User login
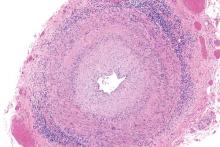
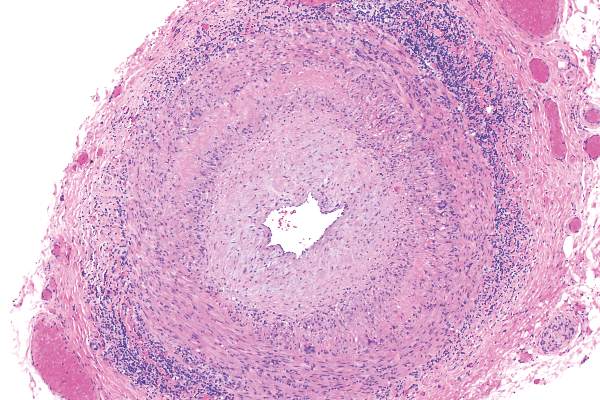
Low vasculitis risk with TNF inhibitors
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
AT RHEUMATOLOGY 2016
Key clinical point: There is a low risk of vasculitis-like events with tumor necrosis factor inhibitors.
Major finding: Crude incidence rates for vasculitis-like events were 16/10,000 person-years with TNF-inhibitor therapy and 7/10,000 person-years with nonbiologic disease-modifying antirheumatic drugs.
Data source: British Society for Rheumatology Biologics Register for Rheumatoid Arthritis of 12,745 TNF-inhibitor and 3,640 nbDMARD users.
Disclosures: The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
Vascular disease linked to sight loss in giant cell arteritis
GLASGOW – People with giant cell arteritis may be more likely to go blind if they have underlying vascular disease, according to an analysis of the Diagnostic and Classification Criteria in Vasculitis Study.
The results of the analysis showed that 7.9% of patients with this common type of vasculitis go blind in at least one eye within 6 months of a diagnosis, and that those with a history of peripheral vascular disease (PVD) could be up to 10 times more at risk than those without additional vascular comorbidity.
“This is the first multinational study for patients with [giant cell arteritis], and it shows that blindness is a significant problem,” said Dr. Max Yates of the University of East Anglia in Norwich, England, who presented the findings at the British Society for Rheumatology annual conference. Blindness was defined as complete visual loss rather than by a full ophthalmology assessment, so the findings probably underplay the problem in patients with some form of visual loss, he observed. Visual disturbance had been noted in 42.9% of the patients who were studied at the first clinic review.
“It is interesting that there is the association with vascular disease,” Dr. Yates said. “Perhaps we need greater vigilance in those people who already have a diagnosis of vascular disease [and] to really watch and monitor those people carefully for sight loss.”
The Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) is an ongoing project designed to develop and then validate new classification and diagnostic criteria for systemic vasculitis that can be used routinely in clinical practice and in clinical trials. So far, more than 3,500 participants over age 18 have been recruited from secondary care clinics, and of those, 2,000 have a new or established diagnosis of vasculitis. The others have a similar presentation but an alternative diagnosis.
A total of 433 patients participating in the DCVAS were identified as having GCA with more than 75% diagnostic certainty, of which 93% fulfilled the 1990 American College of Rheumatology (ACR) criteria for GCA and just over half (54%) had a positive temporal artery biopsy. Visual loss was recorded by completion of the Vasculitis Damage Index (VDI) 6 months after diagnosis.
Two-thirds of patients studied were female, the median age at diagnosis was 73 years, 40% had jaw claudication, 34% had lost weight, and 16% presented with a fever. In addition, 9.2% had diabetes, 3.2% a prior stroke, and 2.5% had PVD.
Looking for predictive factors, baseline laboratory findings such as the presence of anemia, the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, or platelet counts were not associated with sight loss. Dr. Yates noted in discussion that the baseline ESR range was 35-120 mm/h and the CRP ranged from 12 to over 100 mg/dL in the patients studied.
However, prior vascular disease was found to be predictive of later blindness. The odds ratio (OR) for being blind in at least one eye 6 months after a GCA diagnosis was 10.44 for PVD, with the 95% confidence interval (CI) ranging from 2.94 to 37.03. A prior diagnosis of cerebral vascular accident (OR, 4.47; 95% CI, 1.30-15.41), and diabetes (OR, 2.48; 95% CI, 0.98-6.25) also upped the risk for complete sight loss at 6 months.
Dr. Yates noted that patients were selected from multiple clinics across secondary care, so there should be better generalizability than in prior, single-center studies. However, there could be some residual referral bias.
During discussion, it was pointed out that it would be helpful to know the rate of blindness in patients taking steroids, as this was one of the major reasons for emergency rheumatology calls at one clinic, a delegate observed.
“Giant cell arteritis is really the major rheumatology emergency for practicing clinicians. We recently set up a rheumatology day service and get usually 8-10 calls about it per day,” the delegate said. “It’s often said that once a patient is on any dose of steroids, there is not risk of them going blind.” There is a lot of angst about whether it is safe to use higher doses (60 mg vs. 40 mg) and “it’s important for us as clinicians to be able to reassure people.”
Dr. Yates noted that a prospective trial would be needed to answer the question and that trials were planned. “We don’t have any data on treatment,” he said. “So we’re unable to say whether steroids were started instantly or whether there was any improvement in the visual function of these people.” Long-term complications also would be something to look at, particularly in older people who have an increased risk for eye problems such as cataracts, and could be at higher risk for visual problems if treated with steroids or other agents.
The DCVAS study is supported by the ACR and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
GLASGOW – People with giant cell arteritis may be more likely to go blind if they have underlying vascular disease, according to an analysis of the Diagnostic and Classification Criteria in Vasculitis Study.
The results of the analysis showed that 7.9% of patients with this common type of vasculitis go blind in at least one eye within 6 months of a diagnosis, and that those with a history of peripheral vascular disease (PVD) could be up to 10 times more at risk than those without additional vascular comorbidity.
“This is the first multinational study for patients with [giant cell arteritis], and it shows that blindness is a significant problem,” said Dr. Max Yates of the University of East Anglia in Norwich, England, who presented the findings at the British Society for Rheumatology annual conference. Blindness was defined as complete visual loss rather than by a full ophthalmology assessment, so the findings probably underplay the problem in patients with some form of visual loss, he observed. Visual disturbance had been noted in 42.9% of the patients who were studied at the first clinic review.
“It is interesting that there is the association with vascular disease,” Dr. Yates said. “Perhaps we need greater vigilance in those people who already have a diagnosis of vascular disease [and] to really watch and monitor those people carefully for sight loss.”
The Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) is an ongoing project designed to develop and then validate new classification and diagnostic criteria for systemic vasculitis that can be used routinely in clinical practice and in clinical trials. So far, more than 3,500 participants over age 18 have been recruited from secondary care clinics, and of those, 2,000 have a new or established diagnosis of vasculitis. The others have a similar presentation but an alternative diagnosis.
A total of 433 patients participating in the DCVAS were identified as having GCA with more than 75% diagnostic certainty, of which 93% fulfilled the 1990 American College of Rheumatology (ACR) criteria for GCA and just over half (54%) had a positive temporal artery biopsy. Visual loss was recorded by completion of the Vasculitis Damage Index (VDI) 6 months after diagnosis.
Two-thirds of patients studied were female, the median age at diagnosis was 73 years, 40% had jaw claudication, 34% had lost weight, and 16% presented with a fever. In addition, 9.2% had diabetes, 3.2% a prior stroke, and 2.5% had PVD.
Looking for predictive factors, baseline laboratory findings such as the presence of anemia, the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, or platelet counts were not associated with sight loss. Dr. Yates noted in discussion that the baseline ESR range was 35-120 mm/h and the CRP ranged from 12 to over 100 mg/dL in the patients studied.
However, prior vascular disease was found to be predictive of later blindness. The odds ratio (OR) for being blind in at least one eye 6 months after a GCA diagnosis was 10.44 for PVD, with the 95% confidence interval (CI) ranging from 2.94 to 37.03. A prior diagnosis of cerebral vascular accident (OR, 4.47; 95% CI, 1.30-15.41), and diabetes (OR, 2.48; 95% CI, 0.98-6.25) also upped the risk for complete sight loss at 6 months.
Dr. Yates noted that patients were selected from multiple clinics across secondary care, so there should be better generalizability than in prior, single-center studies. However, there could be some residual referral bias.
During discussion, it was pointed out that it would be helpful to know the rate of blindness in patients taking steroids, as this was one of the major reasons for emergency rheumatology calls at one clinic, a delegate observed.
“Giant cell arteritis is really the major rheumatology emergency for practicing clinicians. We recently set up a rheumatology day service and get usually 8-10 calls about it per day,” the delegate said. “It’s often said that once a patient is on any dose of steroids, there is not risk of them going blind.” There is a lot of angst about whether it is safe to use higher doses (60 mg vs. 40 mg) and “it’s important for us as clinicians to be able to reassure people.”
Dr. Yates noted that a prospective trial would be needed to answer the question and that trials were planned. “We don’t have any data on treatment,” he said. “So we’re unable to say whether steroids were started instantly or whether there was any improvement in the visual function of these people.” Long-term complications also would be something to look at, particularly in older people who have an increased risk for eye problems such as cataracts, and could be at higher risk for visual problems if treated with steroids or other agents.
The DCVAS study is supported by the ACR and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
GLASGOW – People with giant cell arteritis may be more likely to go blind if they have underlying vascular disease, according to an analysis of the Diagnostic and Classification Criteria in Vasculitis Study.
The results of the analysis showed that 7.9% of patients with this common type of vasculitis go blind in at least one eye within 6 months of a diagnosis, and that those with a history of peripheral vascular disease (PVD) could be up to 10 times more at risk than those without additional vascular comorbidity.
“This is the first multinational study for patients with [giant cell arteritis], and it shows that blindness is a significant problem,” said Dr. Max Yates of the University of East Anglia in Norwich, England, who presented the findings at the British Society for Rheumatology annual conference. Blindness was defined as complete visual loss rather than by a full ophthalmology assessment, so the findings probably underplay the problem in patients with some form of visual loss, he observed. Visual disturbance had been noted in 42.9% of the patients who were studied at the first clinic review.
“It is interesting that there is the association with vascular disease,” Dr. Yates said. “Perhaps we need greater vigilance in those people who already have a diagnosis of vascular disease [and] to really watch and monitor those people carefully for sight loss.”
The Diagnostic and Classification Criteria in Vasculitis Study (DCVAS) is an ongoing project designed to develop and then validate new classification and diagnostic criteria for systemic vasculitis that can be used routinely in clinical practice and in clinical trials. So far, more than 3,500 participants over age 18 have been recruited from secondary care clinics, and of those, 2,000 have a new or established diagnosis of vasculitis. The others have a similar presentation but an alternative diagnosis.
A total of 433 patients participating in the DCVAS were identified as having GCA with more than 75% diagnostic certainty, of which 93% fulfilled the 1990 American College of Rheumatology (ACR) criteria for GCA and just over half (54%) had a positive temporal artery biopsy. Visual loss was recorded by completion of the Vasculitis Damage Index (VDI) 6 months after diagnosis.
Two-thirds of patients studied were female, the median age at diagnosis was 73 years, 40% had jaw claudication, 34% had lost weight, and 16% presented with a fever. In addition, 9.2% had diabetes, 3.2% a prior stroke, and 2.5% had PVD.
Looking for predictive factors, baseline laboratory findings such as the presence of anemia, the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, or platelet counts were not associated with sight loss. Dr. Yates noted in discussion that the baseline ESR range was 35-120 mm/h and the CRP ranged from 12 to over 100 mg/dL in the patients studied.
However, prior vascular disease was found to be predictive of later blindness. The odds ratio (OR) for being blind in at least one eye 6 months after a GCA diagnosis was 10.44 for PVD, with the 95% confidence interval (CI) ranging from 2.94 to 37.03. A prior diagnosis of cerebral vascular accident (OR, 4.47; 95% CI, 1.30-15.41), and diabetes (OR, 2.48; 95% CI, 0.98-6.25) also upped the risk for complete sight loss at 6 months.
