User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
CHA2DS2-VASc score performs best in assessing atrial fibrillation stroke risk
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.
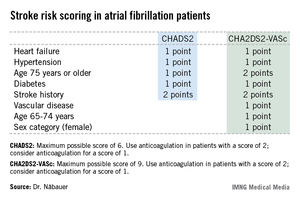
Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.

Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.

Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2013
Major finding: During 5 years of prospective follow-up of patients with nonvalvular atrial fibrillation, 145 patients who had a stroke, transient ischemic attack, or thromboembolism also had a CHADS2 score indicating a low risk for stroke, compared with only 19 who had one of those events and were classified as low risk by the CHA2DS2-VASc stroke risk scoring system.
Data source: The German AFNET registry, a real-world, prospective national registry including 8,847 patients with nonvalvular atrial fibrillation.
Disclosures: The AFNET registry is publically funded by the German Federal Ministry for Education and Research. Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
Diabetics' stroke risk post MI has plummeted
AMSTERDAM – The risk of ischemic stroke following an acute myocardial infarction in diabetes patients dropped markedly during a recent 10-year period, according to a nationwide Swedish study.
Indeed, the reduction in ischemic stroke risk during the first year after an MI was significantly larger in diabetic than in nondiabetic patients over the course of a decade, Stina Jakobsson said at the annual congress of the European Society of Cardiology.
"We believe that the larger risk reduction seen in the diabetic patients may indicate that they have gained more from the increased use of evidence-based secondary preventive treatment," added Ms. Jakobsson, a medical student at Umea (Sweden) University.
She presented an analysis of all 173,233 patients discharged from Swedish coronary care units after an acute MI during 1998-2008. A total of 19% of them had a previous diagnosis of diabetes.
Among diabetes patients with an MI in 1998-2000, ischemic stroke occurred in 7.1% within 1 year after their coronary event. However, the 1-year ischemic stroke rate in such patients whose MI occurred in 2007-2008 dropped to 4.7%. This was a much more impressive improvement than occurred in the same time span among nondiabetic patients, where the ischemic stroke rate during the first year after an acute MI was 4.2% in 1998-2000, nudging downward to 3.7% in 2007-2008.
Ms. Jakobsson stressed that there is definitely room for improvement in the use of reperfusion therapy and secondary preventive medications among diabetes patients with an MI. Although the use of these key interventions increased over time in both diabetic and nondiabetic MI patients, rates still remained lower in the diabetic group in the most recent study years.
The 4.7% 1-year incidence of ischemic stroke among Swedish diabetes patients with an acute MI in 2007-2008 was significantly greater than the 3.7% rate among nondiabetic patients. Moreover, even among patients on optimized secondary prevention therapies, the ischemic stroke rate was higher in the diabetic group. That’s not surprising because they more often had a history of prior cardiovascular disease at the time of their acute MI.
"They were sicker to start with," Ms. Jakobsson observed.
The most powerful predictors of increased risk of ischemic stroke post MI included older age, atrial fibrillation, an ST-elevation MI, and prior ischemic stroke.
This study was supported by Swedish governmental research funds. Ms. Jakobsson reported having no financial conflicts of interest.
AMSTERDAM – The risk of ischemic stroke following an acute myocardial infarction in diabetes patients dropped markedly during a recent 10-year period, according to a nationwide Swedish study.
Indeed, the reduction in ischemic stroke risk during the first year after an MI was significantly larger in diabetic than in nondiabetic patients over the course of a decade, Stina Jakobsson said at the annual congress of the European Society of Cardiology.
"We believe that the larger risk reduction seen in the diabetic patients may indicate that they have gained more from the increased use of evidence-based secondary preventive treatment," added Ms. Jakobsson, a medical student at Umea (Sweden) University.
She presented an analysis of all 173,233 patients discharged from Swedish coronary care units after an acute MI during 1998-2008. A total of 19% of them had a previous diagnosis of diabetes.
Among diabetes patients with an MI in 1998-2000, ischemic stroke occurred in 7.1% within 1 year after their coronary event. However, the 1-year ischemic stroke rate in such patients whose MI occurred in 2007-2008 dropped to 4.7%. This was a much more impressive improvement than occurred in the same time span among nondiabetic patients, where the ischemic stroke rate during the first year after an acute MI was 4.2% in 1998-2000, nudging downward to 3.7% in 2007-2008.
Ms. Jakobsson stressed that there is definitely room for improvement in the use of reperfusion therapy and secondary preventive medications among diabetes patients with an MI. Although the use of these key interventions increased over time in both diabetic and nondiabetic MI patients, rates still remained lower in the diabetic group in the most recent study years.
The 4.7% 1-year incidence of ischemic stroke among Swedish diabetes patients with an acute MI in 2007-2008 was significantly greater than the 3.7% rate among nondiabetic patients. Moreover, even among patients on optimized secondary prevention therapies, the ischemic stroke rate was higher in the diabetic group. That’s not surprising because they more often had a history of prior cardiovascular disease at the time of their acute MI.
"They were sicker to start with," Ms. Jakobsson observed.
The most powerful predictors of increased risk of ischemic stroke post MI included older age, atrial fibrillation, an ST-elevation MI, and prior ischemic stroke.
This study was supported by Swedish governmental research funds. Ms. Jakobsson reported having no financial conflicts of interest.
AMSTERDAM – The risk of ischemic stroke following an acute myocardial infarction in diabetes patients dropped markedly during a recent 10-year period, according to a nationwide Swedish study.
Indeed, the reduction in ischemic stroke risk during the first year after an MI was significantly larger in diabetic than in nondiabetic patients over the course of a decade, Stina Jakobsson said at the annual congress of the European Society of Cardiology.
"We believe that the larger risk reduction seen in the diabetic patients may indicate that they have gained more from the increased use of evidence-based secondary preventive treatment," added Ms. Jakobsson, a medical student at Umea (Sweden) University.
She presented an analysis of all 173,233 patients discharged from Swedish coronary care units after an acute MI during 1998-2008. A total of 19% of them had a previous diagnosis of diabetes.
