User login
National Institute of Mental Health (NIMH): New Clinical Drug Evaluation Unit (NCDEU)
Psychiatrists get low marks in assessing adherence
HOLLYWOOD, FLA. – Many American psychiatrists have a bias against long-acting injectable antipsychotic medications, which constitutes a major barrier to broader use of this often advantageous form of therapy, investigators asserted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
"There’s a lot of anti-shot sentiment in the U.S., particularly on the part of doctors. They think that the therapeutic alliance will be negatively impacted by talking about an injection. Many of my patients have never been told that there is such a thing as injectable medicines," according to Dawn I. Velligan, Ph.D., professor of psychiatry and director of the division of schizophrenia and related disorders at the University of Texas Health Science Center at San Antonio.
She has participated in studies involving structured observation of psychiatrist-patient encounters that she found revealing – and troubling.
"When doctors do offer a long-acting injectable agent, they’re so uncomfortable about doing it that they stutter and are just clearly uncomfortable. You can see it in linguistic fluency measures. Not only that, but they lead with the modality, rather than the potential benefit. They’ll say, ‘You don’t want a shot, do you?’ Well, of course not. What kind of a question is that? A doctor never starts out by saying, ‘I have these horse pills, and they’re really hard to take.’ They start out by explaining what this medication is going to do for your recovery, and then they mention that they’re horse pills and are hard to swallow," Dr. Velligan said.
Roughly 50% of patients are poorly adherent to oral antipsychotic therapy. Nonadherence results in higher rates of relapse, hospitalization, and disability. When asked, most psychiatrists will say they consider long-acting injectables (LAIs) the way to go when patients are nonadherent. Unfortunately, however, they are not good at all at identifying nonadherent patients.
"They don’t have any idea. I think doctors prescribe antipsychotic medication in an atmosphere of unclear adherence. So what they’re doing is raising the dose and raising the dose. I think they don’t know how much to prescribe," Dr. Velligan declared.
She was on a National Institute of Mental Health–sponsored expert panel that concluded all of the standard methods of assessing adherence are error prone (J. Psychosom. Res. 2010;69:591-9). For example, studies show that patient self-report vastly overestimates adherence and that clinicians are poor at judging the level of adherence. Plasma and urine analysis provide good quality information only about the last 4-5 days, not the past 30-60 days. "Smart" pill containers with electronic monitoring technology don’t work if the patient opens the bottle and forgets to put the top back on. Pill counts work only if the patient remembers to bring in his bottles. And pharmacy records, too, are unreliable in assessing adherence.
"I have people who are 100% adherent, but they haven’t picked up their pills from the pharmacy in 7 months because they had a bunch of pills left over from all the times that they weren’t adherent before," she observed.
An LAI offers numerous potential advantages for the prescriber. For one, it eliminates guesswork about compliance status.
"If patients with a prescription for oral therapy come in and they’re not looking well, you don’t know how much of their medication they’ve been taking. It’s an enormous crap shoot, yet we do it every single day," Dr. Velligan charged.
LAI therapy also alerts the psychiatrist when nonadherence starts, and it’s invaluable in disentangling efficacy from problem adherence.
"If I’m giving you an injection and you’re not doing well and you’ve shown up every time for your shots, this medicine is not working so well. It’s not the right antipsychotic for you," she explained.
Together with Dr. Martha Sajatovic, a psychiatrist at Case Western Reserve University in Cleveland, Dr. Velligan has developed what they call the NOB (individuals Not receiving Optimum Benefit from antipsychotic medication) checklist. It’s intended for implementation by overburdened clinicians in community mental health centers. A "yes" answer to any of the five simple questions (see below) should alert the provider that LAIs might in this case help in clinical decision making and improve patient outcomes.
In a separate presentation, Dr. Steven G. Potkin presented a detailed analysis of 69 recorded psychiatrist-patient community mental health center office visits involving 22 patients being treated with oral antipsychotics and 38 on LAIs. Prescribers spent merely a mean 2% of the visit time discussing adherence with their patients. Moreover, psychiatrists made decisions about antipsychotic treatment without patient or caregiver input in two-thirds of the encounters.
"Collectively, these findings suggest that there is an opportunity for prescribers to increase active patient engagement, address resistance about LAIs, and provide better LAI-relevant information for more individualized options and approaches to treating patients with schizophrenia," concluded Dr. Potkin, professor of psychiatry and director of the brain imaging center at the University of California, Irvine.
Dr. Potkin’s study was funded by Otsuka American Pharmaceutical and H. Lundbeck. He reported receiving research support from roughly 20 pharmaceutical companies. Dr. Velligan reported having no financial conflicts.
The NOB Checklist
The NOB checklist is aimed at helping clinicians working in community health centers to quickly determine whether patients are adherent.
Here is a listing of the five simple questions:
1) Based upon the patient’s report, caregiver report, or your prescribing record, has the patient missed doses such that 30% or more of the medication has been missed?
2) Is the patient currently on more than one antipsychotic (not during a switch)?
3) Has the patient been on more than two antipsychotics in the past 12 months?
4) Has the patient been hospitalized or had a crisis visit in the past 12 months?
5) Is the patient not satisfied with the current level of symptom control?
A "yes" answer to any of these questions warrants offering a long-acting injectable antipsychotic agent to this patient.
Source: Dr. Velligan
HOLLYWOOD, FLA. – Many American psychiatrists have a bias against long-acting injectable antipsychotic medications, which constitutes a major barrier to broader use of this often advantageous form of therapy, investigators asserted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
"There’s a lot of anti-shot sentiment in the U.S., particularly on the part of doctors. They think that the therapeutic alliance will be negatively impacted by talking about an injection. Many of my patients have never been told that there is such a thing as injectable medicines," according to Dawn I. Velligan, Ph.D., professor of psychiatry and director of the division of schizophrenia and related disorders at the University of Texas Health Science Center at San Antonio.
She has participated in studies involving structured observation of psychiatrist-patient encounters that she found revealing – and troubling.
"When doctors do offer a long-acting injectable agent, they’re so uncomfortable about doing it that they stutter and are just clearly uncomfortable. You can see it in linguistic fluency measures. Not only that, but they lead with the modality, rather than the potential benefit. They’ll say, ‘You don’t want a shot, do you?’ Well, of course not. What kind of a question is that? A doctor never starts out by saying, ‘I have these horse pills, and they’re really hard to take.’ They start out by explaining what this medication is going to do for your recovery, and then they mention that they’re horse pills and are hard to swallow," Dr. Velligan said.
Roughly 50% of patients are poorly adherent to oral antipsychotic therapy. Nonadherence results in higher rates of relapse, hospitalization, and disability. When asked, most psychiatrists will say they consider long-acting injectables (LAIs) the way to go when patients are nonadherent. Unfortunately, however, they are not good at all at identifying nonadherent patients.
"They don’t have any idea. I think doctors prescribe antipsychotic medication in an atmosphere of unclear adherence. So what they’re doing is raising the dose and raising the dose. I think they don’t know how much to prescribe," Dr. Velligan declared.
She was on a National Institute of Mental Health–sponsored expert panel that concluded all of the standard methods of assessing adherence are error prone (J. Psychosom. Res. 2010;69:591-9). For example, studies show that patient self-report vastly overestimates adherence and that clinicians are poor at judging the level of adherence. Plasma and urine analysis provide good quality information only about the last 4-5 days, not the past 30-60 days. "Smart" pill containers with electronic monitoring technology don’t work if the patient opens the bottle and forgets to put the top back on. Pill counts work only if the patient remembers to bring in his bottles. And pharmacy records, too, are unreliable in assessing adherence.
"I have people who are 100% adherent, but they haven’t picked up their pills from the pharmacy in 7 months because they had a bunch of pills left over from all the times that they weren’t adherent before," she observed.
An LAI offers numerous potential advantages for the prescriber. For one, it eliminates guesswork about compliance status.
"If patients with a prescription for oral therapy come in and they’re not looking well, you don’t know how much of their medication they’ve been taking. It’s an enormous crap shoot, yet we do it every single day," Dr. Velligan charged.
LAI therapy also alerts the psychiatrist when nonadherence starts, and it’s invaluable in disentangling efficacy from problem adherence.
"If I’m giving you an injection and you’re not doing well and you’ve shown up every time for your shots, this medicine is not working so well. It’s not the right antipsychotic for you," she explained.
Together with Dr. Martha Sajatovic, a psychiatrist at Case Western Reserve University in Cleveland, Dr. Velligan has developed what they call the NOB (individuals Not receiving Optimum Benefit from antipsychotic medication) checklist. It’s intended for implementation by overburdened clinicians in community mental health centers. A "yes" answer to any of the five simple questions (see below) should alert the provider that LAIs might in this case help in clinical decision making and improve patient outcomes.
In a separate presentation, Dr. Steven G. Potkin presented a detailed analysis of 69 recorded psychiatrist-patient community mental health center office visits involving 22 patients being treated with oral antipsychotics and 38 on LAIs. Prescribers spent merely a mean 2% of the visit time discussing adherence with their patients. Moreover, psychiatrists made decisions about antipsychotic treatment without patient or caregiver input in two-thirds of the encounters.
"Collectively, these findings suggest that there is an opportunity for prescribers to increase active patient engagement, address resistance about LAIs, and provide better LAI-relevant information for more individualized options and approaches to treating patients with schizophrenia," concluded Dr. Potkin, professor of psychiatry and director of the brain imaging center at the University of California, Irvine.
