User login
Prenatal maternal anxiety linked to hyperactivity in offspring as teenagers
PARIS – Maternal somatic anxiety during pregnancy and early childhood is associated in dose-response fashion with increased hyperactivity symptoms in mothers’ teenage children, according to an update from the landmark Avon Longitudinal Study of Parents and Offspring.
Perhaps the most intriguing finding in the new analysis was that the dose-response effect did not apply to the offspring of mothers in the highest quintile of somatic anxiety as measured during pregnancy and early childhood.
“It’s possible that a ceiling effect exists for those children that are exposed to continuous high levels of anxiety from pregnancy up to 5 years old,” Blanca Bolea-Alamanac, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Bolea-Alamanac of the department of psychiatry at the University of Toronto offered a couple possible explanations for this apparent ceiling effect. One is that children continuously exposed to high levels of maternal stress hormones in utero and through breast feeding become desensitized to the effects of those hormones in ways that affect their future behavior.
Another possibility is that children exposed to very high levels of anxiety in early life later develop internalizing-type symptoms rather than externalizing hyperactivity-type symptoms, she continued.
The Avon Longitudinal Study of Parents and Children is a birth cohort study conducted in Southwest England, where women who were pregnant in 1990 and their 14,062 offspring have been followed prospectively for 26 years.
Dr. Bolea-Alamanac’s analysis included the 8,725 women with maternal anxiety assessments obtained at 18 and 32 weeks of pregnancy, as well as at 8 weeks, 8 months, 2.67 years, and 5 years post partum. Maternal anxiety was measured using the Crown-Crisp Experiential Index, which provided a specific indicator of somatic anxiety via responses to questions including, “Are you troubled by dizziness or shortness of breath?” “Do you experience a tingling or prickling sensation in your body, arms, or legs?” “Have you felt that you may faint, feel sick, or have indigestion?” and “Do you experience extra sweating?” The response options to each question were “never,” “sometimes,” “often,” or “very often.”
All women understandably experienced increased anxiety during pregnancy, peaking in the weeks shortly prior to delivery. But by examining the totality of data obtained at two time points in pregnancy and four points post pregnancy, the psychiatrist was able to construct a model with five distinct quintiles of maternal anxiety.
Hyperactivity symptoms were assessed in 3,417 of their offspring using the Strengths and Difficulties Questionnaire hyperactivity subscale.
Class 1, the reference class, was comprised of women with low anxiety during pregnancy and after delivery. In a logistic regression analysis adjusted for maternal age, alcohol intake, and education, life events during pregnancy, socioeconomic status, and the child’s sex, the women in class 2 were at 1.44-fold increased risk of having a hyperactive teenager. Those who were more anxious, in class 3, were at 1.87-fold increased risk, and those in class 4 were at 2.5-fold greater risk than those in class 1. All those increased risks were statistically significant.
In contrast, women in class 5 – those who scored highest for somatic anxiety both in pregnancy and during the next 5 years – had only a nonsignificant trend toward an increased risk of having a hyperactive teenager.
The association between prenatal and postnatal maternal somatic anxiety and hyperactivity symptoms in their children seen in the Avon study is consistent with Barker’s theory, according to Dr. Bolea-Alamanac. Barker’s theory, developed by the prominent late British epidemiologist David Barker, MD, PhD, holds that intrauterine influences interact with the environment at birth to produce specific disease risks for the child. Dr. Barker’s theory has gained considerable traction over the years, as evidenced by the creation of the multidisciplinary International Society for Developmental Origins of Health and Disease.
Dr. Bolea-Alamanac reported having no relevant financial conflicts of interest.
PARIS – Maternal somatic anxiety during pregnancy and early childhood is associated in dose-response fashion with increased hyperactivity symptoms in mothers’ teenage children, according to an update from the landmark Avon Longitudinal Study of Parents and Offspring.
Perhaps the most intriguing finding in the new analysis was that the dose-response effect did not apply to the offspring of mothers in the highest quintile of somatic anxiety as measured during pregnancy and early childhood.
“It’s possible that a ceiling effect exists for those children that are exposed to continuous high levels of anxiety from pregnancy up to 5 years old,” Blanca Bolea-Alamanac, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Bolea-Alamanac of the department of psychiatry at the University of Toronto offered a couple possible explanations for this apparent ceiling effect. One is that children continuously exposed to high levels of maternal stress hormones in utero and through breast feeding become desensitized to the effects of those hormones in ways that affect their future behavior.
Another possibility is that children exposed to very high levels of anxiety in early life later develop internalizing-type symptoms rather than externalizing hyperactivity-type symptoms, she continued.
The Avon Longitudinal Study of Parents and Children is a birth cohort study conducted in Southwest England, where women who were pregnant in 1990 and their 14,062 offspring have been followed prospectively for 26 years.
Dr. Bolea-Alamanac’s analysis included the 8,725 women with maternal anxiety assessments obtained at 18 and 32 weeks of pregnancy, as well as at 8 weeks, 8 months, 2.67 years, and 5 years post partum. Maternal anxiety was measured using the Crown-Crisp Experiential Index, which provided a specific indicator of somatic anxiety via responses to questions including, “Are you troubled by dizziness or shortness of breath?” “Do you experience a tingling or prickling sensation in your body, arms, or legs?” “Have you felt that you may faint, feel sick, or have indigestion?” and “Do you experience extra sweating?” The response options to each question were “never,” “sometimes,” “often,” or “very often.”
All women understandably experienced increased anxiety during pregnancy, peaking in the weeks shortly prior to delivery. But by examining the totality of data obtained at two time points in pregnancy and four points post pregnancy, the psychiatrist was able to construct a model with five distinct quintiles of maternal anxiety.
Hyperactivity symptoms were assessed in 3,417 of their offspring using the Strengths and Difficulties Questionnaire hyperactivity subscale.
Class 1, the reference class, was comprised of women with low anxiety during pregnancy and after delivery. In a logistic regression analysis adjusted for maternal age, alcohol intake, and education, life events during pregnancy, socioeconomic status, and the child’s sex, the women in class 2 were at 1.44-fold increased risk of having a hyperactive teenager. Those who were more anxious, in class 3, were at 1.87-fold increased risk, and those in class 4 were at 2.5-fold greater risk than those in class 1. All those increased risks were statistically significant.
In contrast, women in class 5 – those who scored highest for somatic anxiety both in pregnancy and during the next 5 years – had only a nonsignificant trend toward an increased risk of having a hyperactive teenager.
The association between prenatal and postnatal maternal somatic anxiety and hyperactivity symptoms in their children seen in the Avon study is consistent with Barker’s theory, according to Dr. Bolea-Alamanac. Barker’s theory, developed by the prominent late British epidemiologist David Barker, MD, PhD, holds that intrauterine influences interact with the environment at birth to produce specific disease risks for the child. Dr. Barker’s theory has gained considerable traction over the years, as evidenced by the creation of the multidisciplinary International Society for Developmental Origins of Health and Disease.
Dr. Bolea-Alamanac reported having no relevant financial conflicts of interest.
PARIS – Maternal somatic anxiety during pregnancy and early childhood is associated in dose-response fashion with increased hyperactivity symptoms in mothers’ teenage children, according to an update from the landmark Avon Longitudinal Study of Parents and Offspring.
Perhaps the most intriguing finding in the new analysis was that the dose-response effect did not apply to the offspring of mothers in the highest quintile of somatic anxiety as measured during pregnancy and early childhood.
“It’s possible that a ceiling effect exists for those children that are exposed to continuous high levels of anxiety from pregnancy up to 5 years old,” Blanca Bolea-Alamanac, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Bolea-Alamanac of the department of psychiatry at the University of Toronto offered a couple possible explanations for this apparent ceiling effect. One is that children continuously exposed to high levels of maternal stress hormones in utero and through breast feeding become desensitized to the effects of those hormones in ways that affect their future behavior.
Another possibility is that children exposed to very high levels of anxiety in early life later develop internalizing-type symptoms rather than externalizing hyperactivity-type symptoms, she continued.
The Avon Longitudinal Study of Parents and Children is a birth cohort study conducted in Southwest England, where women who were pregnant in 1990 and their 14,062 offspring have been followed prospectively for 26 years.
Dr. Bolea-Alamanac’s analysis included the 8,725 women with maternal anxiety assessments obtained at 18 and 32 weeks of pregnancy, as well as at 8 weeks, 8 months, 2.67 years, and 5 years post partum. Maternal anxiety was measured using the Crown-Crisp Experiential Index, which provided a specific indicator of somatic anxiety via responses to questions including, “Are you troubled by dizziness or shortness of breath?” “Do you experience a tingling or prickling sensation in your body, arms, or legs?” “Have you felt that you may faint, feel sick, or have indigestion?” and “Do you experience extra sweating?” The response options to each question were “never,” “sometimes,” “often,” or “very often.”
