User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
VIDEO: Hepatitis C screening recommendations falling on deaf ears
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The call to screen Baby Boomers for hepatitis C virus infections appears to have gone unheeded so far, results from a Chicago primary care clinic show.
Screening increased by only 2% among some 25,000 patients seen in the primary care clinic of the University of Chicago after the 2012 Centers for Disease Control and Prevention recommendation to screen adults born between 1945 and 1965, Dr. Mansi Kothari reported at the annual meeting of the American Association for the Study of Liver Diseases.
On a positive note, Dr. Kothari of the University of Chicago Medical Center noted in an interview that if a patient tested positive for hepatitis C virus, rates of additional testing and referral to a hepatologist remained high.
Dr. Kothari reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2014
VIDEO: Most baby boomers didn’t know their hep C status
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– Almost two-thirds of baby boomers presenting to Alabama emergency departments were unaware of their hepatitis C virus status, despite having such high-risk factors as past intravenous drug use or receipt of a blood transfusion prior to 1992.
Equally concerning, only 48% of patients who knew they were HCV positive were aware of some of the highly efficacious treatments now available, study author and medical student Derek Wells of the University of Alabama-Birmingham said in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
Mr. Wells called for increased awareness among front-line providers to improve screening and help eradicate HCV in the United States.
Mr. Wells reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2014
Interferon-free regimen benefits HCV-infected liver transplant recipients
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
AGA Resource:
HCV Clinical Service Line: http://www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
AGA Resource:
HCV Clinical Service Line: http://www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line
An oral, interferon-free drug regimen produced a 97% rate of sustained virologic response in liver transplant recipients who had recurrent hepatitis C viral infection – “an historically difficult-to-treat population” at high risk of death who have extremely limited treatment options, according to a study reported at the annual meeting of the American Association for the Study of Liver Diseases.
In an industry-sponsored, open-label phase II trial involving 34 adults with recurrent HCV infection following liver transplantation, 24 weeks of daily ombitasvir plus the ritonavir-boosted protease inhibitor ABT-450 (ABT-50/r), added to dasabuvir and ribavirin, eradicated every patient’s HCV RNA levels within 4 months. Only one patient had a relapse during a further 24 weeks of follow-up, said Dr. Parvez Mantry of the Liver Institute at Methodist Dallas, who presented the data at the meeting.*
Results of the study, which was conducted at 10 transplant centers in the United States and Spain, were presented at the meeting and simultaneously published online Nov. 11 in the New England Journal of Medicine (N. Engl. J. Med. 2014 Nov. 11 [doi: 10.1056/NEJMoa1408921]).
The standard of care for treating recurrent HCV infection after liver transplantation has been 48 weeks of peginterferon with ribavirin, but response rates are relatively low (13%-43%) because of interferon’s toxic effects. Moreover, the agent is known to induce graft injury, reducing both graft and patient survival.
The investigators assessed the safety and efficacy of a tablet formulation combining ombitasvir, a potent NS5A inhibitor, with ABT-50/r, a protease inhibitor that increases peak, trough, and overall drug exposure and allows once-daily dosing. To this was added standard dasabuvir and ribavirin, with ribavirin dosing adjusted according to the treating physician’s discretion to avert adverse hematologic effects in these immunosuppressed transplant recipients. Modified doses of standard calcineurin inhibitors (cyclosporine or tacrolimus) also were recommended for all patients, and low-dose glucocorticoids were permitted as needed.
The study participants were 18-70 years of age (mean age, 59.6 years) and had received liver transplants because of chronic HCV infection a minimum of 1 year previously. They had no or only mild liver fibrosis, were receiving stable cyclosporine- or tacrolimus-based immunosuppression, and were not coinfected with HIV or hepatitis B.
The primary efficacy endpoint was a sustained virologic response (SVR) 12 weeks after treatment was completed. All the study participants achieved an SVR by week 4 of treatment, which persisted in all of them until treatment was completed. At that time, 1 patient relapsed, so the overall SVR rate was 97%. This same SVR rate was sustained through final follow-up at post-treatment week 24.
In the patient who relapsed, HCV DNA showed resistance-associated genetic variants that had not been present at baseline. This patient also had been unresponsive to previous peginterferon-ribavirin therapy.
Adverse events were common, although the majority were mild or moderate in severity. Fatigue, headache, and cough were the most frequent adverse events. Grade 2 elevations in total bilirubin developed in two patients (6%), with no jaundice or scleral icterus. Nine patients showed grade 2 decreases in hemoglobin; none required a blood transfusion, and five required erythropoietin. There were no deaths and no cases of graft rejection.
One patient discontinued the study drug at week 18 after developing moderate rash, memory impairment, and anxiety deemed to be possibly drug related. However, that patient had already achieved an SVR before discontinuing treatment, and that SVR persisted at final follow-up 12 weeks later.
However, this study was not large enough to allow adequate assessment of adverse event rates or comparison of them with rates for other treatments, the investigators noted.
The researchers also noted that these study participants were easier to treat than the general population of liver transplant recipients with recurrent HCV, because they did not have advanced fibrosis or comorbid infections. In addition, patients with early, aggressive forms of recurrent HCV, such as fibrosing cholestatic hepatitis, were excluded from this study, as were patients maintained on immunosuppressive agents other than cyclosporine or tacrolimus.
This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Study investigator Dr. Paul Y. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
AGA Resource:
HCV Clinical Service Line: http://www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line
Key clinical point: An oral, interferon-free drug combination produced a 97% sustained virologic response rate in liver transplant recipients with recurrent HCV infection.
Major finding: The primary efficacy endpoint, an SVR 12 weeks after completion of treatment, was 97% (33 of 34 patients).
Data source: An industry-sponsored, multicenter, open-label phase II trial involving 34 adults with chronic HCV infection despite liver transplantation.
Disclosures: This trial was sponsored by AbbVie, whose employees also designed the study, gathered and analyzed the data, and wrote the report. Dr. Kwo reported receiving personal fees and grants from, and serving on advisory boards for, AbbVie, Bristol-Myers Squibb, and other companies. His associates reported ties to numerous industry sources.
Achieving sustained response key to successful HCV treatment
BOSTON – Risks for mortality, hepatocellular carcinoma, and liver transplantations were significantly lower in patients with hepatitis C who achieved a sustained virologic response after treatment, compared with patients without a sustained response, in a meta-analysis of long-term follow-up data on 23,309 patients.
In patients with a sustained virologic response (SVR), the risk of death was 62%-84% lower, the risk of developing hepatocellular carcinoma was 68%-79% lower, and the risk of liver transplantation was 90% lower, compared with patients who did not achieve a SVR, Andrew M. Hill, Ph.D., and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
The cohort included 15,067 patients with hepatitis C without cirrhosis, HIV, or prior transplantation; 4,987 patients with hepatitis C and cirrhosis; 1,170 patients with hepatitis C who had undergone liver transplantation; and 2,085 patients coinfected with hepatitis C and HIV.
Treatment consisted primarily of pegylated interferon plus ribavirin. “These analyses need to be repeated for studies of direct acting antivirals,” said Dr. Hill of the University of Liverpool, England.
In the current study, SVR was associated with a 62% reduction in the risk of death in the general group of mono-infected patients without cirrhosis or transplantation, an 84% reduction in patients with cirrhosis, and a 73% reduction in those coinfected with HIV, multivariate analyses showed.
The 5-year risk of all-cause mortality was 4.5% after SVR and 10.5% with no SVR in the general group of patients with HCV. In those with cirrhosis, mortality rates were 3.6% after SVR or 11.3% with no SVR. In patients who also had HIV, mortality rates were 1.3% after SVR and 10% with no SVR, Dr. Hill reported.