Dr. Yates noted that patients were selected from multiple clinics across secondary care, so there should be better generalizability than in prior, single-center studies. However, there could be some residual referral bias.
During discussion, it was pointed out that it would be helpful to know the rate of blindness in patients taking steroids, as this was one of the major reasons for emergency rheumatology calls at one clinic, a delegate observed.
“Giant cell arteritis is really the major rheumatology emergency for practicing clinicians. We recently set up a rheumatology day service and get usually 8-10 calls about it per day,” the delegate said. “It’s often said that once a patient is on any dose of steroids, there is not risk of them going blind.” There is a lot of angst about whether it is safe to use higher doses (60 mg vs. 40 mg) and “it’s important for us as clinicians to be able to reassure people.”
Dr. Yates noted that a prospective trial would be needed to answer the question and that trials were planned. “We don’t have any data on treatment,” he said. “So we’re unable to say whether steroids were started instantly or whether there was any improvement in the visual function of these people.” Long-term complications also would be something to look at, particularly in older people who have an increased risk for eye problems such as cataracts, and could be at higher risk for visual problems if treated with steroids or other agents.
The DCVAS study is supported by the ACR and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
AT RHEUMATOLOGY 2016
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
TRACTISS ends rituximab hopes for Sjögren’s syndrome
GLASGOW – Rituximab is unlikely to work as a treatment for Sjögren’s syndrome, according to a sneak peak of results from a multicenter study.
The study, the Trial of Anti–B Cell Therapy in Patients With Primary Sjögren’s Syndrome (TRACTISS), showed that there were no significant differences between the active and placebo arms in terms of alleviating oral dryness or fatigue, Dr. Simon J. Bowman, a principal investigator for the trial, observed at the British Society for Rheumatology annual conference. In addition, no difference was found in the EULAR Sjögren’s syndrome disease activity index (ESSDAI).
In TRACTISS, 110 patients with primary Sjögren’s syndrome received two courses of rituximab (Rituxan) or placebo in addition to standard therapy, and they were followed up for up to 48 weeks (BMC Musculoskelet Disord. 2014;15:21).
Dr. Bowman, a consultant rheumatologist and honorary professor of rheumatology at the Queen Elizabeth Hospital, Birmingham, England, noted that there was some indication of an effect of rituximab on fatigue and oral dryness in another large, randomized, controlled trial – the Tolerance and Efficacy of Rituximab in Sjögren’s syndrome (TEARS) study – a couple of years ago (Ann Rheum Dis. 2013;72[6]:1026-31), but these were not the primary endpoints of the study, so those results were essentially negative.
Calling the TRACTISS findings something of disappointment, Dr. Bowman said: “I think we’ll have to wait and see, but having had two big formal trials, I have to say that it doesn’t look like rituximab on its own is going to be any [great] success.”
Hopes were high for rituximab 5 years ago, when recruitment into the TRACTISS trial started, principally because drugs like rituximab already were available for use, Dr. Bowman said at the time. The focus was on anti–B cell therapies, because not only were they available but several features of Sjögren’s syndrome potentially are linked to B-cell activity, such as fatigue in about two-thirds of patients, the presence of anti-Ro and anti-La antibodies in about 40% of patients, and lymphoma in about 5% of patients. Also, several small studies had suggested rituximab might prove beneficial.
Other B-cell therapeutic options
Clearly, other B-cell targets might offer alternative therapeutic approaches. One of those under investigation is using belimumab (Benlysta) to target B cell–activating factor (BAFF) or B-cell lymphocyte stimulator (BLyS). Results of the BELISS study, a small, open-label, phase II trial in 30 patients with Sjögren’s syndrome (Ann Rheum Dis. 2015;74[3]:526-31) showed there were some improvements in the ESSDAI and the EULAR Sjögren’s Syndrome Patient-Reported Index (ESSPRI). The improvements were great enough to justify proceeding to a larger clinical trial.
Perhaps the real hope for biologic therapy lies in targeting the T cells for which there is a very good rationale, said Wan-Fai Ng, Ph.D., professor of rheumatology at Newcastle University, Newcastle upon Tyne, England. Various approaches are available, and in addition, there have been some “encouraging” data from early-phase clinical trials.
Abatacept (Orencia), for example, has been looked at in a few trials, with positive drops in ESSDAI and other endpoints, and currently, there is an ongoing phase III trial in 88 patients with primary Sjögren’s syndrome. There are a couple of ongoing phase II trials, one with the anti-CD40 molecule CFZ533 and another with the anti-B7RP1 molecule AMG 557.
Dr. Ng noted that a trial of efalizumab (Raptiva) had terminated because of an increased risk for progressive multifocal leukoencephalopathy. In addition, a couple of other molecules, rhIL-2 and alefacept (Amevive), are under investigation, he said.
Four additional therapeutic targets
Other novel approaches to developing biologic therapy for Sjögren’s were summarized by Dr. Francesca Barone, a senior lecturer at the Queen Elizabeth Hospital in Birmingham, England. She described four strategies, the first of which was targeting the cross talk between antigen-presenting dendritic cells and T cells using a novel molecule RO5459072 that targets a protein called cathepsin S. Cathepsin S is involved in the assembly of the major histocompatibility complex II protein, and by interfering with this process, the theory is that the effector T-helper cell response will be muted and T-cell interaction with B cells will be decreased. A phase II “proof-of-concept” trial is underway with RO5459072 and will recruit 70 patients, she said.
A second strategy is targeting intracellular B cell signaling by targeting P13 kinase delta with UCB 5857. P13 kinase delta catalyzes B cell activation and “is really at the core of the biology of the B cell,” Dr. Barone observed. A phase II trial with UCB 5857 is planned in 52 patients.
A third approach is to target lymphoneogenesis more generally by interfering with the production of the chemokines CXCL13 and CXCL12. This might be achieved via interleukin (IL)–17 and IL-22 blockade. A trial with the novel agent baminercept, a lymphotoxin beta receptor fusion protein. However, that trial was stopped in 2014 for technical reasons.
A fourth strategy is to use combination “sandwich” treatment, consisting of belimumab, rituximab, and then belimumab again. “This is what is going to come next, putting two drugs together,” Dr. Barone said. A randomized, double-blind, controlled trial now open to recruitment aims to investigate whether there is value in this approach.
“We’ve bombarded you with a lot of targets,” Dr. Barone observed. “What do we believe is going to be the best one is very, very unclear.”
TRACTISS was funded by Arthritis Research UK with rituximab provided by Roche. Dr. Bowman did not provide any disclosures but previously has been a consultant for Roche, UCB Pharma, and Merck-Serono. Dr. Ng reported no conflicts of interest, and Dr. Barone disclosed consultancy or collaboration with UCB Pharma, GlaxoSmithKline, Celgene, Eli Lilly, and Roche.
GLASGOW – Rituximab is unlikely to work as a treatment for Sjögren’s syndrome, according to a sneak peak of results from a multicenter study.
The study, the Trial of Anti–B Cell Therapy in Patients With Primary Sjögren’s Syndrome (TRACTISS), showed that there were no significant differences between the active and placebo arms in terms of alleviating oral dryness or fatigue, Dr. Simon J. Bowman, a principal investigator for the trial, observed at the British Society for Rheumatology annual conference. In addition, no difference was found in the EULAR Sjögren’s syndrome disease activity index (ESSDAI).
In TRACTISS, 110 patients with primary Sjögren’s syndrome received two courses of rituximab (Rituxan) or placebo in addition to standard therapy, and they were followed up for up to 48 weeks (BMC Musculoskelet Disord. 2014;15:21).
Dr. Bowman, a consultant rheumatologist and honorary professor of rheumatology at the Queen Elizabeth Hospital, Birmingham, England, noted that there was some indication of an effect of rituximab on fatigue and oral dryness in another large, randomized, controlled trial – the Tolerance and Efficacy of Rituximab in Sjögren’s syndrome (TEARS) study – a couple of years ago (Ann Rheum Dis. 2013;72[6]:1026-31), but these were not the primary endpoints of the study, so those results were essentially negative.
Calling the TRACTISS findings something of disappointment, Dr. Bowman said: “I think we’ll have to wait and see, but having had two big formal trials, I have to say that it doesn’t look like rituximab on its own is going to be any [great] success.”
Hopes were high for rituximab 5 years ago, when recruitment into the TRACTISS trial started, principally because drugs like rituximab already were available for use, Dr. Bowman said at the time. The focus was on anti–B cell therapies, because not only were they available but several features of Sjögren’s syndrome potentially are linked to B-cell activity, such as fatigue in about two-thirds of patients, the presence of anti-Ro and anti-La antibodies in about 40% of patients, and lymphoma in about 5% of patients. Also, several small studies had suggested rituximab might prove beneficial.
Other B-cell therapeutic options
Clearly, other B-cell targets might offer alternative therapeutic approaches. One of those under investigation is using belimumab (Benlysta) to target B cell–activating factor (BAFF) or B-cell lymphocyte stimulator (BLyS). Results of the BELISS study, a small, open-label, phase II trial in 30 patients with Sjögren’s syndrome (Ann Rheum Dis. 2015;74[3]:526-31) showed there were some improvements in the ESSDAI and the EULAR Sjögren’s Syndrome Patient-Reported Index (ESSPRI). The improvements were great enough to justify proceeding to a larger clinical trial.
Perhaps the real hope for biologic therapy lies in targeting the T cells for which there is a very good rationale, said Wan-Fai Ng, Ph.D., professor of rheumatology at Newcastle University, Newcastle upon Tyne, England. Various approaches are available, and in addition, there have been some “encouraging” data from early-phase clinical trials.
Abatacept (Orencia), for example, has been looked at in a few trials, with positive drops in ESSDAI and other endpoints, and currently, there is an ongoing phase III trial in 88 patients with primary Sjögren’s syndrome. There are a couple of ongoing phase II trials, one with the anti-CD40 molecule CFZ533 and another with the anti-B7RP1 molecule AMG 557.
Dr. Ng noted that a trial of efalizumab (Raptiva) had terminated because of an increased risk for progressive multifocal leukoencephalopathy. In addition, a couple of other molecules, rhIL-2 and alefacept (Amevive), are under investigation, he said.
Four additional therapeutic targets
Other novel approaches to developing biologic therapy for Sjögren’s were summarized by Dr. Francesca Barone, a senior lecturer at the Queen Elizabeth Hospital in Birmingham, England. She described four strategies, the first of which was targeting the cross talk between antigen-presenting dendritic cells and T cells using a novel molecule RO5459072 that targets a protein called cathepsin S. Cathepsin S is involved in the assembly of the major histocompatibility complex II protein, and by interfering with this process, the theory is that the effector T-helper cell response will be muted and T-cell interaction with B cells will be decreased. A phase II “proof-of-concept” trial is underway with RO5459072 and will recruit 70 patients, she said.
A second strategy is targeting intracellular B cell signaling by targeting P13 kinase delta with UCB 5857. P13 kinase delta catalyzes B cell activation and “is really at the core of the biology of the B cell,” Dr. Barone observed. A phase II trial with UCB 5857 is planned in 52 patients.
A third approach is to target lymphoneogenesis more generally by interfering with the production of the chemokines CXCL13 and CXCL12. This might be achieved via interleukin (IL)–17 and IL-22 blockade. A trial with the novel agent baminercept, a lymphotoxin beta receptor fusion protein. However, that trial was stopped in 2014 for technical reasons.
A fourth strategy is to use combination “sandwich” treatment, consisting of belimumab, rituximab, and then belimumab again. “This is what is going to come next, putting two drugs together,” Dr. Barone said. A randomized, double-blind, controlled trial now open to recruitment aims to investigate whether there is value in this approach.
“We’ve bombarded you with a lot of targets,” Dr. Barone observed. “What do we believe is going to be the best one is very, very unclear.”
TRACTISS was funded by Arthritis Research UK with rituximab provided by Roche. Dr. Bowman did not provide any disclosures but previously has been a consultant for Roche, UCB Pharma, and Merck-Serono. Dr. Ng reported no conflicts of interest, and Dr. Barone disclosed consultancy or collaboration with UCB Pharma, GlaxoSmithKline, Celgene, Eli Lilly, and Roche.