All women understandably experienced increased anxiety during pregnancy, peaking in the weeks shortly prior to delivery. But by examining the totality of data obtained at two time points in pregnancy and four points post pregnancy, the psychiatrist was able to construct a model with five distinct quintiles of maternal anxiety.
Hyperactivity symptoms were assessed in 3,417 of their offspring using the Strengths and Difficulties Questionnaire hyperactivity subscale.
Class 1, the reference class, was comprised of women with low anxiety during pregnancy and after delivery. In a logistic regression analysis adjusted for maternal age, alcohol intake, and education, life events during pregnancy, socioeconomic status, and the child’s sex, the women in class 2 were at 1.44-fold increased risk of having a hyperactive teenager. Those who were more anxious, in class 3, were at 1.87-fold increased risk, and those in class 4 were at 2.5-fold greater risk than those in class 1. All those increased risks were statistically significant.
In contrast, women in class 5 – those who scored highest for somatic anxiety both in pregnancy and during the next 5 years – had only a nonsignificant trend toward an increased risk of having a hyperactive teenager.
The association between prenatal and postnatal maternal somatic anxiety and hyperactivity symptoms in their children seen in the Avon study is consistent with Barker’s theory, according to Dr. Bolea-Alamanac. Barker’s theory, developed by the prominent late British epidemiologist David Barker, MD, PhD, holds that intrauterine influences interact with the environment at birth to produce specific disease risks for the child. Dr. Barker’s theory has gained considerable traction over the years, as evidenced by the creation of the multidisciplinary International Society for Developmental Origins of Health and Disease.
Dr. Bolea-Alamanac reported having no relevant financial conflicts of interest.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Women in the fourth quintile of somatic anxiety level during pregnancy and early childhood were at 2.5-fold increased risk of later having a hyperactive 16-year-old, compared with women in the lowest quintile of somatic anxiety.
Data source: Data gathered prospectively in the landmark Avon Longitudinal Study of Parents and Offspring.
Disclosures: The study presenter reported having no relevant financial conflicts of interest.
Adjunctive minocycline for schizophrenia found beneficial
PARIS – A report that adjunctive minocycline was found safe and effective for treatment of schizophrenia must be considered one of the year’s highlights in the field of psychosis, Pascal Steullet, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The report came in the form of a meta-analysis conducted by investigators in China and Australia. This was the largest meta-analysis looking at the topic to date, and the only one to include a search of the Chinese language database, which provided three of the eight randomized, placebo-controlled clinical trials that were examined. Seven of the eight randomized trials were double-blind and deemed high quality by widely used criteria, including the Jadad scale and GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology, noted Dr. Steullet, a neuroscientist at the University of Lausanne (Switzerland).
The primary outcome was change in the PANSS (Positive and Negative Syndrome Scale) total psychopathology score, and the positive, negative, and general symptom subscale scores.
The biggest benefit was on PANSS negative symptoms. Minocycline brought significantly greater improvement in this domain than placebo, with a standard mean difference (SMD) of –0.69 and a P value of less than .00001 (Eur Psychopharmacol. 2017 Jan;27[1]:8-18).
“That’s quite a good effect size,” Dr. Steullet commented.
The benefit on PANSS positive symptoms, while statistically significant, was far less robust, with an SMD of –0.22 in favor of minocycline.
The PANSS total psychopathology score favored minocycline with an SMD of –0.64, which Dr. Steullet again deemed “a quite significant effect size.” The PANSS general symptom score also showed a significant benefit in favor of minocycline, with an SMD of –0.45.
Among various secondary endpoints that were evaluated: A significant benefit was found favoring minocycline on the Clinical Global Impressions scale (SMD of –0.53) and the Abnormal Involuntary Movement Scale (SMD of –0.56). However, no differences were found between the minocycline and control groups on the Global Assessment of Functioning Scale, the Extrapyramidal Symptom Rating Scale, or the Calgary Depression Scale for Schizophrenia.
Although the average dose of minocycline prescribed in the eight trials was 171.9 mg/day, dosing strategies varied widely from trial to trial, and the investigators concluded that future studies are needed in order to pin down the optimal dosing and duration.
There was no increase in adverse drug reactions in the minocycline-treated group.
Neurocognitive function as assessed by the MATRICS Consensus Cognitive Battery showed no differences between the minocycline and placebo groups in working memory, problem solving, attention/vigilance, or other elements.
Proposed mechanisms of minocycline’s benefit as adjunctive therapy alongside antipsychotics for schizophrenia include the antimicrobial’s good CNS penetration and its anti-inflammatory effects, which could reduce neuroinflammation, dampen activated microglia, and enhance glutamate neurotransmitters.
The meta-analysis was supported by the University of Macau. Its authors reported having no financial conflicts of interest. Dr. Steullet, who was not involved in the work, also reported having no financial conflicts of interest.
PARIS – A report that adjunctive minocycline was found safe and effective for treatment of schizophrenia must be considered one of the year’s highlights in the field of psychosis, Pascal Steullet, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The report came in the form of a meta-analysis conducted by investigators in China and Australia. This was the largest meta-analysis looking at the topic to date, and the only one to include a search of the Chinese language database, which provided three of the eight randomized, placebo-controlled clinical trials that were examined. Seven of the eight randomized trials were double-blind and deemed high quality by widely used criteria, including the Jadad scale and GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology, noted Dr. Steullet, a neuroscientist at the University of Lausanne (Switzerland).
The primary outcome was change in the PANSS (Positive and Negative Syndrome Scale) total psychopathology score, and the positive, negative, and general symptom subscale scores.
The biggest benefit was on PANSS negative symptoms. Minocycline brought significantly greater improvement in this domain than placebo, with a standard mean difference (SMD) of –0.69 and a P value of less than .00001 (Eur Psychopharmacol. 2017 Jan;27[1]:8-18).
“That’s quite a good effect size,” Dr. Steullet commented.
The benefit on PANSS positive symptoms, while statistically significant, was far less robust, with an SMD of –0.22 in favor of minocycline.
The PANSS total psychopathology score favored minocycline with an SMD of –0.64, which Dr. Steullet again deemed “a quite significant effect size.” The PANSS general symptom score also showed a significant benefit in favor of minocycline, with an SMD of –0.45.
Among various secondary endpoints that were evaluated: A significant benefit was found favoring minocycline on the Clinical Global Impressions scale (SMD of –0.53) and the Abnormal Involuntary Movement Scale (SMD of –0.56). However, no differences were found between the minocycline and control groups on the Global Assessment of Functioning Scale, the Extrapyramidal Symptom Rating Scale, or the Calgary Depression Scale for Schizophrenia.
Although the average dose of minocycline prescribed in the eight trials was 171.9 mg/day, dosing strategies varied widely from trial to trial, and the investigators concluded that future studies are needed in order to pin down the optimal dosing and duration.
There was no increase in adverse drug reactions in the minocycline-treated group.
Neurocognitive function as assessed by the MATRICS Consensus Cognitive Battery showed no differences between the minocycline and placebo groups in working memory, problem solving, attention/vigilance, or other elements.
Proposed mechanisms of minocycline’s benefit as adjunctive therapy alongside antipsychotics for schizophrenia include the antimicrobial’s good CNS penetration and its anti-inflammatory effects, which could reduce neuroinflammation, dampen activated microglia, and enhance glutamate neurotransmitters.
The meta-analysis was supported by the University of Macau. Its authors reported having no financial conflicts of interest. Dr. Steullet, who was not involved in the work, also reported having no financial conflicts of interest.
PARIS – A report that adjunctive minocycline was found safe and effective for treatment of schizophrenia must be considered one of the year’s highlights in the field of psychosis, Pascal Steullet, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The report came in the form of a meta-analysis conducted by investigators in China and Australia. This was the largest meta-analysis looking at the topic to date, and the only one to include a search of the Chinese language database, which provided three of the eight randomized, placebo-controlled clinical trials that were examined. Seven of the eight randomized trials were double-blind and deemed high quality by widely used criteria, including the Jadad scale and GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology, noted Dr. Steullet, a neuroscientist at the University of Lausanne (Switzerland).
The primary outcome was change in the PANSS (Positive and Negative Syndrome Scale) total psychopathology score, and the positive, negative, and general symptom subscale scores.
The biggest benefit was on PANSS negative symptoms. Minocycline brought significantly greater improvement in this domain than placebo, with a standard mean difference (SMD) of –0.69 and a P value of less than .00001 (Eur Psychopharmacol. 2017 Jan;27[1]:8-18).
“That’s quite a good effect size,” Dr. Steullet commented.
The benefit on PANSS positive symptoms, while statistically significant, was far less robust, with an SMD of –0.22 in favor of minocycline.
The PANSS total psychopathology score favored minocycline with an SMD of –0.64, which Dr. Steullet again deemed “a quite significant effect size.” The PANSS general symptom score also showed a significant benefit in favor of minocycline, with an SMD of –0.45.