Among diabetes patients with an MI in 1998-2000, ischemic stroke occurred in 7.1% within 1 year after their coronary event. However, the 1-year ischemic stroke rate in such patients whose MI occurred in 2007-2008 dropped to 4.7%. This was a much more impressive improvement than occurred in the same time span among nondiabetic patients, where the ischemic stroke rate during the first year after an acute MI was 4.2% in 1998-2000, nudging downward to 3.7% in 2007-2008.
Ms. Jakobsson stressed that there is definitely room for improvement in the use of reperfusion therapy and secondary preventive medications among diabetes patients with an MI. Although the use of these key interventions increased over time in both diabetic and nondiabetic MI patients, rates still remained lower in the diabetic group in the most recent study years.
The 4.7% 1-year incidence of ischemic stroke among Swedish diabetes patients with an acute MI in 2007-2008 was significantly greater than the 3.7% rate among nondiabetic patients. Moreover, even among patients on optimized secondary prevention therapies, the ischemic stroke rate was higher in the diabetic group. That’s not surprising because they more often had a history of prior cardiovascular disease at the time of their acute MI.
"They were sicker to start with," Ms. Jakobsson observed.
The most powerful predictors of increased risk of ischemic stroke post MI included older age, atrial fibrillation, an ST-elevation MI, and prior ischemic stroke.
This study was supported by Swedish governmental research funds. Ms. Jakobsson reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: The rate of ischemic stroke during the first year after an acute MI improved from 7.1% among Swedish diabetic patients whose infarct occurred during 1998-2000 to 4.7% in those with an MI in 2007-2008.
Data source: A retrospective study of 173,233 patients discharged from Swedish coronary care units after an acute MI during 1998-2008. They were followed through national registries in order to learn their incidence of ischemic stroke during the first year post MI.
Disclosures: The presenter reported having no financial conflicts of interest.
How to foil post-CABG aspirin resistance
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AMSTERDAM – Giving low-dose aspirin four times per day in the first days after coronary artery bypass graft surgery suppresses serum thromboxane levels far more effectively than does conventional once-daily dosing at 325 mg, according to a randomized trial.
The clinical implication of this finding is that more frequent dosing of aspirin may prevent the serious problem of premature vein graft failure from the development of aspirin resistance in the postoperative period, although at this point this is a hypothesis that requires testing in a future study, Dr. Jeremy S. Paikin said at the annual congress of the European Society of Cardiology.
He reported on 110 on-pump coronary artery bypass graft (CABG) patients randomized on postoperative day 1 to aspirin either at 81 mg four times daily, the standard 325 mg once daily, or to 81 mg once daily.
The primary study endpoint was the serum thromboxane level on the morning of postoperative day 4. The median level was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
"With 81 mg QD [four times daily], there’s almost complete suppression of serum thromboxane throughout the course of the hospital stay," according to Dr. Paikin of McMaster University, Hamilton, Ont.
Aspirin is known to prevent CABG graft failure, but its effectiveness is limited by the not-infrequent development of aspirin hyporesponsiveness in the postoperative period. The underlying mechanism involved in this aspirin resistance was previously unknown; however, in their randomized trial Dr. Paikin and coinvestigators established that the hyporesponsiveness is caused at least in part by increased platelet turnover in the postoperative period. The investigators showed that platelet turnover per day was increased two- to threefold in the week after CABG, compared with presurgical levels, a finding Dr. Paikin termed "quite exciting."
Recognizing that administration of any drug four times daily raises formidable adherence obstacles, he and his coworkers are just about to start a clinical trial looking at twice-daily aspirin dosing post CABG. They’re also interested in drawing a firm evidentiary connection between serum thromboxane levels and risk of premature graft failure.
Dr. Paikin reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: The median serum thromboxane level on the morning of post CABG day 4 was 13.3 ng/mL in the group on aspirin at 81 mg once daily, 3.4 ng/mL with 325 mg once daily, and significantly lower at 1.1 ng/mL in patients on 81 mg four times daily.
Data source: A randomized clinical trial in which 110 patients who underwent on-pump CABG surgery were randomized on postoperative day 1 to aspirin at either 81 mg four times daily, 325 mg once daily, or 81 mg once daily.
Disclosures: The study presenter reported having no financial conflicts.
High resting heart rate portends cognitive decline
AMSTERDAM – A high resting heart rate proved to be a strong and independent predictor of cognitive decline within the next 4 years in a study of nearly 28,000 patients at high cardiovascular risk.
The clinical implications of this finding, however, remain unclear, according to Dr. Darryl P. Leong.
"What this study cannot answer, and which must be answered, is whether resting heart rate is just a marker of the risk of cognitive decline or whether it exists in the causal pathway. Further research is needed to determine if resting heart rate represents a therapeutic target to prevent cognitive decline. I think the only way to test this is with some intervention to reduce heart rate – whether using a beta-blocker or a medication such as ivabradine – to see whether or not it influences the incidence of cognitive decline," he said in presenting the study findings at the annual congress of the European Society of Cardiology.
"Cognitive dysfunction and decline, I think, are going to be a major scourge over the next decades," he added in explaining the study rationale. "We have an increasingly aged population, we have increasingly better treatment and survival of cardiovascular disease, and as a result more and more people are going to be living long enough to experience the misfortune of having cognitive impairment," said Dr. Leong, a postdoctoral fellow at the population health research institute at McMaster University, Hamilton, Ont.
He presented a post hoc analysis of two major randomized clinical trials – ONTARGET (the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomised Assessment Study in Angiotension-Converting Enzyme Inhibitor–Intolerant Subjects With Cardiovascular Disease). In these two trials, patients with diabetes or vascular disease were assigned to ramipril, telmisartan, both, or placebo. The primary outcomes have already been published. Dr. Leong and his coinvestigators at McMaster used the database for the two trials, which contains information on baseline resting heart rate and subsequent cognitive decline in 27,660 participants.