GLASGOW – Rituximab is unlikely to work as a treatment for Sjögren’s syndrome, according to a sneak peak of results from a multicenter study.
The study, the Trial of Anti–B Cell Therapy in Patients With Primary Sjögren’s Syndrome (TRACTISS), showed that there were no significant differences between the active and placebo arms in terms of alleviating oral dryness or fatigue, Dr. Simon J. Bowman, a principal investigator for the trial, observed at the British Society for Rheumatology annual conference. In addition, no difference was found in the EULAR Sjögren’s syndrome disease activity index (ESSDAI).
In TRACTISS, 110 patients with primary Sjögren’s syndrome received two courses of rituximab (Rituxan) or placebo in addition to standard therapy, and they were followed up for up to 48 weeks (BMC Musculoskelet Disord. 2014;15:21).
Dr. Bowman, a consultant rheumatologist and honorary professor of rheumatology at the Queen Elizabeth Hospital, Birmingham, England, noted that there was some indication of an effect of rituximab on fatigue and oral dryness in another large, randomized, controlled trial – the Tolerance and Efficacy of Rituximab in Sjögren’s syndrome (TEARS) study – a couple of years ago (Ann Rheum Dis. 2013;72[6]:1026-31), but these were not the primary endpoints of the study, so those results were essentially negative.
Calling the TRACTISS findings something of disappointment, Dr. Bowman said: “I think we’ll have to wait and see, but having had two big formal trials, I have to say that it doesn’t look like rituximab on its own is going to be any [great] success.”
Hopes were high for rituximab 5 years ago, when recruitment into the TRACTISS trial started, principally because drugs like rituximab already were available for use, Dr. Bowman said at the time. The focus was on anti–B cell therapies, because not only were they available but several features of Sjögren’s syndrome potentially are linked to B-cell activity, such as fatigue in about two-thirds of patients, the presence of anti-Ro and anti-La antibodies in about 40% of patients, and lymphoma in about 5% of patients. Also, several small studies had suggested rituximab might prove beneficial.
Other B-cell therapeutic options
Clearly, other B-cell targets might offer alternative therapeutic approaches. One of those under investigation is using belimumab (Benlysta) to target B cell–activating factor (BAFF) or B-cell lymphocyte stimulator (BLyS). Results of the BELISS study, a small, open-label, phase II trial in 30 patients with Sjögren’s syndrome (Ann Rheum Dis. 2015;74[3]:526-31) showed there were some improvements in the ESSDAI and the EULAR Sjögren’s Syndrome Patient-Reported Index (ESSPRI). The improvements were great enough to justify proceeding to a larger clinical trial.
Perhaps the real hope for biologic therapy lies in targeting the T cells for which there is a very good rationale, said Wan-Fai Ng, Ph.D., professor of rheumatology at Newcastle University, Newcastle upon Tyne, England. Various approaches are available, and in addition, there have been some “encouraging” data from early-phase clinical trials.
Abatacept (Orencia), for example, has been looked at in a few trials, with positive drops in ESSDAI and other endpoints, and currently, there is an ongoing phase III trial in 88 patients with primary Sjögren’s syndrome. There are a couple of ongoing phase II trials, one with the anti-CD40 molecule CFZ533 and another with the anti-B7RP1 molecule AMG 557.
Dr. Ng noted that a trial of efalizumab (Raptiva) had terminated because of an increased risk for progressive multifocal leukoencephalopathy. In addition, a couple of other molecules, rhIL-2 and alefacept (Amevive), are under investigation, he said.
Four additional therapeutic targets
Other novel approaches to developing biologic therapy for Sjögren’s were summarized by Dr. Francesca Barone, a senior lecturer at the Queen Elizabeth Hospital in Birmingham, England. She described four strategies, the first of which was targeting the cross talk between antigen-presenting dendritic cells and T cells using a novel molecule RO5459072 that targets a protein called cathepsin S. Cathepsin S is involved in the assembly of the major histocompatibility complex II protein, and by interfering with this process, the theory is that the effector T-helper cell response will be muted and T-cell interaction with B cells will be decreased. A phase II “proof-of-concept” trial is underway with RO5459072 and will recruit 70 patients, she said.
A second strategy is targeting intracellular B cell signaling by targeting P13 kinase delta with UCB 5857. P13 kinase delta catalyzes B cell activation and “is really at the core of the biology of the B cell,” Dr. Barone observed. A phase II trial with UCB 5857 is planned in 52 patients.
A third approach is to target lymphoneogenesis more generally by interfering with the production of the chemokines CXCL13 and CXCL12. This might be achieved via interleukin (IL)–17 and IL-22 blockade. A trial with the novel agent baminercept, a lymphotoxin beta receptor fusion protein. However, that trial was stopped in 2014 for technical reasons.
A fourth strategy is to use combination “sandwich” treatment, consisting of belimumab, rituximab, and then belimumab again. “This is what is going to come next, putting two drugs together,” Dr. Barone said. A randomized, double-blind, controlled trial now open to recruitment aims to investigate whether there is value in this approach.
“We’ve bombarded you with a lot of targets,” Dr. Barone observed. “What do we believe is going to be the best one is very, very unclear.”
TRACTISS was funded by Arthritis Research UK with rituximab provided by Roche. Dr. Bowman did not provide any disclosures but previously has been a consultant for Roche, UCB Pharma, and Merck-Serono. Dr. Ng reported no conflicts of interest, and Dr. Barone disclosed consultancy or collaboration with UCB Pharma, GlaxoSmithKline, Celgene, Eli Lilly, and Roche.
AT RHEUMATOLOGY 2016
Key clinical point: Randomized clinical trial data suggest rituximab is unlikely to have any benefit in treating patients with primary Sjögren’s syndrome.
Major finding: There were no significant differences in the study endpoints of sicca syndrome, fatigue, or systemic disease.
Data source: TRACTISS, a U.K. multicenter, double-blind, randomized, controlled, parallel-group trial of rituximab vs. placebo in 110 patients with primary Sjögren’s syndrome.
Disclosures: TRACTISS was funded by Arthritis Research UK, with rituximab provided by Roche. Dr. Bowman did not provide any disclosures but previously has been a consultant for Roche, UCB Pharma, and Merck-Serono. Dr. Ng reported no conflicts of interest, and Dr. Barone disclosed consultancy or collaboration with UCB Pharma, GlaxoSmithKline, Celgene, Eli Lilly, and Roche.
Simple Signs Could Signal Complex Pain Syndrome
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
AT RHEUMATOLOGY 2016
Simple signs could signal complex pain syndrome
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
GLASGOW – Two novel clinical signs can be used to determine whether people who have had a fracture are likely to develop complex regional pain syndrome, a condition for which there is currently no specific diagnostic test.
The way in which patients perceived which of their fingers was being touched when their eyes were closed (finger perception) and the position of different parts of their body in relation to one another generally (body scheme) were found to be reliable ways of identifying those who would experience prolonged pain in the future. The positive and negative predictive values of using those tests together were a respective 84.1% and 76.9%.
“Digit misperception and body scheme combined can be useful in predicting chronic pain,” Dr. Nicholas Shenker of Addenbrooke’s Hospital in Cambridge, England, said in an interview at the British Society for Rheumatology annual conference.
“This may allow a reduction in chronic pain by identifying a cohort requiring more intensive intervention with physiotherapy and intervention,” he added. It also may help to reduce the incidence of complex regional pain syndrome (CRPS) after sustaining a fracture.
CPRS is characterized by prolonged, intractable pain that often follows injury to a limb, such as a fracture or another traumatic event. “It’s a nervy type pain, burning, and shooting, and it’s a horrible condition,” Dr. Shenker explained. “If you ask patients to rate the amount of pain they get from their condition, whether it is rheumatoid arthritis, fibromyalgia, or osteoarthritis, this is the condition that commonly comes top.”
With differential diagnoses spanning many rheumatologic and neurologic conditions, it is perhaps no surprise that patients with CRPS often go undiagnosed for many months. In fact, according to CRPS Registry UK findings, it can take up to 6 months after symptoms start before patients are diagnosed with the pain syndrome (Br J Pain. 2015;9[2]:122-8).
While pain symptoms may resolve by themselves in about one-third of patients by 3 months and three-quarters of patients by 12 months, there are a significant number of patients whose symptoms do not resolve, and their window of opportunity for treatment may have closed. Although treatment may be largely reassurance based for many, some patients may benefit from early physical therapy or pain medication.
“Once you have any chronic pain condition for more than 12 months, the chances of it getting better are pretty slim. So we want to identify these people early, but that is not easy,” Dr. Shenker observed.
Since there is currently no diagnostic test, Dr. Shenker and his associates identified four novel clinical signs – abnormal finger perception, hand laterality identification, body scheme report, and astereognosis – from a cohort of patients with CRPS and developed tests for them.
Finger perception was assessed by asking the subjects to place their hands on their laps, close their eyes and say which finger or thumb was being touched within a 20-second time limit. A positive test was a score of 8 or less out of 10.
Hand laterality was tested using a computer program showing the subjects a series of images of left and right hands and asking them to click whether the image was of a person’s right or left hand. A positive test was a score of fewer than 50 out of 56 or if the subject took longer than 3 minutes to complete the exercise.
Astereognosis, the inability to identify an object by the touch of the hands only, was assessed by asking the subjects to close their eyes and present their hand palms upward, then placing three different objects into it and asking them what they were touching. A test was considered positive if only two out of three objects were recognized, or if the subjects took longer than 6 seconds to identify the objects.
Finally, body scheme is about assessing how patients generally sense the parts of their bodies with their eyes closed, or how one side of the body compares in terms of size, weight, and length to the other. For this test, the body is divided into 17 areas, and over the course of 2 minutes, patients are asked to describe, without moving, how their left side compares to their right side. A test is considered positive if patients’ responses differ by more than 10% in two contiguous areas, such as the arm and elbow.
Only finger perception and body scheme were found to have good positive and negative predictive values individually.
The aim of the prospective observational cohort study Dr. Shenkar presented at the meeting on behalf of colleague Dr. Anoop Kuttikat was to look at those signs to see how common those scenarios were and to determine if these could be used as simple bedside tests to identify those likely to develop CRPS.
Dr. Shenkar reported data on 47 patients aged a mean of 53 years who needed a plaster cast for an upper (wrist) or lower (ankle/tibia) limb fracture who were assessed fewer than 2 weeks after their injury and followed up for 6 months. Their medical records were reviewed 3 years later to see whether chronic pain was present.
One patient developed CRPS. This patient had both a positive finger perception and body scheme test at the baseline assessment. Three patients had persistent pain, and two of those had positive finger perception and body scheme tests. The remaining 43 patients did not have persistent pain, and four of these patients had positive finger perception and body scheme tests. This means that 7 out of the 47 patients would be flagged very early on for further assessment and possible treatment, Dr. Shenker observed.
Future plans are to refine a quicker test for body scheme assessment and to perform a larger prospective multicenter study.
Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
AT RHEUMATOLOGY 2016
Key clinical point: The ways in which patients perceive the position of their fingers and body generally could help identify those with complex regional pain syndrome.
Major finding: Combining the two simple clinical signs had a positive predictive value of 84% and a negative predictive value of 76.9%
Data source: Prospective, single-center observational cohort study of 47 patients with an upper or lower limb fracture followed for up to 6 months and reviewed for pain at 3 years.
Disclosures: Dr. Shenker has received grant or research support from the British Medical Association, the U.K. National Institute for Health Research’s Clinical Research Network, and the Cambridge Arthritis Research Endeavour.
Study backs moderate alcohol intake for MTX patients
GLASGOW – Advising patients with rheumatoid arthritis who are being treated with methotrexate to moderate their alcohol intake remains sound advice, the results of a large clinical database study showed.
Increasing alcohol intake was found to be associated with an increased risk for developing abnormal results on serum liver function tests (LFTs). However, the clinical importance of this was thought to be small if the number of alcoholic units per week remained below 14 – which is general advice for men and women in the United Kingdom.
