User login
Aortomitral continuity calcification predicts new atrial fib after TAVR
PARIS – Aortomitral continuity calcification, a common finding on CT in patients undergoing transcatheter aortic valve replacement, predicts new-onset atrial fibrillation and the need for permanent pacemaker insertion, Marco Spaziano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Increased surveillance for arrhythmias in the 30 days post TAVR is warranted in patients with aortomitral continuity calcification,” declared Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
He presented a single-center retrospective study of 524 patients undergoing TAVR with a self-expandable or balloon-expandable device. Aortomitral continuity calcification (AMCC) was found on CT in 15.8% of them. Dr. Spaziano defined AMCC as the presence of calcium in the curtain linking the aortic and mitral valve annuli. The clinical implications of this common finding were unknown prior to this study.
The 83 patients with AMCC did not differ significantly from the 441 without that CT finding in terms of baseline demographics, Society of Thoracic Surgeons risk score, prevalence of peripheral vascular disease, QRS duration, left ventricular ejection fraction, complete left or right bundle branch block, or aortic valve calcification volume. The prevalence of atrial fibrillation at baseline was 25.6% in the AMCC group and closely similar at 26.3% in the group without AMCC. Sixteen percent of subjects in each group had a previous pacemaker.
Similarly, the two groups didn’t differ in terms of procedural characteristics, including device type, size, or depth of implantation, or need for a second valve, or annular rupture.
However, excluding from consideration the patients with prior AF, the incidence of new AF in the 30 days post-TAVR was 22.7% in patients with AMCC compared with just 7.6% in the no-AMCC group. In addition, 33% of patients with AMCC received a new permanent pacemaker, as did 21% of those with no AMCC.
Other key 30-day outcomes didn’t differ between the two populations, including rates of death, stroke, vascular complications, and moderate or severe paravalvular regurgitation.
In a multivariate regression analysis adjusted for age, sex, device type and implantation depth, preexisting right bundle branch block, and surgical risk score, AMCC was associated with a statistically significant 1.8-fold increased likelihood of new pacemaker insertion and a 3.4-fold greater risk of new AF.
Dr. Spaziano said that in brainstorming with electrophysiology and echocardiography colleagues, the group came up with two hypotheses to explain the study findings. One is that AMCC might be a biologic marker for concomitant mitral stenosis, a known strong predictor of AF.
“Oftentimes it’s very difficult to diagnose mitral stenosis when there is aortic stenosis, because of left ventricular compliance issues, so potentially the patients with this calcium ridge may also have mitral stenosis,” he observed.
The other proposed hypothesis is that AMCC reflects increased calcification and fibrosis in the electrical system of both the AV node and atrium, with a resultant increased risk of developing new AF after the TAVR procedure.
Session chair Mohammad Abdelghani, MD, wasn’t buying either hypothesis. If either were correct, the group with AMCC would be expected to have a higher baseline rate of AF preprocedurally, observed Dr. Abdelghani of the Academic Medical Center at Amsterdam.
He suggested an alternative explanation on the basis of a German study that showed patients with significant calcification of the left coronary cusp were at sixfold greater risk for pacemaker implantation post TAVR. He proposed that calcification in the left sector of the valve landing zone causes the device to end up being positioned a bit off-line.
“I think the device protrudes away from the calcium and towards the right coronary artery commisure, compressing the conduction system that we know lies there,” Dr. Abdelghani said.
Dr. Spaziano reported having no financial conflicts of interest regarding his study.
PARIS – Aortomitral continuity calcification, a common finding on CT in patients undergoing transcatheter aortic valve replacement, predicts new-onset atrial fibrillation and the need for permanent pacemaker insertion, Marco Spaziano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Increased surveillance for arrhythmias in the 30 days post TAVR is warranted in patients with aortomitral continuity calcification,” declared Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
He presented a single-center retrospective study of 524 patients undergoing TAVR with a self-expandable or balloon-expandable device. Aortomitral continuity calcification (AMCC) was found on CT in 15.8% of them. Dr. Spaziano defined AMCC as the presence of calcium in the curtain linking the aortic and mitral valve annuli. The clinical implications of this common finding were unknown prior to this study.