The quartiles of baseline resting heart rate were less than 60 bpm, 60-66, 67-74, and 75 or more bpm. Cognitive decline was defined as at least a 3-point drop from baseline on the Mini-Mental State Exam at a median 4 years of follow-up.
During the follow-up period, 17% of the 27,660 patients exhibited cognitive decline. The investigators noted a stepwise relationship between baseline resting heart rate and cognitive deterioration: Those in the top two quartiles – that is, patients with a resting heart rate of 67 bpm or more – had a 16% greater risk than did those in the lower two quartiles. This was the case even after extensive adjustment in a multivariate regression analysis controlled for baseline demographic variables, cardiovascular comorbidities, depressive symptoms, blood pressure, dietary habits, renal function, medications, lipid levels, left ventricular hypertrophy, alcohol consumption, and physical activity level.
Even after investigators controlled for baseline use of beta-blockers, calcium channel blockers, and digoxin, resting heart rate remained predictive of subsequent cognitive decline, according to Dr. Leong.
He reported having no relevant financial conflicts.
AMSTERDAM – A high resting heart rate proved to be a strong and independent predictor of cognitive decline within the next 4 years in a study of nearly 28,000 patients at high cardiovascular risk.
The clinical implications of this finding, however, remain unclear, according to Dr. Darryl P. Leong.
"What this study cannot answer, and which must be answered, is whether resting heart rate is just a marker of the risk of cognitive decline or whether it exists in the causal pathway. Further research is needed to determine if resting heart rate represents a therapeutic target to prevent cognitive decline. I think the only way to test this is with some intervention to reduce heart rate – whether using a beta-blocker or a medication such as ivabradine – to see whether or not it influences the incidence of cognitive decline," he said in presenting the study findings at the annual congress of the European Society of Cardiology.
"Cognitive dysfunction and decline, I think, are going to be a major scourge over the next decades," he added in explaining the study rationale. "We have an increasingly aged population, we have increasingly better treatment and survival of cardiovascular disease, and as a result more and more people are going to be living long enough to experience the misfortune of having cognitive impairment," said Dr. Leong, a postdoctoral fellow at the population health research institute at McMaster University, Hamilton, Ont.
He presented a post hoc analysis of two major randomized clinical trials – ONTARGET (the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomised Assessment Study in Angiotension-Converting Enzyme Inhibitor–Intolerant Subjects With Cardiovascular Disease). In these two trials, patients with diabetes or vascular disease were assigned to ramipril, telmisartan, both, or placebo. The primary outcomes have already been published. Dr. Leong and his coinvestigators at McMaster used the database for the two trials, which contains information on baseline resting heart rate and subsequent cognitive decline in 27,660 participants.
The quartiles of baseline resting heart rate were less than 60 bpm, 60-66, 67-74, and 75 or more bpm. Cognitive decline was defined as at least a 3-point drop from baseline on the Mini-Mental State Exam at a median 4 years of follow-up.
During the follow-up period, 17% of the 27,660 patients exhibited cognitive decline. The investigators noted a stepwise relationship between baseline resting heart rate and cognitive deterioration: Those in the top two quartiles – that is, patients with a resting heart rate of 67 bpm or more – had a 16% greater risk than did those in the lower two quartiles. This was the case even after extensive adjustment in a multivariate regression analysis controlled for baseline demographic variables, cardiovascular comorbidities, depressive symptoms, blood pressure, dietary habits, renal function, medications, lipid levels, left ventricular hypertrophy, alcohol consumption, and physical activity level.
Even after investigators controlled for baseline use of beta-blockers, calcium channel blockers, and digoxin, resting heart rate remained predictive of subsequent cognitive decline, according to Dr. Leong.
He reported having no relevant financial conflicts.
AMSTERDAM – A high resting heart rate proved to be a strong and independent predictor of cognitive decline within the next 4 years in a study of nearly 28,000 patients at high cardiovascular risk.
The clinical implications of this finding, however, remain unclear, according to Dr. Darryl P. Leong.
"What this study cannot answer, and which must be answered, is whether resting heart rate is just a marker of the risk of cognitive decline or whether it exists in the causal pathway. Further research is needed to determine if resting heart rate represents a therapeutic target to prevent cognitive decline. I think the only way to test this is with some intervention to reduce heart rate – whether using a beta-blocker or a medication such as ivabradine – to see whether or not it influences the incidence of cognitive decline," he said in presenting the study findings at the annual congress of the European Society of Cardiology.
"Cognitive dysfunction and decline, I think, are going to be a major scourge over the next decades," he added in explaining the study rationale. "We have an increasingly aged population, we have increasingly better treatment and survival of cardiovascular disease, and as a result more and more people are going to be living long enough to experience the misfortune of having cognitive impairment," said Dr. Leong, a postdoctoral fellow at the population health research institute at McMaster University, Hamilton, Ont.
He presented a post hoc analysis of two major randomized clinical trials – ONTARGET (the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomised Assessment Study in Angiotension-Converting Enzyme Inhibitor–Intolerant Subjects With Cardiovascular Disease). In these two trials, patients with diabetes or vascular disease were assigned to ramipril, telmisartan, both, or placebo. The primary outcomes have already been published. Dr. Leong and his coinvestigators at McMaster used the database for the two trials, which contains information on baseline resting heart rate and subsequent cognitive decline in 27,660 participants.
The quartiles of baseline resting heart rate were less than 60 bpm, 60-66, 67-74, and 75 or more bpm. Cognitive decline was defined as at least a 3-point drop from baseline on the Mini-Mental State Exam at a median 4 years of follow-up.
During the follow-up period, 17% of the 27,660 patients exhibited cognitive decline. The investigators noted a stepwise relationship between baseline resting heart rate and cognitive deterioration: Those in the top two quartiles – that is, patients with a resting heart rate of 67 bpm or more – had a 16% greater risk than did those in the lower two quartiles. This was the case even after extensive adjustment in a multivariate regression analysis controlled for baseline demographic variables, cardiovascular comorbidities, depressive symptoms, blood pressure, dietary habits, renal function, medications, lipid levels, left ventricular hypertrophy, alcohol consumption, and physical activity level.