The 5-year risk of developing hepatocellular carcinoma as 2.9% after SVR and 9.3% with no SVR in the general group with HCV. In patients with cirrhosis, hepatocellular carcinoma rates were 5.3% with SVR and 13.9% with no SVR. In patients who had HIV, hepatocellular carcinoma rates were 0.9% after SVR and 10% with no SVR.
Reinfection with HCV can complicate the results of treatment, Dr. Hill said. The 5-year reinfection rates were 0.9% in low-risk patients, 8.2% in IV drug users or prisoners, and 23.6% in patients coinfected with HIV.
“The cost-effectiveness of treatment of hepatitis C depends on the extent of reductions in the risk of liver transplantation, hepatocellular carcinoma, and all-cause mortality,” he said.
The World Health Organization and UNITAID funded the study. Dr. Hill reported financial associations with Janssen Pharmaceuticals.
On Twitter @sherryboschert
BOSTON – Risks for mortality, hepatocellular carcinoma, and liver transplantations were significantly lower in patients with hepatitis C who achieved a sustained virologic response after treatment, compared with patients without a sustained response, in a meta-analysis of long-term follow-up data on 23,309 patients.
In patients with a sustained virologic response (SVR), the risk of death was 62%-84% lower, the risk of developing hepatocellular carcinoma was 68%-79% lower, and the risk of liver transplantation was 90% lower, compared with patients who did not achieve a SVR, Andrew M. Hill, Ph.D., and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
The cohort included 15,067 patients with hepatitis C without cirrhosis, HIV, or prior transplantation; 4,987 patients with hepatitis C and cirrhosis; 1,170 patients with hepatitis C who had undergone liver transplantation; and 2,085 patients coinfected with hepatitis C and HIV.
Treatment consisted primarily of pegylated interferon plus ribavirin. “These analyses need to be repeated for studies of direct acting antivirals,” said Dr. Hill of the University of Liverpool, England.
In the current study, SVR was associated with a 62% reduction in the risk of death in the general group of mono-infected patients without cirrhosis or transplantation, an 84% reduction in patients with cirrhosis, and a 73% reduction in those coinfected with HIV, multivariate analyses showed.
The 5-year risk of all-cause mortality was 4.5% after SVR and 10.5% with no SVR in the general group of patients with HCV. In those with cirrhosis, mortality rates were 3.6% after SVR or 11.3% with no SVR. In patients who also had HIV, mortality rates were 1.3% after SVR and 10% with no SVR, Dr. Hill reported.
The 5-year risk of developing hepatocellular carcinoma as 2.9% after SVR and 9.3% with no SVR in the general group with HCV. In patients with cirrhosis, hepatocellular carcinoma rates were 5.3% with SVR and 13.9% with no SVR. In patients who had HIV, hepatocellular carcinoma rates were 0.9% after SVR and 10% with no SVR.
Reinfection with HCV can complicate the results of treatment, Dr. Hill said. The 5-year reinfection rates were 0.9% in low-risk patients, 8.2% in IV drug users or prisoners, and 23.6% in patients coinfected with HIV.
“The cost-effectiveness of treatment of hepatitis C depends on the extent of reductions in the risk of liver transplantation, hepatocellular carcinoma, and all-cause mortality,” he said.
The World Health Organization and UNITAID funded the study. Dr. Hill reported financial associations with Janssen Pharmaceuticals.
On Twitter @sherryboschert
BOSTON – Risks for mortality, hepatocellular carcinoma, and liver transplantations were significantly lower in patients with hepatitis C who achieved a sustained virologic response after treatment, compared with patients without a sustained response, in a meta-analysis of long-term follow-up data on 23,309 patients.
In patients with a sustained virologic response (SVR), the risk of death was 62%-84% lower, the risk of developing hepatocellular carcinoma was 68%-79% lower, and the risk of liver transplantation was 90% lower, compared with patients who did not achieve a SVR, Andrew M. Hill, Ph.D., and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
The cohort included 15,067 patients with hepatitis C without cirrhosis, HIV, or prior transplantation; 4,987 patients with hepatitis C and cirrhosis; 1,170 patients with hepatitis C who had undergone liver transplantation; and 2,085 patients coinfected with hepatitis C and HIV.
Treatment consisted primarily of pegylated interferon plus ribavirin. “These analyses need to be repeated for studies of direct acting antivirals,” said Dr. Hill of the University of Liverpool, England.
In the current study, SVR was associated with a 62% reduction in the risk of death in the general group of mono-infected patients without cirrhosis or transplantation, an 84% reduction in patients with cirrhosis, and a 73% reduction in those coinfected with HIV, multivariate analyses showed.
The 5-year risk of all-cause mortality was 4.5% after SVR and 10.5% with no SVR in the general group of patients with HCV. In those with cirrhosis, mortality rates were 3.6% after SVR or 11.3% with no SVR. In patients who also had HIV, mortality rates were 1.3% after SVR and 10% with no SVR, Dr. Hill reported.
The 5-year risk of developing hepatocellular carcinoma as 2.9% after SVR and 9.3% with no SVR in the general group with HCV. In patients with cirrhosis, hepatocellular carcinoma rates were 5.3% with SVR and 13.9% with no SVR. In patients who had HIV, hepatocellular carcinoma rates were 0.9% after SVR and 10% with no SVR.
Reinfection with HCV can complicate the results of treatment, Dr. Hill said. The 5-year reinfection rates were 0.9% in low-risk patients, 8.2% in IV drug users or prisoners, and 23.6% in patients coinfected with HIV.
“The cost-effectiveness of treatment of hepatitis C depends on the extent of reductions in the risk of liver transplantation, hepatocellular carcinoma, and all-cause mortality,” he said.
The World Health Organization and UNITAID funded the study. Dr. Hill reported financial associations with Janssen Pharmaceuticals.
On Twitter @sherryboschert
AT THE LIVER MEETING 2014
Key clinical point: Achieving a sustained virologic response to hepatitis C treatment improved long-term outcomes.
Major finding: Achieving a sustained virologic response was associated with a 62%-84% lower risk of death.
Data source: A meta-analysis of data on 23,309 patients with hepatitis C infection in 129 studies.
Disclosures: The World Health Organization and UNITAID funded the study. Dr. Hill reported ties with Janssen Pharmaceuticals.
VIDEO: Hepatitis C burden could wallop Medicare
BOSTON – The graying of America will add at least 1 million more patients with chronic hepatitis C virus infection into the Medicare system between 2010 and 2024.
“If all of these patients were treated with an all-oral high efficacy regimen, however, it could save 33,922 lives and increase the number of years lived by an estimated 200,000 years,” study author David Rein, Ph.D., reported at the annual meeting of the American Association for the Study of Liver Diseases.
The study is the first to estimate the number of chronically infected HCV patients in the Medicare system, and the data suggest that as of 2009, there were already 407,786 such patients in the system.
Most patients (68%) were diagnosed with chronic disease only, 24% were diagnosed with some form of end-stage liver disease, and 8% died in 2009.
Co-morbities were commona among the cohort, with 64% having at least one other chronic condition, such as diabetes, renal disease, alcohol/substance abuse, or mental health conditions.
The annual cost of their HCV treatment in 2009 was $2.7 billion in 2014 dollars.
Over the next 10 years, that number could rise to $6.7 billion if these patients aren’t treated, Dr. Rein, a principal research scientist in the Atlanta office of NORC at the University of Chicago, said.