Dr. Potkin’s study was funded by Otsuka American Pharmaceutical and H. Lundbeck. He reported receiving research support from roughly 20 pharmaceutical companies. Dr. Velligan reported having no financial conflicts.
The NOB Checklist
The NOB checklist is aimed at helping clinicians working in community health centers to quickly determine whether patients are adherent.
Here is a listing of the five simple questions:
1) Based upon the patient’s report, caregiver report, or your prescribing record, has the patient missed doses such that 30% or more of the medication has been missed?
2) Is the patient currently on more than one antipsychotic (not during a switch)?
3) Has the patient been on more than two antipsychotics in the past 12 months?
4) Has the patient been hospitalized or had a crisis visit in the past 12 months?
5) Is the patient not satisfied with the current level of symptom control?
A "yes" answer to any of these questions warrants offering a long-acting injectable antipsychotic agent to this patient.
Source: Dr. Velligan
HOLLYWOOD, FLA. – Many American psychiatrists have a bias against long-acting injectable antipsychotic medications, which constitutes a major barrier to broader use of this often advantageous form of therapy, investigators asserted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
"There’s a lot of anti-shot sentiment in the U.S., particularly on the part of doctors. They think that the therapeutic alliance will be negatively impacted by talking about an injection. Many of my patients have never been told that there is such a thing as injectable medicines," according to Dawn I. Velligan, Ph.D., professor of psychiatry and director of the division of schizophrenia and related disorders at the University of Texas Health Science Center at San Antonio.
She has participated in studies involving structured observation of psychiatrist-patient encounters that she found revealing – and troubling.
"When doctors do offer a long-acting injectable agent, they’re so uncomfortable about doing it that they stutter and are just clearly uncomfortable. You can see it in linguistic fluency measures. Not only that, but they lead with the modality, rather than the potential benefit. They’ll say, ‘You don’t want a shot, do you?’ Well, of course not. What kind of a question is that? A doctor never starts out by saying, ‘I have these horse pills, and they’re really hard to take.’ They start out by explaining what this medication is going to do for your recovery, and then they mention that they’re horse pills and are hard to swallow," Dr. Velligan said.
Roughly 50% of patients are poorly adherent to oral antipsychotic therapy. Nonadherence results in higher rates of relapse, hospitalization, and disability. When asked, most psychiatrists will say they consider long-acting injectables (LAIs) the way to go when patients are nonadherent. Unfortunately, however, they are not good at all at identifying nonadherent patients.
"They don’t have any idea. I think doctors prescribe antipsychotic medication in an atmosphere of unclear adherence. So what they’re doing is raising the dose and raising the dose. I think they don’t know how much to prescribe," Dr. Velligan declared.
She was on a National Institute of Mental Health–sponsored expert panel that concluded all of the standard methods of assessing adherence are error prone (J. Psychosom. Res. 2010;69:591-9). For example, studies show that patient self-report vastly overestimates adherence and that clinicians are poor at judging the level of adherence. Plasma and urine analysis provide good quality information only about the last 4-5 days, not the past 30-60 days. "Smart" pill containers with electronic monitoring technology don’t work if the patient opens the bottle and forgets to put the top back on. Pill counts work only if the patient remembers to bring in his bottles. And pharmacy records, too, are unreliable in assessing adherence.
"I have people who are 100% adherent, but they haven’t picked up their pills from the pharmacy in 7 months because they had a bunch of pills left over from all the times that they weren’t adherent before," she observed.
An LAI offers numerous potential advantages for the prescriber. For one, it eliminates guesswork about compliance status.
"If patients with a prescription for oral therapy come in and they’re not looking well, you don’t know how much of their medication they’ve been taking. It’s an enormous crap shoot, yet we do it every single day," Dr. Velligan charged.
LAI therapy also alerts the psychiatrist when nonadherence starts, and it’s invaluable in disentangling efficacy from problem adherence.
"If I’m giving you an injection and you’re not doing well and you’ve shown up every time for your shots, this medicine is not working so well. It’s not the right antipsychotic for you," she explained.
Together with Dr. Martha Sajatovic, a psychiatrist at Case Western Reserve University in Cleveland, Dr. Velligan has developed what they call the NOB (individuals Not receiving Optimum Benefit from antipsychotic medication) checklist. It’s intended for implementation by overburdened clinicians in community mental health centers. A "yes" answer to any of the five simple questions (see below) should alert the provider that LAIs might in this case help in clinical decision making and improve patient outcomes.
In a separate presentation, Dr. Steven G. Potkin presented a detailed analysis of 69 recorded psychiatrist-patient community mental health center office visits involving 22 patients being treated with oral antipsychotics and 38 on LAIs. Prescribers spent merely a mean 2% of the visit time discussing adherence with their patients. Moreover, psychiatrists made decisions about antipsychotic treatment without patient or caregiver input in two-thirds of the encounters.
"Collectively, these findings suggest that there is an opportunity for prescribers to increase active patient engagement, address resistance about LAIs, and provide better LAI-relevant information for more individualized options and approaches to treating patients with schizophrenia," concluded Dr. Potkin, professor of psychiatry and director of the brain imaging center at the University of California, Irvine.
Dr. Potkin’s study was funded by Otsuka American Pharmaceutical and H. Lundbeck. He reported receiving research support from roughly 20 pharmaceutical companies. Dr. Velligan reported having no financial conflicts.
The NOB Checklist
The NOB checklist is aimed at helping clinicians working in community health centers to quickly determine whether patients are adherent.
Here is a listing of the five simple questions:
1) Based upon the patient’s report, caregiver report, or your prescribing record, has the patient missed doses such that 30% or more of the medication has been missed?
2) Is the patient currently on more than one antipsychotic (not during a switch)?
3) Has the patient been on more than two antipsychotics in the past 12 months?
4) Has the patient been hospitalized or had a crisis visit in the past 12 months?
5) Is the patient not satisfied with the current level of symptom control?
A "yes" answer to any of these questions warrants offering a long-acting injectable antipsychotic agent to this patient.
Source: Dr. Velligan
EXPERT ANALYSIS FROM THE NCDEU MEETING
Novel intervention improves outcomes in homeless schizophrenia patients
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health
The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
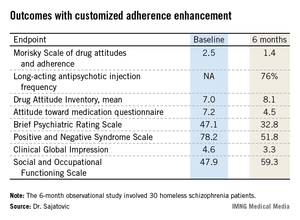
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health

The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health

The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
AT THE NCDEU MEETING
Major finding: Homeless patients with schizophrenia missed 46% of their oral medication in the month prior to a customized psychosocial intervention. After the intervention, the percentage of missed medication dropped sharply to just 10% in the final month of a 6-month study.
Data source: This was a 6-month, prospective, uncontrolled study involving 30 homeless individuals with schizophrenia or schizoaffective disorder.
Disclosures: The study was supported by a grant from the Reuter Foundation. The presenter reported having no financial conflicts.
Levomilnacipran SR for major depression also improves attention deficits
HOLLYWOOD, FLA. – Levomilnacipran SR resulted in significant improvement in cognitive measures of attention and information processing in a phase 3 trial conducted in patients with major depressive disorder; the drug was approved July 25 by the Food and Drug Administration under the trade name Fetzima.*
Attention deficits are commonplace in patients with major depressive disorder (MDD). In the phase 3 study, improvement in MDD in levomilnacipran SR–treated patients was accompanied by gains in measures of attention. A question that will need to be addressed in future studies is whether the observed improvement in attention deficits results from a direct drug effect upon brain structures controlling attention or is a secondary by-product of reduced depressive symptoms, Keith A. Wesnes, Ph.D., noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Levomilnacipran SR is a selective serotonin and norepinephrine reuptake inhibitor. Its norepinephrine reuptake potency is twice as great as its serotonin reuptake potency. In addition, levomilnacipran SR is greater than 10-fold more selective for norepinephrine reuptake inhibition than either duloxetine or venlafaxine.
The 8-week, double-blind, phase 3 clinical trial included 429 patients with MDD who were randomized to once-daily levomilnacipran SR at 40-120 mg or placebo. This was a severely depressed population as reflected in their mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score of 35, said Dr. Wesnes, professor of psychology at Northumbria University in Newcastle, England, and an employee of Bracket, a pharmaceutical industry consulting firm.
A MADRS total score reduction of at least 50% was achieved in 42% of the levomilnacipran SR group compared with 29% of controls. These MADRS responders on levomilnacipran SR also displayed significant improvement from baseline in several measures of attention. In contrast, placebo-treated controls and MADRS nonresponders on levomilnacipran SR did not.
During the course of the 8-week study, cognitive assessments carried out using the Cognitive Drug Research System for Attention demonstrated that MADRS responders on levomilnacipran SR had significant improvement over time on computerized tasks measuring power of attention, continuity of attention, reaction time variability, and digit vigilance task accuracy.
For example, levomilnacipran SR–treated MADRS responders improved their digit vigilance task accuracy by 3.1-U over baseline, while levomilnacipran SR–treated MADRS nonresponders had a 0.8-U decrease in performance, placebo-treated MADRS nonresponders averaged a 1.8-U decrease, and placebo-treated MADRS responders had a 1.5-U decline.