Even after investigators controlled for baseline use of beta-blockers, calcium channel blockers, and digoxin, resting heart rate remained predictive of subsequent cognitive decline, according to Dr. Leong.
He reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2013
Major finding: Individuals at high cardiovascular risk who had a baseline resting heart rate of 67 bpm or more had a 16% greater risk of clinically meaningful cognitive decline during the next 4 years than did those with a resting heart rate below that threshold.
Data source: A post hoc secondary analysis of data on the nearly 28,000 participants in the randomized, double-blind ONTARGET and TRANSCEND clinical trials.
Disclosures: The post hoc analysis was funded by the population health research institute at McMaster University. The presenter reported having no relevant financial conflicts.
Tour de France riders live longer
AMSTERDAM – French bicyclists who have competed in the Tour de France have a 41% lower mortality rate, compared with the age-matched male French general population, according to a first-of-its-kind study carried out on the 100th anniversary of the world’s most venerable professional bike race.
Causes of death were consistently lower across the board among the ex-racers. They had a 33% reduction in mortality owing to cardiovascular diseases, a 44% decrease in fatal cancers, a 72% reduction in deaths from respiratory disease, and a 78% decrease in deaths from digestive diseases, compared with controls. The sole cause-of-death category that was not significantly less common among the former Tour de France athletes was trauma, mostly from bike or auto crashes, Dr. Xavier Jouven reported at the annual congress of the European Society of Cardiology.
The study included all 786 French professional bike racers who competed in the Tour de France during 1947-2012. They participated in a mean 2.5 tours. By 2012, there had been 208 deaths.
The mortality advantage favoring the ex-racers was consistent across all 5-year age groups. It’s estimated that the Tour de France participants are averaging a 6- to 7-year greater longevity than do other Frenchmen, according to Dr. Jouven of Georges Pompidou Hospital, Paris.
He confessed to being surprised by the study results. He had anticipated finding an above-average mortality rate in the ex-racers because of the deleterious long-term effects of the various performance-enhancing drugs widely used over the decades, coupled with the sheer physical grind of participating in an event described as similar to racing a marathon per day for nearly 3 weeks and the crashes that are part of the job description.
"If there was really a danger in doing high-level exercise, then we should have observed an increased mortality rate. That’s actually what we’d expected to find," the cardiologist said.
The cyclists’ mortality reduction was consistent across all eras of the race: the 1950s and 1960s, when the use of amphetamines was common; the 1970s and 1980s, when anabolic steroids were the performance-enhancing drugs of choice; and most recently, the era of erythropoietin and human growth hormone, he noted.
The study was restricted to French cyclists because the investigators knew they could capture every single death through French registries.
Discussant Sanjay Sharma took issue with Dr. Jouven’s interpretation of the Tour de France data, which he said do not really address one of the hottest controversies in all of sports medicine: Is too much exercise cardiotoxic?
"I would like to have some data on comorbidities. I would like to have known how many of the surviving individuals have atrial fibrillation, compared with the general population. How many have implanted pacemakers? I would urge the investigators to go back and look at the survivors and produce [these] very important data," said Dr. Sharma of St. George’s Hospital, London.
He added that he was unimpressed by the investigators’ choice of a comparison group.
"They have compared possibly the fittest human beings in the world, people who are probably genetically, physically, physiologically, and psychologically superior to the general population, in which there is of course a much higher burden of comorbidities, a higher burden of risk factors for cardiovascular disease, a higher burden of adverse lifestyle, as well as a lower socioeconomic class. So I don’t believe that the Tour de France per se increases longevity of life; what I believe they have shown is, if you are capable of doing the Tour de France, then you may live 6-7 years longer than the average individual in the community," the cardiologist continued.
The benefits of physical exercise in the range of 40 metabolic equivalents (METs) per week spread over five or six workouts are well established. This level of exercise confers a 40%-50% reduction in cardiovascular mortality. But elite endurance athletes exercise at intensities 10- to 20-fold greater than that.
"There is emerging evidence that too much exercise may be deleterious to the heart," according to Dr. Sharma. "We know that marathon runners have high circulating markers of cardiac damage. Animal studies show increased inflammation and fibrosis within the myocardium. Recent cardiac magnetic resonance imaging studies have shown fibrosis in veteran athletes, and [electrophysiologists] continue to talk about the high prevalence of atrial fibrillation amongst veteran athletes."
Dr. Jouven’s presentation was big news in daily newspapers across cycling-crazy Europe. Simultaneous with his report on the Tour de France study in Amsterdam, the findings were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht347]) with an accompanying editorial coauthored by Dr. Sharma (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht373]).
Neither cardiologist had any relevant financial interests. The study was funded by a research grant from the Sudden Death Expertise Center in Paris.
AMSTERDAM – French bicyclists who have competed in the Tour de France have a 41% lower mortality rate, compared with the age-matched male French general population, according to a first-of-its-kind study carried out on the 100th anniversary of the world’s most venerable professional bike race.
Causes of death were consistently lower across the board among the ex-racers. They had a 33% reduction in mortality owing to cardiovascular diseases, a 44% decrease in fatal cancers, a 72% reduction in deaths from respiratory disease, and a 78% decrease in deaths from digestive diseases, compared with controls. The sole cause-of-death category that was not significantly less common among the former Tour de France athletes was trauma, mostly from bike or auto crashes, Dr. Xavier Jouven reported at the annual congress of the European Society of Cardiology.
The study included all 786 French professional bike racers who competed in the Tour de France during 1947-2012. They participated in a mean 2.5 tours. By 2012, there had been 208 deaths.
The mortality advantage favoring the ex-racers was consistent across all 5-year age groups. It’s estimated that the Tour de France participants are averaging a 6- to 7-year greater longevity than do other Frenchmen, according to Dr. Jouven of Georges Pompidou Hospital, Paris.
He confessed to being surprised by the study results. He had anticipated finding an above-average mortality rate in the ex-racers because of the deleterious long-term effects of the various performance-enhancing drugs widely used over the decades, coupled with the sheer physical grind of participating in an event described as similar to racing a marathon per day for nearly 3 weeks and the crashes that are part of the job description.