To hear more about this study and whether Medicare can afford this level of care, click here to hear our interview with Dr. Rein.
The study was funded by an unrestricted research grant from Gilead Sciences. Dr. Rein and his co-authors reported having no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The graying of America will add at least 1 million more patients with chronic hepatitis C virus infection into the Medicare system between 2010 and 2024.
“If all of these patients were treated with an all-oral high efficacy regimen, however, it could save 33,922 lives and increase the number of years lived by an estimated 200,000 years,” study author David Rein, Ph.D., reported at the annual meeting of the American Association for the Study of Liver Diseases.
The study is the first to estimate the number of chronically infected HCV patients in the Medicare system, and the data suggest that as of 2009, there were already 407,786 such patients in the system.
Most patients (68%) were diagnosed with chronic disease only, 24% were diagnosed with some form of end-stage liver disease, and 8% died in 2009.
Co-morbities were commona among the cohort, with 64% having at least one other chronic condition, such as diabetes, renal disease, alcohol/substance abuse, or mental health conditions.
The annual cost of their HCV treatment in 2009 was $2.7 billion in 2014 dollars.
Over the next 10 years, that number could rise to $6.7 billion if these patients aren’t treated, Dr. Rein, a principal research scientist in the Atlanta office of NORC at the University of Chicago, said.
To hear more about this study and whether Medicare can afford this level of care, click here to hear our interview with Dr. Rein.
The study was funded by an unrestricted research grant from Gilead Sciences. Dr. Rein and his co-authors reported having no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – The graying of America will add at least 1 million more patients with chronic hepatitis C virus infection into the Medicare system between 2010 and 2024.
“If all of these patients were treated with an all-oral high efficacy regimen, however, it could save 33,922 lives and increase the number of years lived by an estimated 200,000 years,” study author David Rein, Ph.D., reported at the annual meeting of the American Association for the Study of Liver Diseases.
The study is the first to estimate the number of chronically infected HCV patients in the Medicare system, and the data suggest that as of 2009, there were already 407,786 such patients in the system.
Most patients (68%) were diagnosed with chronic disease only, 24% were diagnosed with some form of end-stage liver disease, and 8% died in 2009.
Co-morbities were commona among the cohort, with 64% having at least one other chronic condition, such as diabetes, renal disease, alcohol/substance abuse, or mental health conditions.
The annual cost of their HCV treatment in 2009 was $2.7 billion in 2014 dollars.
Over the next 10 years, that number could rise to $6.7 billion if these patients aren’t treated, Dr. Rein, a principal research scientist in the Atlanta office of NORC at the University of Chicago, said.
To hear more about this study and whether Medicare can afford this level of care, click here to hear our interview with Dr. Rein.
The study was funded by an unrestricted research grant from Gilead Sciences. Dr. Rein and his co-authors reported having no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE LIVER MEETING 2014
Sofosbuvir/ledipasvir effective for relapsed hep C patients
Patients with chronic hepatitis C genotype 1 infection who relapse after sofosbuvir plus ribavirin may be successfully retreated with sofosbuvir plus ledipasvir, a new study demonstrated.
During the study, investigators approached patients treated with sofosbuvir plus ribavirin for 24 weeks in the National Institute of Allergy and Infectious Diseases (NIAID) SPARE study who relapsed after treatment. The participants, mostly black men with an interleukin-28B non-CC genotype, were offered retreatment with sofosbuvir plus ledipasvir for 12 weeks in the ongoing, phase IIa, open-label NIAID SYNERGY study. Fourteen enrolled. The medication was a single daily tablet of 400 mg of sofosbuvir and 90 mg of ledipasvir.
All patients achieved sustained viral response to treatment (SVR12), including seven who had advanced liver disease and one with a detectable NS5B S282T mutation, according to work directed by Dr. Anu Osinusi of Gilead Sciences, manufacturer of the combination drug. Most adverse events, including loose stool, constipation, headache, myalgia, nasal congestion, and pruritic rash, were mild.
The research suggests that patients who have viral relapse after sofosbuvir/ribavirin “can be successfully retreated with sofosbuvir/ledipasvir for 12 weeks,” the authors wrote. “The low incidence of adverse events, low pill burden, short treatment duration, and high efficacy demonstrated in this group and other populations make this drug combination attractive in a real-world setting.”
The study was supported by NIAID, the National Institutes of Health, the National Cancer Institute, and Gilead Sciences (manufacturer of sofosbuvir/ledipasvir). Two authors are employed by Gilead; two others disclosed other company-sponsored findings during the study period.
Patients with chronic hepatitis C genotype 1 infection who relapse after sofosbuvir plus ribavirin may be successfully retreated with sofosbuvir plus ledipasvir, a new study demonstrated.
During the study, investigators approached patients treated with sofosbuvir plus ribavirin for 24 weeks in the National Institute of Allergy and Infectious Diseases (NIAID) SPARE study who relapsed after treatment. The participants, mostly black men with an interleukin-28B non-CC genotype, were offered retreatment with sofosbuvir plus ledipasvir for 12 weeks in the ongoing, phase IIa, open-label NIAID SYNERGY study. Fourteen enrolled. The medication was a single daily tablet of 400 mg of sofosbuvir and 90 mg of ledipasvir.
All patients achieved sustained viral response to treatment (SVR12), including seven who had advanced liver disease and one with a detectable NS5B S282T mutation, according to work directed by Dr. Anu Osinusi of Gilead Sciences, manufacturer of the combination drug. Most adverse events, including loose stool, constipation, headache, myalgia, nasal congestion, and pruritic rash, were mild.
The research suggests that patients who have viral relapse after sofosbuvir/ribavirin “can be successfully retreated with sofosbuvir/ledipasvir for 12 weeks,” the authors wrote. “The low incidence of adverse events, low pill burden, short treatment duration, and high efficacy demonstrated in this group and other populations make this drug combination attractive in a real-world setting.”
The study was supported by NIAID, the National Institutes of Health, the National Cancer Institute, and Gilead Sciences (manufacturer of sofosbuvir/ledipasvir). Two authors are employed by Gilead; two others disclosed other company-sponsored findings during the study period.
Patients with chronic hepatitis C genotype 1 infection who relapse after sofosbuvir plus ribavirin may be successfully retreated with sofosbuvir plus ledipasvir, a new study demonstrated.
During the study, investigators approached patients treated with sofosbuvir plus ribavirin for 24 weeks in the National Institute of Allergy and Infectious Diseases (NIAID) SPARE study who relapsed after treatment. The participants, mostly black men with an interleukin-28B non-CC genotype, were offered retreatment with sofosbuvir plus ledipasvir for 12 weeks in the ongoing, phase IIa, open-label NIAID SYNERGY study. Fourteen enrolled. The medication was a single daily tablet of 400 mg of sofosbuvir and 90 mg of ledipasvir.
All patients achieved sustained viral response to treatment (SVR12), including seven who had advanced liver disease and one with a detectable NS5B S282T mutation, according to work directed by Dr. Anu Osinusi of Gilead Sciences, manufacturer of the combination drug. Most adverse events, including loose stool, constipation, headache, myalgia, nasal congestion, and pruritic rash, were mild.
The research suggests that patients who have viral relapse after sofosbuvir/ribavirin “can be successfully retreated with sofosbuvir/ledipasvir for 12 weeks,” the authors wrote. “The low incidence of adverse events, low pill burden, short treatment duration, and high efficacy demonstrated in this group and other populations make this drug combination attractive in a real-world setting.”