“We advise our patients to restrict their alcohol consumption, because we know that alcohol is hepatotoxic …, methotrexate is hepatotoxic, and there is a presumed interaction there,” the presenting study author, Dr. Jenny H. Humphreys, said in an interview at the British Society for Rheumatology annual conference. “But actually, the data to support that advice [are] fairly limited and may not reflect our current patient population,” added Dr. Humphreys of the Arthritis Research U.K. Centre for Epidemiology at the University of Manchester (England).
In a poster presentation, Dr. Humphreys aimed to quantify the association between alcohol intake and serum alanine or aspartate aminotransferase (ALT/AST) levels using routinely collected clinical data from the Clinical Practice Research Datalink (CPRD). Data on more than 8,000 patients starting methotrexate (MTX) for treating rheumatoid arthritis (RA) between 1987 and 2011 were obtained and analyzed.
Patients were included in the analysis if they had information on their alcohol consumption recorded and if serum LFTs had been assessed routinely by their primary care physician at least six times per year. They were then grouped based on their weekly reported alcohol consumption from none (0 units) to excessive (more than 28 units). In the United Kingdom, a unit of alcohol is defined as 10 mL (8 g) of pure alcohol, and the typical alcoholic beverage may contain 1-3 units of alcohol. The mean age of participants in the study was 58 years, and 71% were female.
When 38,000 person-years of follow-up were evaluated, 241 abnormal LFT events, defined as ALT/AST levels more than three times the upper limit of normal, were identified.
Crude incidence rates of abnormal LFTs per 1,000 person-years were 5.58 in nondrinkers, 5.57 in those who drank 1-7 units of alcohol per week, 5.99 in those who drank 8-14 units, 7.58 in those who drank 15-21 units, 8.61 in those who drank 22-28 units, and 9.05 in those who drank more than 28 units. Although the hazard ratios adjusted for age and sex also increased with increasing alcohol intake, from 1.02 in the lightest drinkers to 1.92 in the heaviest drinkers, no statistical significance was found overall between the drinkers and nondrinkers.
“When treated as a continuous variable, there was a statistically significant increase of 1% abnormal LFTs for every unit of alcohol consumed, but you wonder how clinically relevant is a 1% increase,” Dr. Humphreys said.
To look at the data another way, a clinically relevant increase was set for abnormal LFTs; then the data for increasing alcohol consumption were used to see how many patients had crossed that boundary. The investigators found an increase with higher alcohol consumption levels, but that power was limited to patients who drank 14 units a week or fewer. “So essentially, increasing alcohol consumption does increase your risk of abnormal LFTs. But when you are drinking less than about 14 units a week, there is little clinical significance of that, “ Dr. Humphreys said. So for patients who do want to continue drinking while on MTX, it should be reasonably safe to advise them that they can do so as long as they moderate their intake.
Separate research presented in a poster by Dr. Ravi Narang of Frimley Health NHS Foundation Trust in Surrey, England, and associates looked at the use of hepatic elastography (FibroScan) in MTX-treated patients and found 91% of 44 patients had normal ALT levels before their liver was scanned. Of 23 patients who were scanned, 11 (48%) had abnormal findings, and 9 (39%) showed signs of fatty liver. Seven (16%) patients underwent a liver biopsy based on the liver scan findings, and five stopped treatment with MTX based on the findings. Methotrexate also was stopped in nine other patients who did not undergo biopsy but had raised liver stiffness, which is a sign of fibrosis. Meanwhile, three patients continued at a reduced dose.
The patients in their retrospective case note study included those who had undergone FibroScan between 2011 and 2015 while on MTX. Most (56%) patients were taking MTX for RA; 27%, for psoriatic arthritis; 9%, for psoriasis; and the remainder, for other rheumatologic or dermatologic conditions. The median age at the time of the scan was 64 years. Eight patients had comorbid diabetes mellitus, and 11% consumed more than 14 units of alcohol per week.
“FibroScan led to a change in management in many of our patients confirming its value as a noninvasive adjunct for assessing hepatic fibrosis,” said Dr. Narang. He and his associates noted that the imaging technique was “guiding practice” and confirmed the “relative insensitivity of current methotrexate liver monitoring.”
Dr. Humphreys, Dr. Narang, and their associates had no relevant financial disclosures.
GLASGOW – Advising patients with rheumatoid arthritis who are being treated with methotrexate to moderate their alcohol intake remains sound advice, the results of a large clinical database study showed.
Increasing alcohol intake was found to be associated with an increased risk for developing abnormal results on serum liver function tests (LFTs). However, the clinical importance of this was thought to be small if the number of alcoholic units per week remained below 14 – which is general advice for men and women in the United Kingdom.
“We advise our patients to restrict their alcohol consumption, because we know that alcohol is hepatotoxic …, methotrexate is hepatotoxic, and there is a presumed interaction there,” the presenting study author, Dr. Jenny H. Humphreys, said in an interview at the British Society for Rheumatology annual conference. “But actually, the data to support that advice [are] fairly limited and may not reflect our current patient population,” added Dr. Humphreys of the Arthritis Research U.K. Centre for Epidemiology at the University of Manchester (England).
In a poster presentation, Dr. Humphreys aimed to quantify the association between alcohol intake and serum alanine or aspartate aminotransferase (ALT/AST) levels using routinely collected clinical data from the Clinical Practice Research Datalink (CPRD). Data on more than 8,000 patients starting methotrexate (MTX) for treating rheumatoid arthritis (RA) between 1987 and 2011 were obtained and analyzed.
Patients were included in the analysis if they had information on their alcohol consumption recorded and if serum LFTs had been assessed routinely by their primary care physician at least six times per year. They were then grouped based on their weekly reported alcohol consumption from none (0 units) to excessive (more than 28 units). In the United Kingdom, a unit of alcohol is defined as 10 mL (8 g) of pure alcohol, and the typical alcoholic beverage may contain 1-3 units of alcohol. The mean age of participants in the study was 58 years, and 71% were female.
When 38,000 person-years of follow-up were evaluated, 241 abnormal LFT events, defined as ALT/AST levels more than three times the upper limit of normal, were identified.
Crude incidence rates of abnormal LFTs per 1,000 person-years were 5.58 in nondrinkers, 5.57 in those who drank 1-7 units of alcohol per week, 5.99 in those who drank 8-14 units, 7.58 in those who drank 15-21 units, 8.61 in those who drank 22-28 units, and 9.05 in those who drank more than 28 units. Although the hazard ratios adjusted for age and sex also increased with increasing alcohol intake, from 1.02 in the lightest drinkers to 1.92 in the heaviest drinkers, no statistical significance was found overall between the drinkers and nondrinkers.
“When treated as a continuous variable, there was a statistically significant increase of 1% abnormal LFTs for every unit of alcohol consumed, but you wonder how clinically relevant is a 1% increase,” Dr. Humphreys said.
To look at the data another way, a clinically relevant increase was set for abnormal LFTs; then the data for increasing alcohol consumption were used to see how many patients had crossed that boundary. The investigators found an increase with higher alcohol consumption levels, but that power was limited to patients who drank 14 units a week or fewer. “So essentially, increasing alcohol consumption does increase your risk of abnormal LFTs. But when you are drinking less than about 14 units a week, there is little clinical significance of that, “ Dr. Humphreys said. So for patients who do want to continue drinking while on MTX, it should be reasonably safe to advise them that they can do so as long as they moderate their intake.
Separate research presented in a poster by Dr. Ravi Narang of Frimley Health NHS Foundation Trust in Surrey, England, and associates looked at the use of hepatic elastography (FibroScan) in MTX-treated patients and found 91% of 44 patients had normal ALT levels before their liver was scanned. Of 23 patients who were scanned, 11 (48%) had abnormal findings, and 9 (39%) showed signs of fatty liver. Seven (16%) patients underwent a liver biopsy based on the liver scan findings, and five stopped treatment with MTX based on the findings. Methotrexate also was stopped in nine other patients who did not undergo biopsy but had raised liver stiffness, which is a sign of fibrosis. Meanwhile, three patients continued at a reduced dose.
The patients in their retrospective case note study included those who had undergone FibroScan between 2011 and 2015 while on MTX. Most (56%) patients were taking MTX for RA; 27%, for psoriatic arthritis; 9%, for psoriasis; and the remainder, for other rheumatologic or dermatologic conditions. The median age at the time of the scan was 64 years. Eight patients had comorbid diabetes mellitus, and 11% consumed more than 14 units of alcohol per week.
“FibroScan led to a change in management in many of our patients confirming its value as a noninvasive adjunct for assessing hepatic fibrosis,” said Dr. Narang. He and his associates noted that the imaging technique was “guiding practice” and confirmed the “relative insensitivity of current methotrexate liver monitoring.”
Dr. Humphreys, Dr. Narang, and their associates had no relevant financial disclosures.
GLASGOW – Advising patients with rheumatoid arthritis who are being treated with methotrexate to moderate their alcohol intake remains sound advice, the results of a large clinical database study showed.
Increasing alcohol intake was found to be associated with an increased risk for developing abnormal results on serum liver function tests (LFTs). However, the clinical importance of this was thought to be small if the number of alcoholic units per week remained below 14 – which is general advice for men and women in the United Kingdom.
“We advise our patients to restrict their alcohol consumption, because we know that alcohol is hepatotoxic …, methotrexate is hepatotoxic, and there is a presumed interaction there,” the presenting study author, Dr. Jenny H. Humphreys, said in an interview at the British Society for Rheumatology annual conference. “But actually, the data to support that advice [are] fairly limited and may not reflect our current patient population,” added Dr. Humphreys of the Arthritis Research U.K. Centre for Epidemiology at the University of Manchester (England).
In a poster presentation, Dr. Humphreys aimed to quantify the association between alcohol intake and serum alanine or aspartate aminotransferase (ALT/AST) levels using routinely collected clinical data from the Clinical Practice Research Datalink (CPRD). Data on more than 8,000 patients starting methotrexate (MTX) for treating rheumatoid arthritis (RA) between 1987 and 2011 were obtained and analyzed.
Patients were included in the analysis if they had information on their alcohol consumption recorded and if serum LFTs had been assessed routinely by their primary care physician at least six times per year. They were then grouped based on their weekly reported alcohol consumption from none (0 units) to excessive (more than 28 units). In the United Kingdom, a unit of alcohol is defined as 10 mL (8 g) of pure alcohol, and the typical alcoholic beverage may contain 1-3 units of alcohol. The mean age of participants in the study was 58 years, and 71% were female.
When 38,000 person-years of follow-up were evaluated, 241 abnormal LFT events, defined as ALT/AST levels more than three times the upper limit of normal, were identified.
Crude incidence rates of abnormal LFTs per 1,000 person-years were 5.58 in nondrinkers, 5.57 in those who drank 1-7 units of alcohol per week, 5.99 in those who drank 8-14 units, 7.58 in those who drank 15-21 units, 8.61 in those who drank 22-28 units, and 9.05 in those who drank more than 28 units. Although the hazard ratios adjusted for age and sex also increased with increasing alcohol intake, from 1.02 in the lightest drinkers to 1.92 in the heaviest drinkers, no statistical significance was found overall between the drinkers and nondrinkers.
“When treated as a continuous variable, there was a statistically significant increase of 1% abnormal LFTs for every unit of alcohol consumed, but you wonder how clinically relevant is a 1% increase,” Dr. Humphreys said.
To look at the data another way, a clinically relevant increase was set for abnormal LFTs; then the data for increasing alcohol consumption were used to see how many patients had crossed that boundary. The investigators found an increase with higher alcohol consumption levels, but that power was limited to patients who drank 14 units a week or fewer. “So essentially, increasing alcohol consumption does increase your risk of abnormal LFTs. But when you are drinking less than about 14 units a week, there is little clinical significance of that, “ Dr. Humphreys said. So for patients who do want to continue drinking while on MTX, it should be reasonably safe to advise them that they can do so as long as they moderate their intake.