Arguing in favor of the possibility that the antidepressant medication improves attention deficits in MDD through direct action upon brain structures controlling attention is the observation that baseline measures of cognition correlated poorly with baseline MADRS depression symptoms or with changes in depressive symptoms during treatment, according to Dr. Wesnes.
He is an employee of Bracket, which was funded by Forest Laboratories and Pierre Fabre to conduct this analysis.
*This story was updated 7/29/2013.
HOLLYWOOD, FLA. – Levomilnacipran SR resulted in significant improvement in cognitive measures of attention and information processing in a phase 3 trial conducted in patients with major depressive disorder; the drug was approved July 25 by the Food and Drug Administration under the trade name Fetzima.*
Attention deficits are commonplace in patients with major depressive disorder (MDD). In the phase 3 study, improvement in MDD in levomilnacipran SR–treated patients was accompanied by gains in measures of attention. A question that will need to be addressed in future studies is whether the observed improvement in attention deficits results from a direct drug effect upon brain structures controlling attention or is a secondary by-product of reduced depressive symptoms, Keith A. Wesnes, Ph.D., noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Levomilnacipran SR is a selective serotonin and norepinephrine reuptake inhibitor. Its norepinephrine reuptake potency is twice as great as its serotonin reuptake potency. In addition, levomilnacipran SR is greater than 10-fold more selective for norepinephrine reuptake inhibition than either duloxetine or venlafaxine.
The 8-week, double-blind, phase 3 clinical trial included 429 patients with MDD who were randomized to once-daily levomilnacipran SR at 40-120 mg or placebo. This was a severely depressed population as reflected in their mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score of 35, said Dr. Wesnes, professor of psychology at Northumbria University in Newcastle, England, and an employee of Bracket, a pharmaceutical industry consulting firm.
A MADRS total score reduction of at least 50% was achieved in 42% of the levomilnacipran SR group compared with 29% of controls. These MADRS responders on levomilnacipran SR also displayed significant improvement from baseline in several measures of attention. In contrast, placebo-treated controls and MADRS nonresponders on levomilnacipran SR did not.
During the course of the 8-week study, cognitive assessments carried out using the Cognitive Drug Research System for Attention demonstrated that MADRS responders on levomilnacipran SR had significant improvement over time on computerized tasks measuring power of attention, continuity of attention, reaction time variability, and digit vigilance task accuracy.
For example, levomilnacipran SR–treated MADRS responders improved their digit vigilance task accuracy by 3.1-U over baseline, while levomilnacipran SR–treated MADRS nonresponders had a 0.8-U decrease in performance, placebo-treated MADRS nonresponders averaged a 1.8-U decrease, and placebo-treated MADRS responders had a 1.5-U decline.
Arguing in favor of the possibility that the antidepressant medication improves attention deficits in MDD through direct action upon brain structures controlling attention is the observation that baseline measures of cognition correlated poorly with baseline MADRS depression symptoms or with changes in depressive symptoms during treatment, according to Dr. Wesnes.
He is an employee of Bracket, which was funded by Forest Laboratories and Pierre Fabre to conduct this analysis.
*This story was updated 7/29/2013.
HOLLYWOOD, FLA. – Levomilnacipran SR resulted in significant improvement in cognitive measures of attention and information processing in a phase 3 trial conducted in patients with major depressive disorder; the drug was approved July 25 by the Food and Drug Administration under the trade name Fetzima.*
Attention deficits are commonplace in patients with major depressive disorder (MDD). In the phase 3 study, improvement in MDD in levomilnacipran SR–treated patients was accompanied by gains in measures of attention. A question that will need to be addressed in future studies is whether the observed improvement in attention deficits results from a direct drug effect upon brain structures controlling attention or is a secondary by-product of reduced depressive symptoms, Keith A. Wesnes, Ph.D., noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Levomilnacipran SR is a selective serotonin and norepinephrine reuptake inhibitor. Its norepinephrine reuptake potency is twice as great as its serotonin reuptake potency. In addition, levomilnacipran SR is greater than 10-fold more selective for norepinephrine reuptake inhibition than either duloxetine or venlafaxine.
The 8-week, double-blind, phase 3 clinical trial included 429 patients with MDD who were randomized to once-daily levomilnacipran SR at 40-120 mg or placebo. This was a severely depressed population as reflected in their mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score of 35, said Dr. Wesnes, professor of psychology at Northumbria University in Newcastle, England, and an employee of Bracket, a pharmaceutical industry consulting firm.
A MADRS total score reduction of at least 50% was achieved in 42% of the levomilnacipran SR group compared with 29% of controls. These MADRS responders on levomilnacipran SR also displayed significant improvement from baseline in several measures of attention. In contrast, placebo-treated controls and MADRS nonresponders on levomilnacipran SR did not.
During the course of the 8-week study, cognitive assessments carried out using the Cognitive Drug Research System for Attention demonstrated that MADRS responders on levomilnacipran SR had significant improvement over time on computerized tasks measuring power of attention, continuity of attention, reaction time variability, and digit vigilance task accuracy.
For example, levomilnacipran SR–treated MADRS responders improved their digit vigilance task accuracy by 3.1-U over baseline, while levomilnacipran SR–treated MADRS nonresponders had a 0.8-U decrease in performance, placebo-treated MADRS nonresponders averaged a 1.8-U decrease, and placebo-treated MADRS responders had a 1.5-U decline.
Arguing in favor of the possibility that the antidepressant medication improves attention deficits in MDD through direct action upon brain structures controlling attention is the observation that baseline measures of cognition correlated poorly with baseline MADRS depression symptoms or with changes in depressive symptoms during treatment, according to Dr. Wesnes.
He is an employee of Bracket, which was funded by Forest Laboratories and Pierre Fabre to conduct this analysis.
*This story was updated 7/29/2013.
AT THE NCDEU MEETING
Major finding: Patients with severe major depressive disorder who had a significant clinical response to the investigational antidepressant levomilnacipran SR also showed significant improvement in baseline attention deficits.
Data source: An 8-week, double-blind, phase 3 clinical trial involving 429 patients with major depressive disorder who were randomized to once-daily levomilnacipran SR or placebo.
Disclosures: Dr. Wesnes is an employee of Bracket, which was funded by Forest Laboratories and Pierre Fabre to conduct this analysis.
Two antipsychotic switching strategies yield different early results
HOLLYWOOD, FLA. – An immediate and abrupt switch to iloperidone from another atypical antipsychotic agent provides a markedly greater clinical response rate within the first 2 weeks than does a gradual switch with stepwise dose reduction of the drug being discontinued, according to data from the i-FANS trial.
This superior early clinical response rate comes at the price of a modest increase in dizziness. However, the rates of no other antipsychotic side effects in the Iloperidone Flexible-Dose Study Assessing Efficacy and Tolerability of Two Switch Approaches in Schizophrenia patients, or i-FANS trial, were affected by switching strategy, Dr. Leslie Citrome reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The i-FANS study was a multicenter, open-label trial involving 500 adults with schizophrenia randomized to a gradual or immediate switch from risperidone (Risperdal), olanzapine (Zyprexa), or aripiprazole (Abilify) to iloperidone (Fanapt). The switch was made because of inadequate efficacy and/or emergence of tolerability problems with the drug being discontinued.
In the gradual-switch group, the baseline dose of the first antipsychotic was reduced by 50% on day 1 and by 75% after 1 week, with a complete halt of the drug at the end of week 2. In contrast, current therapy was discontinued on day 0 in the immediate-switch group. Patients in both study arms received iloperidone at 1 mg b.i.d. on day 1, titrated over 4 days to 6 mg b.i.d., then further increasing by no more than 4 mg per day up to 12 mg b.i.d. as warranted, explained Dr. Citrome, professor of psychiatry at New York Medical College, Valhalla.
The primary outcome for this analysis was a rating of "much" or "very much" improved on the Integrated Clinical Global Impression of Change (I-CGI-C) at the end of week 2. This endpoint was achieved in 5.4% of the gradual switch and 11.1% of the immediate switch group at week 1, and by 17.5% and 26.1%, respectively, in the two groups at week 2. The significant advantage favoring the immediate switch strategy was similar in magnitude across the individual subgroups switching from risperidone, olanzapine, and aripiprazole.
The most common treatment emergent adverse event seen in i-FANS was dizziness. The incidence was significantly lower in the gradual-switch group during week 1, at 8.8% compared to 14.2% in the immediate-switch group. The rate during week 2 was 1.3% in the gradual- and 3.2% in the immediate switch group during week 2.
Iloperidone is a mixed dopamine D2, serotonin 5HT-2A, and alpha-adrenergic antagonist approved for the treatment of schizophrenia.
The i-FANS study was supported by Novartis. Dr. Citrome reported receiving research support and/or consulting fees from Novartis and 17 other pharmaceutical companies.
HOLLYWOOD, FLA. – An immediate and abrupt switch to iloperidone from another atypical antipsychotic agent provides a markedly greater clinical response rate within the first 2 weeks than does a gradual switch with stepwise dose reduction of the drug being discontinued, according to data from the i-FANS trial.
This superior early clinical response rate comes at the price of a modest increase in dizziness. However, the rates of no other antipsychotic side effects in the Iloperidone Flexible-Dose Study Assessing Efficacy and Tolerability of Two Switch Approaches in Schizophrenia patients, or i-FANS trial, were affected by switching strategy, Dr. Leslie Citrome reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The i-FANS study was a multicenter, open-label trial involving 500 adults with schizophrenia randomized to a gradual or immediate switch from risperidone (Risperdal), olanzapine (Zyprexa), or aripiprazole (Abilify) to iloperidone (Fanapt). The switch was made because of inadequate efficacy and/or emergence of tolerability problems with the drug being discontinued.
