cirrhosis with a Child-Turcotte-Pugh score no greater than 7, who were all wait-listed to receive liver transplantations. Subjects received up to 48 weeks of treatment with 400 mg of sofosbuvir, and a separate dose of ribavirin prior to liver transplantation, while 43 patients received transplantations alone. The primary outcome sought by investigators was HCV-RNA levels less than 25 IU/mL at 12 weeks after transplantation among patients that had this level prior to the operation.
The investigators found that 43 subjects had the desired HCV-RNA levels; of that population, 49% had a posttransplantation virologic response, with the most frequent side effects reported by subjects being fatigue (38%), headache (23%), and anemia (21%). Of the 43 applicable subjects, 30 (70% of the population) had a posttransplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died.
“This study provides proof of concept that virologic suppression without interferon significantly can reduce the rate of recurrent HCV after liver transplantation,” the study says, adding that the results “compare favorably with those observed in other trials of pretransplantation antiviral therapy.”
In the second study, the authors ascertained that combination therapy consisting of sofosbuvir and ribavirin for 24 weeks is effective at preventing hepatitis C virus recurrence in patients who undergo liver transplantations.
“Recurrent HCV infection is the most common cause of mortality and graft loss following transplantation, and up to 30% of patients with recurrent infection develop cirrhosis within 5 years,” wrote the study’s authors, led by Dr. Michael Charlton of the Mayo Clinic in Rochester, Minn.
Using a prospective, multicenter, open-label pilot study, investigators enrolled and treated 40 patients with a 24-week regimen of 400 mg sofosbuvir and ribavirin starting at 400 mg, which was subsequently adjusted per patient based on individual creatinine clearance and hemoglobin levels. Subjects were 78% male and 85% white, with 83% having HCV genotype 1, 40% having cirrhosis, and 88% having been previously treated with interferon. The primary outcome investigators looked for was “sustained virologic response 12 weeks after treatment (SVR12).”
Data showed that SVR12 was achieved by 28 of the 40 subjects that received treatment, or 70%. The most commonly reported adverse effects were fatigue (30%), diarrhea (28%), headache (25%), and anemia (20%). No patients exhibited detectable viral resistance during or after treatment, and although two patients terminated their treatment because of adverse events, investigators reported no deaths, graft losses, or episodes of rejection.
“In contrast,” Dr. Charlton and his coauthors noted, “interferon-based treatments have been associated with posttreatment immunological dysfunction (particularly plasma cell hepatitis) and even hepatic decompensation in LT [liver transplant] recipients.”
The authors of the first study disclosed that Dr. Curry has received grants from and been affiliated with Gilead, which was a sponsor of the study. The authors of the second study reported no relevant financial disclosures.
Sofosbuvir and ribavirin treatments should be administered to patients with hepatitis C virus who undergo liver transplantations in order to significantly decrease the risks of posttransplant HCV recurrence, according to two new studies published in the January issue of Gastroenterology (10.1053/j.gastro.2014.09.023 and 10.1053/j.gastro.2014.10.001).
“In clinical trials, administration of sofosbuvir with ribavirin was associated with rapid decreases of HCV RNA to undetectable levels in patients with HCV genotype 1, 2, 3, 4, and 6 infections,” wrote lead author Dr. Michael P. Curry of the Beth Israel Deaconess Medical Center in Boston, and his coauthors on the first of these two studies. “In more than 3,000 patients treated to date, sofosbuvir has been shown to be safe, viral breakthrough during treatment has been rare (and associated with nonadherence), and few drug interactions have been observed.”
In a phase II, open-label study, Dr. Curry and his coinvestigators enrolled 61 patients with HCV of any genotype, and cirrhosis with a Child-Turcotte-Pugh score no greater than 7, who were all wait-listed to receive liver transplantations. Subjects received up to 48 weeks of treatment with 400 mg of sofosbuvir, and a separate dose of ribavirin prior to liver transplantation, while 43 patients received transplantations alone. The primary outcome sought by investigators was HCV-RNA levels less than 25 IU/mL at 12 weeks after transplantation among patients that had this level prior to the operation.
The investigators found that 43 subjects had the desired HCV-RNA levels; of that population, 49% had a posttransplantation virologic response, with the most frequent side effects reported by subjects being fatigue (38%), headache (23%), and anemia (21%). Of the 43 applicable subjects, 30 (70% of the population) had a posttransplantation virologic response at 12 weeks, 10 (23%) had recurrent infection, and 3 (7%) died.
“This study provides proof of concept that virologic suppression without interferon significantly can reduce the rate of recurrent HCV after liver transplantation,” the study says, adding that the results “compare favorably with those observed in other trials of pretransplantation antiviral therapy.”