Among various secondary endpoints that were evaluated: A significant benefit was found favoring minocycline on the Clinical Global Impressions scale (SMD of –0.53) and the Abnormal Involuntary Movement Scale (SMD of –0.56). However, no differences were found between the minocycline and control groups on the Global Assessment of Functioning Scale, the Extrapyramidal Symptom Rating Scale, or the Calgary Depression Scale for Schizophrenia.
Although the average dose of minocycline prescribed in the eight trials was 171.9 mg/day, dosing strategies varied widely from trial to trial, and the investigators concluded that future studies are needed in order to pin down the optimal dosing and duration.
There was no increase in adverse drug reactions in the minocycline-treated group.
Neurocognitive function as assessed by the MATRICS Consensus Cognitive Battery showed no differences between the minocycline and placebo groups in working memory, problem solving, attention/vigilance, or other elements.
Proposed mechanisms of minocycline’s benefit as adjunctive therapy alongside antipsychotics for schizophrenia include the antimicrobial’s good CNS penetration and its anti-inflammatory effects, which could reduce neuroinflammation, dampen activated microglia, and enhance glutamate neurotransmitters.
The meta-analysis was supported by the University of Macau. Its authors reported having no financial conflicts of interest. Dr. Steullet, who was not involved in the work, also reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Rethinking lithium: It keeps patients with unipolar depression out of the hospital
PARIS – What’s the most effective medication for preventing rehospitalization in patients previously admitted for severe unipolar depression?
The answer, based upon a study of all 123,712 patients hospitalized for severe unipolar depression in Finland during 1987-2012, might come as a surprise. The medication that most effectively kept patients from returning to the hospital wasn’t a selective serotonin reuptake inhibitor (SSRI), a serotonin-norepinephrine reuptake inhibitor (SNRI), or an atypical antipsychotic. It was lithium, Markku Lahteenvuo, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive nationwide databases, he and his coinvestigators followed these 123,712 hospitalized patients for a mean of 7.9 years, during which 49,146 of them had a total of 153,784 rehospitalizations for mental disorders. The investigators analyzed what psychotropic drugs the patients were taking when rehospitalized, as well as the drugs they were taking when they were out of hospital. Individuals served as their own controls in order to eliminate selection bias.
In an analysis adjusted for time since diagnosis of unipolar depression, the temporal order of prescribed drugs, and other concomitant medications, the use of lithium was associated with a 53% reduction in the risk of readmission, compared with nonuse.
Patients using lithium as monotherapy for their depression were 69% less likely to be rehospitalized than if they were not, whereas patients on lithium with antidepressants had a more modest 50% reduction in rehospitalization risk, reported Dr. Lahteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland in Kuopio.
After lithium, clozapine was the drug associated with the next lowest risk of rehospitalization for a mental disorder. Patients on that drug were 35% less likely to be rehospitalized; however, clozapine wasn’t widely prescribed in patients with severe unipolar depression.
Antidepressants as a broad class were associated with a statistically significant 10% increase in risk of rehospitalization for a mental disorder. But within that category there were a couple of exceptions: Amitriptyline, which was widely prescribed, particularly in the early years of the study period, was associated with a 25% reduction in rehospitalization, compared with no use. And doxepin was associated with a 15% reduction.
Antipsychotics were associated with an overall 16% increased risk of rehospitalization, compared with no use. But there were some notable exceptions within this class of drugs. Both quetiapine and aripiprazole were associated with an 18% risk reduction.
The investigators also analyzed all-cause rehospitalizations, including acute myocardial infarction and all other somatic diseases, as well as mental disorders. During follow-up, 98,662 patients who had been hospitalized for severe unipolar depression had a collective total of 592,926 all-cause rehospitalizations.
Dr. Lahteenvuo said he had expected that lithium’s strong showing in preventing psychiatric rehospitalization might be offset by an increased risk of hospitalization for other conditions, because of the drug’s well-established association with thyroid, renal, and other toxicities. But that was not the case. Patients on lithium without antidepressants were 49% less likely to have an all-cause hospitalization than when they were not using the drug, and patients on lithium with antidepressants had a 42% reduction in risk.
”It seems that lithium also shielded the patients from going to the hospital for any somatic reason. So lithium seems to be safe and prevents various somatic hospitalizations as well. Whether that’s due to the drug’s enhancement of your ability to live your life, eat healthy, exercise, and give you energy to have good lifestyle habits, or it actually has an anti-inflammatory effect or other disease-modifying effect, we do not know,” Dr. Lahteenvuo said.
Lithium has traditionally been prescribed mainly as third-line therapy in treatment-refractory patients for severe unipolar depression. The Finnish data suggest it’s time to reconsider that strategy.
“We think lithium should be considered for a wider audience, keeping in mind of course that it can cause thyroid and kidney toxicity,” Dr. Lahteenvuo said.
Patients taking clozapine were 30% less likely to have an all-cause hospitalization than nonusers. Aripiprazole was associated with a 21% reduction in risk, quetiapine had a 12% risk reduction, amitriptyline had an 11% risk reduction, and doxepin had a 12% risk reduction.
In contrast, all-cause hospitalization was 12% more likely when patients were taking chronic benzodiazepines and 8% more likely while on hypnotics.
The full study results were published in the Lancet Psychiatry (2017 Jul;4[7]:547-53).
Dr. Lahteenvuo reported having no financial conflicts of interest related to the study, which was funded by the Finnish Ministry of Health.
PARIS – What’s the most effective medication for preventing rehospitalization in patients previously admitted for severe unipolar depression?
The answer, based upon a study of all 123,712 patients hospitalized for severe unipolar depression in Finland during 1987-2012, might come as a surprise. The medication that most effectively kept patients from returning to the hospital wasn’t a selective serotonin reuptake inhibitor (SSRI), a serotonin-norepinephrine reuptake inhibitor (SNRI), or an atypical antipsychotic. It was lithium, Markku Lahteenvuo, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive nationwide databases, he and his coinvestigators followed these 123,712 hospitalized patients for a mean of 7.9 years, during which 49,146 of them had a total of 153,784 rehospitalizations for mental disorders. The investigators analyzed what psychotropic drugs the patients were taking when rehospitalized, as well as the drugs they were taking when they were out of hospital. Individuals served as their own controls in order to eliminate selection bias.
In an analysis adjusted for time since diagnosis of unipolar depression, the temporal order of prescribed drugs, and other concomitant medications, the use of lithium was associated with a 53% reduction in the risk of readmission, compared with nonuse.
Patients using lithium as monotherapy for their depression were 69% less likely to be rehospitalized than if they were not, whereas patients on lithium with antidepressants had a more modest 50% reduction in rehospitalization risk, reported Dr. Lahteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland in Kuopio.
After lithium, clozapine was the drug associated with the next lowest risk of rehospitalization for a mental disorder. Patients on that drug were 35% less likely to be rehospitalized; however, clozapine wasn’t widely prescribed in patients with severe unipolar depression.
Antidepressants as a broad class were associated with a statistically significant 10% increase in risk of rehospitalization for a mental disorder. But within that category there were a couple of exceptions: Amitriptyline, which was widely prescribed, particularly in the early years of the study period, was associated with a 25% reduction in rehospitalization, compared with no use. And doxepin was associated with a 15% reduction.
Antipsychotics were associated with an overall 16% increased risk of rehospitalization, compared with no use. But there were some notable exceptions within this class of drugs. Both quetiapine and aripiprazole were associated with an 18% risk reduction.
The investigators also analyzed all-cause rehospitalizations, including acute myocardial infarction and all other somatic diseases, as well as mental disorders. During follow-up, 98,662 patients who had been hospitalized for severe unipolar depression had a collective total of 592,926 all-cause rehospitalizations.
Dr. Lahteenvuo said he had expected that lithium’s strong showing in preventing psychiatric rehospitalization might be offset by an increased risk of hospitalization for other conditions, because of the drug’s well-established association with thyroid, renal, and other toxicities. But that was not the case. Patients on lithium without antidepressants were 49% less likely to have an all-cause hospitalization than when they were not using the drug, and patients on lithium with antidepressants had a 42% reduction in risk.
”It seems that lithium also shielded the patients from going to the hospital for any somatic reason. So lithium seems to be safe and prevents various somatic hospitalizations as well. Whether that’s due to the drug’s enhancement of your ability to live your life, eat healthy, exercise, and give you energy to have good lifestyle habits, or it actually has an anti-inflammatory effect or other disease-modifying effect, we do not know,” Dr. Lahteenvuo said.
Lithium has traditionally been prescribed mainly as third-line therapy in treatment-refractory patients for severe unipolar depression. The Finnish data suggest it’s time to reconsider that strategy.
“We think lithium should be considered for a wider audience, keeping in mind of course that it can cause thyroid and kidney toxicity,” Dr. Lahteenvuo said.
Patients taking clozapine were 30% less likely to have an all-cause hospitalization than nonusers. Aripiprazole was associated with a 21% reduction in risk, quetiapine had a 12% risk reduction, amitriptyline had an 11% risk reduction, and doxepin had a 12% risk reduction.