The study was supported by NIAID, the National Institutes of Health, the National Cancer Institute, and Gilead Sciences (manufacturer of sofosbuvir/ledipasvir). Two authors are employed by Gilead; two others disclosed other company-sponsored findings during the study period.
Key clinical point: Patients with chronic hepatitis C genotype 1 infection who relapse after sofosbuvir/ribavirin treatment may be successfully retreated with sofosbuvir/ledipasvir.
Major finding: All patients achieved sustained viral response to treatment.
Data source: A phase IIa, open-label study of 14 patients given sofosbuvir plus ledipasvir for 12 weeks after relapsing from 24 weeks of treatment with sofosbuvir plus ribavirin.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the National Cancer Institute, and Gilead Sciences (manufacturer of sofosbuvir/ledipasvir). Two authors are employed by Gilead; two others disclosed other company-sponsored findings during the study period.
Women with depression more susceptible to obesity
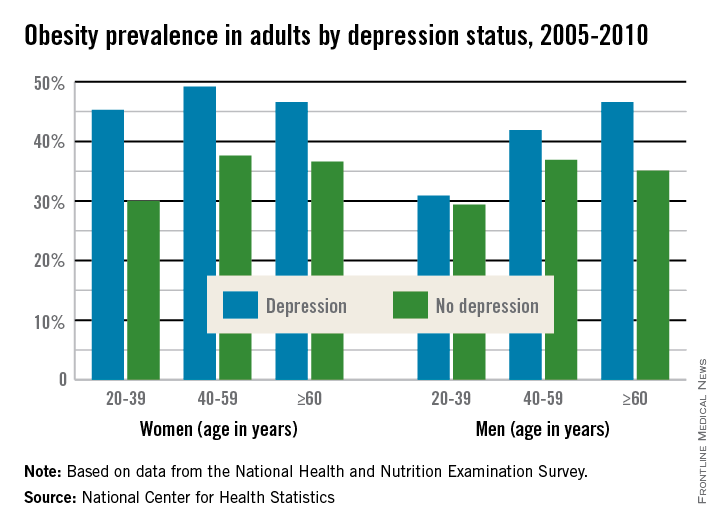
Adult women who are depressed are more likely to be obese than women who are not depressed at any age. Depressed adult men over 60 are more likely to be obese than nondepressed men, a recent report from the National Center for Health Statistics shows.
A significant statistical difference was found in obesity rates for all identified age groups of women. For women aged 20-39, 45% of those who were depressed were obese, compared with 30% of nondepressed women. Among women aged 40-59, 49% of depressed women were obese, compared with 38% of nondepressed women, and among women over 60, 47% of depressed women were obese, compared with 37% of nondepressed women.
Among depressed men, only those over 60 had a significant difference in obesity rates, at 47%, compared with 35% of nondepressed men, the NCHS found. Overall, 43% of adults with depression were obese, compared with 33% of adults who were not depressed.
The report cited no statistical differences in obesity rates by ethnicity or race except for non-Hispanic white women. Among that group, 45% of those who were depressed were obese, and 32% of those who were not depressed were obese.
In addition, the NCHS found, adults who were taking antidepressants were more likely to be obese than those who were not taking antidepressants, regardless of the severity or presence of depressive symptoms. The report cited an obesity rate of nearly 55% for adults with moderate to severe depressive symptoms who also were taking antidepressants; that rate was 16% higher than it was for people with similar symptoms but not taking antidepressants, the NCHS reported. People with mild to no depressive symptoms who were taking antidepressants had an obesity rate of 38%. That was 5% higher than it was for those not on antidepressants but with similar symptoms.
It could not be determined whether depression or obesity occurred first, the report said, because “they were both measured at the same time.”
The NCHS study used data collected from the National Health and Nutrition Examination Survey.
Adult women who are depressed are more likely to be obese than women who are not depressed at any age. Depressed adult men over 60 are more likely to be obese than nondepressed men, a recent report from the National Center for Health Statistics shows.
A significant statistical difference was found in obesity rates for all identified age groups of women. For women aged 20-39, 45% of those who were depressed were obese, compared with 30% of nondepressed women. Among women aged 40-59, 49% of depressed women were obese, compared with 38% of nondepressed women, and among women over 60, 47% of depressed women were obese, compared with 37% of nondepressed women.
Among depressed men, only those over 60 had a significant difference in obesity rates, at 47%, compared with 35% of nondepressed men, the NCHS found. Overall, 43% of adults with depression were obese, compared with 33% of adults who were not depressed.
The report cited no statistical differences in obesity rates by ethnicity or race except for non-Hispanic white women. Among that group, 45% of those who were depressed were obese, and 32% of those who were not depressed were obese.
In addition, the NCHS found, adults who were taking antidepressants were more likely to be obese than those who were not taking antidepressants, regardless of the severity or presence of depressive symptoms. The report cited an obesity rate of nearly 55% for adults with moderate to severe depressive symptoms who also were taking antidepressants; that rate was 16% higher than it was for people with similar symptoms but not taking antidepressants, the NCHS reported. People with mild to no depressive symptoms who were taking antidepressants had an obesity rate of 38%. That was 5% higher than it was for those not on antidepressants but with similar symptoms.
It could not be determined whether depression or obesity occurred first, the report said, because “they were both measured at the same time.”
The NCHS study used data collected from the National Health and Nutrition Examination Survey.
Adult women who are depressed are more likely to be obese than women who are not depressed at any age. Depressed adult men over 60 are more likely to be obese than nondepressed men, a recent report from the National Center for Health Statistics shows.
A significant statistical difference was found in obesity rates for all identified age groups of women. For women aged 20-39, 45% of those who were depressed were obese, compared with 30% of nondepressed women. Among women aged 40-59, 49% of depressed women were obese, compared with 38% of nondepressed women, and among women over 60, 47% of depressed women were obese, compared with 37% of nondepressed women.
Among depressed men, only those over 60 had a significant difference in obesity rates, at 47%, compared with 35% of nondepressed men, the NCHS found. Overall, 43% of adults with depression were obese, compared with 33% of adults who were not depressed.
The report cited no statistical differences in obesity rates by ethnicity or race except for non-Hispanic white women. Among that group, 45% of those who were depressed were obese, and 32% of those who were not depressed were obese.
In addition, the NCHS found, adults who were taking antidepressants were more likely to be obese than those who were not taking antidepressants, regardless of the severity or presence of depressive symptoms. The report cited an obesity rate of nearly 55% for adults with moderate to severe depressive symptoms who also were taking antidepressants; that rate was 16% higher than it was for people with similar symptoms but not taking antidepressants, the NCHS reported. People with mild to no depressive symptoms who were taking antidepressants had an obesity rate of 38%. That was 5% higher than it was for those not on antidepressants but with similar symptoms.
It could not be determined whether depression or obesity occurred first, the report said, because “they were both measured at the same time.”
The NCHS study used data collected from the National Health and Nutrition Examination Survey.
FDA approves hepatitis C combination pill
The first combination pill to treat chronic hepatitis C virus genotype 1 infection was approved by the Food and Drug Administration Oct. 10.
The once-daily tablet under the trade name Harvoni combines 400 mg of the already-approved hepatitis C drug sofosbuvir (Sovaldi) with 90 mg of a new drug, ledipasvir, and is the first approved regimen that does not require coadministration of interferon or ribaviron to treat hepatitis C in adults, according to the FDA.
In three clinical trials with a total of 1,518 patients, 94%-99% reached a sustained virologic response (no virus detected in the blood) at least 12 weeks (SVR 12) after finishing treatment, according to an FDA statement. The agency gave the drug fast-track consideration and approval under its breakthrough therapy designation.