In the gradual-switch group, the baseline dose of the first antipsychotic was reduced by 50% on day 1 and by 75% after 1 week, with a complete halt of the drug at the end of week 2. In contrast, current therapy was discontinued on day 0 in the immediate-switch group. Patients in both study arms received iloperidone at 1 mg b.i.d. on day 1, titrated over 4 days to 6 mg b.i.d., then further increasing by no more than 4 mg per day up to 12 mg b.i.d. as warranted, explained Dr. Citrome, professor of psychiatry at New York Medical College, Valhalla.
The primary outcome for this analysis was a rating of "much" or "very much" improved on the Integrated Clinical Global Impression of Change (I-CGI-C) at the end of week 2. This endpoint was achieved in 5.4% of the gradual switch and 11.1% of the immediate switch group at week 1, and by 17.5% and 26.1%, respectively, in the two groups at week 2. The significant advantage favoring the immediate switch strategy was similar in magnitude across the individual subgroups switching from risperidone, olanzapine, and aripiprazole.
The most common treatment emergent adverse event seen in i-FANS was dizziness. The incidence was significantly lower in the gradual-switch group during week 1, at 8.8% compared to 14.2% in the immediate-switch group. The rate during week 2 was 1.3% in the gradual- and 3.2% in the immediate switch group during week 2.
Iloperidone is a mixed dopamine D2, serotonin 5HT-2A, and alpha-adrenergic antagonist approved for the treatment of schizophrenia.
The i-FANS study was supported by Novartis. Dr. Citrome reported receiving research support and/or consulting fees from Novartis and 17 other pharmaceutical companies.
HOLLYWOOD, FLA. – An immediate and abrupt switch to iloperidone from another atypical antipsychotic agent provides a markedly greater clinical response rate within the first 2 weeks than does a gradual switch with stepwise dose reduction of the drug being discontinued, according to data from the i-FANS trial.
This superior early clinical response rate comes at the price of a modest increase in dizziness. However, the rates of no other antipsychotic side effects in the Iloperidone Flexible-Dose Study Assessing Efficacy and Tolerability of Two Switch Approaches in Schizophrenia patients, or i-FANS trial, were affected by switching strategy, Dr. Leslie Citrome reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The i-FANS study was a multicenter, open-label trial involving 500 adults with schizophrenia randomized to a gradual or immediate switch from risperidone (Risperdal), olanzapine (Zyprexa), or aripiprazole (Abilify) to iloperidone (Fanapt). The switch was made because of inadequate efficacy and/or emergence of tolerability problems with the drug being discontinued.
In the gradual-switch group, the baseline dose of the first antipsychotic was reduced by 50% on day 1 and by 75% after 1 week, with a complete halt of the drug at the end of week 2. In contrast, current therapy was discontinued on day 0 in the immediate-switch group. Patients in both study arms received iloperidone at 1 mg b.i.d. on day 1, titrated over 4 days to 6 mg b.i.d., then further increasing by no more than 4 mg per day up to 12 mg b.i.d. as warranted, explained Dr. Citrome, professor of psychiatry at New York Medical College, Valhalla.
The primary outcome for this analysis was a rating of "much" or "very much" improved on the Integrated Clinical Global Impression of Change (I-CGI-C) at the end of week 2. This endpoint was achieved in 5.4% of the gradual switch and 11.1% of the immediate switch group at week 1, and by 17.5% and 26.1%, respectively, in the two groups at week 2. The significant advantage favoring the immediate switch strategy was similar in magnitude across the individual subgroups switching from risperidone, olanzapine, and aripiprazole.
The most common treatment emergent adverse event seen in i-FANS was dizziness. The incidence was significantly lower in the gradual-switch group during week 1, at 8.8% compared to 14.2% in the immediate-switch group. The rate during week 2 was 1.3% in the gradual- and 3.2% in the immediate switch group during week 2.
Iloperidone is a mixed dopamine D2, serotonin 5HT-2A, and alpha-adrenergic antagonist approved for the treatment of schizophrenia.
The i-FANS study was supported by Novartis. Dr. Citrome reported receiving research support and/or consulting fees from Novartis and 17 other pharmaceutical companies.
AT THE NCDEU MEETING
Major finding: Patients with schizophrenia who discontinued risperidone, olanzapine, or aripiprazole and started on iloperidone the following day had an 11.1% rate of being scored much or very much improved after 1 week and a 26.1% rate after 2 weeks, compared to 5.4% and 17.5%, respectively, when the first atypical antipsychotic was gradually discontinued over the course of 2 weeks while iloperidone was being uptitrated.
Data source: The i-FANS trial, a multicenter, open-label study involving 500 adults with schizophrenia who were switched to iloperidone because of efficacy and/or tolerability problems with their current antipsychotic.
Disclosures: The presenter has received research support and serves as a consultant to Novartis, which funded the study.
Novel antipsychotic shows early promise
HOLLYWOOD, FLA. – A novel oral antipsychotic, RP5063, displayed broad safety and efficacy for the treatment of schizophrenia and schizoaffective disorder in a phase II study.
RP5063 is a dopamine-serotonin system stabilizer. The agent is a potent partial agonist at the dopamine D2, D3, and D4 receptors and the serotonin 5-HT1A and 5-HT2A receptors, as well as an antagonist at the serotonin 5-HT6 and 5-HT7 receptors. Several of those sites have never been targeted by other medications, Dr. Marc Cantillon noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Sixty-one percent of the drug’s metabolism is by the CYP3A4 pathway, the rest by the CYP2D6 pathway, an arrangement that provides a potential for low drug-drug interaction.
The agent was designed to provide safe, less side effect laden, and more broadly effective alternatives to current atypical antipsychotics. RP5063 has minimal effects on off-target receptors, such as the histamine receptor, which are responsible for the common side effects of atypical antipsychotics that often result in poor treatment adherence, Dr. Cantillon explained.
He presented the results of the REFRESH trial, a 4-week, double-blind study of 234 patients with acute exacerbation of schizophrenia or schizoaffective disorder in the United States, Europe, and Asia. The patients were randomized 3:3:3:2:1 to once-daily RP5063 at 15, 30, or 50 mg/day, placebo, or aripiprazole (Abilify) at 15 mg/day.
Andreasen schizophrenia remission criteria (Am. J. Psychiatry 2005;162:441-9) were met by 34% of patients on RP5063 at 15 mg, 30% at 30 mg, and 46% at 50 mg, all significantly better than the 22% rate with placebo. The aripiprazole group was too small to include in the results comparison. Efficacy in the RP5063 group became significantly better than placebo within the first week and continued to steadily improve throughout the 4-week investigation, reported Dr. Cantillon, a psychiatrist and geriatrician in Livingston, N.J., and chief medical officer at Reviva Pharmaceuticals, which is developing RP5063.
Efficacy was defined as at least a 20% improvement over baseline on the Positive and Negative Syndrome Scale (PANSS) plus a 2-point improvement on the Clinical Global Impression Severity (CGI-S) scale; 46% of patients assigned to RP5063 at 15 mg/day met this bar, as did 32% of those on RP5063 at 30 mg/day and 33% on 50 mg/day, compared with 19% of placebo-treated controls.
"These remission efficacy levels within such a short study place RP5063 among the robust antipsychotics," Dr. Cantillon said.
While comparisons between different placebo-controlled randomized trials must be taken with a grain of salt, he said, the effect sizes for changes in PANSS scores with the three doses of RP5063 used in the REFRESH study are considerably bigger than in published meta-analyses of placebo-controlled trials of amisulpride, aripiprazole, quetiapine, olanzapine, or risperidone (Arch. Gen. Psychiatry 2003;60:553-64 and Mol. Psychiatry 2009;14:429-47).
On the safety front, 4 weeks of RP5063 resulted in no differences compared with placebo in terms of body weight, lipids, or blood glucose, suggesting the drug may produce fewer of the metabolic problems common of current atypical antipsychotics, noted Dr. Cantillon. Also, there were no significant differences between active treatment and control patients in terms of movement side effects or ECG changes. Serum prolactin levels declined by nearly 50% in all RP5063 treatment arms, then climbed back after the study ended.
Based upon the findings of REFRESH, a large phase III clinical trial of RP5063 for schizophrenia and schizoaffective disorder will start later this year.
"We’ll probably be including a lower dose for use in pediatric and geriatric populations in phase III, since 15 mg performed so well in REFRESH," Dr. Cantillon said.
Because of its favorable balance of agonism and antagonism of key dopaminergic and serotonergic receptors and minimal off-target effects, RP5063 is also under development for the treatment of major depressive disorder, bipolar disorder, Tourette syndrome, autism, attention-deficit/hyperactivity disorder, and psychosis in Alzheimer’s and Parkinson’s disease.
The REFRESH trial was funded by Reviva Pharmaceuticals.
HOLLYWOOD, FLA. – A novel oral antipsychotic, RP5063, displayed broad safety and efficacy for the treatment of schizophrenia and schizoaffective disorder in a phase II study.