In contrast, all-cause hospitalization was 12% more likely when patients were taking chronic benzodiazepines and 8% more likely while on hypnotics.
The full study results were published in the Lancet Psychiatry (2017 Jul;4[7]:547-53).
Dr. Lahteenvuo reported having no financial conflicts of interest related to the study, which was funded by the Finnish Ministry of Health.
PARIS – What’s the most effective medication for preventing rehospitalization in patients previously admitted for severe unipolar depression?
The answer, based upon a study of all 123,712 patients hospitalized for severe unipolar depression in Finland during 1987-2012, might come as a surprise. The medication that most effectively kept patients from returning to the hospital wasn’t a selective serotonin reuptake inhibitor (SSRI), a serotonin-norepinephrine reuptake inhibitor (SNRI), or an atypical antipsychotic. It was lithium, Markku Lahteenvuo, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive nationwide databases, he and his coinvestigators followed these 123,712 hospitalized patients for a mean of 7.9 years, during which 49,146 of them had a total of 153,784 rehospitalizations for mental disorders. The investigators analyzed what psychotropic drugs the patients were taking when rehospitalized, as well as the drugs they were taking when they were out of hospital. Individuals served as their own controls in order to eliminate selection bias.
In an analysis adjusted for time since diagnosis of unipolar depression, the temporal order of prescribed drugs, and other concomitant medications, the use of lithium was associated with a 53% reduction in the risk of readmission, compared with nonuse.
Patients using lithium as monotherapy for their depression were 69% less likely to be rehospitalized than if they were not, whereas patients on lithium with antidepressants had a more modest 50% reduction in rehospitalization risk, reported Dr. Lahteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland in Kuopio.
After lithium, clozapine was the drug associated with the next lowest risk of rehospitalization for a mental disorder. Patients on that drug were 35% less likely to be rehospitalized; however, clozapine wasn’t widely prescribed in patients with severe unipolar depression.
Antidepressants as a broad class were associated with a statistically significant 10% increase in risk of rehospitalization for a mental disorder. But within that category there were a couple of exceptions: Amitriptyline, which was widely prescribed, particularly in the early years of the study period, was associated with a 25% reduction in rehospitalization, compared with no use. And doxepin was associated with a 15% reduction.
Antipsychotics were associated with an overall 16% increased risk of rehospitalization, compared with no use. But there were some notable exceptions within this class of drugs. Both quetiapine and aripiprazole were associated with an 18% risk reduction.
The investigators also analyzed all-cause rehospitalizations, including acute myocardial infarction and all other somatic diseases, as well as mental disorders. During follow-up, 98,662 patients who had been hospitalized for severe unipolar depression had a collective total of 592,926 all-cause rehospitalizations.
Dr. Lahteenvuo said he had expected that lithium’s strong showing in preventing psychiatric rehospitalization might be offset by an increased risk of hospitalization for other conditions, because of the drug’s well-established association with thyroid, renal, and other toxicities. But that was not the case. Patients on lithium without antidepressants were 49% less likely to have an all-cause hospitalization than when they were not using the drug, and patients on lithium with antidepressants had a 42% reduction in risk.
”It seems that lithium also shielded the patients from going to the hospital for any somatic reason. So lithium seems to be safe and prevents various somatic hospitalizations as well. Whether that’s due to the drug’s enhancement of your ability to live your life, eat healthy, exercise, and give you energy to have good lifestyle habits, or it actually has an anti-inflammatory effect or other disease-modifying effect, we do not know,” Dr. Lahteenvuo said.
Lithium has traditionally been prescribed mainly as third-line therapy in treatment-refractory patients for severe unipolar depression. The Finnish data suggest it’s time to reconsider that strategy.
“We think lithium should be considered for a wider audience, keeping in mind of course that it can cause thyroid and kidney toxicity,” Dr. Lahteenvuo said.
Patients taking clozapine were 30% less likely to have an all-cause hospitalization than nonusers. Aripiprazole was associated with a 21% reduction in risk, quetiapine had a 12% risk reduction, amitriptyline had an 11% risk reduction, and doxepin had a 12% risk reduction.
In contrast, all-cause hospitalization was 12% more likely when patients were taking chronic benzodiazepines and 8% more likely while on hypnotics.
The full study results were published in the Lancet Psychiatry (2017 Jul;4[7]:547-53).
Dr. Lahteenvuo reported having no financial conflicts of interest related to the study, which was funded by the Finnish Ministry of Health.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Patients on lithium alone were 69% less likely to be rehospitalized for a mental disorder than nonusers.
Data source: This cohort study included all 123,712 Finnish patients who were hospitalized for severe unipolar depression during 1987-2012, along with rehospitalizations during a mean 7.9 years of follow-up.
Disclosures: The study was funded by the Finnish Ministry of Health. The presenter reported having no financial conflicts.
Lumateperone shows broad phase 3 potential for psychiatric disorders
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Physical inactivity in youth is an independent risk factor for schizophrenia
PARIS – Low physical activity in childhood and adolescence was independently associated with later development of schizophrenia and other nonaffective psychotic disorders in the large, prospective, population-based Cardiovascular Risk in Young Finns cohort study, Jarmo Hietala, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The key question now: Is this risk factor remediable? That is, will a pediatric exercise intervention that results in improved physical fitness also reduce the risk of later nonaffective psychosis? Given that there are really no downsides to physical activity, the Finnish data make a strong case for including exercise and physical activity interventions in investigational psychosis prevention programs targeting high-risk youth, according to Dr. Hietala, professor of psychiatry at the University of Turku (Finland).
Dr. Hietala and his coinvestigators tapped into comprehensive national registries in order to identify all study participants with a psychiatric diagnosis of sufficient severity to have resulted in hospitalization up to 2012. Forty-one patients were hospitalized for schizophrenia spectrum disorders, 47 for other forms of nonaffective psychosis, 43 for personality disorders, 111 for affective disorders, and 49 with alcohol and other substance use disorders.
In a multivariate analysis adjusted for sex, age, body mass index, birth weight, non-preterm birth, and maternal mental disorders, each 1-point decrement in the pediatric physical activity index was associated with a 26% increase in the risk of developing any nonaffective psychosis and, more specifically, a 43% increased risk of schizophrenia.
Moreover, nonparticipation in organized sports competitions was independently associated with a 2.58-fold increased risk of any nonaffective psychosis and a 4.88-fold increased risk of schizophrenia. And social isolation as reflected in spending less time in common activities with friends during leisure time was associated with a 71% increased risk of nonaffective psychosis and a 76% increased risk of schizophrenia.
Of note, schizophrenia was the only psychiatric disorder associated with low physical activity in childhood and adolescence. Sedentary youths were not at increased risk of later hospitalization for affective disorders or other forms of mental illness.
“Our results have relevance for preemptive psychiatry and provide rationale for including exercise in early interventions for psychosis,” the psychiatrist said.
Current programs aimed at preventing schizophrenia in youth at high risk because of family history typically emphasize avoidance of street drugs, the importance of seeking out constructive social interactions, stress reduction techniques, and cognitive-behavioral therapy aimed at promoting a positive world view.
Formal evaluation of physical activity as part of a preventive approach has a sound theoretic basis, according to Dr. Hietala. He cited an influential essay called “Rethinking Schizophrenia” by the then-director of the National Institute of Mental Health, Thomas R. Insel, MD. In that article, Dr. Insel highlights the past half-century of largely unsatisfactory results with pharmacotherapy and goes on to make the case for considering schizophrenia as a neurodevelopmental disorder in which, he argues, “psychosis is a late, potentially preventable stage of the illness” (Nature. 2010 Nov 11;468[7321]:187-93).
This view of schizophrenia as a neurodevelopmental disorder has quickly come to dominate thinking within the field. Dr. Hietala noted that the schizophrenia spectrum chapter in the DSM-5 includes a greater focus on abnormal behavior and catatonia as a core domain alongside classic features, such as delusions, hallucinations, negative symptoms, and disorganized speech.
“My view of schizophrenia is that the psychotic symptoms are a secondary phenomenon, a complication of the disease that has been going on for a while. It’s a pity that we focus so much on the psychotic symptoms rather than the cognitive or negative or affective symptoms,” he said.
The hope is that a long-term physical activity intervention in at-risk youth will stimulate neurodevelopmental catch-up, thereby thwarting their predisposition to schizophrenia.
“Human development is not a linear process; it happens in spurts of rapid growth followed by consolidation periods,” Dr. Hietala said.
However, even if it turns out that an early physical activity intervention does not reduce the risk of developing schizophrenia, it might favorably alter its course in important ways, according to Dr. Hietala.
Individuals with schizophrenia are known to be at increased risk for metabolic syndrome and premature death tied to cardiovascular disease. A recent meta-analysis of 16 prospective cohort studies totaling more than 1 million men and women found that mortality during follow-up was 59% greater in those who sat for more than 8 hours per day and were in the lowest quartile of physical activity, compared with those sitting for less than 4 hours per day who were in the top quartile of physical activity, at more than 35.5 metabolic equivalent hours per week.