“With the development and approval of new treatments for hepatitis C virus, we are changing the treatment paradigm for Americans living with the disease,” Dr. Edward Cox, director of the FDA Office of Antimicrobial Products said in a statement. “Until last year, the only available treatments for hepatitis C virus required administration with interferon and ribavirin. Now, patients and health care professionals have multiple treatment options, including a combination pill to help simplify treatment regimens,.”
The FDA approved sofosbuvir and another new drug to treat hepatitis C, simeprevir (Olysio), in late 2013. Since then, the high costs of these new treatments have generated controversy. Even before Harvoni’s approval, one analysis estimated that the new treatments could increase 2015 spending in Medicare’s Part D program by up to $5.8 billion.
Gilead, which markets sofosbuvir and the new combination pill, said in a statement that a typical 12-week course of Harvoni will cost $94,500, compared with Sovaldi’s price tag of $84,000 for 12 weeks, the New York Times reported. Some patients may be successfully treated after 8 weeks.
In the first of Harvoni’s three pivotal trials, cure rates were 94% after 8 weeks of treatment and 96% after 12 weeks of treatment in previously untreated patients with chronic hepatitis C infection. In the second trial including patients with and without cirrhosis, 99% were cured after 12 weeks of treatment. In the third trial of treatment-experience patients with or without cirrhosis, 94% were cured after 12 weeks of treatment and 99% after 24 weeks of treatment. Adding ribavirin did not improve response rates in any of the trials.
The FDA recommended a dosage duration of 12 weeks in patients with chronic hepatitis who have not previously been treated or who failed treatment but don’t have cirrhosis, or 24 weeks in patients with cirrhosis who failed previous treatment. Limiting treatment to 8 weeks may be considered in treatment-naive patients without cirrhosis who have pretreatment hepatitis C virus RNA levels below 6 million IU/mL.
The most common adverse reactions reported with Harvoni use included fatigue in 13%-18%, headache in 11%-17%, nausea in 6%-9%, diarrhea in 3%-7%, and insomnia in 3%-6%.
Both drugs in Harvoni interfere with enzymes needed by hepatitis C virus to multiply. Ledipasvir is a hepatitis C virus NS5A inhibitor, and sofosbuvir is a hepatitis C virus nucleotide analog NS5B polymerase inhibitor.
On Twitter @sherryboschert
The first combination pill to treat chronic hepatitis C virus genotype 1 infection was approved by the Food and Drug Administration Oct. 10.
The once-daily tablet under the trade name Harvoni combines 400 mg of the already-approved hepatitis C drug sofosbuvir (Sovaldi) with 90 mg of a new drug, ledipasvir, and is the first approved regimen that does not require coadministration of interferon or ribaviron to treat hepatitis C in adults, according to the FDA.
In three clinical trials with a total of 1,518 patients, 94%-99% reached a sustained virologic response (no virus detected in the blood) at least 12 weeks (SVR 12) after finishing treatment, according to an FDA statement. The agency gave the drug fast-track consideration and approval under its breakthrough therapy designation.
“With the development and approval of new treatments for hepatitis C virus, we are changing the treatment paradigm for Americans living with the disease,” Dr. Edward Cox, director of the FDA Office of Antimicrobial Products said in a statement. “Until last year, the only available treatments for hepatitis C virus required administration with interferon and ribavirin. Now, patients and health care professionals have multiple treatment options, including a combination pill to help simplify treatment regimens,.”
The FDA approved sofosbuvir and another new drug to treat hepatitis C, simeprevir (Olysio), in late 2013. Since then, the high costs of these new treatments have generated controversy. Even before Harvoni’s approval, one analysis estimated that the new treatments could increase 2015 spending in Medicare’s Part D program by up to $5.8 billion.
Gilead, which markets sofosbuvir and the new combination pill, said in a statement that a typical 12-week course of Harvoni will cost $94,500, compared with Sovaldi’s price tag of $84,000 for 12 weeks, the New York Times reported. Some patients may be successfully treated after 8 weeks.
In the first of Harvoni’s three pivotal trials, cure rates were 94% after 8 weeks of treatment and 96% after 12 weeks of treatment in previously untreated patients with chronic hepatitis C infection. In the second trial including patients with and without cirrhosis, 99% were cured after 12 weeks of treatment. In the third trial of treatment-experience patients with or without cirrhosis, 94% were cured after 12 weeks of treatment and 99% after 24 weeks of treatment. Adding ribavirin did not improve response rates in any of the trials.
The FDA recommended a dosage duration of 12 weeks in patients with chronic hepatitis who have not previously been treated or who failed treatment but don’t have cirrhosis, or 24 weeks in patients with cirrhosis who failed previous treatment. Limiting treatment to 8 weeks may be considered in treatment-naive patients without cirrhosis who have pretreatment hepatitis C virus RNA levels below 6 million IU/mL.
The most common adverse reactions reported with Harvoni use included fatigue in 13%-18%, headache in 11%-17%, nausea in 6%-9%, diarrhea in 3%-7%, and insomnia in 3%-6%.
Both drugs in Harvoni interfere with enzymes needed by hepatitis C virus to multiply. Ledipasvir is a hepatitis C virus NS5A inhibitor, and sofosbuvir is a hepatitis C virus nucleotide analog NS5B polymerase inhibitor.
On Twitter @sherryboschert
The first combination pill to treat chronic hepatitis C virus genotype 1 infection was approved by the Food and Drug Administration Oct. 10.
The once-daily tablet under the trade name Harvoni combines 400 mg of the already-approved hepatitis C drug sofosbuvir (Sovaldi) with 90 mg of a new drug, ledipasvir, and is the first approved regimen that does not require coadministration of interferon or ribaviron to treat hepatitis C in adults, according to the FDA.
In three clinical trials with a total of 1,518 patients, 94%-99% reached a sustained virologic response (no virus detected in the blood) at least 12 weeks (SVR 12) after finishing treatment, according to an FDA statement. The agency gave the drug fast-track consideration and approval under its breakthrough therapy designation.
“With the development and approval of new treatments for hepatitis C virus, we are changing the treatment paradigm for Americans living with the disease,” Dr. Edward Cox, director of the FDA Office of Antimicrobial Products said in a statement. “Until last year, the only available treatments for hepatitis C virus required administration with interferon and ribavirin. Now, patients and health care professionals have multiple treatment options, including a combination pill to help simplify treatment regimens,.”
The FDA approved sofosbuvir and another new drug to treat hepatitis C, simeprevir (Olysio), in late 2013. Since then, the high costs of these new treatments have generated controversy. Even before Harvoni’s approval, one analysis estimated that the new treatments could increase 2015 spending in Medicare’s Part D program by up to $5.8 billion.
Gilead, which markets sofosbuvir and the new combination pill, said in a statement that a typical 12-week course of Harvoni will cost $94,500, compared with Sovaldi’s price tag of $84,000 for 12 weeks, the New York Times reported. Some patients may be successfully treated after 8 weeks.
In the first of Harvoni’s three pivotal trials, cure rates were 94% after 8 weeks of treatment and 96% after 12 weeks of treatment in previously untreated patients with chronic hepatitis C infection. In the second trial including patients with and without cirrhosis, 99% were cured after 12 weeks of treatment. In the third trial of treatment-experience patients with or without cirrhosis, 94% were cured after 12 weeks of treatment and 99% after 24 weeks of treatment. Adding ribavirin did not improve response rates in any of the trials.