RP5063 is a dopamine-serotonin system stabilizer. The agent is a potent partial agonist at the dopamine D2, D3, and D4 receptors and the serotonin 5-HT1A and 5-HT2A receptors, as well as an antagonist at the serotonin 5-HT6 and 5-HT7 receptors. Several of those sites have never been targeted by other medications, Dr. Marc Cantillon noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Sixty-one percent of the drug’s metabolism is by the CYP3A4 pathway, the rest by the CYP2D6 pathway, an arrangement that provides a potential for low drug-drug interaction.
The agent was designed to provide safe, less side effect laden, and more broadly effective alternatives to current atypical antipsychotics. RP5063 has minimal effects on off-target receptors, such as the histamine receptor, which are responsible for the common side effects of atypical antipsychotics that often result in poor treatment adherence, Dr. Cantillon explained.
He presented the results of the REFRESH trial, a 4-week, double-blind study of 234 patients with acute exacerbation of schizophrenia or schizoaffective disorder in the United States, Europe, and Asia. The patients were randomized 3:3:3:2:1 to once-daily RP5063 at 15, 30, or 50 mg/day, placebo, or aripiprazole (Abilify) at 15 mg/day.
Andreasen schizophrenia remission criteria (Am. J. Psychiatry 2005;162:441-9) were met by 34% of patients on RP5063 at 15 mg, 30% at 30 mg, and 46% at 50 mg, all significantly better than the 22% rate with placebo. The aripiprazole group was too small to include in the results comparison. Efficacy in the RP5063 group became significantly better than placebo within the first week and continued to steadily improve throughout the 4-week investigation, reported Dr. Cantillon, a psychiatrist and geriatrician in Livingston, N.J., and chief medical officer at Reviva Pharmaceuticals, which is developing RP5063.
Efficacy was defined as at least a 20% improvement over baseline on the Positive and Negative Syndrome Scale (PANSS) plus a 2-point improvement on the Clinical Global Impression Severity (CGI-S) scale; 46% of patients assigned to RP5063 at 15 mg/day met this bar, as did 32% of those on RP5063 at 30 mg/day and 33% on 50 mg/day, compared with 19% of placebo-treated controls.
"These remission efficacy levels within such a short study place RP5063 among the robust antipsychotics," Dr. Cantillon said.
While comparisons between different placebo-controlled randomized trials must be taken with a grain of salt, he said, the effect sizes for changes in PANSS scores with the three doses of RP5063 used in the REFRESH study are considerably bigger than in published meta-analyses of placebo-controlled trials of amisulpride, aripiprazole, quetiapine, olanzapine, or risperidone (Arch. Gen. Psychiatry 2003;60:553-64 and Mol. Psychiatry 2009;14:429-47).
On the safety front, 4 weeks of RP5063 resulted in no differences compared with placebo in terms of body weight, lipids, or blood glucose, suggesting the drug may produce fewer of the metabolic problems common of current atypical antipsychotics, noted Dr. Cantillon. Also, there were no significant differences between active treatment and control patients in terms of movement side effects or ECG changes. Serum prolactin levels declined by nearly 50% in all RP5063 treatment arms, then climbed back after the study ended.
Based upon the findings of REFRESH, a large phase III clinical trial of RP5063 for schizophrenia and schizoaffective disorder will start later this year.
"We’ll probably be including a lower dose for use in pediatric and geriatric populations in phase III, since 15 mg performed so well in REFRESH," Dr. Cantillon said.
Because of its favorable balance of agonism and antagonism of key dopaminergic and serotonergic receptors and minimal off-target effects, RP5063 is also under development for the treatment of major depressive disorder, bipolar disorder, Tourette syndrome, autism, attention-deficit/hyperactivity disorder, and psychosis in Alzheimer’s and Parkinson’s disease.
The REFRESH trial was funded by Reviva Pharmaceuticals.
HOLLYWOOD, FLA. – A novel oral antipsychotic, RP5063, displayed broad safety and efficacy for the treatment of schizophrenia and schizoaffective disorder in a phase II study.
RP5063 is a dopamine-serotonin system stabilizer. The agent is a potent partial agonist at the dopamine D2, D3, and D4 receptors and the serotonin 5-HT1A and 5-HT2A receptors, as well as an antagonist at the serotonin 5-HT6 and 5-HT7 receptors. Several of those sites have never been targeted by other medications, Dr. Marc Cantillon noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
Sixty-one percent of the drug’s metabolism is by the CYP3A4 pathway, the rest by the CYP2D6 pathway, an arrangement that provides a potential for low drug-drug interaction.
The agent was designed to provide safe, less side effect laden, and more broadly effective alternatives to current atypical antipsychotics. RP5063 has minimal effects on off-target receptors, such as the histamine receptor, which are responsible for the common side effects of atypical antipsychotics that often result in poor treatment adherence, Dr. Cantillon explained.
He presented the results of the REFRESH trial, a 4-week, double-blind study of 234 patients with acute exacerbation of schizophrenia or schizoaffective disorder in the United States, Europe, and Asia. The patients were randomized 3:3:3:2:1 to once-daily RP5063 at 15, 30, or 50 mg/day, placebo, or aripiprazole (Abilify) at 15 mg/day.
Andreasen schizophrenia remission criteria (Am. J. Psychiatry 2005;162:441-9) were met by 34% of patients on RP5063 at 15 mg, 30% at 30 mg, and 46% at 50 mg, all significantly better than the 22% rate with placebo. The aripiprazole group was too small to include in the results comparison. Efficacy in the RP5063 group became significantly better than placebo within the first week and continued to steadily improve throughout the 4-week investigation, reported Dr. Cantillon, a psychiatrist and geriatrician in Livingston, N.J., and chief medical officer at Reviva Pharmaceuticals, which is developing RP5063.
Efficacy was defined as at least a 20% improvement over baseline on the Positive and Negative Syndrome Scale (PANSS) plus a 2-point improvement on the Clinical Global Impression Severity (CGI-S) scale; 46% of patients assigned to RP5063 at 15 mg/day met this bar, as did 32% of those on RP5063 at 30 mg/day and 33% on 50 mg/day, compared with 19% of placebo-treated controls.
"These remission efficacy levels within such a short study place RP5063 among the robust antipsychotics," Dr. Cantillon said.
While comparisons between different placebo-controlled randomized trials must be taken with a grain of salt, he said, the effect sizes for changes in PANSS scores with the three doses of RP5063 used in the REFRESH study are considerably bigger than in published meta-analyses of placebo-controlled trials of amisulpride, aripiprazole, quetiapine, olanzapine, or risperidone (Arch. Gen. Psychiatry 2003;60:553-64 and Mol. Psychiatry 2009;14:429-47).
On the safety front, 4 weeks of RP5063 resulted in no differences compared with placebo in terms of body weight, lipids, or blood glucose, suggesting the drug may produce fewer of the metabolic problems common of current atypical antipsychotics, noted Dr. Cantillon. Also, there were no significant differences between active treatment and control patients in terms of movement side effects or ECG changes. Serum prolactin levels declined by nearly 50% in all RP5063 treatment arms, then climbed back after the study ended.
Based upon the findings of REFRESH, a large phase III clinical trial of RP5063 for schizophrenia and schizoaffective disorder will start later this year.
"We’ll probably be including a lower dose for use in pediatric and geriatric populations in phase III, since 15 mg performed so well in REFRESH," Dr. Cantillon said.
Because of its favorable balance of agonism and antagonism of key dopaminergic and serotonergic receptors and minimal off-target effects, RP5063 is also under development for the treatment of major depressive disorder, bipolar disorder, Tourette syndrome, autism, attention-deficit/hyperactivity disorder, and psychosis in Alzheimer’s and Parkinson’s disease.
The REFRESH trial was funded by Reviva Pharmaceuticals.
AT THE NCDEU MEETING
Major Finding: Forty-five percent of patients with schizophrenia or schizoaffective disorder randomized to RP5063 responded with at least a 2-point reduction from baseline on the CGI-S scale plus a 20% improvement on PANSS, compared with 19% on placebo.
Data Source: The phase-II REFRESH study, a 4-week, double-blind trial of 234 patients with acute exacerbation of schizophrenia or schizoaffective disorder who were randomized to RP5063 at one of three daily doses, aripiprazole at 15 mg/day, or placebo.
Disclosures: REFRESH was funded by Reviva Pharmaceuticals. Dr. Cantillon is the company’s chief medical officer.
Lurasidone shows efficacy in bipolar depression
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
AT THE NCDEU MEETING
Major finding: Patients with bipolar I depression responded to 6 weeks of lurasidone at either a lower or higher dose range with identical 15.4-point improvements from a baseline score of 30 on the Montgomery-Åsberg Depression Rating Scale, significantly better than the 10.7-point improvement with placebo.
Data source: PREVAIL 2 was a phase III, double-blind, 6-week randomized trial involving 505 patients with bipolar I depression.
Disclosures: The study was sponsored by Sunovion. The presenter is a company employee.
Selegiline transdermal system promising for social phobia
HOLLYWOOD, FLA. – Patients with social phobia responded favorably to treatment with the selegiline transdermal system, showing significant improvement in the anxiety component of the Liebowitz Social Anxiety Scale and other efficacy endpoints in a small phase II study.