"If there was really a danger in doing high-level exercise, then we should have observed an increased mortality rate. That’s actually what we’d expected to find," the cardiologist said.
The cyclists’ mortality reduction was consistent across all eras of the race: the 1950s and 1960s, when the use of amphetamines was common; the 1970s and 1980s, when anabolic steroids were the performance-enhancing drugs of choice; and most recently, the era of erythropoietin and human growth hormone, he noted.
The study was restricted to French cyclists because the investigators knew they could capture every single death through French registries.
Discussant Sanjay Sharma took issue with Dr. Jouven’s interpretation of the Tour de France data, which he said do not really address one of the hottest controversies in all of sports medicine: Is too much exercise cardiotoxic?
"I would like to have some data on comorbidities. I would like to have known how many of the surviving individuals have atrial fibrillation, compared with the general population. How many have implanted pacemakers? I would urge the investigators to go back and look at the survivors and produce [these] very important data," said Dr. Sharma of St. George’s Hospital, London.
He added that he was unimpressed by the investigators’ choice of a comparison group.
"They have compared possibly the fittest human beings in the world, people who are probably genetically, physically, physiologically, and psychologically superior to the general population, in which there is of course a much higher burden of comorbidities, a higher burden of risk factors for cardiovascular disease, a higher burden of adverse lifestyle, as well as a lower socioeconomic class. So I don’t believe that the Tour de France per se increases longevity of life; what I believe they have shown is, if you are capable of doing the Tour de France, then you may live 6-7 years longer than the average individual in the community," the cardiologist continued.
The benefits of physical exercise in the range of 40 metabolic equivalents (METs) per week spread over five or six workouts are well established. This level of exercise confers a 40%-50% reduction in cardiovascular mortality. But elite endurance athletes exercise at intensities 10- to 20-fold greater than that.
"There is emerging evidence that too much exercise may be deleterious to the heart," according to Dr. Sharma. "We know that marathon runners have high circulating markers of cardiac damage. Animal studies show increased inflammation and fibrosis within the myocardium. Recent cardiac magnetic resonance imaging studies have shown fibrosis in veteran athletes, and [electrophysiologists] continue to talk about the high prevalence of atrial fibrillation amongst veteran athletes."
Dr. Jouven’s presentation was big news in daily newspapers across cycling-crazy Europe. Simultaneous with his report on the Tour de France study in Amsterdam, the findings were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht347]) with an accompanying editorial coauthored by Dr. Sharma (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht373]).
Neither cardiologist had any relevant financial interests. The study was funded by a research grant from the Sudden Death Expertise Center in Paris.
AMSTERDAM – French bicyclists who have competed in the Tour de France have a 41% lower mortality rate, compared with the age-matched male French general population, according to a first-of-its-kind study carried out on the 100th anniversary of the world’s most venerable professional bike race.
Causes of death were consistently lower across the board among the ex-racers. They had a 33% reduction in mortality owing to cardiovascular diseases, a 44% decrease in fatal cancers, a 72% reduction in deaths from respiratory disease, and a 78% decrease in deaths from digestive diseases, compared with controls. The sole cause-of-death category that was not significantly less common among the former Tour de France athletes was trauma, mostly from bike or auto crashes, Dr. Xavier Jouven reported at the annual congress of the European Society of Cardiology.
The study included all 786 French professional bike racers who competed in the Tour de France during 1947-2012. They participated in a mean 2.5 tours. By 2012, there had been 208 deaths.
The mortality advantage favoring the ex-racers was consistent across all 5-year age groups. It’s estimated that the Tour de France participants are averaging a 6- to 7-year greater longevity than do other Frenchmen, according to Dr. Jouven of Georges Pompidou Hospital, Paris.
He confessed to being surprised by the study results. He had anticipated finding an above-average mortality rate in the ex-racers because of the deleterious long-term effects of the various performance-enhancing drugs widely used over the decades, coupled with the sheer physical grind of participating in an event described as similar to racing a marathon per day for nearly 3 weeks and the crashes that are part of the job description.
"If there was really a danger in doing high-level exercise, then we should have observed an increased mortality rate. That’s actually what we’d expected to find," the cardiologist said.
The cyclists’ mortality reduction was consistent across all eras of the race: the 1950s and 1960s, when the use of amphetamines was common; the 1970s and 1980s, when anabolic steroids were the performance-enhancing drugs of choice; and most recently, the era of erythropoietin and human growth hormone, he noted.
The study was restricted to French cyclists because the investigators knew they could capture every single death through French registries.
Discussant Sanjay Sharma took issue with Dr. Jouven’s interpretation of the Tour de France data, which he said do not really address one of the hottest controversies in all of sports medicine: Is too much exercise cardiotoxic?
"I would like to have some data on comorbidities. I would like to have known how many of the surviving individuals have atrial fibrillation, compared with the general population. How many have implanted pacemakers? I would urge the investigators to go back and look at the survivors and produce [these] very important data," said Dr. Sharma of St. George’s Hospital, London.
He added that he was unimpressed by the investigators’ choice of a comparison group.
"They have compared possibly the fittest human beings in the world, people who are probably genetically, physically, physiologically, and psychologically superior to the general population, in which there is of course a much higher burden of comorbidities, a higher burden of risk factors for cardiovascular disease, a higher burden of adverse lifestyle, as well as a lower socioeconomic class. So I don’t believe that the Tour de France per se increases longevity of life; what I believe they have shown is, if you are capable of doing the Tour de France, then you may live 6-7 years longer than the average individual in the community," the cardiologist continued.
The benefits of physical exercise in the range of 40 metabolic equivalents (METs) per week spread over five or six workouts are well established. This level of exercise confers a 40%-50% reduction in cardiovascular mortality. But elite endurance athletes exercise at intensities 10- to 20-fold greater than that.