But there was no increased risk of mortality among those who sat for more than 8 hours per day and were also in the highest quartile of physical activity. The implication is that high levels of moderate-intensity physical activity eliminates the increased risk of death associated with high sitting time (Lancet. 2016 Sep 24;388[10051]:1302-10). That’s a finding that could be applicable to patients with schizophrenia.
Dr. Hietala reported having no financial conflicts regarding the Cardiovascular Risk in Young Finns study, which is supported by the Academy of Finland, the Social Insurance Institution of Finland, and grants from nonprofit foundations.
PARIS – Low physical activity in childhood and adolescence was independently associated with later development of schizophrenia and other nonaffective psychotic disorders in the large, prospective, population-based Cardiovascular Risk in Young Finns cohort study, Jarmo Hietala, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The key question now: Is this risk factor remediable? That is, will a pediatric exercise intervention that results in improved physical fitness also reduce the risk of later nonaffective psychosis? Given that there are really no downsides to physical activity, the Finnish data make a strong case for including exercise and physical activity interventions in investigational psychosis prevention programs targeting high-risk youth, according to Dr. Hietala, professor of psychiatry at the University of Turku (Finland).
Dr. Hietala and his coinvestigators tapped into comprehensive national registries in order to identify all study participants with a psychiatric diagnosis of sufficient severity to have resulted in hospitalization up to 2012. Forty-one patients were hospitalized for schizophrenia spectrum disorders, 47 for other forms of nonaffective psychosis, 43 for personality disorders, 111 for affective disorders, and 49 with alcohol and other substance use disorders.
In a multivariate analysis adjusted for sex, age, body mass index, birth weight, non-preterm birth, and maternal mental disorders, each 1-point decrement in the pediatric physical activity index was associated with a 26% increase in the risk of developing any nonaffective psychosis and, more specifically, a 43% increased risk of schizophrenia.
Moreover, nonparticipation in organized sports competitions was independently associated with a 2.58-fold increased risk of any nonaffective psychosis and a 4.88-fold increased risk of schizophrenia. And social isolation as reflected in spending less time in common activities with friends during leisure time was associated with a 71% increased risk of nonaffective psychosis and a 76% increased risk of schizophrenia.
Of note, schizophrenia was the only psychiatric disorder associated with low physical activity in childhood and adolescence. Sedentary youths were not at increased risk of later hospitalization for affective disorders or other forms of mental illness.
“Our results have relevance for preemptive psychiatry and provide rationale for including exercise in early interventions for psychosis,” the psychiatrist said.
Current programs aimed at preventing schizophrenia in youth at high risk because of family history typically emphasize avoidance of street drugs, the importance of seeking out constructive social interactions, stress reduction techniques, and cognitive-behavioral therapy aimed at promoting a positive world view.
Formal evaluation of physical activity as part of a preventive approach has a sound theoretic basis, according to Dr. Hietala. He cited an influential essay called “Rethinking Schizophrenia” by the then-director of the National Institute of Mental Health, Thomas R. Insel, MD. In that article, Dr. Insel highlights the past half-century of largely unsatisfactory results with pharmacotherapy and goes on to make the case for considering schizophrenia as a neurodevelopmental disorder in which, he argues, “psychosis is a late, potentially preventable stage of the illness” (Nature. 2010 Nov 11;468[7321]:187-93).
This view of schizophrenia as a neurodevelopmental disorder has quickly come to dominate thinking within the field. Dr. Hietala noted that the schizophrenia spectrum chapter in the DSM-5 includes a greater focus on abnormal behavior and catatonia as a core domain alongside classic features, such as delusions, hallucinations, negative symptoms, and disorganized speech.
“My view of schizophrenia is that the psychotic symptoms are a secondary phenomenon, a complication of the disease that has been going on for a while. It’s a pity that we focus so much on the psychotic symptoms rather than the cognitive or negative or affective symptoms,” he said.
The hope is that a long-term physical activity intervention in at-risk youth will stimulate neurodevelopmental catch-up, thereby thwarting their predisposition to schizophrenia.
“Human development is not a linear process; it happens in spurts of rapid growth followed by consolidation periods,” Dr. Hietala said.
However, even if it turns out that an early physical activity intervention does not reduce the risk of developing schizophrenia, it might favorably alter its course in important ways, according to Dr. Hietala.
Individuals with schizophrenia are known to be at increased risk for metabolic syndrome and premature death tied to cardiovascular disease. A recent meta-analysis of 16 prospective cohort studies totaling more than 1 million men and women found that mortality during follow-up was 59% greater in those who sat for more than 8 hours per day and were in the lowest quartile of physical activity, compared with those sitting for less than 4 hours per day who were in the top quartile of physical activity, at more than 35.5 metabolic equivalent hours per week.
But there was no increased risk of mortality among those who sat for more than 8 hours per day and were also in the highest quartile of physical activity. The implication is that high levels of moderate-intensity physical activity eliminates the increased risk of death associated with high sitting time (Lancet. 2016 Sep 24;388[10051]:1302-10). That’s a finding that could be applicable to patients with schizophrenia.
Dr. Hietala reported having no financial conflicts regarding the Cardiovascular Risk in Young Finns study, which is supported by the Academy of Finland, the Social Insurance Institution of Finland, and grants from nonprofit foundations.
PARIS – Low physical activity in childhood and adolescence was independently associated with later development of schizophrenia and other nonaffective psychotic disorders in the large, prospective, population-based Cardiovascular Risk in Young Finns cohort study, Jarmo Hietala, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
The key question now: Is this risk factor remediable? That is, will a pediatric exercise intervention that results in improved physical fitness also reduce the risk of later nonaffective psychosis? Given that there are really no downsides to physical activity, the Finnish data make a strong case for including exercise and physical activity interventions in investigational psychosis prevention programs targeting high-risk youth, according to Dr. Hietala, professor of psychiatry at the University of Turku (Finland).
Dr. Hietala and his coinvestigators tapped into comprehensive national registries in order to identify all study participants with a psychiatric diagnosis of sufficient severity to have resulted in hospitalization up to 2012. Forty-one patients were hospitalized for schizophrenia spectrum disorders, 47 for other forms of nonaffective psychosis, 43 for personality disorders, 111 for affective disorders, and 49 with alcohol and other substance use disorders.
In a multivariate analysis adjusted for sex, age, body mass index, birth weight, non-preterm birth, and maternal mental disorders, each 1-point decrement in the pediatric physical activity index was associated with a 26% increase in the risk of developing any nonaffective psychosis and, more specifically, a 43% increased risk of schizophrenia.
Moreover, nonparticipation in organized sports competitions was independently associated with a 2.58-fold increased risk of any nonaffective psychosis and a 4.88-fold increased risk of schizophrenia. And social isolation as reflected in spending less time in common activities with friends during leisure time was associated with a 71% increased risk of nonaffective psychosis and a 76% increased risk of schizophrenia.
Of note, schizophrenia was the only psychiatric disorder associated with low physical activity in childhood and adolescence. Sedentary youths were not at increased risk of later hospitalization for affective disorders or other forms of mental illness.
“Our results have relevance for preemptive psychiatry and provide rationale for including exercise in early interventions for psychosis,” the psychiatrist said.
Current programs aimed at preventing schizophrenia in youth at high risk because of family history typically emphasize avoidance of street drugs, the importance of seeking out constructive social interactions, stress reduction techniques, and cognitive-behavioral therapy aimed at promoting a positive world view.
Formal evaluation of physical activity as part of a preventive approach has a sound theoretic basis, according to Dr. Hietala. He cited an influential essay called “Rethinking Schizophrenia” by the then-director of the National Institute of Mental Health, Thomas R. Insel, MD. In that article, Dr. Insel highlights the past half-century of largely unsatisfactory results with pharmacotherapy and goes on to make the case for considering schizophrenia as a neurodevelopmental disorder in which, he argues, “psychosis is a late, potentially preventable stage of the illness” (Nature. 2010 Nov 11;468[7321]:187-93).
This view of schizophrenia as a neurodevelopmental disorder has quickly come to dominate thinking within the field. Dr. Hietala noted that the schizophrenia spectrum chapter in the DSM-5 includes a greater focus on abnormal behavior and catatonia as a core domain alongside classic features, such as delusions, hallucinations, negative symptoms, and disorganized speech.
“My view of schizophrenia is that the psychotic symptoms are a secondary phenomenon, a complication of the disease that has been going on for a while. It’s a pity that we focus so much on the psychotic symptoms rather than the cognitive or negative or affective symptoms,” he said.
The hope is that a long-term physical activity intervention in at-risk youth will stimulate neurodevelopmental catch-up, thereby thwarting their predisposition to schizophrenia.
“Human development is not a linear process; it happens in spurts of rapid growth followed by consolidation periods,” Dr. Hietala said.
However, even if it turns out that an early physical activity intervention does not reduce the risk of developing schizophrenia, it might favorably alter its course in important ways, according to Dr. Hietala.