Twenty patients participated in this 12-week, open-label, pilot study. They were treated with the selegiline transdermal system (Emsam) at a dose of 6 mg/24 hours. They demonstrated a significant mean 7.1-point drop from a baseline score of 17.6 on the avoidance component of the Liebowitz Social Anxiety Scale, as well as a 6.6-point drop on the scale’s anxiety component, a result that just missed statistical significance (P = .053), Kimberly Portland, Ph.D., reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The selegiline transdermal system is approved for the treatment of major depression. Selegiline is an MAO-A and -B inhibitor. Its transdermal administration bypasses the GI tract and liver, thereby essentially eliminating the risks of hypertensive crisis and dietary interactions with tyramine-rich foods, which have demoted the traditional oral monoamine oxidase (MAO) inhibitors in antidepressant treatment hierarchies.
Oral MAO inhibitors previously have been shown in clinical trials to be at least as effective as cognitive-behavioral therapy or benzodiazepines in treating social phobia. So it seemed appropriate to examine the safety and efficacy of the selegiline transdermal system for this condition, which has an estimated prevalence in the United States of 5%-12%. The study was particularly timely because the selegiline transdermal system has dopaminergic effects, and recent evidence points to dopamine systems as playing a role in social phobia, explained Dr. Portland of Mylan Specialty, Basking Ridge, N.J., which markets Emsam.
In addition to the favorable results on the Liebowitz Social Anxiety Scale over the course of 12 weeks, participants also showed statistically significant improvements on the Social Life/Leisure Activities domain of the Sheehan Disability Scale, the Clinical Global Impression Severity of Illness Scale, and the Clinical Global Impression Change Scale.
Three patients dropped out of the study because of adverse events. The most common adverse events in the study were headache, reported by 25% of participants, and application site reactions, reported by 20%.
The major limitation of this pilot study is its small size, Dr. Portland noted. Further studies incorporating larger patient numbers, a double-blind format, and higher doses of the MAO inhibitor are planned.
HOLLYWOOD, FLA. – Patients with social phobia responded favorably to treatment with the selegiline transdermal system, showing significant improvement in the anxiety component of the Liebowitz Social Anxiety Scale and other efficacy endpoints in a small phase II study.
Twenty patients participated in this 12-week, open-label, pilot study. They were treated with the selegiline transdermal system (Emsam) at a dose of 6 mg/24 hours. They demonstrated a significant mean 7.1-point drop from a baseline score of 17.6 on the avoidance component of the Liebowitz Social Anxiety Scale, as well as a 6.6-point drop on the scale’s anxiety component, a result that just missed statistical significance (P = .053), Kimberly Portland, Ph.D., reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The selegiline transdermal system is approved for the treatment of major depression. Selegiline is an MAO-A and -B inhibitor. Its transdermal administration bypasses the GI tract and liver, thereby essentially eliminating the risks of hypertensive crisis and dietary interactions with tyramine-rich foods, which have demoted the traditional oral monoamine oxidase (MAO) inhibitors in antidepressant treatment hierarchies.
Oral MAO inhibitors previously have been shown in clinical trials to be at least as effective as cognitive-behavioral therapy or benzodiazepines in treating social phobia. So it seemed appropriate to examine the safety and efficacy of the selegiline transdermal system for this condition, which has an estimated prevalence in the United States of 5%-12%. The study was particularly timely because the selegiline transdermal system has dopaminergic effects, and recent evidence points to dopamine systems as playing a role in social phobia, explained Dr. Portland of Mylan Specialty, Basking Ridge, N.J., which markets Emsam.
In addition to the favorable results on the Liebowitz Social Anxiety Scale over the course of 12 weeks, participants also showed statistically significant improvements on the Social Life/Leisure Activities domain of the Sheehan Disability Scale, the Clinical Global Impression Severity of Illness Scale, and the Clinical Global Impression Change Scale.
Three patients dropped out of the study because of adverse events. The most common adverse events in the study were headache, reported by 25% of participants, and application site reactions, reported by 20%.
The major limitation of this pilot study is its small size, Dr. Portland noted. Further studies incorporating larger patient numbers, a double-blind format, and higher doses of the MAO inhibitor are planned.
HOLLYWOOD, FLA. – Patients with social phobia responded favorably to treatment with the selegiline transdermal system, showing significant improvement in the anxiety component of the Liebowitz Social Anxiety Scale and other efficacy endpoints in a small phase II study.
Twenty patients participated in this 12-week, open-label, pilot study. They were treated with the selegiline transdermal system (Emsam) at a dose of 6 mg/24 hours. They demonstrated a significant mean 7.1-point drop from a baseline score of 17.6 on the avoidance component of the Liebowitz Social Anxiety Scale, as well as a 6.6-point drop on the scale’s anxiety component, a result that just missed statistical significance (P = .053), Kimberly Portland, Ph.D., reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The selegiline transdermal system is approved for the treatment of major depression. Selegiline is an MAO-A and -B inhibitor. Its transdermal administration bypasses the GI tract and liver, thereby essentially eliminating the risks of hypertensive crisis and dietary interactions with tyramine-rich foods, which have demoted the traditional oral monoamine oxidase (MAO) inhibitors in antidepressant treatment hierarchies.
Oral MAO inhibitors previously have been shown in clinical trials to be at least as effective as cognitive-behavioral therapy or benzodiazepines in treating social phobia. So it seemed appropriate to examine the safety and efficacy of the selegiline transdermal system for this condition, which has an estimated prevalence in the United States of 5%-12%. The study was particularly timely because the selegiline transdermal system has dopaminergic effects, and recent evidence points to dopamine systems as playing a role in social phobia, explained Dr. Portland of Mylan Specialty, Basking Ridge, N.J., which markets Emsam.
In addition to the favorable results on the Liebowitz Social Anxiety Scale over the course of 12 weeks, participants also showed statistically significant improvements on the Social Life/Leisure Activities domain of the Sheehan Disability Scale, the Clinical Global Impression Severity of Illness Scale, and the Clinical Global Impression Change Scale.
Three patients dropped out of the study because of adverse events. The most common adverse events in the study were headache, reported by 25% of participants, and application site reactions, reported by 20%.
The major limitation of this pilot study is its small size, Dr. Portland noted. Further studies incorporating larger patient numbers, a double-blind format, and higher doses of the MAO inhibitor are planned.
AT THE NCDEU MEETING
Major finding: Patients with social phobia showed significant improvement on multiple efficacy measures in response to treatment with the selegiline transdermal system.
Data source: This was an open-label, 20-patient, 12-week phase II study.
Disclosures: The study was sponsored by Mylan Specialty, which markets the selegiline transdermal system for the treatment of major depression. The presenter is a company employee.
Novel intranasal antidepressant shows results after 1 week
HOLLYWOOD, FLA. – An investigational intranasal spray antidepressant known for now as PH10 shows early promise in addressing two major unmet needs in the treatment of major depressive disorder: faster-acting drugs with novel mechanisms of action.
PH10 showed a large antidepressant effect in a small phase II study after just 1 week, when the first scheduled assessment took place. Future studies will look for an antidepressant effect even sooner, perhaps as early as day 1 of treatment, Dr. Michael R. Liebowitz said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
PH10 is a proprietary pherine. The pherines are a class of intranasally administered psychoactive therapeutic agents that bind locally on nasal chemosensory receptors and trigger responses in the hypothalamus, amygdala, prefrontal cortex, and hippocampus. They have an excellent safety and tolerability profile, are effective in nanogram quantities, and do not circulate systemically in the blood. Instead, they initiate neural impulses that follow defined pathways in order to directly affect brain function, explained Dr. Liebowitz, professor of clinical psychiatry at Columbia University, New York.
He presented an 8-week, phase II, double-blind, single-site pilot randomized trial involving 30 patients with major depressive disorder. None had treatment-resistant depression. The participants were randomized to two self-administered inhalations in each nostril twice daily at a dose of 3.2 mcg/day of PH10 from a metered-dose spray device, or a high-dose group receiving 6.4 mcg/day, or placebo spray.
Baseline scores on the 17-item Hamilton Rating Scale for Depression (HAM-D) were in the low to mid 20s. After 8 weeks of treatment, mean HAM-D scores dropped by 10.9 points in the placebo-treated controls, 16.3 points in the low-dose PH10 group, and 17.8 points in the high-dose arm, said Dr. Liebowitz, also managing director and founder of the Medical Research Network.
Particularly intriguing were the results after just 1 week: a 4.2-point drop with placebo, compared with decreases of 8.4 and 10.1 points, respectively, in the low- and high-dose PH10 arms.
"The effect sizes are pretty substantial," Dr. Liebowitz noted. He cited the Cohen’s d value of 1.01 for the comparison between high-dose PH10 and placebo, indicative of a large effect size; and a Cohen’s d of 0.71, indicative of a moderate to large effect size, for low-dose PH10 vs. placebo.
Baseline scores on the patient self-rated Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) averaged 40 but improved by a mean of 20.3 points in the high-dose PH10 group, 15.3 points in the low-dose group, and 10.1 points in controls.
Remission as defined by a final HAM-D score of 7 or less occurred in 80% of the low-dose PH10 group in this small study, in 60% of those on high-dose therapy, and 20% on placebo.
The most common side effects reported in patients on PH10 were daytime sleepiness, nasal irritation, and headache. Three patients on high-dose PH10 reported an increase in appetite, as did one patient in the low-dose arm and two patients on placebo. No significant changes in body weight were seen in the 8-week study.