The 83 patients with AMCC did not differ significantly from the 441 without that CT finding in terms of baseline demographics, Society of Thoracic Surgeons risk score, prevalence of peripheral vascular disease, QRS duration, left ventricular ejection fraction, complete left or right bundle branch block, or aortic valve calcification volume. The prevalence of atrial fibrillation at baseline was 25.6% in the AMCC group and closely similar at 26.3% in the group without AMCC. Sixteen percent of subjects in each group had a previous pacemaker.
Similarly, the two groups didn’t differ in terms of procedural characteristics, including device type, size, or depth of implantation, or need for a second valve, or annular rupture.
However, excluding from consideration the patients with prior AF, the incidence of new AF in the 30 days post-TAVR was 22.7% in patients with AMCC compared with just 7.6% in the no-AMCC group. In addition, 33% of patients with AMCC received a new permanent pacemaker, as did 21% of those with no AMCC.
Other key 30-day outcomes didn’t differ between the two populations, including rates of death, stroke, vascular complications, and moderate or severe paravalvular regurgitation.
In a multivariate regression analysis adjusted for age, sex, device type and implantation depth, preexisting right bundle branch block, and surgical risk score, AMCC was associated with a statistically significant 1.8-fold increased likelihood of new pacemaker insertion and a 3.4-fold greater risk of new AF.
Dr. Spaziano said that in brainstorming with electrophysiology and echocardiography colleagues, the group came up with two hypotheses to explain the study findings. One is that AMCC might be a biologic marker for concomitant mitral stenosis, a known strong predictor of AF.
“Oftentimes it’s very difficult to diagnose mitral stenosis when there is aortic stenosis, because of left ventricular compliance issues, so potentially the patients with this calcium ridge may also have mitral stenosis,” he observed.
The other proposed hypothesis is that AMCC reflects increased calcification and fibrosis in the electrical system of both the AV node and atrium, with a resultant increased risk of developing new AF after the TAVR procedure.
Session chair Mohammad Abdelghani, MD, wasn’t buying either hypothesis. If either were correct, the group with AMCC would be expected to have a higher baseline rate of AF preprocedurally, observed Dr. Abdelghani of the Academic Medical Center at Amsterdam.
He suggested an alternative explanation on the basis of a German study that showed patients with significant calcification of the left coronary cusp were at sixfold greater risk for pacemaker implantation post TAVR. He proposed that calcification in the left sector of the valve landing zone causes the device to end up being positioned a bit off-line.
“I think the device protrudes away from the calcium and towards the right coronary artery commisure, compressing the conduction system that we know lies there,” Dr. Abdelghani said.
Dr. Spaziano reported having no financial conflicts of interest regarding his study.
PARIS – Aortomitral continuity calcification, a common finding on CT in patients undergoing transcatheter aortic valve replacement, predicts new-onset atrial fibrillation and the need for permanent pacemaker insertion, Marco Spaziano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Increased surveillance for arrhythmias in the 30 days post TAVR is warranted in patients with aortomitral continuity calcification,” declared Dr. Spaziano of the Paris South Cardiovascular Institute in Massy, France.
He presented a single-center retrospective study of 524 patients undergoing TAVR with a self-expandable or balloon-expandable device. Aortomitral continuity calcification (AMCC) was found on CT in 15.8% of them. Dr. Spaziano defined AMCC as the presence of calcium in the curtain linking the aortic and mitral valve annuli. The clinical implications of this common finding were unknown prior to this study.
The 83 patients with AMCC did not differ significantly from the 441 without that CT finding in terms of baseline demographics, Society of Thoracic Surgeons risk score, prevalence of peripheral vascular disease, QRS duration, left ventricular ejection fraction, complete left or right bundle branch block, or aortic valve calcification volume. The prevalence of atrial fibrillation at baseline was 25.6% in the AMCC group and closely similar at 26.3% in the group without AMCC. Sixteen percent of subjects in each group had a previous pacemaker.