"There is emerging evidence that too much exercise may be deleterious to the heart," according to Dr. Sharma. "We know that marathon runners have high circulating markers of cardiac damage. Animal studies show increased inflammation and fibrosis within the myocardium. Recent cardiac magnetic resonance imaging studies have shown fibrosis in veteran athletes, and [electrophysiologists] continue to talk about the high prevalence of atrial fibrillation amongst veteran athletes."
Dr. Jouven’s presentation was big news in daily newspapers across cycling-crazy Europe. Simultaneous with his report on the Tour de France study in Amsterdam, the findings were published online (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht347]) with an accompanying editorial coauthored by Dr. Sharma (Eur. Heart J. 2013 [doi:10.1093/eurheartj/eht373]).
Neither cardiologist had any relevant financial interests. The study was funded by a research grant from the Sudden Death Expertise Center in Paris.
AT THE ESC CONGRESS 2013
Major finding: French professional cyclists who participated in the Tour de France during 1947-2012 had a 41% lower mortality rate than did the age-matched general French male population.
Data source: This was a retrospective study that captured all 208 deaths that have occurred among the 786 French cyclists.
Disclosures: The study was funded by a research grant from the Sudden Death Expertise Center in Paris. The presenter had no financial conflicts.
New heart failure inotrope could be ‘Holy Grail’
AMSTERDAM – The novel intravenous inotropic agent omecamtiv mecarbil achieved greater reduction in dyspnea scores and less likelihood of worsening heart failure at the highest dose studied, compared with placebo, in patients with acute decompensated heart failure in a phase II, dose-ranging study.
The drug also demonstrated a strong dose-dependent increase in systolic ejection time, no evidence of a proarrhythmic effect, and a greater reduction in heart rate with a larger increase in blood pressure than in placebo-treated controls, Dr. John R. Teerlink reported at the annual congress of the European Society of Cardiology.
In the overall population on omecamtiv mecarbil, however, which included the patients in the low- and intermediate-dose arms of this dose-ranging study, the dyspnea response rate wasn’t better than with placebo. Hence, the drug’s future is uncertain.
In a press release following Dr. Teerlink’s presentation, Amgen, cosponsor of the phase II ATOMIC-AHF (Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure) trial, said that no decision has been made as to whether to move omecamtiv mecarbil into phase III clinical trials. Dr. Teerlink thinks the drug has earned a shot.
"I personally believe this drug has continued to demonstrate its promise all along the way. It could replace milrinone and dobutamine in the hospital to improve cardiac performance," predicted Dr. Teerlink, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the San Francisco Veterans Affairs Medical Center.
Omecamtiv mecarbil is a novel selective cardiac myosin activator. It increases the entry rate of myosin into the force-producing state with actin. It boosts cardiac output through prolongation of the duration of systole and increased stroke volume, with no change in myocardial contractility and no increases in myocardial oxygen demand or myocyte calcium.
"It’s essentially producing more hands pulling on the rope," the cardiologist explained.
Discussant Theresa A. McDonagh said omecamtiv mecarbil has drawn great interest within the heart failure field because current inotropes are recognized as "very inadequate."
"It is truly a new inotrope, a drug that acts on the sarcomere itself. It does seem to be free of the nemesis of other inotropes: increased heart rate, greater myocardial oxygen consumption, arrhythmia production, and increased mortality in heart failure. It appears to be the Holy Grail, and may be capable of replacing dobutamine and milrinone," said Dr. McDonagh, professor of heart failure at King’s College London.
"I think this [ATOMIC-AHF trial] is a very promising start for omecamtiv mecarbil in acute heart failure," she added.
ATOMIC-AHF included 613 patients with a left ventricular ejection fraction of 40% or less who were hospitalized for acute heart failure with dyspnea at rest or with minimal exertion despite intravenous diuretic therapy. The study was conducted at 106 sites on three continents. Participants were randomized to 48 hours of intravenous placebo or omecamtiv mecarbil in three different ascending dose regimens.
The primary efficacy endpoint, dyspnea at 6, 24, and 48 hours across the three doses, compared with the pooled placebo group, was not met.
However, patients in the highest-dose omecamtiv mecarbil study arm had a 51% rate of significant improvement in dyspnea on a self-rated 7-point Likert scale at 48 hours, a significantly better response than the 37% rate in matched placebo-treated controls (P = .03). This comparison was a prespecified secondary endpoint in the study.
In addition, patients in the middle- and high-dose omecamtiv mecarbil arms had roughly a 45% reduction in the 7-day incidence of worsening heart failure, compared with the 17% rate in controls.
Not only was there no evidence of a proarrhythmic effect with omecamtiv mecarbil, the drug was associated with a 50% reduction in the incidence of supraventricular tachyarrhythmias, compared with placebo, he continued.
A modest increase in troponin I levels was noted in the omecamtiv mecarbil–treated group, but it was unrelated to plasma drug concentrations, which Dr. Teerlink found reassuring. Nevertheless, Dr. McDonagh said the small increases in troponin "should herald some caution amid the enthusiasm here."
The drug’s mechanism of action, which results in greater systolic ejection time, always raises the concern that it might also shorten diastole and compromise coronary perfusion. However, the observed reduction in heart rate makes that seem less likely, she added.
The ATOMIC-AHF study population was narrowly defined and of a profile commonly seen in clinical practice. They had an estimated glomerular filtration rate of about 50 mL/min, and they presented with dyspnea but didn’t have an acute coronary syndrome.
"This study population is an area of great unmet need in heart failure, with mortality rates of about 10% during their hospitalization and 30% at 1 year following discharge," according to Dr. McDonagh.
Nevertheless, it will be important in phase III testing to see how the drug performs in a broader group of heart failure patients, including those with lower estimated glomerular filtration rates, she added.
In an interview, Dr. Teerlink said that before a decision is reached regarding advancement of omecamtiv mecarbil to phase III studies, investigators and the study sponsors first want to see the data from an ongoing phase II dose-finding study of an oral formulation of the drug in chronic heart failure patients. This study, COSMIC-HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure), is still enrolling patients.