Separate research presented in a poster by Dr. Ravi Narang of Frimley Health NHS Foundation Trust in Surrey, England, and associates looked at the use of hepatic elastography (FibroScan) in MTX-treated patients and found 91% of 44 patients had normal ALT levels before their liver was scanned. Of 23 patients who were scanned, 11 (48%) had abnormal findings, and 9 (39%) showed signs of fatty liver. Seven (16%) patients underwent a liver biopsy based on the liver scan findings, and five stopped treatment with MTX based on the findings. Methotrexate also was stopped in nine other patients who did not undergo biopsy but had raised liver stiffness, which is a sign of fibrosis. Meanwhile, three patients continued at a reduced dose.
The patients in their retrospective case note study included those who had undergone FibroScan between 2011 and 2015 while on MTX. Most (56%) patients were taking MTX for RA; 27%, for psoriatic arthritis; 9%, for psoriasis; and the remainder, for other rheumatologic or dermatologic conditions. The median age at the time of the scan was 64 years. Eight patients had comorbid diabetes mellitus, and 11% consumed more than 14 units of alcohol per week.
“FibroScan led to a change in management in many of our patients confirming its value as a noninvasive adjunct for assessing hepatic fibrosis,” said Dr. Narang. He and his associates noted that the imaging technique was “guiding practice” and confirmed the “relative insensitivity of current methotrexate liver monitoring.”
Dr. Humphreys, Dr. Narang, and their associates had no relevant financial disclosures.
Key clinical point: Patients who drink moderately while taking methotrexate appear to be at no greater risk for liver damage than nondrinkers.
Major finding: The hazard ratio for abnormal serum liver function tests comparing drinkers and nondrinkers was not significant at 1.12 (95% confidence interval, 0.82-1.51).
Data source: A clinical database study of more than 8,000 patients starting MTX for the treatment of rheumatoid arthritis between 1987 and 2011.
Disclosures: Dr. Humphreys, Dr. Narang, and their associates had no relevant financial disclosures.
Adolescent knee pain ‘not benign,’ linked to later OA
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
GLASGOW – Older adults are more than seven times as likely to develop knee osteoarthritis if they had anterior knee pain as adolescents, according to the results of a case-control study.
The adjusted odds ratio for patellofemoral osteoarthritis (PFOA) was 7.5 if there was prior adolescent anterior knee pain. Although the 95% confidence interval was wide (1.51-36.94) the association was significant (P = .014). Adjustment had been made for the potential confounding factors of previous patellar dislocation, prior surgery, and patient-reported knee instability; before this adjustment the OR was 20.2 (95% CI, 3.34-11.67).
Patellar dislocation during adolescence also was found to be a significant risk factor for later PFOA (aOR, 3.2; 95% CI, 1.25-9.18; P = .016).
“Adolescent anterior knee pain represents a constellation of symptoms and had always been thought of as benign and self-limiting,” Henry Conchie, a medical student at the University of Bristol (England), said at the British Society for Rheumatology annual conference.
“I think the take-home message from our research is really that this traditional view of benign pathology associated with adolescent anterior knee pain and patellar dislocation must be challenged and when seen in clinical practice we now encourage the acknowledgment of the potentially severe consequences in the future,” Mr. Conchie said.
A link between adolescent anterior knee pain and later PFOA has previously been suggested but there are few data to support this observation, he explained. So the aim of the current study was to look at this in more detail in a group of patients from the knee arthroplasty database at Southmead Hospital in Bristol.
Questionnaires that asked about a variety of symptoms and knee pain were sent to 190 patients in the database who had undergone patellofemoral arthroplasty and so had severe, isolated, and radiologically confirmed PFOA. Questionnaires also were sent to 445 patients who had undergone arthroplasty for unicompartmental tibiofemoral arthritis to serve as the control group.
A subanalysis was performed to look at the mean age of the first dislocation and the investigators found that patients with PFOA were likely to be much younger than controls, with a 44-year difference observed between the groups.
“This adds some weight to the theory that this process [PFOA] begins much earlier than once thought – at a younger age,” Mr. Conchie suggested.
The study subjects were surveyed 1-4 years after their operation, so patient recall could have affected the results, but the use of the unicompartmental tibiofemoral arthritis patients as controls should have reduced this potential bias, he said. The fact that they had gone through an arthroplasty meant that they would have had very similar experiences to the PFOA group in terms of pain.
Although only severe OA cases and arthritis controls were used, the team believes that the findings are robust as these were clearly defined patient groups, albeit at the end of the disease spectrum.
“Thought can now turn to etiological mechanisms underlying these relationships, and I think it is likely that anatomical etiologies such as patellar outer and trochlear dysplasia can define both the pain and instability in youth as well as the patellofemoral osteoarthritis in later life,” Mr. Conchie proposed. Further research to look at this would be needed in future.
During the Q&A following his presentation, Dr. Eileen Baildam of Alder Hey Children’s Hospital in Liverpool, England, commented that she had looked at the persistence of pain in patients with adolescent anterior knee pain some years ago and found that, 10-20 years later, 60% were still experiencing pain.
The chair of the session, Dr. Joyce Davidson of the Royal Hospital for Sick Children in Glasgow, summed up by saying: “I think we do see lots of patients and maybe we just need to be aware that this may not be as benign as we think, and certainly we should be looking for abnormal patellae and being very aware of it in young people.”
Mr. Conchie and his coauthors had nothing to disclose.
AT RHEUMATOLOGY 2016
Key clinical point: Knee pain in adolescence was directly linked to later development of knee osteoarthritis.
Major finding: Adolescent knee pain increased the likelihood for developing OA, with an adjusted odds ratio of 7.5 (P = .014).
Data source: Case-control study of 190 adults with patellofemoral OA and 445 controls without patellofemoral OA who had arthroplasty.
Disclosures: Mr. Conchie and his coauthors had nothing to disclose.
Remicade to infliximab biosimilar switches fare well in real-life practice
GLASGOW – Switching patients on the anti–tumor necrosis factor drug Remicade to a biosimilar infliximab product resulted in good efficacy and tolerability with substantial cost savings in two real-world studies from the United Kingdom.
A total of 52 (88%) of 59 patients who had switched to the biosimilar infliximab CT-P13 (Inflectra) for the treatment of various indications for which Remicade is approved remained on the biosimilar after 10 months of follow-up and experienced comparable adverse events and similar levels of efficacy before and after switching, Dr. Lucy Parker of University Hospital Southampton NHS Foundation Trust reported at the British Society for Rheumatology annual conference. A second smaller study reported at the conference also showed similar results with the same infliximab biosimilar, which is also marketed as Remsima in Europe.
CT-P13 had efficacy, immunogenicity, and pharmacokinetic and pharmacodynamic parameters comparable to Remicade in 1-year follow-up data from the phase III randomized PLANETRAstudy (Arthritis Res Ther. 2016;18:82. doi: 10.1186/s13075-016-0981-6), but whether these study findings hold in a routine practice setting remains to be determined, Dr. Parker noted.
In Dr. Parker and colleagues’ study of data from the Southampton Biological Therapies Review Service, every patient was initially “seen in clinic and had the opportunity to speak to their consultant rheumatologist about the potential switchover from the originator drug to the new version,” she explained. Patients were then contacted directly by letter to explain the potential switch and given an information leaflet on Inflectra. They were also given access to a dedicated helpline number that could be used to re-explain the switch and discuss any concerns after switching had occurred.
In May 2015, all 59 patients being treated with Remicade were gradually switched over to the biosimilar version. Patients were reviewed after 3 months and then at 6-month intervals.
“Every single patient agreed to switch,” Dr. Parker observed. No patient used the helpline service or asked to talk with the consultant rheumatologist. One delegate noted during discussion that it was impressive that all patients agreed to switch, but Dr. Parker suggested that it was probably important that people were invited to switch rather being told that they would be switched. “If anyone had refused, then they would have been kept on Remicade,” she observed.
Dr. Parker noted that the patients were taking Remicade for various rheumatologic conditions, including 29 with rheumatoid arthritis (RA), 14 with ankylosing spondylitis (AS), 14 with psoriatic arthritis (PsA), and 2 with enteropathic arthritis. The mean age was 58.9 years, 51% of patients were female, mean disease duration was 18 years, and there was a mean of 5.7 years on Remicade before the switch. Patients had been diagnosed an average of 10 years before the first use of a biologic agent, 56% were also taking methotrexate, and 17% were on another disease-modifying antirheumatic drug (DMARD).
There was no significant difference in disease activity before or after switching, with respective mean 28-joint Disease Activity Scores of 3.4 and 3.3 and Bath Ankylosing Spondylitis Disease Activity Index scores of 3.7 and 3.6.
Dr. Parker noted that two 6-month periods before switching were compared to a 6-month period after switching and there were a similarly low number of cases reporting inefficacy, which was defined as any increase in symptoms or disease activity measure. Inefficacy was seen in one patient 12 months before the switch, two patients 6 months before the switch, and three patients after the switch. All three of the latter patients were switched back to Remicade, with one being then further switched to rituximab (Rituxan).
There were four adverse events in patients switched to the biosimilar versus three and four cases in the two 6-month periods before the switch. Adverse events after switching were widespread pain or myalgia and arthralgia after two infusions in two patients with PsA, multiple subjective symptoms such as dizziness and labile blood pressure and forgetfulness in another patient with PsA who also had these symptoms before the switch, and a case of chronic osteomyelitic foot infection in a patient with RA that also predated the switch. Biologic therapy was stopped in the RA patient, one of the PsA patients switched back to Remicade, and the other two were switched to ustekinumab (Stelara).
Dr. Parker reported that switching to the biosimilar has significantly cut the cost of treatment by £197,974 (about $291,000 USD) or 41.5% in their practice.
A team from St. George’s University Hospitals NHS Foundation Trust in London reported in a poster session similar findings after switching 31 patients to Remsima (Rheumatology [Oxford]. 2016;55[suppl 1]:i125-i126). Most switches occurred in RA patients (n = 18), followed by seven with AS and five with PsA. One patient did not switch following a consultant decision after the patient in question developed septic arthritis.
Dr. Ritu Malaiya and associates found equivalent efficacy responses in the vast majority of cases. Only one patient switched back to Remicade. Dr. Malaiya said that their experience of switching was, “on the whole, positive.” Effective planning and education of patients and staff around the switch was again considered vital and “instrumental to our early success,” the team reported. “Overall, patients were keen for others to benefit from more cost-effective drugs.”
Dr. Parker and Dr. Malaiya reported having no financial disclosures. Coauthors of the studies disclosed acting as consultants or receiving research grants from manufacturers of anti-TNF therapies.
GLASGOW – Switching patients on the anti–tumor necrosis factor drug Remicade to a biosimilar infliximab product resulted in good efficacy and tolerability with substantial cost savings in two real-world studies from the United Kingdom.
A total of 52 (88%) of 59 patients who had switched to the biosimilar infliximab CT-P13 (Inflectra) for the treatment of various indications for which Remicade is approved remained on the biosimilar after 10 months of follow-up and experienced comparable adverse events and similar levels of efficacy before and after switching, Dr. Lucy Parker of University Hospital Southampton NHS Foundation Trust reported at the British Society for Rheumatology annual conference. A second smaller study reported at the conference also showed similar results with the same infliximab biosimilar, which is also marketed as Remsima in Europe.
CT-P13 had efficacy, immunogenicity, and pharmacokinetic and pharmacodynamic parameters comparable to Remicade in 1-year follow-up data from the phase III randomized PLANETRAstudy (Arthritis Res Ther. 2016;18:82. doi: 10.1186/s13075-016-0981-6), but whether these study findings hold in a routine practice setting remains to be determined, Dr. Parker noted.
In Dr. Parker and colleagues’ study of data from the Southampton Biological Therapies Review Service, every patient was initially “seen in clinic and had the opportunity to speak to their consultant rheumatologist about the potential switchover from the originator drug to the new version,” she explained. Patients were then contacted directly by letter to explain the potential switch and given an information leaflet on Inflectra. They were also given access to a dedicated helpline number that could be used to re-explain the switch and discuss any concerns after switching had occurred.
In May 2015, all 59 patients being treated with Remicade were gradually switched over to the biosimilar version. Patients were reviewed after 3 months and then at 6-month intervals.