Individuals with schizophrenia are known to be at increased risk for metabolic syndrome and premature death tied to cardiovascular disease. A recent meta-analysis of 16 prospective cohort studies totaling more than 1 million men and women found that mortality during follow-up was 59% greater in those who sat for more than 8 hours per day and were in the lowest quartile of physical activity, compared with those sitting for less than 4 hours per day who were in the top quartile of physical activity, at more than 35.5 metabolic equivalent hours per week.
But there was no increased risk of mortality among those who sat for more than 8 hours per day and were also in the highest quartile of physical activity. The implication is that high levels of moderate-intensity physical activity eliminates the increased risk of death associated with high sitting time (Lancet. 2016 Sep 24;388[10051]:1302-10). That’s a finding that could be applicable to patients with schizophrenia.
Dr. Hietala reported having no financial conflicts regarding the Cardiovascular Risk in Young Finns study, which is supported by the Academy of Finland, the Social Insurance Institution of Finland, and grants from nonprofit foundations.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Each 1-point decrement on a physical activity score recorded during childhood and adolescence was independently associated with a 43% increased risk of later development of schizophrenia.
Data source: The Cardiovascular Risk in Young Finns study is an ongoing prospective, population-based study of nearly 3,600 Finns who were aged 3-18 years old when the study began in 1980.
Disclosures: The presenter reported having no financial conflicts regarding the study, which is supported by the Academy of Finland, the Social Insurance Institution of Finland, and grants from nonprofit foundations.
Maternal antepartum depression creates bevy of long-term risks in offspring
PARIS – Maternal depression during pregnancy is a common occurrence that can have far-reaching effects in the offspring, according to Tiina Taka-Eilola, MD, of the University of Oulu (Finland).
Indeed, maternal antepartum depression may best be thought of as an adverse environmental factor that exacerbates the impact of any underlying genetic vulnerability to severe mental disorder that may be present in the offspring, she said at the annual congress of the European College of Neuropsychopharmacology.
At midgestation, back in the mid-1960s, 13.9% of the mothers of the Northern Finland Birth Cohort acknowledged feeling “depressed” or “very depressed,” rates consistent with those reported in other studies using standardized depression assessment instruments. Their offspring, by age 43 years, were 1.6-fold more likely to have a history of a current or past nonpsychotic mood disorder and 2-fold more likely to have had a psychotic mood disorder than did the offspring of mothers free of antepartum depression.
These risks were greatly amplified if either parent experienced a hospital-treated severe mental disorder before, during, or up to 18 years after the pregnancy. Offspring who had both a mother who experienced antepartum depression and a parent with a severe, hospital-treated mental disorder were at 3.9-fold increased risk for being diagnosed with nonpsychotic depression by age 43 years in an analysis adjusted for sex, perinatal complications, and other potential confounders. They also were at 5.6-fold increased risk for psychotic depression and a whopping 7.8-fold greater risk of bipolar disorder than offspring with neither risk factor.
Moreover, in an earlier study, among men in the Northern Finland cohort who were assessed at age 33 years, investigators found that maternal depression during pregnancy was associated with an adjusted 1.4-fold increased likelihood of having a criminal record for a nonviolent offense, a 1.6-fold increased risk of violent crime, and a 1.7-fold increase in violent recidivism. In contrast, women whose mothers were depressed during pregnancy didn’t have a significantly higher rate of criminality, compared with those whose mothers weren’t depressed (J Affect Disord. 2003 May;74[3]:273-8).
In another earlier analysis, Dr. Taka-Eilola’s senior coinvestigators demonstrated that the risk of schizophrenia in the Northern Finland offspring was 2.6-fold greater if there was parental psychosis but no maternal antepartum depression than if neither was present, while the risk was 9.4-fold higher when both risk factors were present (Am J Psychiatry. 2010 Jan;167[1]:70-7).
Dr. Taka-Eilola, a primary care physician, said that postpartum depression garners news headlines and is far more extensively researched than is antepartum depression, but as the Finnish data show, antepartum depression is at least as common and deserves to be taken seriously. It’s important to screen for it and to treat it in an effort to prevent adverse effects in the offspring, as well as out of concern for the mother’s well-being, she emphasized. She believes this is now more likely to happen as a consequence of a recent World Psychiatric Association report calling for greater clinician attention to perinatal mental health.
Dr. Taka-Eilola reported having no financial conflicts of interest regarding the Northern Finland Birth Cohort Study, which is supported by the Academy of Finland, the Finnish Cultural Foundation Lapland Regional Fund, and grants from various nonprofit foundations.
PARIS – Maternal depression during pregnancy is a common occurrence that can have far-reaching effects in the offspring, according to Tiina Taka-Eilola, MD, of the University of Oulu (Finland).
Indeed, maternal antepartum depression may best be thought of as an adverse environmental factor that exacerbates the impact of any underlying genetic vulnerability to severe mental disorder that may be present in the offspring, she said at the annual congress of the European College of Neuropsychopharmacology.
At midgestation, back in the mid-1960s, 13.9% of the mothers of the Northern Finland Birth Cohort acknowledged feeling “depressed” or “very depressed,” rates consistent with those reported in other studies using standardized depression assessment instruments. Their offspring, by age 43 years, were 1.6-fold more likely to have a history of a current or past nonpsychotic mood disorder and 2-fold more likely to have had a psychotic mood disorder than did the offspring of mothers free of antepartum depression.
These risks were greatly amplified if either parent experienced a hospital-treated severe mental disorder before, during, or up to 18 years after the pregnancy. Offspring who had both a mother who experienced antepartum depression and a parent with a severe, hospital-treated mental disorder were at 3.9-fold increased risk for being diagnosed with nonpsychotic depression by age 43 years in an analysis adjusted for sex, perinatal complications, and other potential confounders. They also were at 5.6-fold increased risk for psychotic depression and a whopping 7.8-fold greater risk of bipolar disorder than offspring with neither risk factor.
Moreover, in an earlier study, among men in the Northern Finland cohort who were assessed at age 33 years, investigators found that maternal depression during pregnancy was associated with an adjusted 1.4-fold increased likelihood of having a criminal record for a nonviolent offense, a 1.6-fold increased risk of violent crime, and a 1.7-fold increase in violent recidivism. In contrast, women whose mothers were depressed during pregnancy didn’t have a significantly higher rate of criminality, compared with those whose mothers weren’t depressed (J Affect Disord. 2003 May;74[3]:273-8).
In another earlier analysis, Dr. Taka-Eilola’s senior coinvestigators demonstrated that the risk of schizophrenia in the Northern Finland offspring was 2.6-fold greater if there was parental psychosis but no maternal antepartum depression than if neither was present, while the risk was 9.4-fold higher when both risk factors were present (Am J Psychiatry. 2010 Jan;167[1]:70-7).
Dr. Taka-Eilola, a primary care physician, said that postpartum depression garners news headlines and is far more extensively researched than is antepartum depression, but as the Finnish data show, antepartum depression is at least as common and deserves to be taken seriously. It’s important to screen for it and to treat it in an effort to prevent adverse effects in the offspring, as well as out of concern for the mother’s well-being, she emphasized. She believes this is now more likely to happen as a consequence of a recent World Psychiatric Association report calling for greater clinician attention to perinatal mental health.
Dr. Taka-Eilola reported having no financial conflicts of interest regarding the Northern Finland Birth Cohort Study, which is supported by the Academy of Finland, the Finnish Cultural Foundation Lapland Regional Fund, and grants from various nonprofit foundations.
PARIS – Maternal depression during pregnancy is a common occurrence that can have far-reaching effects in the offspring, according to Tiina Taka-Eilola, MD, of the University of Oulu (Finland).
Indeed, maternal antepartum depression may best be thought of as an adverse environmental factor that exacerbates the impact of any underlying genetic vulnerability to severe mental disorder that may be present in the offspring, she said at the annual congress of the European College of Neuropsychopharmacology.
At midgestation, back in the mid-1960s, 13.9% of the mothers of the Northern Finland Birth Cohort acknowledged feeling “depressed” or “very depressed,” rates consistent with those reported in other studies using standardized depression assessment instruments. Their offspring, by age 43 years, were 1.6-fold more likely to have a history of a current or past nonpsychotic mood disorder and 2-fold more likely to have had a psychotic mood disorder than did the offspring of mothers free of antepartum depression.
These risks were greatly amplified if either parent experienced a hospital-treated severe mental disorder before, during, or up to 18 years after the pregnancy. Offspring who had both a mother who experienced antepartum depression and a parent with a severe, hospital-treated mental disorder were at 3.9-fold increased risk for being diagnosed with nonpsychotic depression by age 43 years in an analysis adjusted for sex, perinatal complications, and other potential confounders. They also were at 5.6-fold increased risk for psychotic depression and a whopping 7.8-fold greater risk of bipolar disorder than offspring with neither risk factor.