"We’ll get the combined data from ATOMIC-AHF and COSMIC-HF and then decide what phase III should look like. There are a lot of options here: IV [intravenous] alone, IV transitioning to chronic oral therapy, or even oral alone," he explained.
"One of the greatest challenges of the ATOMIC-AHF trial has been managing expectations," according to Dr. Teerlink. "Omecamtiv mecarbil is probably one of the most interesting new chemical entities in cardiovascular medicine now, and consequently expectations are very high in the scientific and lay communities."
The studies are sponsored by Amgen and Cytokinetics. Dr. Teerlink has received research grants and/or consulting fees from those companies as well as a handful of others. Dr. McDonagh reported no financial conflicts.
AMSTERDAM – The novel intravenous inotropic agent omecamtiv mecarbil achieved greater reduction in dyspnea scores and less likelihood of worsening heart failure at the highest dose studied, compared with placebo, in patients with acute decompensated heart failure in a phase II, dose-ranging study.
The drug also demonstrated a strong dose-dependent increase in systolic ejection time, no evidence of a proarrhythmic effect, and a greater reduction in heart rate with a larger increase in blood pressure than in placebo-treated controls, Dr. John R. Teerlink reported at the annual congress of the European Society of Cardiology.
In the overall population on omecamtiv mecarbil, however, which included the patients in the low- and intermediate-dose arms of this dose-ranging study, the dyspnea response rate wasn’t better than with placebo. Hence, the drug’s future is uncertain.
In a press release following Dr. Teerlink’s presentation, Amgen, cosponsor of the phase II ATOMIC-AHF (Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure) trial, said that no decision has been made as to whether to move omecamtiv mecarbil into phase III clinical trials. Dr. Teerlink thinks the drug has earned a shot.
"I personally believe this drug has continued to demonstrate its promise all along the way. It could replace milrinone and dobutamine in the hospital to improve cardiac performance," predicted Dr. Teerlink, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the San Francisco Veterans Affairs Medical Center.
Omecamtiv mecarbil is a novel selective cardiac myosin activator. It increases the entry rate of myosin into the force-producing state with actin. It boosts cardiac output through prolongation of the duration of systole and increased stroke volume, with no change in myocardial contractility and no increases in myocardial oxygen demand or myocyte calcium.
"It’s essentially producing more hands pulling on the rope," the cardiologist explained.
Discussant Theresa A. McDonagh said omecamtiv mecarbil has drawn great interest within the heart failure field because current inotropes are recognized as "very inadequate."
"It is truly a new inotrope, a drug that acts on the sarcomere itself. It does seem to be free of the nemesis of other inotropes: increased heart rate, greater myocardial oxygen consumption, arrhythmia production, and increased mortality in heart failure. It appears to be the Holy Grail, and may be capable of replacing dobutamine and milrinone," said Dr. McDonagh, professor of heart failure at King’s College London.
"I think this [ATOMIC-AHF trial] is a very promising start for omecamtiv mecarbil in acute heart failure," she added.
ATOMIC-AHF included 613 patients with a left ventricular ejection fraction of 40% or less who were hospitalized for acute heart failure with dyspnea at rest or with minimal exertion despite intravenous diuretic therapy. The study was conducted at 106 sites on three continents. Participants were randomized to 48 hours of intravenous placebo or omecamtiv mecarbil in three different ascending dose regimens.
The primary efficacy endpoint, dyspnea at 6, 24, and 48 hours across the three doses, compared with the pooled placebo group, was not met.
However, patients in the highest-dose omecamtiv mecarbil study arm had a 51% rate of significant improvement in dyspnea on a self-rated 7-point Likert scale at 48 hours, a significantly better response than the 37% rate in matched placebo-treated controls (P = .03). This comparison was a prespecified secondary endpoint in the study.
In addition, patients in the middle- and high-dose omecamtiv mecarbil arms had roughly a 45% reduction in the 7-day incidence of worsening heart failure, compared with the 17% rate in controls.
Not only was there no evidence of a proarrhythmic effect with omecamtiv mecarbil, the drug was associated with a 50% reduction in the incidence of supraventricular tachyarrhythmias, compared with placebo, he continued.
A modest increase in troponin I levels was noted in the omecamtiv mecarbil–treated group, but it was unrelated to plasma drug concentrations, which Dr. Teerlink found reassuring. Nevertheless, Dr. McDonagh said the small increases in troponin "should herald some caution amid the enthusiasm here."
The drug’s mechanism of action, which results in greater systolic ejection time, always raises the concern that it might also shorten diastole and compromise coronary perfusion. However, the observed reduction in heart rate makes that seem less likely, she added.
The ATOMIC-AHF study population was narrowly defined and of a profile commonly seen in clinical practice. They had an estimated glomerular filtration rate of about 50 mL/min, and they presented with dyspnea but didn’t have an acute coronary syndrome.
"This study population is an area of great unmet need in heart failure, with mortality rates of about 10% during their hospitalization and 30% at 1 year following discharge," according to Dr. McDonagh.
Nevertheless, it will be important in phase III testing to see how the drug performs in a broader group of heart failure patients, including those with lower estimated glomerular filtration rates, she added.
In an interview, Dr. Teerlink said that before a decision is reached regarding advancement of omecamtiv mecarbil to phase III studies, investigators and the study sponsors first want to see the data from an ongoing phase II dose-finding study of an oral formulation of the drug in chronic heart failure patients. This study, COSMIC-HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure), is still enrolling patients.
"We’ll get the combined data from ATOMIC-AHF and COSMIC-HF and then decide what phase III should look like. There are a lot of options here: IV [intravenous] alone, IV transitioning to chronic oral therapy, or even oral alone," he explained.
"One of the greatest challenges of the ATOMIC-AHF trial has been managing expectations," according to Dr. Teerlink. "Omecamtiv mecarbil is probably one of the most interesting new chemical entities in cardiovascular medicine now, and consequently expectations are very high in the scientific and lay communities."
The studies are sponsored by Amgen and Cytokinetics. Dr. Teerlink has received research grants and/or consulting fees from those companies as well as a handful of others. Dr. McDonagh reported no financial conflicts.