“Every single patient agreed to switch,” Dr. Parker observed. No patient used the helpline service or asked to talk with the consultant rheumatologist. One delegate noted during discussion that it was impressive that all patients agreed to switch, but Dr. Parker suggested that it was probably important that people were invited to switch rather being told that they would be switched. “If anyone had refused, then they would have been kept on Remicade,” she observed.
Dr. Parker noted that the patients were taking Remicade for various rheumatologic conditions, including 29 with rheumatoid arthritis (RA), 14 with ankylosing spondylitis (AS), 14 with psoriatic arthritis (PsA), and 2 with enteropathic arthritis. The mean age was 58.9 years, 51% of patients were female, mean disease duration was 18 years, and there was a mean of 5.7 years on Remicade before the switch. Patients had been diagnosed an average of 10 years before the first use of a biologic agent, 56% were also taking methotrexate, and 17% were on another disease-modifying antirheumatic drug (DMARD).
There was no significant difference in disease activity before or after switching, with respective mean 28-joint Disease Activity Scores of 3.4 and 3.3 and Bath Ankylosing Spondylitis Disease Activity Index scores of 3.7 and 3.6.
Dr. Parker noted that two 6-month periods before switching were compared to a 6-month period after switching and there were a similarly low number of cases reporting inefficacy, which was defined as any increase in symptoms or disease activity measure. Inefficacy was seen in one patient 12 months before the switch, two patients 6 months before the switch, and three patients after the switch. All three of the latter patients were switched back to Remicade, with one being then further switched to rituximab (Rituxan).
There were four adverse events in patients switched to the biosimilar versus three and four cases in the two 6-month periods before the switch. Adverse events after switching were widespread pain or myalgia and arthralgia after two infusions in two patients with PsA, multiple subjective symptoms such as dizziness and labile blood pressure and forgetfulness in another patient with PsA who also had these symptoms before the switch, and a case of chronic osteomyelitic foot infection in a patient with RA that also predated the switch. Biologic therapy was stopped in the RA patient, one of the PsA patients switched back to Remicade, and the other two were switched to ustekinumab (Stelara).
Dr. Parker reported that switching to the biosimilar has significantly cut the cost of treatment by £197,974 (about $291,000 USD) or 41.5% in their practice.
A team from St. George’s University Hospitals NHS Foundation Trust in London reported in a poster session similar findings after switching 31 patients to Remsima (Rheumatology [Oxford]. 2016;55[suppl 1]:i125-i126). Most switches occurred in RA patients (n = 18), followed by seven with AS and five with PsA. One patient did not switch following a consultant decision after the patient in question developed septic arthritis.
Dr. Ritu Malaiya and associates found equivalent efficacy responses in the vast majority of cases. Only one patient switched back to Remicade. Dr. Malaiya said that their experience of switching was, “on the whole, positive.” Effective planning and education of patients and staff around the switch was again considered vital and “instrumental to our early success,” the team reported. “Overall, patients were keen for others to benefit from more cost-effective drugs.”
Dr. Parker and Dr. Malaiya reported having no financial disclosures. Coauthors of the studies disclosed acting as consultants or receiving research grants from manufacturers of anti-TNF therapies.
GLASGOW – Switching patients on the anti–tumor necrosis factor drug Remicade to a biosimilar infliximab product resulted in good efficacy and tolerability with substantial cost savings in two real-world studies from the United Kingdom.
A total of 52 (88%) of 59 patients who had switched to the biosimilar infliximab CT-P13 (Inflectra) for the treatment of various indications for which Remicade is approved remained on the biosimilar after 10 months of follow-up and experienced comparable adverse events and similar levels of efficacy before and after switching, Dr. Lucy Parker of University Hospital Southampton NHS Foundation Trust reported at the British Society for Rheumatology annual conference. A second smaller study reported at the conference also showed similar results with the same infliximab biosimilar, which is also marketed as Remsima in Europe.
CT-P13 had efficacy, immunogenicity, and pharmacokinetic and pharmacodynamic parameters comparable to Remicade in 1-year follow-up data from the phase III randomized PLANETRAstudy (Arthritis Res Ther. 2016;18:82. doi: 10.1186/s13075-016-0981-6), but whether these study findings hold in a routine practice setting remains to be determined, Dr. Parker noted.
In Dr. Parker and colleagues’ study of data from the Southampton Biological Therapies Review Service, every patient was initially “seen in clinic and had the opportunity to speak to their consultant rheumatologist about the potential switchover from the originator drug to the new version,” she explained. Patients were then contacted directly by letter to explain the potential switch and given an information leaflet on Inflectra. They were also given access to a dedicated helpline number that could be used to re-explain the switch and discuss any concerns after switching had occurred.
In May 2015, all 59 patients being treated with Remicade were gradually switched over to the biosimilar version. Patients were reviewed after 3 months and then at 6-month intervals.
“Every single patient agreed to switch,” Dr. Parker observed. No patient used the helpline service or asked to talk with the consultant rheumatologist. One delegate noted during discussion that it was impressive that all patients agreed to switch, but Dr. Parker suggested that it was probably important that people were invited to switch rather being told that they would be switched. “If anyone had refused, then they would have been kept on Remicade,” she observed.
Dr. Parker noted that the patients were taking Remicade for various rheumatologic conditions, including 29 with rheumatoid arthritis (RA), 14 with ankylosing spondylitis (AS), 14 with psoriatic arthritis (PsA), and 2 with enteropathic arthritis. The mean age was 58.9 years, 51% of patients were female, mean disease duration was 18 years, and there was a mean of 5.7 years on Remicade before the switch. Patients had been diagnosed an average of 10 years before the first use of a biologic agent, 56% were also taking methotrexate, and 17% were on another disease-modifying antirheumatic drug (DMARD).
There was no significant difference in disease activity before or after switching, with respective mean 28-joint Disease Activity Scores of 3.4 and 3.3 and Bath Ankylosing Spondylitis Disease Activity Index scores of 3.7 and 3.6.
Dr. Parker noted that two 6-month periods before switching were compared to a 6-month period after switching and there were a similarly low number of cases reporting inefficacy, which was defined as any increase in symptoms or disease activity measure. Inefficacy was seen in one patient 12 months before the switch, two patients 6 months before the switch, and three patients after the switch. All three of the latter patients were switched back to Remicade, with one being then further switched to rituximab (Rituxan).
There were four adverse events in patients switched to the biosimilar versus three and four cases in the two 6-month periods before the switch. Adverse events after switching were widespread pain or myalgia and arthralgia after two infusions in two patients with PsA, multiple subjective symptoms such as dizziness and labile blood pressure and forgetfulness in another patient with PsA who also had these symptoms before the switch, and a case of chronic osteomyelitic foot infection in a patient with RA that also predated the switch. Biologic therapy was stopped in the RA patient, one of the PsA patients switched back to Remicade, and the other two were switched to ustekinumab (Stelara).
Dr. Parker reported that switching to the biosimilar has significantly cut the cost of treatment by £197,974 (about $291,000 USD) or 41.5% in their practice.
A team from St. George’s University Hospitals NHS Foundation Trust in London reported in a poster session similar findings after switching 31 patients to Remsima (Rheumatology [Oxford]. 2016;55[suppl 1]:i125-i126). Most switches occurred in RA patients (n = 18), followed by seven with AS and five with PsA. One patient did not switch following a consultant decision after the patient in question developed septic arthritis.
Dr. Ritu Malaiya and associates found equivalent efficacy responses in the vast majority of cases. Only one patient switched back to Remicade. Dr. Malaiya said that their experience of switching was, “on the whole, positive.” Effective planning and education of patients and staff around the switch was again considered vital and “instrumental to our early success,” the team reported. “Overall, patients were keen for others to benefit from more cost-effective drugs.”
Dr. Parker and Dr. Malaiya reported having no financial disclosures. Coauthors of the studies disclosed acting as consultants or receiving research grants from manufacturers of anti-TNF therapies.
Key clinical point: Both biosimilar versions of infliximab available in Europe appear to be as effective and tolerated as Remicade after switching from it.
Major finding: Of 59 patients who had switched to Inflectra, 88% remained on the biosimilar after 10 months of follow-up.
Data source: Two real-world studies of switching to biosimilar infliximab in the United Kingdom.
Disclosures: Dr. Parker and Dr. Malaiya reported having no financial disclosures. Coauthors of the studies disclosed acting as consultants or receiving research grants from manufacturers of anti-TNF therapies.
Risk of arthritis in children with Down syndrome higher than previously reported
GLASGOW – Children with Down syndrome are at increased risk for arthritis that often goes unrecognized and leads to treatment delays and potential chronic disability.
Research presented at the British Society for Rheumatology annual conference highlighted how Down arthropathy is not only more prevalent than idiopathic juvenile arthritis (JIA), but also has distinct clinical and radiographic features.
“Our research to date has shown that there is a significant increased risk of arthritis in children with trisomy 21, and higher than that previously reported,” said Dr. Charlene Foley, a research fellow at Our Lady’s Children’s Hospital in Dublin.
“There is a significant delay in diagnosis, which may be a cause of the x-ray changes at diagnosis, or it may be in fact that Down arthropathy is more aggressive,” than other childhood forms of arthritis, she observed.
Dr. Foley noted that Down arthropathy was first reported in the medical literature about 30 years ago and crude estimates suggested a prevalence of around 8.7 cases per 1,000 children versus 1 per 1,000 for JIA. However, the research she presented put the crude point prevalence at 18-21 per 1,000 children.
Dr. Foley presented the findings of an observational study conducted in the Republic of Ireland in which children with trisomy 21 and their families were identified from a variety of sources and invited to participate. After completion of a screening questionnaire and an appointment local to the participants, children who were suspected of having arthritis were invited to attend a consultant appointment. They underwent a clinical management pathway developed for JIA because no specific pathway had been developed for the children at that time, with follow-up appointments held every 3-6 months depending on the child’s needs.
Over an 18-month period, 503 children with trisomy 21 and a mean age of 8 years were screened. They had a range of musculoskeletal anomalies, the most common of which were flat feet in almost all the children (91.1%), inflammatory arthritis in 7.1%, and scoliosis in 4.8%. Many other problems occurred, with an incidence of 1.5% or less for each.
A total of 22 new cases of Down arthropathy have been identified to date, in addition to 11 at the clinic who predated the start of the study. About 75% have come through the screening clinics and the rest through pediatricians’ referral.
“It is a challenging disease both in terms of diagnosis and management,” Dr. Foley said. Of all the identified children, 91% had poor language skills or nonverbal communication and 15% had autism spectrum disorder.
On average, the time to diagnosis of the arthropathy was 1.7 years versus 0.74 years for a control group of 33 children with JIA. This is likely an underestimation, however, as 42% of the children or parents in the Down arthropathy cohort were unable to give a date on which symptoms had started.
Dr. Foley reported that the majority of trisomy 21 children had presented with polyarticular arthritis, mostly involving the proximal interphalangeal joints of the hands (78.6% of cases), or the wrists (53.6% of cases). There was significant small joint involvement (88% vs. 43% of the JIA cohort), and higher restricted joint counts (4.5 vs. 2.0). There were also differences in erythrocyte sedimentation rate and C-reactive protein at diagnosis, with these being “barely raised” in children with Down arthropathy versus children with JIA, so unlikely to aid a diagnosis. Children were also found to be rheumatoid factor negative.
Two-thirds of Down arthropathy cases had x-ray changes at presentation versus 24% of the JIA group, of which 29% versus 9.5% were erosive.
Treatment is complicated by drug-related side effects, with many children unable to tolerate methotrexate, Dr. Foley said. In the Irish cohort, treatment with methotrexate led to nausea in 75%, compared with 7.1% of the JIA children. Although reports are limited, methotrexate intolerance has been shown in children with trisomy 21, so there could be a genetic or metabolic reason behind this. Dr. Foley noted that they manage this problem by starting methotrexate on the lowest possible doses (10 mg/m2) and co-administering the antiemetic ondansetron. They have a low threshold for switching to an anti-TNF drug if needed, and have also started giving biologic drugs to some newly diagnosed children.