Moreover, in an earlier study, among men in the Northern Finland cohort who were assessed at age 33 years, investigators found that maternal depression during pregnancy was associated with an adjusted 1.4-fold increased likelihood of having a criminal record for a nonviolent offense, a 1.6-fold increased risk of violent crime, and a 1.7-fold increase in violent recidivism. In contrast, women whose mothers were depressed during pregnancy didn’t have a significantly higher rate of criminality, compared with those whose mothers weren’t depressed (J Affect Disord. 2003 May;74[3]:273-8).
In another earlier analysis, Dr. Taka-Eilola’s senior coinvestigators demonstrated that the risk of schizophrenia in the Northern Finland offspring was 2.6-fold greater if there was parental psychosis but no maternal antepartum depression than if neither was present, while the risk was 9.4-fold higher when both risk factors were present (Am J Psychiatry. 2010 Jan;167[1]:70-7).
Dr. Taka-Eilola, a primary care physician, said that postpartum depression garners news headlines and is far more extensively researched than is antepartum depression, but as the Finnish data show, antepartum depression is at least as common and deserves to be taken seriously. It’s important to screen for it and to treat it in an effort to prevent adverse effects in the offspring, as well as out of concern for the mother’s well-being, she emphasized. She believes this is now more likely to happen as a consequence of a recent World Psychiatric Association report calling for greater clinician attention to perinatal mental health.
Dr. Taka-Eilola reported having no financial conflicts of interest regarding the Northern Finland Birth Cohort Study, which is supported by the Academy of Finland, the Finnish Cultural Foundation Lapland Regional Fund, and grants from various nonprofit foundations.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: By midlife, offspring of an mother who had antepartum depression and a parent with a severe, hospital-treated mental disorder were at an adjusted 5.6-fold increased risk for psychotic depression and 7.8-fold greater risk of bipolar disorder than the offspring with neither risk factor.
Data source: The Northern Finland Birth Cohort 1966 is an ongoing, observational, prospective, general population-based study of the 12,058 individuals born in the two northernmost provinces of Finland during 1966.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, which is supported by the Academy of Finland, the Finnish Cultural Foundation Lapland Regional Fund, and grants from various nonprofit foundations.
Evenamide impresses for schizophrenia
PARIS – The oral selective glutamate inhibitor evenamide is moving on to advanced clinical trials on the strength of positive results in a phase 2 study, Ravi Anand, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Evenamide is a highly selective inhibitor of voltage-gated sodium channels that also attenuates glutamate release by hyperexcited neurons, explained Dr. Anand, chief medical officer at Newron Pharmaceuticals, a company based in Bresso, Italy, that is developing this novel antipsychotic agent.
The 4-week, randomized, double-blind, placebo-controlled, multicenter study included 89 patients with schizophrenia of a mean 18 years’ duration. All participants showed baseline evidence of breakthrough psychosis despite being on stable therapeutic doses of previously effective risperidone or aripiprazole. They were randomized either to add-on oral evenamide at a starting dose of 15 mg twice daily titrated to 20 or 25 mg twice daily or to placebo.
As a phase 2 study, the primary focus was on safety and tolerability. Evenamide showed no dose-limiting toxicities. Nor did those in the evenamide group experience any of the common dopaminergic side effects seen with currently available antipsychotics, such as sedation, weight gain, sexual dysfunction, cardiac abnormalities, or extrapyramidal symptoms.
The only adverse events more common in the evenamide-treated group than in placebo-treated controls were insomnia and headache, which occurred in 5 and 3 of the 50 patients on evenamide, respectively.
In terms of efficacy, 75% of the evenamide group showed improvement over baseline in terms of the Positive and Negative Syndrome Scale, compared with 44% of controls. From a mean baseline total PANSS score of 62.7, the evenamide group experienced a 5.1-point reduction at day 28, compared with a 3.7-point improvement in controls. The difference between the two study arms was already significant at the first assessment, which took place on day 8.
Furthermore, 55% of patients in the evenamide group showed significant improvement on the Clinical Global Impression Severity score, compared with 36% of controls.
Because evenamide’s efficacy was greater in younger patients, the next round of larger, longer clinical trials will focus on younger patients with more severe symptoms, Dr. Anand said.
PARIS – The oral selective glutamate inhibitor evenamide is moving on to advanced clinical trials on the strength of positive results in a phase 2 study, Ravi Anand, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Evenamide is a highly selective inhibitor of voltage-gated sodium channels that also attenuates glutamate release by hyperexcited neurons, explained Dr. Anand, chief medical officer at Newron Pharmaceuticals, a company based in Bresso, Italy, that is developing this novel antipsychotic agent.
The 4-week, randomized, double-blind, placebo-controlled, multicenter study included 89 patients with schizophrenia of a mean 18 years’ duration. All participants showed baseline evidence of breakthrough psychosis despite being on stable therapeutic doses of previously effective risperidone or aripiprazole. They were randomized either to add-on oral evenamide at a starting dose of 15 mg twice daily titrated to 20 or 25 mg twice daily or to placebo.
As a phase 2 study, the primary focus was on safety and tolerability. Evenamide showed no dose-limiting toxicities. Nor did those in the evenamide group experience any of the common dopaminergic side effects seen with currently available antipsychotics, such as sedation, weight gain, sexual dysfunction, cardiac abnormalities, or extrapyramidal symptoms.
The only adverse events more common in the evenamide-treated group than in placebo-treated controls were insomnia and headache, which occurred in 5 and 3 of the 50 patients on evenamide, respectively.
In terms of efficacy, 75% of the evenamide group showed improvement over baseline in terms of the Positive and Negative Syndrome Scale, compared with 44% of controls. From a mean baseline total PANSS score of 62.7, the evenamide group experienced a 5.1-point reduction at day 28, compared with a 3.7-point improvement in controls. The difference between the two study arms was already significant at the first assessment, which took place on day 8.
Furthermore, 55% of patients in the evenamide group showed significant improvement on the Clinical Global Impression Severity score, compared with 36% of controls.
Because evenamide’s efficacy was greater in younger patients, the next round of larger, longer clinical trials will focus on younger patients with more severe symptoms, Dr. Anand said.
PARIS – The oral selective glutamate inhibitor evenamide is moving on to advanced clinical trials on the strength of positive results in a phase 2 study, Ravi Anand, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Evenamide is a highly selective inhibitor of voltage-gated sodium channels that also attenuates glutamate release by hyperexcited neurons, explained Dr. Anand, chief medical officer at Newron Pharmaceuticals, a company based in Bresso, Italy, that is developing this novel antipsychotic agent.
The 4-week, randomized, double-blind, placebo-controlled, multicenter study included 89 patients with schizophrenia of a mean 18 years’ duration. All participants showed baseline evidence of breakthrough psychosis despite being on stable therapeutic doses of previously effective risperidone or aripiprazole. They were randomized either to add-on oral evenamide at a starting dose of 15 mg twice daily titrated to 20 or 25 mg twice daily or to placebo.
As a phase 2 study, the primary focus was on safety and tolerability. Evenamide showed no dose-limiting toxicities. Nor did those in the evenamide group experience any of the common dopaminergic side effects seen with currently available antipsychotics, such as sedation, weight gain, sexual dysfunction, cardiac abnormalities, or extrapyramidal symptoms.
The only adverse events more common in the evenamide-treated group than in placebo-treated controls were insomnia and headache, which occurred in 5 and 3 of the 50 patients on evenamide, respectively.
In terms of efficacy, 75% of the evenamide group showed improvement over baseline in terms of the Positive and Negative Syndrome Scale, compared with 44% of controls. From a mean baseline total PANSS score of 62.7, the evenamide group experienced a 5.1-point reduction at day 28, compared with a 3.7-point improvement in controls. The difference between the two study arms was already significant at the first assessment, which took place on day 8.
Furthermore, 55% of patients in the evenamide group showed significant improvement on the Clinical Global Impression Severity score, compared with 36% of controls.
Because evenamide’s efficacy was greater in younger patients, the next round of larger, longer clinical trials will focus on younger patients with more severe symptoms, Dr. Anand said.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Three-quarters of schizophrenia patients on the oral glutamate modulator evenamide showed improvement over baseline in terms of the Positive and Negative Syndrome Scale, compared with 44% of controls.
Data source: This 4-week, randomized, double-blind, placebo-controlled, multicenter study included 89 patients with schizophrenia.
Disclosures: The study presenter is the chief medical officer at Newron Pharmaceuticals, which is developing the novel antipsychotic.
Methylphenidate shows enduring sleep benefits in pediatric ADHD
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.
Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.
Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
PARIS – Methylphenidate therapy for attention-deficit/hyperactivity disorder in medication-naive boys significantly improved their sleep quality, timing, and duration in a double-blind randomized trial, Michelle M. Solleveld, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Moreover, these salutary effects on sleep persisted for at least 1 week after methylphenidate was stopped at the end of the 16-week study, added Dr. Solleveld of the University of Amsterdam.
Indeed, while parents embrace the improvement in behavioral symptoms of ADHD provided by methylphenidate, they often express concern about the possible adverse effects of stimulant medication on their child’s sleep. The new study findings are reassuring on that score.