The FDA recommended a dosage duration of 12 weeks in patients with chronic hepatitis who have not previously been treated or who failed treatment but don’t have cirrhosis, or 24 weeks in patients with cirrhosis who failed previous treatment. Limiting treatment to 8 weeks may be considered in treatment-naive patients without cirrhosis who have pretreatment hepatitis C virus RNA levels below 6 million IU/mL.
The most common adverse reactions reported with Harvoni use included fatigue in 13%-18%, headache in 11%-17%, nausea in 6%-9%, diarrhea in 3%-7%, and insomnia in 3%-6%.
Both drugs in Harvoni interfere with enzymes needed by hepatitis C virus to multiply. Ledipasvir is a hepatitis C virus NS5A inhibitor, and sofosbuvir is a hepatitis C virus nucleotide analog NS5B polymerase inhibitor.
On Twitter @sherryboschert
TURQUOISE regimen active against HCV-HIV coinfection
WASHINGTON – Early studies show that a new, combined antiviral regimen seems to suppress hepatitis C viremia while keeping HIV viremia stable in coinfected patients.
Dr. Joseph Eron presented phase II data from 63 patients who were enrolled in TURQUOISE-I. A total of 94% (59/63) of patients achieved a sustained virologic response (SVR) 12 weeks after completing 12 weeks of therapy, and 61 of 63 patients achieved an SVR 4 weeks after completing 12 or 24 weeks of therapy, Dr. Eron reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The regimen included ABT-450 (an NS3-4A protease inhibitor that was identified by AbbVie and Enanta), which was combined with ritonavir and ombitasvir (another AbbVie drug, also known as ABT-267) into a single tablet, plus dasabuvir (ABT-333) and ribavirin. Ombitasvir is an NS5A inhibitor and dasabuvir is a non-nucleoside NS5B polymerase inhibitor.
To be included in TURQUOISE-I, patients have to be aged 18-70 years; be treatment naive or have previous pegylated interferon/ribavirin treatment experience; have hepatitis C virus (HCV) genotype 1 infection; have plasma HIV-1 RNA less than 40 copies/mL; and be taking a stable, qualifying HIV-1 antiretroviral therapy regimen, said Dr. Eron, a professor of medicine at the University of North Carolina, Chapel Hill.
Patients with cirrhosis are not excluded, but will not be enrolled if they have had prior therapy with direct-acting antivirals for HCV, or any current or past clinical evidence of liver decompensation.
All patients will be followed for 48 weeks after therapy is stopped. Thirty-one patients were given therapy for 12 weeks and then assessed for SVR at 12 weeks. Thirty-two patients were given therapy for 24 weeks and assessed 4 weeks later. Patients also were evaluated for end-of-treatment response, on-treatment virologic failure, and post-treatment HCV viral relapse.
At baseline, 59 patients in each arm were male, and 16 were African American. The mean age was 51 years. Six patients in each arm (19%) had cirrhosis. A high proportion in each arm (53/63 in the 12-week and 49/63 in the 24-week arm) had the interleukin-28B genotype, which is known to indicate a much harder-to-treat disease, Dr. Eron said.
At the end of treatment, only one patient in each arm was not responsive. Four weeks post therapy, 29 of 31 patients in the 12-week arm and 31 of 32 patients in the 24-week arm had an SVR.
Two patients had virologic failure. Both had been unresponsive to previous therapies, and both had the IL-28B genotype and resistance-associated variants at the time of failure.
No patient withdrew because of adverse events, but about 90% (57/63) experienced some side effect, with the most common being fatigue and insomnia. Six patients had to reduce their ribavirin dose because of a decline in hemoglobin levels, but they were still responsive to therapy. Five patients had a confirmed increase in HIV RNA higher than 40 copies/mL, but not above 200 copies/mL, said Dr. Eron. And all the patients remained suppressed on the same HIV regimen and without interrupting their HCV therapy, he said.
Soon, a cohort of patients on stable darunavir antiretroviral therapy will be enrolled and given the three-drug HCV regimen for 12 weeks. The full global TURQUOISE study will begin later in 2014, Dr. Eron said.
The study was sponsored by AbbVie. Dr. Eron received grant and research support from, and/or served as a consultant to, AbbVie, Bristol-Myers Squibb, and other companies. Other authors had numerous additional disclosures.
On Twitter @aliciaault
WASHINGTON – Early studies show that a new, combined antiviral regimen seems to suppress hepatitis C viremia while keeping HIV viremia stable in coinfected patients.
Dr. Joseph Eron presented phase II data from 63 patients who were enrolled in TURQUOISE-I. A total of 94% (59/63) of patients achieved a sustained virologic response (SVR) 12 weeks after completing 12 weeks of therapy, and 61 of 63 patients achieved an SVR 4 weeks after completing 12 or 24 weeks of therapy, Dr. Eron reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The regimen included ABT-450 (an NS3-4A protease inhibitor that was identified by AbbVie and Enanta), which was combined with ritonavir and ombitasvir (another AbbVie drug, also known as ABT-267) into a single tablet, plus dasabuvir (ABT-333) and ribavirin. Ombitasvir is an NS5A inhibitor and dasabuvir is a non-nucleoside NS5B polymerase inhibitor.
To be included in TURQUOISE-I, patients have to be aged 18-70 years; be treatment naive or have previous pegylated interferon/ribavirin treatment experience; have hepatitis C virus (HCV) genotype 1 infection; have plasma HIV-1 RNA less than 40 copies/mL; and be taking a stable, qualifying HIV-1 antiretroviral therapy regimen, said Dr. Eron, a professor of medicine at the University of North Carolina, Chapel Hill.
Patients with cirrhosis are not excluded, but will not be enrolled if they have had prior therapy with direct-acting antivirals for HCV, or any current or past clinical evidence of liver decompensation.
All patients will be followed for 48 weeks after therapy is stopped. Thirty-one patients were given therapy for 12 weeks and then assessed for SVR at 12 weeks. Thirty-two patients were given therapy for 24 weeks and assessed 4 weeks later. Patients also were evaluated for end-of-treatment response, on-treatment virologic failure, and post-treatment HCV viral relapse.
At baseline, 59 patients in each arm were male, and 16 were African American. The mean age was 51 years. Six patients in each arm (19%) had cirrhosis. A high proportion in each arm (53/63 in the 12-week and 49/63 in the 24-week arm) had the interleukin-28B genotype, which is known to indicate a much harder-to-treat disease, Dr. Eron said.
At the end of treatment, only one patient in each arm was not responsive. Four weeks post therapy, 29 of 31 patients in the 12-week arm and 31 of 32 patients in the 24-week arm had an SVR.
Two patients had virologic failure. Both had been unresponsive to previous therapies, and both had the IL-28B genotype and resistance-associated variants at the time of failure.
No patient withdrew because of adverse events, but about 90% (57/63) experienced some side effect, with the most common being fatigue and insomnia. Six patients had to reduce their ribavirin dose because of a decline in hemoglobin levels, but they were still responsive to therapy. Five patients had a confirmed increase in HIV RNA higher than 40 copies/mL, but not above 200 copies/mL, said Dr. Eron. And all the patients remained suppressed on the same HIV regimen and without interrupting their HCV therapy, he said.
Soon, a cohort of patients on stable darunavir antiretroviral therapy will be enrolled and given the three-drug HCV regimen for 12 weeks. The full global TURQUOISE study will begin later in 2014, Dr. Eron said.
The study was sponsored by AbbVie. Dr. Eron received grant and research support from, and/or served as a consultant to, AbbVie, Bristol-Myers Squibb, and other companies. Other authors had numerous additional disclosures.