Similarly, the two groups didn’t differ in terms of procedural characteristics, including device type, size, or depth of implantation, or need for a second valve, or annular rupture.
However, excluding from consideration the patients with prior AF, the incidence of new AF in the 30 days post-TAVR was 22.7% in patients with AMCC compared with just 7.6% in the no-AMCC group. In addition, 33% of patients with AMCC received a new permanent pacemaker, as did 21% of those with no AMCC.
Other key 30-day outcomes didn’t differ between the two populations, including rates of death, stroke, vascular complications, and moderate or severe paravalvular regurgitation.
In a multivariate regression analysis adjusted for age, sex, device type and implantation depth, preexisting right bundle branch block, and surgical risk score, AMCC was associated with a statistically significant 1.8-fold increased likelihood of new pacemaker insertion and a 3.4-fold greater risk of new AF.
Dr. Spaziano said that in brainstorming with electrophysiology and echocardiography colleagues, the group came up with two hypotheses to explain the study findings. One is that AMCC might be a biologic marker for concomitant mitral stenosis, a known strong predictor of AF.
“Oftentimes it’s very difficult to diagnose mitral stenosis when there is aortic stenosis, because of left ventricular compliance issues, so potentially the patients with this calcium ridge may also have mitral stenosis,” he observed.
The other proposed hypothesis is that AMCC reflects increased calcification and fibrosis in the electrical system of both the AV node and atrium, with a resultant increased risk of developing new AF after the TAVR procedure.
Session chair Mohammad Abdelghani, MD, wasn’t buying either hypothesis. If either were correct, the group with AMCC would be expected to have a higher baseline rate of AF preprocedurally, observed Dr. Abdelghani of the Academic Medical Center at Amsterdam.
He suggested an alternative explanation on the basis of a German study that showed patients with significant calcification of the left coronary cusp were at sixfold greater risk for pacemaker implantation post TAVR. He proposed that calcification in the left sector of the valve landing zone causes the device to end up being positioned a bit off-line.
“I think the device protrudes away from the calcium and towards the right coronary artery commisure, compressing the conduction system that we know lies there,” Dr. Abdelghani said.
Dr. Spaziano reported having no financial conflicts of interest regarding his study.
AT europcr 2016
Key clinical point: Aortomitral continuity calcification is associated with a markedly increased risk of new atrial fibrillation in patients undergoing transcatheter aortic valve replacement.
Major finding: The CT finding of aortomitral continuity calcification in patients undergoing transcatheter aortic valve replacement was associated with a 3.4-fold increased likelihood of new atrial fibrillation arising during the first 30 days post procedure.
Data source: A retrospective single-center study in 524 patients undergoing transcatheter aortic valve replacement, nearly 16% of whom were found to have aortomitral continuity calcification.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study.
Same-day discharge after PCI gets a boost
PARIS – Same-day discharge after uncomplicated transradial-access percutaneous coronary intervention (PCI) in patients with stable coronary artery disease is both feasible and safe, according to the findings of a multicenter prospective Spanish registry study.
Under the Spanish investigators’ protocol for same-day discharge, roughly three-quarters of patients successfully completed the 4- to 12-hour post-PCI surveillance period and were expeditiously sent home without spending a night in the hospital, Juan Gabriel Cordoba Soriano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The other 26% of patients were admitted, most often because they showed clinical instability during the surveillance period, less frequently due to a suboptimal angiographic result, explained Dr. Cordoba Soriano of the University of Albacete, Spain.
The rationale for same-day discharge post PCI – provided it has first been shown to be safe, as was the case using the Spanish criteria – is that it reduces costs by avoiding an expensive hospital bed. Also, most patients prefer to sleep in their own bed and avoid a hospital stay, he continued.
Eligibility for same-day discharge in the Spanish study was restricted to patients with stable coronary artery disease undergoing elective transradial PCI with no complications during the procedure and with clinical stability during the subsequent 4- to 12-hour observation period. Patients undergoing complex PCIs – for example, treatment of left main lesions, complex bifurcation lesions, or chronic total occlusions – were ineligible.