In the second study, the authors ascertained that combination therapy consisting of sofosbuvir and ribavirin for 24 weeks is effective at preventing hepatitis C virus recurrence in patients who undergo liver transplantations.
“Recurrent HCV infection is the most common cause of mortality and graft loss following transplantation, and up to 30% of patients with recurrent infection develop cirrhosis within 5 years,” wrote the study’s authors, led by Dr. Michael Charlton of the Mayo Clinic in Rochester, Minn.
Using a prospective, multicenter, open-label pilot study, investigators enrolled and treated 40 patients with a 24-week regimen of 400 mg sofosbuvir and ribavirin starting at 400 mg, which was subsequently adjusted per patient based on individual creatinine clearance and hemoglobin levels. Subjects were 78% male and 85% white, with 83% having HCV genotype 1, 40% having cirrhosis, and 88% having been previously treated with interferon. The primary outcome investigators looked for was “sustained virologic response 12 weeks after treatment (SVR12).”
Data showed that SVR12 was achieved by 28 of the 40 subjects that received treatment, or 70%. The most commonly reported adverse effects were fatigue (30%), diarrhea (28%), headache (25%), and anemia (20%). No patients exhibited detectable viral resistance during or after treatment, and although two patients terminated their treatment because of adverse events, investigators reported no deaths, graft losses, or episodes of rejection.
“In contrast,” Dr. Charlton and his coauthors noted, “interferon-based treatments have been associated with posttreatment immunological dysfunction (particularly plasma cell hepatitis) and even hepatic decompensation in LT [liver transplant] recipients.”
The authors of the first study disclosed that Dr. Curry has received grants from and been affiliated with Gilead, which was a sponsor of the study. The authors of the second study reported no relevant financial disclosures.
FROM GASTROENTEROLOGY
VIDEO: Hepatitis C screening rises, but where are the positive cases?
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE LIVER MEETING 2014
VIDEO: Will new HCV drugs’ costs kill health care budgets?
BOSTON – The estimated cost of treating all eligible U.S. hepatitis C patients with a new generation of high-priced medications may be breathtaking, but would the resulting savings over those cured patients’ lifetimes offset the initial financial blow?
A new analysis unveiled at the annual meeting of the American Association for the Study of Liver Diseases calculated the impact of the new drugs on treatment costs and compared the cost of treatment with new drugs to the old standard of care.
“We found that the cost of treatment is very high, as expected,” explained lead investigator Jagpreet Chhatwal, Ph.D., of MD Anderson Cancer Center, Houston. In fact, if everyone who was eligible for the new drugs were treated, the cost over the next 5 years would be $136 billion.
“This is clearly unsustainable for any payer,” he noted. “So the question is: How can we manage to treat people who need this treatment?”
In a video interview, Dr. Chhatwal outlined how the researchers calculated their cost estimates, what cost savings could be gained with the new drugs, and how patients and payers alike could manage the price of treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The estimated cost of treating all eligible U.S. hepatitis C patients with a new generation of high-priced medications may be breathtaking, but would the resulting savings over those cured patients’ lifetimes offset the initial financial blow?
A new analysis unveiled at the annual meeting of the American Association for the Study of Liver Diseases calculated the impact of the new drugs on treatment costs and compared the cost of treatment with new drugs to the old standard of care.
“We found that the cost of treatment is very high, as expected,” explained lead investigator Jagpreet Chhatwal, Ph.D., of MD Anderson Cancer Center, Houston. In fact, if everyone who was eligible for the new drugs were treated, the cost over the next 5 years would be $136 billion.
“This is clearly unsustainable for any payer,” he noted. “So the question is: How can we manage to treat people who need this treatment?”
In a video interview, Dr. Chhatwal outlined how the researchers calculated their cost estimates, what cost savings could be gained with the new drugs, and how patients and payers alike could manage the price of treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The estimated cost of treating all eligible U.S. hepatitis C patients with a new generation of high-priced medications may be breathtaking, but would the resulting savings over those cured patients’ lifetimes offset the initial financial blow?
A new analysis unveiled at the annual meeting of the American Association for the Study of Liver Diseases calculated the impact of the new drugs on treatment costs and compared the cost of treatment with new drugs to the old standard of care.
“We found that the cost of treatment is very high, as expected,” explained lead investigator Jagpreet Chhatwal, Ph.D., of MD Anderson Cancer Center, Houston. In fact, if everyone who was eligible for the new drugs were treated, the cost over the next 5 years would be $136 billion.
“This is clearly unsustainable for any payer,” he noted. “So the question is: How can we manage to treat people who need this treatment?”
In a video interview, Dr. Chhatwal outlined how the researchers calculated their cost estimates, what cost savings could be gained with the new drugs, and how patients and payers alike could manage the price of treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE LIVER MEETING 2014