“The take-home message is to think outside of the Down syndrome box and don’t just blame everything on Down syndrome,” Dr. Foley said. As it may be challenging to examine a child, she suggested looking at the hands first because they are the most likely to be affected.
“We feel that a musculoskeletal assessment should be part of the annual surveillance for all children with Down syndrome,” she concluded.
As for who should conduct such an assessment, Dr. Foley suggested that general pediatricians who are regularly seeing these children for other health checks should perform it. However, as one delegate observed, nonrheumatology professionals may need a little training and guidance, as musculoskeletal assessments can be difficult. Looking only at the hands, and potentially the feet, may be one solution.
The study has raised a number of questions and future research will be needed to further characterize the arthritis and to determine how best to diagnose and treat it, noted Dr. Foley, who indicated that she had no conflicts of interest.
GLASGOW – Children with Down syndrome are at increased risk for arthritis that often goes unrecognized and leads to treatment delays and potential chronic disability.
Research presented at the British Society for Rheumatology annual conference highlighted how Down arthropathy is not only more prevalent than idiopathic juvenile arthritis (JIA), but also has distinct clinical and radiographic features.
“Our research to date has shown that there is a significant increased risk of arthritis in children with trisomy 21, and higher than that previously reported,” said Dr. Charlene Foley, a research fellow at Our Lady’s Children’s Hospital in Dublin.
“There is a significant delay in diagnosis, which may be a cause of the x-ray changes at diagnosis, or it may be in fact that Down arthropathy is more aggressive,” than other childhood forms of arthritis, she observed.
Dr. Foley noted that Down arthropathy was first reported in the medical literature about 30 years ago and crude estimates suggested a prevalence of around 8.7 cases per 1,000 children versus 1 per 1,000 for JIA. However, the research she presented put the crude point prevalence at 18-21 per 1,000 children.
Dr. Foley presented the findings of an observational study conducted in the Republic of Ireland in which children with trisomy 21 and their families were identified from a variety of sources and invited to participate. After completion of a screening questionnaire and an appointment local to the participants, children who were suspected of having arthritis were invited to attend a consultant appointment. They underwent a clinical management pathway developed for JIA because no specific pathway had been developed for the children at that time, with follow-up appointments held every 3-6 months depending on the child’s needs.
Over an 18-month period, 503 children with trisomy 21 and a mean age of 8 years were screened. They had a range of musculoskeletal anomalies, the most common of which were flat feet in almost all the children (91.1%), inflammatory arthritis in 7.1%, and scoliosis in 4.8%. Many other problems occurred, with an incidence of 1.5% or less for each.
A total of 22 new cases of Down arthropathy have been identified to date, in addition to 11 at the clinic who predated the start of the study. About 75% have come through the screening clinics and the rest through pediatricians’ referral.
“It is a challenging disease both in terms of diagnosis and management,” Dr. Foley said. Of all the identified children, 91% had poor language skills or nonverbal communication and 15% had autism spectrum disorder.
On average, the time to diagnosis of the arthropathy was 1.7 years versus 0.74 years for a control group of 33 children with JIA. This is likely an underestimation, however, as 42% of the children or parents in the Down arthropathy cohort were unable to give a date on which symptoms had started.
Dr. Foley reported that the majority of trisomy 21 children had presented with polyarticular arthritis, mostly involving the proximal interphalangeal joints of the hands (78.6% of cases), or the wrists (53.6% of cases). There was significant small joint involvement (88% vs. 43% of the JIA cohort), and higher restricted joint counts (4.5 vs. 2.0). There were also differences in erythrocyte sedimentation rate and C-reactive protein at diagnosis, with these being “barely raised” in children with Down arthropathy versus children with JIA, so unlikely to aid a diagnosis. Children were also found to be rheumatoid factor negative.
Two-thirds of Down arthropathy cases had x-ray changes at presentation versus 24% of the JIA group, of which 29% versus 9.5% were erosive.
Treatment is complicated by drug-related side effects, with many children unable to tolerate methotrexate, Dr. Foley said. In the Irish cohort, treatment with methotrexate led to nausea in 75%, compared with 7.1% of the JIA children. Although reports are limited, methotrexate intolerance has been shown in children with trisomy 21, so there could be a genetic or metabolic reason behind this. Dr. Foley noted that they manage this problem by starting methotrexate on the lowest possible doses (10 mg/m2) and co-administering the antiemetic ondansetron. They have a low threshold for switching to an anti-TNF drug if needed, and have also started giving biologic drugs to some newly diagnosed children.
“The take-home message is to think outside of the Down syndrome box and don’t just blame everything on Down syndrome,” Dr. Foley said. As it may be challenging to examine a child, she suggested looking at the hands first because they are the most likely to be affected.
“We feel that a musculoskeletal assessment should be part of the annual surveillance for all children with Down syndrome,” she concluded.
As for who should conduct such an assessment, Dr. Foley suggested that general pediatricians who are regularly seeing these children for other health checks should perform it. However, as one delegate observed, nonrheumatology professionals may need a little training and guidance, as musculoskeletal assessments can be difficult. Looking only at the hands, and potentially the feet, may be one solution.
The study has raised a number of questions and future research will be needed to further characterize the arthritis and to determine how best to diagnose and treat it, noted Dr. Foley, who indicated that she had no conflicts of interest.
GLASGOW – Children with Down syndrome are at increased risk for arthritis that often goes unrecognized and leads to treatment delays and potential chronic disability.
Research presented at the British Society for Rheumatology annual conference highlighted how Down arthropathy is not only more prevalent than idiopathic juvenile arthritis (JIA), but also has distinct clinical and radiographic features.
“Our research to date has shown that there is a significant increased risk of arthritis in children with trisomy 21, and higher than that previously reported,” said Dr. Charlene Foley, a research fellow at Our Lady’s Children’s Hospital in Dublin.
“There is a significant delay in diagnosis, which may be a cause of the x-ray changes at diagnosis, or it may be in fact that Down arthropathy is more aggressive,” than other childhood forms of arthritis, she observed.
Dr. Foley noted that Down arthropathy was first reported in the medical literature about 30 years ago and crude estimates suggested a prevalence of around 8.7 cases per 1,000 children versus 1 per 1,000 for JIA. However, the research she presented put the crude point prevalence at 18-21 per 1,000 children.
Dr. Foley presented the findings of an observational study conducted in the Republic of Ireland in which children with trisomy 21 and their families were identified from a variety of sources and invited to participate. After completion of a screening questionnaire and an appointment local to the participants, children who were suspected of having arthritis were invited to attend a consultant appointment. They underwent a clinical management pathway developed for JIA because no specific pathway had been developed for the children at that time, with follow-up appointments held every 3-6 months depending on the child’s needs.
Over an 18-month period, 503 children with trisomy 21 and a mean age of 8 years were screened. They had a range of musculoskeletal anomalies, the most common of which were flat feet in almost all the children (91.1%), inflammatory arthritis in 7.1%, and scoliosis in 4.8%. Many other problems occurred, with an incidence of 1.5% or less for each.
A total of 22 new cases of Down arthropathy have been identified to date, in addition to 11 at the clinic who predated the start of the study. About 75% have come through the screening clinics and the rest through pediatricians’ referral.
“It is a challenging disease both in terms of diagnosis and management,” Dr. Foley said. Of all the identified children, 91% had poor language skills or nonverbal communication and 15% had autism spectrum disorder.
On average, the time to diagnosis of the arthropathy was 1.7 years versus 0.74 years for a control group of 33 children with JIA. This is likely an underestimation, however, as 42% of the children or parents in the Down arthropathy cohort were unable to give a date on which symptoms had started.
Dr. Foley reported that the majority of trisomy 21 children had presented with polyarticular arthritis, mostly involving the proximal interphalangeal joints of the hands (78.6% of cases), or the wrists (53.6% of cases). There was significant small joint involvement (88% vs. 43% of the JIA cohort), and higher restricted joint counts (4.5 vs. 2.0). There were also differences in erythrocyte sedimentation rate and C-reactive protein at diagnosis, with these being “barely raised” in children with Down arthropathy versus children with JIA, so unlikely to aid a diagnosis. Children were also found to be rheumatoid factor negative.
Two-thirds of Down arthropathy cases had x-ray changes at presentation versus 24% of the JIA group, of which 29% versus 9.5% were erosive.
Treatment is complicated by drug-related side effects, with many children unable to tolerate methotrexate, Dr. Foley said. In the Irish cohort, treatment with methotrexate led to nausea in 75%, compared with 7.1% of the JIA children. Although reports are limited, methotrexate intolerance has been shown in children with trisomy 21, so there could be a genetic or metabolic reason behind this. Dr. Foley noted that they manage this problem by starting methotrexate on the lowest possible doses (10 mg/m2) and co-administering the antiemetic ondansetron. They have a low threshold for switching to an anti-TNF drug if needed, and have also started giving biologic drugs to some newly diagnosed children.
“The take-home message is to think outside of the Down syndrome box and don’t just blame everything on Down syndrome,” Dr. Foley said. As it may be challenging to examine a child, she suggested looking at the hands first because they are the most likely to be affected.
“We feel that a musculoskeletal assessment should be part of the annual surveillance for all children with Down syndrome,” she concluded.
As for who should conduct such an assessment, Dr. Foley suggested that general pediatricians who are regularly seeing these children for other health checks should perform it. However, as one delegate observed, nonrheumatology professionals may need a little training and guidance, as musculoskeletal assessments can be difficult. Looking only at the hands, and potentially the feet, may be one solution.
The study has raised a number of questions and future research will be needed to further characterize the arthritis and to determine how best to diagnose and treat it, noted Dr. Foley, who indicated that she had no conflicts of interest.
AT RHEUMATOLOGY 2016
Key clinical point:A musculoskeletal assessment should be part of the annual surveillance for all children with Down syndrome to look for arthritis.
Major finding: Arthritis in children with Down syndrome typically presents as polyarticular inflammation in the hands and wrists.
Data source: Observational study of 33 children with Down arthropathy and 33 with juvenile idiopathic arthritis living in Ireland.
Disclosures: Dr. Foley had no conflicts of interest.
Long-term Use of Opioids for Musculoskeletal Pain Incurs Major Risks
GLASGOW, SCOTLAND – Adverse events ranging from major trauma to osteoporosis to death from any cause occurred at significantly higher rates among people who were prescribed opioids long term for musculoskeletal pain in comparison to those who used a single prescription, in a large retrospective matched cohort study.
The analysis of almost 200,000 patient records from 190 primary care practices held within the U.K. Clinical Practice Research Datalink found that use of opioids to control pain for more than 3 months was associated with an increased risk for major trauma and almost doubled the risk of overdose of nonopioid drugs, compared with short-term use.
The absolute risk for major trauma, which included bone fracture, joint dislocation, ligament or tendon rupture, or head trauma, was 387.4 per 10,000 person-years with long-term opioid use and 269.7 per 10,000 person-years for short-term opioid use, with a hazard ratio (HR) of 1.26 and a 95% confident interval (CI) of 1.20 to 1.33. The absolute risk for overdose with drugs other than opioids was 37.7 and 12.9 per 10,000 person-years, respectively (HR 2.03; 95% CI 1.63-2.53).
Long-term use of opioids led to a significant increase in the risk of accidental poisoning (HR 4.12; 95% CI 1.66-10.19) and becoming newly addicted (HR 2.76; 95% CI 1.76-4.35) in comparison with short-term opioid use. The risk of addiction significantly rose with long-term users of nonopioid drugs (HR 2.16; 95% CI 1.78-2.62), compared with short-term users.
Other risks included an increase in falls (HR 1.20; 95% CI 1.14-1.25) and new cases of osteoporosis (HR 1.65; 95% CI 1.52-1.79), incident depression (HR 1.45; 95% CI 1.37-1.53), new gastric (HR 1.38; 95% CI 1.20-1.60) and nongastric (1.33; 95% CI 1.21-1.45) gastrointestinal bleeding, incident iron deficiency anemia (HR 1.22; 95% CI 1.12-1.33), and death from any cause (HR 1.20; 95% CI 1.12-1.29).