Why restrict eligibility to patients undergoing transradial PCI? Multiple studies convincingly show it is safer than femoral access. And outside of the United States, it is by far the more popular access route. In a show of hands, virtually all of Dr. Cordoba Soriano’s audience indicated they perform more than 70% of their PCIs via transradial access. And patients with stable CAD are less likely to experience stent thrombosis or acute occlusion of the treated artery or side branches, he continued.
Of 989 patients who presented to the three participating Spanish medical centers for elective PCI, 257 were immediately excluded from the registry because they underwent elective femoral access. That left 732 patients, 74% of whom got same-day discharge.
The same-day discharge and hospital admission groups were closely similar in terms of baseline characteristics with two exceptions: The prevalence of peripheral arterial disease in the same-day discharge group was less than half of the 10% figure in the hospitalized group, and kidney function was better in patients who ultimately received same-day discharge as evidenced by a serum creatinine of 0.9 mg/dL, half that of the hospitalized patients.
Procedural characteristics were mostly similar for the two groups as well. Although the same-day discharge group had a 26-minute shorter median procedure time, were less likely to undergo multivessel PCI, and had fewer stents implanted per patient, in a multivariate regression analysis the only independent predictors of admission post PCI were the presence of peripheral arterial disease, with an associated 2.2-fold increased risk; multivessel PCI, with a 1.8-fold risk; ad hoc as opposed to a scheduled PCI, with a 4.0-fold increased risk; and a history of prior transradial catheterization, which cut the risk of hospitalization in half.
Turning to the safety of same-day discharge, the cardiologist deemed the rate of major complications in the first 24 hours to be acceptable at 0.18% for a single case of significant bleeding. Minor complications were confined to a 1.8% incidence of hematomas greater than 5 cm in size.
The major complication rate from 24 hours to 30 days post PCI was 0.54% (two deaths, one stroke), with a 2.2% incidence of minor complications.
Dr. Cordoba Soriano noted that investigators at the Quebec Heart and Lung Institute have published a meta-analysis of 13 studies of same-day discharge after PCI totaling more than 111,000 patients (JACC Cardiovasc Interv. 2013 Feb;6[2]:99-112). The investigators concluded that a definitive randomized trial would require more than 17,000 subjects, and in the absence of such evidence same-day discharge after uncomplicated PCI “seems a reasonable approach in selected patients.”
Stanford University investigators have published a separate meta-analysis of same-day discharge after PCI in nearly 13,000 patients in 30 observational and 7 randomized controlled trials. They concluded that it appears to be as safe as overnight observation (J Am Coll Cardiol. 2013 Jul 23;62[4]:275-85).
Nevertheless, the Society for Cardiovascular Angiography and Interventions has yet to update its 2009 expert consensus document stating that the standard of care is an overnight stay following PCI (Catheter Cardiovasc Interv. 2009 Jun 1;73[7]:847-58), Dr. Cordoba Soriano observed.
He reported having no financial conflicts regarding the registry study, which was conducted with university research funds.
PARIS – Same-day discharge after uncomplicated transradial-access percutaneous coronary intervention (PCI) in patients with stable coronary artery disease is both feasible and safe, according to the findings of a multicenter prospective Spanish registry study.
Under the Spanish investigators’ protocol for same-day discharge, roughly three-quarters of patients successfully completed the 4- to 12-hour post-PCI surveillance period and were expeditiously sent home without spending a night in the hospital, Juan Gabriel Cordoba Soriano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The other 26% of patients were admitted, most often because they showed clinical instability during the surveillance period, less frequently due to a suboptimal angiographic result, explained Dr. Cordoba Soriano of the University of Albacete, Spain.
The rationale for same-day discharge post PCI – provided it has first been shown to be safe, as was the case using the Spanish criteria – is that it reduces costs by avoiding an expensive hospital bed. Also, most patients prefer to sleep in their own bed and avoid a hospital stay, he continued.