AMSTERDAM – The novel intravenous inotropic agent omecamtiv mecarbil achieved greater reduction in dyspnea scores and less likelihood of worsening heart failure at the highest dose studied, compared with placebo, in patients with acute decompensated heart failure in a phase II, dose-ranging study.
The drug also demonstrated a strong dose-dependent increase in systolic ejection time, no evidence of a proarrhythmic effect, and a greater reduction in heart rate with a larger increase in blood pressure than in placebo-treated controls, Dr. John R. Teerlink reported at the annual congress of the European Society of Cardiology.
In the overall population on omecamtiv mecarbil, however, which included the patients in the low- and intermediate-dose arms of this dose-ranging study, the dyspnea response rate wasn’t better than with placebo. Hence, the drug’s future is uncertain.
In a press release following Dr. Teerlink’s presentation, Amgen, cosponsor of the phase II ATOMIC-AHF (Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure) trial, said that no decision has been made as to whether to move omecamtiv mecarbil into phase III clinical trials. Dr. Teerlink thinks the drug has earned a shot.
"I personally believe this drug has continued to demonstrate its promise all along the way. It could replace milrinone and dobutamine in the hospital to improve cardiac performance," predicted Dr. Teerlink, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the San Francisco Veterans Affairs Medical Center.
Omecamtiv mecarbil is a novel selective cardiac myosin activator. It increases the entry rate of myosin into the force-producing state with actin. It boosts cardiac output through prolongation of the duration of systole and increased stroke volume, with no change in myocardial contractility and no increases in myocardial oxygen demand or myocyte calcium.
"It’s essentially producing more hands pulling on the rope," the cardiologist explained.
Discussant Theresa A. McDonagh said omecamtiv mecarbil has drawn great interest within the heart failure field because current inotropes are recognized as "very inadequate."
"It is truly a new inotrope, a drug that acts on the sarcomere itself. It does seem to be free of the nemesis of other inotropes: increased heart rate, greater myocardial oxygen consumption, arrhythmia production, and increased mortality in heart failure. It appears to be the Holy Grail, and may be capable of replacing dobutamine and milrinone," said Dr. McDonagh, professor of heart failure at King’s College London.
"I think this [ATOMIC-AHF trial] is a very promising start for omecamtiv mecarbil in acute heart failure," she added.
ATOMIC-AHF included 613 patients with a left ventricular ejection fraction of 40% or less who were hospitalized for acute heart failure with dyspnea at rest or with minimal exertion despite intravenous diuretic therapy. The study was conducted at 106 sites on three continents. Participants were randomized to 48 hours of intravenous placebo or omecamtiv mecarbil in three different ascending dose regimens.
The primary efficacy endpoint, dyspnea at 6, 24, and 48 hours across the three doses, compared with the pooled placebo group, was not met.
However, patients in the highest-dose omecamtiv mecarbil study arm had a 51% rate of significant improvement in dyspnea on a self-rated 7-point Likert scale at 48 hours, a significantly better response than the 37% rate in matched placebo-treated controls (P = .03). This comparison was a prespecified secondary endpoint in the study.
In addition, patients in the middle- and high-dose omecamtiv mecarbil arms had roughly a 45% reduction in the 7-day incidence of worsening heart failure, compared with the 17% rate in controls.
Not only was there no evidence of a proarrhythmic effect with omecamtiv mecarbil, the drug was associated with a 50% reduction in the incidence of supraventricular tachyarrhythmias, compared with placebo, he continued.
A modest increase in troponin I levels was noted in the omecamtiv mecarbil–treated group, but it was unrelated to plasma drug concentrations, which Dr. Teerlink found reassuring. Nevertheless, Dr. McDonagh said the small increases in troponin "should herald some caution amid the enthusiasm here."
The drug’s mechanism of action, which results in greater systolic ejection time, always raises the concern that it might also shorten diastole and compromise coronary perfusion. However, the observed reduction in heart rate makes that seem less likely, she added.
The ATOMIC-AHF study population was narrowly defined and of a profile commonly seen in clinical practice. They had an estimated glomerular filtration rate of about 50 mL/min, and they presented with dyspnea but didn’t have an acute coronary syndrome.
"This study population is an area of great unmet need in heart failure, with mortality rates of about 10% during their hospitalization and 30% at 1 year following discharge," according to Dr. McDonagh.
Nevertheless, it will be important in phase III testing to see how the drug performs in a broader group of heart failure patients, including those with lower estimated glomerular filtration rates, she added.
In an interview, Dr. Teerlink said that before a decision is reached regarding advancement of omecamtiv mecarbil to phase III studies, investigators and the study sponsors first want to see the data from an ongoing phase II dose-finding study of an oral formulation of the drug in chronic heart failure patients. This study, COSMIC-HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure), is still enrolling patients.
"We’ll get the combined data from ATOMIC-AHF and COSMIC-HF and then decide what phase III should look like. There are a lot of options here: IV [intravenous] alone, IV transitioning to chronic oral therapy, or even oral alone," he explained.
"One of the greatest challenges of the ATOMIC-AHF trial has been managing expectations," according to Dr. Teerlink. "Omecamtiv mecarbil is probably one of the most interesting new chemical entities in cardiovascular medicine now, and consequently expectations are very high in the scientific and lay communities."
The studies are sponsored by Amgen and Cytokinetics. Dr. Teerlink has received research grants and/or consulting fees from those companies as well as a handful of others. Dr. McDonagh reported no financial conflicts.
AT THE ESC CONGRESS 2013
Major finding: Fifty-one percent of patients with acute heart failure assigned to the high-dose arm in a study of intravenous omecamtiv mecarbil showed significant improvement in dyspnea scores at 48 hours, compared with 37% of placebo-treated controls.
Data source: The ATOMIC-AHF trial was a 613-patient, phase II, randomized, placebo-controlled, double-blind, dose-finding study.
Disclosures: The study was sponsored by Amgen and Cytokinetics. The presenter has received research grants and consultant fees from the companies.