There was no increase in the risk for attempted suicide or self-harm, however, nor in a couple of control outcomes (new cases of eczema or psoriasis) that were tested.
“Long-term users [of opioids] appear to be a very vulnerable group at high risk of experiencing quite a lot of adverse events,” said Dr. John Bedson of Keele (England) University who presented the findings at the British Society for Rheumatology annual conference. “So GPs [general practitioners], naturally, need to be actively managing these patients and ... as this is in the very early stages of prescribing, they need to be assessing the benefit and whether there is a need to carry on taking these medications and stop them if appropriate.”
The use of opioids in patients with musculoskeletal conditions has been increasing in the past 10 years, particularly the use of more potent and long-acting opioids, Dr. Bedson observed. He noted that of all the adults who newly present with musculoskeletal conditions in the United Kingdom – estimated to be 20% of all primary care consulters annually – one in seven will be prescribed an opioid analgesic. In fact, as it was noted during the discussion, U.K. clinicians are now often opting to use opioids over nonsteroidal anti-inflammatory drugs (NSAIDs) to avoid gastrointestinal bleeding.
While there has been some evidence obtained on the risks associated with long-term opioid use in the United States, there has not been data on the risks in a U.K. population. With differing health systems, guidelines, and practices between the two countries, the aim of the retrospective matched cohort study was to compare the adverse event profiles of long- and short-term opioids users over a period of 12 months in a U.K. primary care practice population.
Between 2002 and 2013 there were 98,140 patients with musculoskeletal conditions who had been prescribed opioids and had received two or more further opioid prescriptions within a 90-day period. These were the long-term opioid users; they were matched according to age, gender, and practice to 98,140 short-term opioid users who were patients with musculoskeletal conditions who did not fulfill the criteria for the long-term definition. The median age of patients was 61 years and 41% were male.
Numerous covariates were taken into account, including previous adverse events in the 15 months prior to the start of follow-up, smoking status, alcohol consumption, body mass index, where patients lived and their socioeconomic status, the presence of any comorbidities, and the coprescription of NSAIDs.
Further research into how and why patients on long-term opioids experience more adverse effects needs to be conducted, Dr. Bedson noted, adding that causality had not been found in this study. The next step would be to look at the morphine equivalent doses of the opioids used to see if that had an effect.
But how does the risk compare to long-term use of NSAIDs? Has the preference for opioids been mistaken as being safer?
“The problem is that we have started using these drugs and we don’t know if they work,” Dr. Bedson said. “So we are now at the point of identifying more and more risks, but do they do any good? So I think the jury is still out, and I think GPs have very little else to use at the moment because of the worries over anti-inflammatories, but from an anecdotal point of view I think people are beginning to swing back to using them again.”
Dr. Bedson had no conflicts of interest to disclose.
GLASGOW, SCOTLAND – Adverse events ranging from major trauma to osteoporosis to death from any cause occurred at significantly higher rates among people who were prescribed opioids long term for musculoskeletal pain in comparison to those who used a single prescription, in a large retrospective matched cohort study.
The analysis of almost 200,000 patient records from 190 primary care practices held within the U.K. Clinical Practice Research Datalink found that use of opioids to control pain for more than 3 months was associated with an increased risk for major trauma and almost doubled the risk of overdose of nonopioid drugs, compared with short-term use.
The absolute risk for major trauma, which included bone fracture, joint dislocation, ligament or tendon rupture, or head trauma, was 387.4 per 10,000 person-years with long-term opioid use and 269.7 per 10,000 person-years for short-term opioid use, with a hazard ratio (HR) of 1.26 and a 95% confident interval (CI) of 1.20 to 1.33. The absolute risk for overdose with drugs other than opioids was 37.7 and 12.9 per 10,000 person-years, respectively (HR 2.03; 95% CI 1.63-2.53).
Long-term use of opioids led to a significant increase in the risk of accidental poisoning (HR 4.12; 95% CI 1.66-10.19) and becoming newly addicted (HR 2.76; 95% CI 1.76-4.35) in comparison with short-term opioid use. The risk of addiction significantly rose with long-term users of nonopioid drugs (HR 2.16; 95% CI 1.78-2.62), compared with short-term users.
Other risks included an increase in falls (HR 1.20; 95% CI 1.14-1.25) and new cases of osteoporosis (HR 1.65; 95% CI 1.52-1.79), incident depression (HR 1.45; 95% CI 1.37-1.53), new gastric (HR 1.38; 95% CI 1.20-1.60) and nongastric (1.33; 95% CI 1.21-1.45) gastrointestinal bleeding, incident iron deficiency anemia (HR 1.22; 95% CI 1.12-1.33), and death from any cause (HR 1.20; 95% CI 1.12-1.29).
There was no increase in the risk for attempted suicide or self-harm, however, nor in a couple of control outcomes (new cases of eczema or psoriasis) that were tested.
“Long-term users [of opioids] appear to be a very vulnerable group at high risk of experiencing quite a lot of adverse events,” said Dr. John Bedson of Keele (England) University who presented the findings at the British Society for Rheumatology annual conference. “So GPs [general practitioners], naturally, need to be actively managing these patients and ... as this is in the very early stages of prescribing, they need to be assessing the benefit and whether there is a need to carry on taking these medications and stop them if appropriate.”
The use of opioids in patients with musculoskeletal conditions has been increasing in the past 10 years, particularly the use of more potent and long-acting opioids, Dr. Bedson observed. He noted that of all the adults who newly present with musculoskeletal conditions in the United Kingdom – estimated to be 20% of all primary care consulters annually – one in seven will be prescribed an opioid analgesic. In fact, as it was noted during the discussion, U.K. clinicians are now often opting to use opioids over nonsteroidal anti-inflammatory drugs (NSAIDs) to avoid gastrointestinal bleeding.
While there has been some evidence obtained on the risks associated with long-term opioid use in the United States, there has not been data on the risks in a U.K. population. With differing health systems, guidelines, and practices between the two countries, the aim of the retrospective matched cohort study was to compare the adverse event profiles of long- and short-term opioids users over a period of 12 months in a U.K. primary care practice population.
Between 2002 and 2013 there were 98,140 patients with musculoskeletal conditions who had been prescribed opioids and had received two or more further opioid prescriptions within a 90-day period. These were the long-term opioid users; they were matched according to age, gender, and practice to 98,140 short-term opioid users who were patients with musculoskeletal conditions who did not fulfill the criteria for the long-term definition. The median age of patients was 61 years and 41% were male.
Numerous covariates were taken into account, including previous adverse events in the 15 months prior to the start of follow-up, smoking status, alcohol consumption, body mass index, where patients lived and their socioeconomic status, the presence of any comorbidities, and the coprescription of NSAIDs.
Further research into how and why patients on long-term opioids experience more adverse effects needs to be conducted, Dr. Bedson noted, adding that causality had not been found in this study. The next step would be to look at the morphine equivalent doses of the opioids used to see if that had an effect.
But how does the risk compare to long-term use of NSAIDs? Has the preference for opioids been mistaken as being safer?
“The problem is that we have started using these drugs and we don’t know if they work,” Dr. Bedson said. “So we are now at the point of identifying more and more risks, but do they do any good? So I think the jury is still out, and I think GPs have very little else to use at the moment because of the worries over anti-inflammatories, but from an anecdotal point of view I think people are beginning to swing back to using them again.”
Dr. Bedson had no conflicts of interest to disclose.
GLASGOW, SCOTLAND – Adverse events ranging from major trauma to osteoporosis to death from any cause occurred at significantly higher rates among people who were prescribed opioids long term for musculoskeletal pain in comparison to those who used a single prescription, in a large retrospective matched cohort study.
The analysis of almost 200,000 patient records from 190 primary care practices held within the U.K. Clinical Practice Research Datalink found that use of opioids to control pain for more than 3 months was associated with an increased risk for major trauma and almost doubled the risk of overdose of nonopioid drugs, compared with short-term use.
The absolute risk for major trauma, which included bone fracture, joint dislocation, ligament or tendon rupture, or head trauma, was 387.4 per 10,000 person-years with long-term opioid use and 269.7 per 10,000 person-years for short-term opioid use, with a hazard ratio (HR) of 1.26 and a 95% confident interval (CI) of 1.20 to 1.33. The absolute risk for overdose with drugs other than opioids was 37.7 and 12.9 per 10,000 person-years, respectively (HR 2.03; 95% CI 1.63-2.53).
Long-term use of opioids led to a significant increase in the risk of accidental poisoning (HR 4.12; 95% CI 1.66-10.19) and becoming newly addicted (HR 2.76; 95% CI 1.76-4.35) in comparison with short-term opioid use. The risk of addiction significantly rose with long-term users of nonopioid drugs (HR 2.16; 95% CI 1.78-2.62), compared with short-term users.
Other risks included an increase in falls (HR 1.20; 95% CI 1.14-1.25) and new cases of osteoporosis (HR 1.65; 95% CI 1.52-1.79), incident depression (HR 1.45; 95% CI 1.37-1.53), new gastric (HR 1.38; 95% CI 1.20-1.60) and nongastric (1.33; 95% CI 1.21-1.45) gastrointestinal bleeding, incident iron deficiency anemia (HR 1.22; 95% CI 1.12-1.33), and death from any cause (HR 1.20; 95% CI 1.12-1.29).
There was no increase in the risk for attempted suicide or self-harm, however, nor in a couple of control outcomes (new cases of eczema or psoriasis) that were tested.
“Long-term users [of opioids] appear to be a very vulnerable group at high risk of experiencing quite a lot of adverse events,” said Dr. John Bedson of Keele (England) University who presented the findings at the British Society for Rheumatology annual conference. “So GPs [general practitioners], naturally, need to be actively managing these patients and ... as this is in the very early stages of prescribing, they need to be assessing the benefit and whether there is a need to carry on taking these medications and stop them if appropriate.”
The use of opioids in patients with musculoskeletal conditions has been increasing in the past 10 years, particularly the use of more potent and long-acting opioids, Dr. Bedson observed. He noted that of all the adults who newly present with musculoskeletal conditions in the United Kingdom – estimated to be 20% of all primary care consulters annually – one in seven will be prescribed an opioid analgesic. In fact, as it was noted during the discussion, U.K. clinicians are now often opting to use opioids over nonsteroidal anti-inflammatory drugs (NSAIDs) to avoid gastrointestinal bleeding.
While there has been some evidence obtained on the risks associated with long-term opioid use in the United States, there has not been data on the risks in a U.K. population. With differing health systems, guidelines, and practices between the two countries, the aim of the retrospective matched cohort study was to compare the adverse event profiles of long- and short-term opioids users over a period of 12 months in a U.K. primary care practice population.
Between 2002 and 2013 there were 98,140 patients with musculoskeletal conditions who had been prescribed opioids and had received two or more further opioid prescriptions within a 90-day period. These were the long-term opioid users; they were matched according to age, gender, and practice to 98,140 short-term opioid users who were patients with musculoskeletal conditions who did not fulfill the criteria for the long-term definition. The median age of patients was 61 years and 41% were male.
Numerous covariates were taken into account, including previous adverse events in the 15 months prior to the start of follow-up, smoking status, alcohol consumption, body mass index, where patients lived and their socioeconomic status, the presence of any comorbidities, and the coprescription of NSAIDs.
Further research into how and why patients on long-term opioids experience more adverse effects needs to be conducted, Dr. Bedson noted, adding that causality had not been found in this study. The next step would be to look at the morphine equivalent doses of the opioids used to see if that had an effect.
But how does the risk compare to long-term use of NSAIDs? Has the preference for opioids been mistaken as being safer?
“The problem is that we have started using these drugs and we don’t know if they work,” Dr. Bedson said. “So we are now at the point of identifying more and more risks, but do they do any good? So I think the jury is still out, and I think GPs have very little else to use at the moment because of the worries over anti-inflammatories, but from an anecdotal point of view I think people are beginning to swing back to using them again.”
Dr. Bedson had no conflicts of interest to disclose.
AT RHEUMATOLOGY 2016