As was frequently noted at the NCDEU meeting, depression is the mental illness with the largest prescription drug market in the United States. The need for antidepressants with new mechanisms of action is underscored by the observation that 50%-70% of patients do not experience remission on selective serotonin reuptake inhibitor/selective norepinephrine reuptake inhibitor therapy.
Pherin Pharmaceuticals, which is developing PH10 as a treatment for depression, also has a handful of other intranasal pherines in its pipeline. Furthest along is aloradine, now in phase III clinical trials for social anxiety disorder. Meanwhile, phase II studies have been completed for an intranasal spray used to treat premenstrual dysphoric disorder called PH80-PMD, and phase III studies for this product are gearing up. Salubrin-HF is currently in phase II studies for menopausal hot flashes. And PH15 is in preclinical testing as a cognitive enhancement agent.
Dr. Liebowitz serves as a consultant to and holds stock options in Pherin Pharmaceuticals. He receives research grants from more than 20 pharmaceutical companies.
HOLLYWOOD, FLA. – An investigational intranasal spray antidepressant known for now as PH10 shows early promise in addressing two major unmet needs in the treatment of major depressive disorder: faster-acting drugs with novel mechanisms of action.
PH10 showed a large antidepressant effect in a small phase II study after just 1 week, when the first scheduled assessment took place. Future studies will look for an antidepressant effect even sooner, perhaps as early as day 1 of treatment, Dr. Michael R. Liebowitz said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
PH10 is a proprietary pherine. The pherines are a class of intranasally administered psychoactive therapeutic agents that bind locally on nasal chemosensory receptors and trigger responses in the hypothalamus, amygdala, prefrontal cortex, and hippocampus. They have an excellent safety and tolerability profile, are effective in nanogram quantities, and do not circulate systemically in the blood. Instead, they initiate neural impulses that follow defined pathways in order to directly affect brain function, explained Dr. Liebowitz, professor of clinical psychiatry at Columbia University, New York.
He presented an 8-week, phase II, double-blind, single-site pilot randomized trial involving 30 patients with major depressive disorder. None had treatment-resistant depression. The participants were randomized to two self-administered inhalations in each nostril twice daily at a dose of 3.2 mcg/day of PH10 from a metered-dose spray device, or a high-dose group receiving 6.4 mcg/day, or placebo spray.
Baseline scores on the 17-item Hamilton Rating Scale for Depression (HAM-D) were in the low to mid 20s. After 8 weeks of treatment, mean HAM-D scores dropped by 10.9 points in the placebo-treated controls, 16.3 points in the low-dose PH10 group, and 17.8 points in the high-dose arm, said Dr. Liebowitz, also managing director and founder of the Medical Research Network.
Particularly intriguing were the results after just 1 week: a 4.2-point drop with placebo, compared with decreases of 8.4 and 10.1 points, respectively, in the low- and high-dose PH10 arms.
"The effect sizes are pretty substantial," Dr. Liebowitz noted. He cited the Cohen’s d value of 1.01 for the comparison between high-dose PH10 and placebo, indicative of a large effect size; and a Cohen’s d of 0.71, indicative of a moderate to large effect size, for low-dose PH10 vs. placebo.
Baseline scores on the patient self-rated Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) averaged 40 but improved by a mean of 20.3 points in the high-dose PH10 group, 15.3 points in the low-dose group, and 10.1 points in controls.
Remission as defined by a final HAM-D score of 7 or less occurred in 80% of the low-dose PH10 group in this small study, in 60% of those on high-dose therapy, and 20% on placebo.
The most common side effects reported in patients on PH10 were daytime sleepiness, nasal irritation, and headache. Three patients on high-dose PH10 reported an increase in appetite, as did one patient in the low-dose arm and two patients on placebo. No significant changes in body weight were seen in the 8-week study.
As was frequently noted at the NCDEU meeting, depression is the mental illness with the largest prescription drug market in the United States. The need for antidepressants with new mechanisms of action is underscored by the observation that 50%-70% of patients do not experience remission on selective serotonin reuptake inhibitor/selective norepinephrine reuptake inhibitor therapy.
Pherin Pharmaceuticals, which is developing PH10 as a treatment for depression, also has a handful of other intranasal pherines in its pipeline. Furthest along is aloradine, now in phase III clinical trials for social anxiety disorder. Meanwhile, phase II studies have been completed for an intranasal spray used to treat premenstrual dysphoric disorder called PH80-PMD, and phase III studies for this product are gearing up. Salubrin-HF is currently in phase II studies for menopausal hot flashes. And PH15 is in preclinical testing as a cognitive enhancement agent.
Dr. Liebowitz serves as a consultant to and holds stock options in Pherin Pharmaceuticals. He receives research grants from more than 20 pharmaceutical companies.
HOLLYWOOD, FLA. – An investigational intranasal spray antidepressant known for now as PH10 shows early promise in addressing two major unmet needs in the treatment of major depressive disorder: faster-acting drugs with novel mechanisms of action.
PH10 showed a large antidepressant effect in a small phase II study after just 1 week, when the first scheduled assessment took place. Future studies will look for an antidepressant effect even sooner, perhaps as early as day 1 of treatment, Dr. Michael R. Liebowitz said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
PH10 is a proprietary pherine. The pherines are a class of intranasally administered psychoactive therapeutic agents that bind locally on nasal chemosensory receptors and trigger responses in the hypothalamus, amygdala, prefrontal cortex, and hippocampus. They have an excellent safety and tolerability profile, are effective in nanogram quantities, and do not circulate systemically in the blood. Instead, they initiate neural impulses that follow defined pathways in order to directly affect brain function, explained Dr. Liebowitz, professor of clinical psychiatry at Columbia University, New York.
He presented an 8-week, phase II, double-blind, single-site pilot randomized trial involving 30 patients with major depressive disorder. None had treatment-resistant depression. The participants were randomized to two self-administered inhalations in each nostril twice daily at a dose of 3.2 mcg/day of PH10 from a metered-dose spray device, or a high-dose group receiving 6.4 mcg/day, or placebo spray.
Baseline scores on the 17-item Hamilton Rating Scale for Depression (HAM-D) were in the low to mid 20s. After 8 weeks of treatment, mean HAM-D scores dropped by 10.9 points in the placebo-treated controls, 16.3 points in the low-dose PH10 group, and 17.8 points in the high-dose arm, said Dr. Liebowitz, also managing director and founder of the Medical Research Network.
Particularly intriguing were the results after just 1 week: a 4.2-point drop with placebo, compared with decreases of 8.4 and 10.1 points, respectively, in the low- and high-dose PH10 arms.
"The effect sizes are pretty substantial," Dr. Liebowitz noted. He cited the Cohen’s d value of 1.01 for the comparison between high-dose PH10 and placebo, indicative of a large effect size; and a Cohen’s d of 0.71, indicative of a moderate to large effect size, for low-dose PH10 vs. placebo.
Baseline scores on the patient self-rated Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) averaged 40 but improved by a mean of 20.3 points in the high-dose PH10 group, 15.3 points in the low-dose group, and 10.1 points in controls.
Remission as defined by a final HAM-D score of 7 or less occurred in 80% of the low-dose PH10 group in this small study, in 60% of those on high-dose therapy, and 20% on placebo.
The most common side effects reported in patients on PH10 were daytime sleepiness, nasal irritation, and headache. Three patients on high-dose PH10 reported an increase in appetite, as did one patient in the low-dose arm and two patients on placebo. No significant changes in body weight were seen in the 8-week study.
As was frequently noted at the NCDEU meeting, depression is the mental illness with the largest prescription drug market in the United States. The need for antidepressants with new mechanisms of action is underscored by the observation that 50%-70% of patients do not experience remission on selective serotonin reuptake inhibitor/selective norepinephrine reuptake inhibitor therapy.
Pherin Pharmaceuticals, which is developing PH10 as a treatment for depression, also has a handful of other intranasal pherines in its pipeline. Furthest along is aloradine, now in phase III clinical trials for social anxiety disorder. Meanwhile, phase II studies have been completed for an intranasal spray used to treat premenstrual dysphoric disorder called PH80-PMD, and phase III studies for this product are gearing up. Salubrin-HF is currently in phase II studies for menopausal hot flashes. And PH15 is in preclinical testing as a cognitive enhancement agent.
Dr. Liebowitz serves as a consultant to and holds stock options in Pherin Pharmaceuticals. He receives research grants from more than 20 pharmaceutical companies.
AT THE NCDEU MEETING
Major finding: A self-administered intranasal spray antidepressant resulted in a mean 10.1-point reduction in scores on the Hamilton Rating Scale for Depression after just 1 week of high-dose therapy, compared to an 8.4-point decrease in patient on the low-dose regimen and 4.2 points in placebo-treated controls.
Data source: An 8-week, double-blind, single-center, phase II study involving 30 patients with major depressive disorder.
Disclosures: The clinical trial was sponsored by Pherin Pharmaceuticals. The presenter is a consultant to and holds stock options in the company.
Novel procognitive drug promising in schizophrenia, Alzheimer's
HOLLYWOOD, FLA. – A novel procognitive medication has entered pivotal phase III, randomized clinical trials in patients with chronic schizophrenia, with a separate phase III trial due to start later this year in Alzheimer’s disease.