Eligibility for same-day discharge in the Spanish study was restricted to patients with stable coronary artery disease undergoing elective transradial PCI with no complications during the procedure and with clinical stability during the subsequent 4- to 12-hour observation period. Patients undergoing complex PCIs – for example, treatment of left main lesions, complex bifurcation lesions, or chronic total occlusions – were ineligible.
Why restrict eligibility to patients undergoing transradial PCI? Multiple studies convincingly show it is safer than femoral access. And outside of the United States, it is by far the more popular access route. In a show of hands, virtually all of Dr. Cordoba Soriano’s audience indicated they perform more than 70% of their PCIs via transradial access. And patients with stable CAD are less likely to experience stent thrombosis or acute occlusion of the treated artery or side branches, he continued.
Of 989 patients who presented to the three participating Spanish medical centers for elective PCI, 257 were immediately excluded from the registry because they underwent elective femoral access. That left 732 patients, 74% of whom got same-day discharge.
The same-day discharge and hospital admission groups were closely similar in terms of baseline characteristics with two exceptions: The prevalence of peripheral arterial disease in the same-day discharge group was less than half of the 10% figure in the hospitalized group, and kidney function was better in patients who ultimately received same-day discharge as evidenced by a serum creatinine of 0.9 mg/dL, half that of the hospitalized patients.
Procedural characteristics were mostly similar for the two groups as well. Although the same-day discharge group had a 26-minute shorter median procedure time, were less likely to undergo multivessel PCI, and had fewer stents implanted per patient, in a multivariate regression analysis the only independent predictors of admission post PCI were the presence of peripheral arterial disease, with an associated 2.2-fold increased risk; multivessel PCI, with a 1.8-fold risk; ad hoc as opposed to a scheduled PCI, with a 4.0-fold increased risk; and a history of prior transradial catheterization, which cut the risk of hospitalization in half.
Turning to the safety of same-day discharge, the cardiologist deemed the rate of major complications in the first 24 hours to be acceptable at 0.18% for a single case of significant bleeding. Minor complications were confined to a 1.8% incidence of hematomas greater than 5 cm in size.
The major complication rate from 24 hours to 30 days post PCI was 0.54% (two deaths, one stroke), with a 2.2% incidence of minor complications.
Dr. Cordoba Soriano noted that investigators at the Quebec Heart and Lung Institute have published a meta-analysis of 13 studies of same-day discharge after PCI totaling more than 111,000 patients (JACC Cardiovasc Interv. 2013 Feb;6[2]:99-112). The investigators concluded that a definitive randomized trial would require more than 17,000 subjects, and in the absence of such evidence same-day discharge after uncomplicated PCI “seems a reasonable approach in selected patients.”
Stanford University investigators have published a separate meta-analysis of same-day discharge after PCI in nearly 13,000 patients in 30 observational and 7 randomized controlled trials. They concluded that it appears to be as safe as overnight observation (J Am Coll Cardiol. 2013 Jul 23;62[4]:275-85).
Nevertheless, the Society for Cardiovascular Angiography and Interventions has yet to update its 2009 expert consensus document stating that the standard of care is an overnight stay following PCI (Catheter Cardiovasc Interv. 2009 Jun 1;73[7]:847-58), Dr. Cordoba Soriano observed.
He reported having no financial conflicts regarding the registry study, which was conducted with university research funds.
PARIS – Same-day discharge after uncomplicated transradial-access percutaneous coronary intervention (PCI) in patients with stable coronary artery disease is both feasible and safe, according to the findings of a multicenter prospective Spanish registry study.
Under the Spanish investigators’ protocol for same-day discharge, roughly three-quarters of patients successfully completed the 4- to 12-hour post-PCI surveillance period and were expeditiously sent home without spending a night in the hospital, Juan Gabriel Cordoba Soriano, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The other 26% of patients were admitted, most often because they showed clinical instability during the surveillance period, less frequently due to a suboptimal angiographic result, explained Dr. Cordoba Soriano of the University of Albacete, Spain.