On Twitter @aliciaault
WASHINGTON – Early studies show that a new, combined antiviral regimen seems to suppress hepatitis C viremia while keeping HIV viremia stable in coinfected patients.
Dr. Joseph Eron presented phase II data from 63 patients who were enrolled in TURQUOISE-I. A total of 94% (59/63) of patients achieved a sustained virologic response (SVR) 12 weeks after completing 12 weeks of therapy, and 61 of 63 patients achieved an SVR 4 weeks after completing 12 or 24 weeks of therapy, Dr. Eron reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The regimen included ABT-450 (an NS3-4A protease inhibitor that was identified by AbbVie and Enanta), which was combined with ritonavir and ombitasvir (another AbbVie drug, also known as ABT-267) into a single tablet, plus dasabuvir (ABT-333) and ribavirin. Ombitasvir is an NS5A inhibitor and dasabuvir is a non-nucleoside NS5B polymerase inhibitor.
To be included in TURQUOISE-I, patients have to be aged 18-70 years; be treatment naive or have previous pegylated interferon/ribavirin treatment experience; have hepatitis C virus (HCV) genotype 1 infection; have plasma HIV-1 RNA less than 40 copies/mL; and be taking a stable, qualifying HIV-1 antiretroviral therapy regimen, said Dr. Eron, a professor of medicine at the University of North Carolina, Chapel Hill.
Patients with cirrhosis are not excluded, but will not be enrolled if they have had prior therapy with direct-acting antivirals for HCV, or any current or past clinical evidence of liver decompensation.
All patients will be followed for 48 weeks after therapy is stopped. Thirty-one patients were given therapy for 12 weeks and then assessed for SVR at 12 weeks. Thirty-two patients were given therapy for 24 weeks and assessed 4 weeks later. Patients also were evaluated for end-of-treatment response, on-treatment virologic failure, and post-treatment HCV viral relapse.
At baseline, 59 patients in each arm were male, and 16 were African American. The mean age was 51 years. Six patients in each arm (19%) had cirrhosis. A high proportion in each arm (53/63 in the 12-week and 49/63 in the 24-week arm) had the interleukin-28B genotype, which is known to indicate a much harder-to-treat disease, Dr. Eron said.
At the end of treatment, only one patient in each arm was not responsive. Four weeks post therapy, 29 of 31 patients in the 12-week arm and 31 of 32 patients in the 24-week arm had an SVR.
Two patients had virologic failure. Both had been unresponsive to previous therapies, and both had the IL-28B genotype and resistance-associated variants at the time of failure.
No patient withdrew because of adverse events, but about 90% (57/63) experienced some side effect, with the most common being fatigue and insomnia. Six patients had to reduce their ribavirin dose because of a decline in hemoglobin levels, but they were still responsive to therapy. Five patients had a confirmed increase in HIV RNA higher than 40 copies/mL, but not above 200 copies/mL, said Dr. Eron. And all the patients remained suppressed on the same HIV regimen and without interrupting their HCV therapy, he said.
Soon, a cohort of patients on stable darunavir antiretroviral therapy will be enrolled and given the three-drug HCV regimen for 12 weeks. The full global TURQUOISE study will begin later in 2014, Dr. Eron said.
The study was sponsored by AbbVie. Dr. Eron received grant and research support from, and/or served as a consultant to, AbbVie, Bristol-Myers Squibb, and other companies. Other authors had numerous additional disclosures.
On Twitter @aliciaault
AT ICAAC 2014
Key clinical point: A new therapy is on the horizon for HCV-HIV coinfected patients that achieves high virologic response with few severe side effects.
Major finding: A three-drug regimen that includes ABT-450 and ombitasvir in one pill, plus dasabuvir and ribavirin, achieved high SVR in patients coinfected with HCV GT1 and HIV.
Data source: A 63-patient, two-arm, open-label prospective study.
Disclosures: The study was sponsored by AbbVie. Dr. Eron received grant and research support from, and/or served as a consultant to, AbbVie, Bristol-Myers Squibb, and other companies. Other authors had numerous additional disclosures.
HCV infection raises risk of death after kidney transplant
SAN FRANCISCO – Infection with hepatitis C virus is a risk factor for poor outcomes after kidney transplantation, but infection with human immunodeficiency virus is not, finds a cohort study reported at the 2014 World Transplant Congress.
"Centers should be more selective in transplanting HCV-positive kidney transplant candidates. But controlled HIV infection should no longer be perceived as a barrier to kidney transplantation," said first author Dr. Deirdre L. Sawinski of the University of Pennsylvania in Philadelphia.
Further, control of hepatitis C with treatment should be a priority in hopes of improving outcomes, she added.
Researchers studied 111,990 patients from the United Network for Organ Sharing (UNOS) database who had a known serostatus and underwent kidney transplantation in 1996 or later when highly active antiretroviral therapy became widespread. Overall, 4.6% were infected with HCV alone, 0.4% were infected with HIV alone, and 0.1% were coinfected.
Multivariate analyses adjusted for a variety of donor and recipient characteristics, and included a variable for transplant after 2001. This time point "reflects both the year in which more than half of patients were discharged on tacrolimus maintenance therapy as well as the year in which interferon therapy was approved for treatment for hepatitis C," Dr. Sawinski said.
HCV-infected patients and especially HCV and HIV-coinfected patients had significantly higher risks of death (hazard ratio, 1.52 and 3.83, respectively) and of graft loss (HR, 1.48 and 3.40, respectively), compared with uninfected patients. In contrast, patients infected with HIV alone were not at higher risk of death or graft loss.
"The main cause of death for the reference [uninfected] group and the hep C–positive patients was listed as cardiovascular disease, whereas the HIV-positive and coinfected patients most often had infection as their causes of death," Dr. Sawinski said. "However, you have to take that with a grain of salt as 40% of patients across all four groups had missing data [regarding] cause of death."
The risk persisted even after researchers corrected for the impact of antibody-depleting therapy during transplantation on graft survival. However, the UNOS database does not provide information about treatment with interferon before and after direct-acting antiretroviral drugs became available. Additionally, Dr. Sawinski noted, "the UNOS data set does not identify which patients are actually viremic vs. antibody positive." Data are similarly nonspecific for deceased donors but are detailed (antibody positive vs. viremic) for living donors.
A secondary analysis considered a cohort of 180,177 patients with unknown serostatus. In this analysis, the risks of death and graft loss were elevated among patients with dual unknown status relative to those known to be dually uninfected (HR, 1.06 and 1.02), according to data reported at the 2014 World Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. However, these small elevations of risk are "probably not clinically meaningful," commented Dr. Sawinski, who disclosed no relevant conflicts of interest. Risks were not significantly elevated for HCV-positive patients vs. HCV-positive, HIV-unknown patients, or for HIV-positive patients vs. HIV-positive, HCV-unknown patients.
Additionally, main study findings were essentially the same when the cohort with known serostatus and the cohort with an unknown serostatus were combined and the researchers assumed the unknown status patients were uninfected.
This study was selected for a plenary session because it provides information about coinfected patients. Yet, reporting of hepatitis C status was missing in quite a number of patients. Hence, they had to be excluded from this study, which was retrospective in nature. In spite of this, the results do make one think hard about transplanting a coinfected patient. This could all change with the new hepatitis-C therapies, and make outcomes better.
Dr. Roslyn B. Mannon was the session cochair at the meeting and is a professor of medicine and surgery and director of research at the Comprehensive Transplant Institute, University of Alabama, Birmingham. She made her remarks in an interview after the meeting. She has no financial conflicts.