Sleep difficulties are a major problem in patients with ADHD: They tend to fall asleep later and have more frequent awakenings during the night, which results in decreased total sleep time and sleep efficiency, Dr. Solleveld noted.
Prior studies of methylphenidate’s effects on sleep in pediatric ADHD have yielded mixed results. The negative studies were too brief to provide meaningful results, according to Dr. Solleveld, who said at least 8 weeks of treatment are required in order to evaluate the drug’s effect on sleep problems properly.
She presented a randomized, double-blind, 16-week, placebo-controlled clinical trial involving 50 medication-naive boys with ADHD who were 10-12 years old. Their sleep was assessed via actigraphy measurements taken over 5 consecutive nights, keeping a sleep diary, and answering questionnaires, including the Epworth Sleepiness Scale, at three time points: prior to randomization, 8 weeks into the trial, and finally 1 week after the study ended.
Sleep efficiency – the primary study outcome – showed a strong 5% improvement in the methylphenidate group but was unchanged from baseline in placebo-treated controls. The boys who received methylphenidate fell asleep earlier, had a shorter latency of sleep onset, and slept for longer, compared with their baseline measures or with the sleep results in controls.
The finding that the methylphenidate-induced improvements in sleep persisted for a week after drug clearance is consistent with brain imaging studies carried out by Dr. Solleveld and her coinvestigators. They believe that the effects of stimulant therapy may be age dependent. The investigators previously have shown that adults with ADHD who began treatment with stimulants before age 16 years – when brain development is still ongoing – had lower levels of basal gamma-aminobutyric acid (GABA) and higher GABA response to an oral methylphenidate than did those who began treatment with stimulants after age 23 years. This is thought to be attributable to prolonged reductions in dopamine turnover induced by methylphenidate in the developing brain (Neuroimage Clin. 2017 Jun 2;15:812-8).
The Dutch investigators also have reported that methylphenidate therapy in children with ADHD – but not in affected adults – increased the cerebral blood flow response within the thalamus to a dopamine challenge (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
The clinical ramifications of these apparently long-lasting, drug-related alterations in GABA neurotransmission are the subject of ongoing research.
Dr. Solleveld reported having no financial conflicts of interest.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Sleep efficiency in boys with ADHD improved significantly by 5% in response to methylphenidate therapy.
Data source: This randomized, double-blind, placebo-controlled clinical trial included 50 medication-naive boys aged 10-12 years with ADHD.
Disclosures: The study presenter reported having no financial conflicts of interest.
Add aggressiveness to mixed features specifier for major depressive episode
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.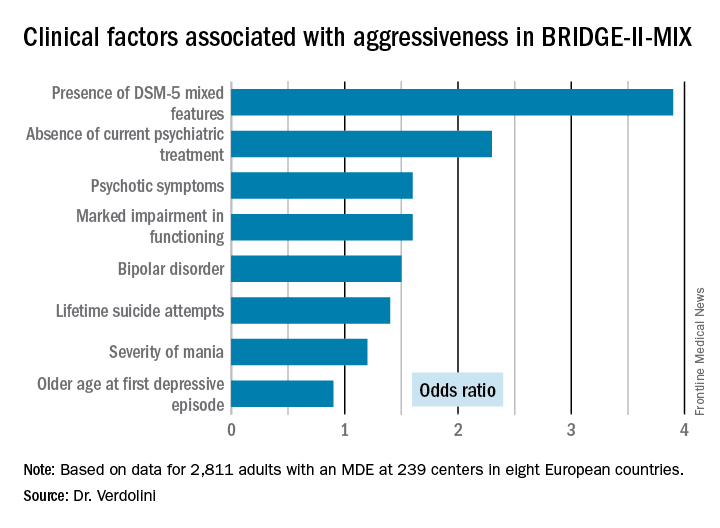
“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.
“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
PARIS – Aggressiveness deserves to be incorporated in the next Diagnostic and Statistical Manual of Mental Disorders update as a new clinical criterion triggering application of the “with mixed features” specifier in patients diagnosed with a major depressive episode, Norma Verdolini, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Aggressiveness might be a trait component of bipolarity and a diagnostic indicator of ‘mixicity’ in patients with a major depressive episode. This has implications for the therapeutic strategy,” said Dr. Verdolini of the bipolar disorders unit at the University of Barcelona Institute of Neurosciences.
The BRIDGE-II-MIX study was a cross-sectional observational study of 2,811 adults with MDE at 239 centers in eight European countries (J Clin Psychiatry. 2015 Mar;76[3]:e351-8). Three hundred ninety-nine participants (14.2%) met the operational definition of physical or verbal aggressiveness used in Dr. Verdolini’s new post-hoc analysis.
Statistically significant and clinically meaningful differences were found between MDE patients with aggressiveness (MDE-aggro) and MDE without aggressiveness. For example, the MDE-aggro group was twice as likely to meet DSM-IV-TR criteria for bipolar disorder I. Twenty-seven percent of the MDE-aggro group met DSM-5 criteria for a mixed state, meaning both depressed mood and mania in the same episode, compared with just 4% of the MDE-no-aggro group.
The MDE-aggro patients also had a strikingly greater prevalence of comorbid borderline personality disorder, by a margin of 20% versus 4%. They had a younger mean age at their first depressive episode: 29.9 years old, compared with 36.1 in the MDE-no-aggro group. The MDE-aggro patients had more prior mood episodes and a greater number of lifetime suicide attempts. In addition, they had significantly more severe depression, mania, and bipolar disorder scores on the Clinical Global Impression Scale for Bipolar Disorder.
“Our results should prompt reconsideration of the diagnostic criteria for the mixed features specifier. The detection of aggression in MDE could represent a therapeutic target in personalized pharmacological treatment for bipolar disorder,” Dr. Verdolini concluded.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Verdolini reported receiving research funding from the company.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Patients who fulfilled the DSM-5 criteria for a major depressive episode with mixed features were 3.9-fold more likely to meet investigators’ operational definition of aggressiveness.
Data source: This was a post-hoc analysis of the BRIDGE-II-MIX study, an observational cross-sectional study of 2,811 adults experiencing a major depressive episode.
Disclosures: The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. The presenter reported receiving research funding from the company.
ADHD meds don’t raise seizure risk in epilepsy patients
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.
“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.
“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
PARIS – The use of attention-deficit/hyperactivity disorder medications is not associated with increased risk of epileptic seizures in patients with both disorders, according to an analysis of Swedish national registry data.
“Seizure history should not exempt patients from ADHD medication treatment,” Isabell Brikell stated at the annual congress of the European College of Neuropsychopharmacology.
“That’s why it’s such an important question, whether ADHD medications increase the risk of seizures,” observed Ms. Brikell, a PhD candidate in psychiatric genetics and epidemiology at the Karolinska Institute in Stockholm.
Swedish health care registries are famously comprehensive. For example, the Swedish prescription medication registry that Ms. Brikell and her coinvestigators tapped into for their ADHD/epilepsy study contains information on 99% of all prescriptions ordered in the country since 2005.
She reported on 38,247 Swedish patients with epilepsy born during 1976-2008 and followed during 2006-2013. Forty-eight percent were female. They collectively experienced 30,093 acute epileptic seizures of sufficient severity that they presented to a hospital for an unplanned visit. When the investigators compared the rate of seizures while the patients with ADHD were on a collective 4,248 ADHD medication exposure periods to that of epilepsy patients without ADHD, they found that the seizure risk was actually 17% lower in ADHD patients while on medication. This difference fell just shy of statistical significance. The analysis was adjusted for gender, age, and time on ADHD medications.
However, Ms. Brikell and her coworkers also performed a separate analysis for each individual with ADHD in which they compared seizure rates when a given patient was on ADHD medication versus off medication, a design that controls for many of the potential confounding factors that can occur with observational data. The seizure risk proved to be 19% lower while an individual was on ADHD medication – and this difference was indeed statistically significant.
In an interview, Ms. Brikell noted that the Swedish data are confirmed by a much larger National Institute of Mental Health–sponsored American study she was involved with that is now under review for publication. The U.S. study, which used the enormous MarketScan private health insurance database, demonstrated with the power provided by very large patient numbers that the seizure risk was convincingly lower while dual-diagnosis patients were on ADHD medication than when they were off.
“It’s reassuring to see the same effect across two countries with such different health care systems,” she commented.
Epilepsy is known to be inherently associated with a threefold increased prevalence of ADHD.
Ms. Brikell’s study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. She reported having no financial conflicts of interest.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Patients with ADHD and a history of epilepsy were at 19% lower risk of experiencing seizures while on ADHD medication than when off it.
Data source: This was an observational study of prospectively collected data on all of the nearly 40,000 Swedish patients with epilepsy born during 1976-2008.
Disclosures: The study was funded by the Swedish Research Council, the U.S. National Institute of Mental Health, and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences. The presenter reported having no financial conflicts of interest.