The drug, known for now as EVP-6124, is an oral alpha-7 nicotinic partial agonist. The alpha-7 nicotinic receptor is an acetylcholine receptor highly localized to the cortical and hippocampal regions of the brain. EVP-6124 acts as a coagonist in combination with acetylcholine to activate the receptor and enhance cognition, Dr. Ilise Lombardo explained at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
In a phase IIa randomized, double-blind placebo-controlled study in patients with stable chronic schizophrenia who remained on their atypical antipsychotic therapy, EVP-6124 normalized three EEG biomarkers for cognitive processing: p50, p300, and MMN, or mismatch negativity. This encouraging finding, coupled with clinical evidence of a procognitive effect on the CogState computerized test of cognition, led to a subsequent 319-patient, 12-week, double-blind phase IIb study.
The phase IIb trial showed that adding once-daily EVP-6124 to standard antipsychotic medication resulted in statistically significant and clinically meaningful benefits compared with placebo in terms of global cognitive function on the CogState test as well as on the Positive and Negative Syndrome Scale (PANSS) cognitive impairment domain, the PANSS negative symptoms domain, and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB). The drug had no effect on PANSS positive symptoms.
EVP-6124 also resulted in significant improvement in clinical function as measured on the interview-based Schizophrenia Cognition Rating Scale (SCoRS). That’s a key finding, because the Food and Drug Administration now requires evidence of improved clinical function as a condition for approval of any new treatment for schizophrenia, added Dr. Lombardo, vice president for clinical research at EnVivo Pharmaceuticals, which is developing the drug.
There are currently no approved treatments for the cognitive or negative symptoms of schizophrenia, making this a major unmet need in mental health care.
EVP-6124 proved safe and well tolerated in phase II testing. There were fewer adverse events and study dropouts than with placebo.
Based on these encouraging results, two phase III studies are underway. Each of the 26-week studies will randomize roughly 700 patients with schizophrenia. The MCCB and SCoRS tests will be the coprimary endpoints.
The recently completed double-blind, placebo-controlled, 24-week phase IIb study in Alzheimer’s disease involved 409 randomized patients. A dose-response effect was seen. The 0.3-mg dose of EVP-6124 was not significantly more effective than placebo. The 1.0-mg dose showed intermediate efficacy. And the 2.0-mg dose showed statistically significant benefits across the board, compared with placebo.
Moreover, these benefits were clinically meaningful in size. For example, the effect size for EVP-6124 at 2.0 mg/day was a solid 0.39 for the primary endpoint, which was improvement on the Alzheimer’s Disease Assessment Scale, 13-item subscale (ADAS-cog-13), Dr. Lombardo observed.
The top dose also resulted in significant improvement on numerous secondary endpoints, including the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), the Controlled Oral Word Association Test (COWAT), the Mini-Mental State Examination, and the ADAS-cog-11.
HOLLYWOOD, FLA. – A novel procognitive medication has entered pivotal phase III, randomized clinical trials in patients with chronic schizophrenia, with a separate phase III trial due to start later this year in Alzheimer’s disease.
The drug, known for now as EVP-6124, is an oral alpha-7 nicotinic partial agonist. The alpha-7 nicotinic receptor is an acetylcholine receptor highly localized to the cortical and hippocampal regions of the brain. EVP-6124 acts as a coagonist in combination with acetylcholine to activate the receptor and enhance cognition, Dr. Ilise Lombardo explained at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
In a phase IIa randomized, double-blind placebo-controlled study in patients with stable chronic schizophrenia who remained on their atypical antipsychotic therapy, EVP-6124 normalized three EEG biomarkers for cognitive processing: p50, p300, and MMN, or mismatch negativity. This encouraging finding, coupled with clinical evidence of a procognitive effect on the CogState computerized test of cognition, led to a subsequent 319-patient, 12-week, double-blind phase IIb study.
The phase IIb trial showed that adding once-daily EVP-6124 to standard antipsychotic medication resulted in statistically significant and clinically meaningful benefits compared with placebo in terms of global cognitive function on the CogState test as well as on the Positive and Negative Syndrome Scale (PANSS) cognitive impairment domain, the PANSS negative symptoms domain, and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB). The drug had no effect on PANSS positive symptoms.
EVP-6124 also resulted in significant improvement in clinical function as measured on the interview-based Schizophrenia Cognition Rating Scale (SCoRS). That’s a key finding, because the Food and Drug Administration now requires evidence of improved clinical function as a condition for approval of any new treatment for schizophrenia, added Dr. Lombardo, vice president for clinical research at EnVivo Pharmaceuticals, which is developing the drug.
There are currently no approved treatments for the cognitive or negative symptoms of schizophrenia, making this a major unmet need in mental health care.
EVP-6124 proved safe and well tolerated in phase II testing. There were fewer adverse events and study dropouts than with placebo.
Based on these encouraging results, two phase III studies are underway. Each of the 26-week studies will randomize roughly 700 patients with schizophrenia. The MCCB and SCoRS tests will be the coprimary endpoints.
The recently completed double-blind, placebo-controlled, 24-week phase IIb study in Alzheimer’s disease involved 409 randomized patients. A dose-response effect was seen. The 0.3-mg dose of EVP-6124 was not significantly more effective than placebo. The 1.0-mg dose showed intermediate efficacy. And the 2.0-mg dose showed statistically significant benefits across the board, compared with placebo.
Moreover, these benefits were clinically meaningful in size. For example, the effect size for EVP-6124 at 2.0 mg/day was a solid 0.39 for the primary endpoint, which was improvement on the Alzheimer’s Disease Assessment Scale, 13-item subscale (ADAS-cog-13), Dr. Lombardo observed.
The top dose also resulted in significant improvement on numerous secondary endpoints, including the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), the Controlled Oral Word Association Test (COWAT), the Mini-Mental State Examination, and the ADAS-cog-11.
HOLLYWOOD, FLA. – A novel procognitive medication has entered pivotal phase III, randomized clinical trials in patients with chronic schizophrenia, with a separate phase III trial due to start later this year in Alzheimer’s disease.
The drug, known for now as EVP-6124, is an oral alpha-7 nicotinic partial agonist. The alpha-7 nicotinic receptor is an acetylcholine receptor highly localized to the cortical and hippocampal regions of the brain. EVP-6124 acts as a coagonist in combination with acetylcholine to activate the receptor and enhance cognition, Dr. Ilise Lombardo explained at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
In a phase IIa randomized, double-blind placebo-controlled study in patients with stable chronic schizophrenia who remained on their atypical antipsychotic therapy, EVP-6124 normalized three EEG biomarkers for cognitive processing: p50, p300, and MMN, or mismatch negativity. This encouraging finding, coupled with clinical evidence of a procognitive effect on the CogState computerized test of cognition, led to a subsequent 319-patient, 12-week, double-blind phase IIb study.
The phase IIb trial showed that adding once-daily EVP-6124 to standard antipsychotic medication resulted in statistically significant and clinically meaningful benefits compared with placebo in terms of global cognitive function on the CogState test as well as on the Positive and Negative Syndrome Scale (PANSS) cognitive impairment domain, the PANSS negative symptoms domain, and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB). The drug had no effect on PANSS positive symptoms.
EVP-6124 also resulted in significant improvement in clinical function as measured on the interview-based Schizophrenia Cognition Rating Scale (SCoRS). That’s a key finding, because the Food and Drug Administration now requires evidence of improved clinical function as a condition for approval of any new treatment for schizophrenia, added Dr. Lombardo, vice president for clinical research at EnVivo Pharmaceuticals, which is developing the drug.
There are currently no approved treatments for the cognitive or negative symptoms of schizophrenia, making this a major unmet need in mental health care.
EVP-6124 proved safe and well tolerated in phase II testing. There were fewer adverse events and study dropouts than with placebo.
Based on these encouraging results, two phase III studies are underway. Each of the 26-week studies will randomize roughly 700 patients with schizophrenia. The MCCB and SCoRS tests will be the coprimary endpoints.
The recently completed double-blind, placebo-controlled, 24-week phase IIb study in Alzheimer’s disease involved 409 randomized patients. A dose-response effect was seen. The 0.3-mg dose of EVP-6124 was not significantly more effective than placebo. The 1.0-mg dose showed intermediate efficacy. And the 2.0-mg dose showed statistically significant benefits across the board, compared with placebo.
Moreover, these benefits were clinically meaningful in size. For example, the effect size for EVP-6124 at 2.0 mg/day was a solid 0.39 for the primary endpoint, which was improvement on the Alzheimer’s Disease Assessment Scale, 13-item subscale (ADAS-cog-13), Dr. Lombardo observed.
The top dose also resulted in significant improvement on numerous secondary endpoints, including the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), the Controlled Oral Word Association Test (COWAT), the Mini-Mental State Examination, and the ADAS-cog-11.
EXPERT ANALYSIS FROM THE NCDEU MEETING
TMS may bring remission in bipolar depression
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
AT THE NCDEU MEETING
Major finding: Nine of 14 patients with highly treatment-resistant bipolar depression achieved a clinically meaningful antidepressant response to transcranial magnetic stimulation, including 4 who reached and maintained remission. Two of the four in remission were prior nonresponders to ECT.
Data source: This observational study was a naturalistic case series with no control group. Patients had been unresponsive to and/or intolerant of a mean of 6.4 antidepressant medications during their current episode of bipolar depression, which had been ongoing for more than 18 months in nine cases.
Disclosures: Dr. Gilmer is on the speakers’ bureau for Neuronetics, which markets the transcranial magnetic stimulation device used in the investigation.