This study was selected for a plenary session because it provides information about coinfected patients. Yet, reporting of hepatitis C status was missing in quite a number of patients. Hence, they had to be excluded from this study, which was retrospective in nature. In spite of this, the results do make one think hard about transplanting a coinfected patient. This could all change with the new hepatitis-C therapies, and make outcomes better.
Dr. Roslyn B. Mannon was the session cochair at the meeting and is a professor of medicine and surgery and director of research at the Comprehensive Transplant Institute, University of Alabama, Birmingham. She made her remarks in an interview after the meeting. She has no financial conflicts.
This study was selected for a plenary session because it provides information about coinfected patients. Yet, reporting of hepatitis C status was missing in quite a number of patients. Hence, they had to be excluded from this study, which was retrospective in nature. In spite of this, the results do make one think hard about transplanting a coinfected patient. This could all change with the new hepatitis-C therapies, and make outcomes better.
Dr. Roslyn B. Mannon was the session cochair at the meeting and is a professor of medicine and surgery and director of research at the Comprehensive Transplant Institute, University of Alabama, Birmingham. She made her remarks in an interview after the meeting. She has no financial conflicts.
SAN FRANCISCO – Infection with hepatitis C virus is a risk factor for poor outcomes after kidney transplantation, but infection with human immunodeficiency virus is not, finds a cohort study reported at the 2014 World Transplant Congress.
"Centers should be more selective in transplanting HCV-positive kidney transplant candidates. But controlled HIV infection should no longer be perceived as a barrier to kidney transplantation," said first author Dr. Deirdre L. Sawinski of the University of Pennsylvania in Philadelphia.
Further, control of hepatitis C with treatment should be a priority in hopes of improving outcomes, she added.
Researchers studied 111,990 patients from the United Network for Organ Sharing (UNOS) database who had a known serostatus and underwent kidney transplantation in 1996 or later when highly active antiretroviral therapy became widespread. Overall, 4.6% were infected with HCV alone, 0.4% were infected with HIV alone, and 0.1% were coinfected.
Multivariate analyses adjusted for a variety of donor and recipient characteristics, and included a variable for transplant after 2001. This time point "reflects both the year in which more than half of patients were discharged on tacrolimus maintenance therapy as well as the year in which interferon therapy was approved for treatment for hepatitis C," Dr. Sawinski said.
HCV-infected patients and especially HCV and HIV-coinfected patients had significantly higher risks of death (hazard ratio, 1.52 and 3.83, respectively) and of graft loss (HR, 1.48 and 3.40, respectively), compared with uninfected patients. In contrast, patients infected with HIV alone were not at higher risk of death or graft loss.
"The main cause of death for the reference [uninfected] group and the hep C–positive patients was listed as cardiovascular disease, whereas the HIV-positive and coinfected patients most often had infection as their causes of death," Dr. Sawinski said. "However, you have to take that with a grain of salt as 40% of patients across all four groups had missing data [regarding] cause of death."
The risk persisted even after researchers corrected for the impact of antibody-depleting therapy during transplantation on graft survival. However, the UNOS database does not provide information about treatment with interferon before and after direct-acting antiretroviral drugs became available. Additionally, Dr. Sawinski noted, "the UNOS data set does not identify which patients are actually viremic vs. antibody positive." Data are similarly nonspecific for deceased donors but are detailed (antibody positive vs. viremic) for living donors.
A secondary analysis considered a cohort of 180,177 patients with unknown serostatus. In this analysis, the risks of death and graft loss were elevated among patients with dual unknown status relative to those known to be dually uninfected (HR, 1.06 and 1.02), according to data reported at the 2014 World Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. However, these small elevations of risk are "probably not clinically meaningful," commented Dr. Sawinski, who disclosed no relevant conflicts of interest. Risks were not significantly elevated for HCV-positive patients vs. HCV-positive, HIV-unknown patients, or for HIV-positive patients vs. HIV-positive, HCV-unknown patients.
Additionally, main study findings were essentially the same when the cohort with known serostatus and the cohort with an unknown serostatus were combined and the researchers assumed the unknown status patients were uninfected.
SAN FRANCISCO – Infection with hepatitis C virus is a risk factor for poor outcomes after kidney transplantation, but infection with human immunodeficiency virus is not, finds a cohort study reported at the 2014 World Transplant Congress.
"Centers should be more selective in transplanting HCV-positive kidney transplant candidates. But controlled HIV infection should no longer be perceived as a barrier to kidney transplantation," said first author Dr. Deirdre L. Sawinski of the University of Pennsylvania in Philadelphia.
Further, control of hepatitis C with treatment should be a priority in hopes of improving outcomes, she added.
Researchers studied 111,990 patients from the United Network for Organ Sharing (UNOS) database who had a known serostatus and underwent kidney transplantation in 1996 or later when highly active antiretroviral therapy became widespread. Overall, 4.6% were infected with HCV alone, 0.4% were infected with HIV alone, and 0.1% were coinfected.
Multivariate analyses adjusted for a variety of donor and recipient characteristics, and included a variable for transplant after 2001. This time point "reflects both the year in which more than half of patients were discharged on tacrolimus maintenance therapy as well as the year in which interferon therapy was approved for treatment for hepatitis C," Dr. Sawinski said.
HCV-infected patients and especially HCV and HIV-coinfected patients had significantly higher risks of death (hazard ratio, 1.52 and 3.83, respectively) and of graft loss (HR, 1.48 and 3.40, respectively), compared with uninfected patients. In contrast, patients infected with HIV alone were not at higher risk of death or graft loss.
"The main cause of death for the reference [uninfected] group and the hep C–positive patients was listed as cardiovascular disease, whereas the HIV-positive and coinfected patients most often had infection as their causes of death," Dr. Sawinski said. "However, you have to take that with a grain of salt as 40% of patients across all four groups had missing data [regarding] cause of death."
The risk persisted even after researchers corrected for the impact of antibody-depleting therapy during transplantation on graft survival. However, the UNOS database does not provide information about treatment with interferon before and after direct-acting antiretroviral drugs became available. Additionally, Dr. Sawinski noted, "the UNOS data set does not identify which patients are actually viremic vs. antibody positive." Data are similarly nonspecific for deceased donors but are detailed (antibody positive vs. viremic) for living donors.
A secondary analysis considered a cohort of 180,177 patients with unknown serostatus. In this analysis, the risks of death and graft loss were elevated among patients with dual unknown status relative to those known to be dually uninfected (HR, 1.06 and 1.02), according to data reported at the 2014 World Transplant Congress, which was sponsored by the American Society of Transplant Surgeons. However, these small elevations of risk are "probably not clinically meaningful," commented Dr. Sawinski, who disclosed no relevant conflicts of interest. Risks were not significantly elevated for HCV-positive patients vs. HCV-positive, HIV-unknown patients, or for HIV-positive patients vs. HIV-positive, HCV-unknown patients.
Additionally, main study findings were essentially the same when the cohort with known serostatus and the cohort with an unknown serostatus were combined and the researchers assumed the unknown status patients were uninfected.
2014 WORLD TRANSPLANT CONGRESS
Key clinical point: Centers should be selective when kidney transplant candidates are HCV positive, but controlled HIV infection should no longer be perceived as a barrier.
Major finding: Relative to uninfected peers, HCV-infected patients and HIV/HCV-coinfected patients had higher risks of death (HR, 1.52 and 3.83) and graft loss (HR, 1.48 and 3.40), but HIV-infected patients did not.
Data source: Cohort study of 111,990 patients from the UNOS database who underwent kidney transplant.
Disclosures: Dr. Sawinski disclosed no relevant conflicts of interest.