The rationale for same-day discharge post PCI – provided it has first been shown to be safe, as was the case using the Spanish criteria – is that it reduces costs by avoiding an expensive hospital bed. Also, most patients prefer to sleep in their own bed and avoid a hospital stay, he continued.
Eligibility for same-day discharge in the Spanish study was restricted to patients with stable coronary artery disease undergoing elective transradial PCI with no complications during the procedure and with clinical stability during the subsequent 4- to 12-hour observation period. Patients undergoing complex PCIs – for example, treatment of left main lesions, complex bifurcation lesions, or chronic total occlusions – were ineligible.
Why restrict eligibility to patients undergoing transradial PCI? Multiple studies convincingly show it is safer than femoral access. And outside of the United States, it is by far the more popular access route. In a show of hands, virtually all of Dr. Cordoba Soriano’s audience indicated they perform more than 70% of their PCIs via transradial access. And patients with stable CAD are less likely to experience stent thrombosis or acute occlusion of the treated artery or side branches, he continued.
Of 989 patients who presented to the three participating Spanish medical centers for elective PCI, 257 were immediately excluded from the registry because they underwent elective femoral access. That left 732 patients, 74% of whom got same-day discharge.
The same-day discharge and hospital admission groups were closely similar in terms of baseline characteristics with two exceptions: The prevalence of peripheral arterial disease in the same-day discharge group was less than half of the 10% figure in the hospitalized group, and kidney function was better in patients who ultimately received same-day discharge as evidenced by a serum creatinine of 0.9 mg/dL, half that of the hospitalized patients.
Procedural characteristics were mostly similar for the two groups as well. Although the same-day discharge group had a 26-minute shorter median procedure time, were less likely to undergo multivessel PCI, and had fewer stents implanted per patient, in a multivariate regression analysis the only independent predictors of admission post PCI were the presence of peripheral arterial disease, with an associated 2.2-fold increased risk; multivessel PCI, with a 1.8-fold risk; ad hoc as opposed to a scheduled PCI, with a 4.0-fold increased risk; and a history of prior transradial catheterization, which cut the risk of hospitalization in half.
Turning to the safety of same-day discharge, the cardiologist deemed the rate of major complications in the first 24 hours to be acceptable at 0.18% for a single case of significant bleeding. Minor complications were confined to a 1.8% incidence of hematomas greater than 5 cm in size.
The major complication rate from 24 hours to 30 days post PCI was 0.54% (two deaths, one stroke), with a 2.2% incidence of minor complications.
Dr. Cordoba Soriano noted that investigators at the Quebec Heart and Lung Institute have published a meta-analysis of 13 studies of same-day discharge after PCI totaling more than 111,000 patients (JACC Cardiovasc Interv. 2013 Feb;6[2]:99-112). The investigators concluded that a definitive randomized trial would require more than 17,000 subjects, and in the absence of such evidence same-day discharge after uncomplicated PCI “seems a reasonable approach in selected patients.”
Stanford University investigators have published a separate meta-analysis of same-day discharge after PCI in nearly 13,000 patients in 30 observational and 7 randomized controlled trials. They concluded that it appears to be as safe as overnight observation (J Am Coll Cardiol. 2013 Jul 23;62[4]:275-85).
Nevertheless, the Society for Cardiovascular Angiography and Interventions has yet to update its 2009 expert consensus document stating that the standard of care is an overnight stay following PCI (Catheter Cardiovasc Interv. 2009 Jun 1;73[7]:847-58), Dr. Cordoba Soriano observed.
He reported having no financial conflicts regarding the registry study, which was conducted with university research funds.
AT EUROPCR 2016
Key clinical point: Same-day discharge following uncomplicated elective transradial PCI is feasible and safe.
Major finding: The rates of major and minor complications in the 24 hours following PCI with same-day discharge were 0.18% and 1.8%, respectively.
Data source: A prospective observational registry study including 989 PCI patients at three Spanish university hospitals.
Disclosures: The presenter reported having no financial conflicts regarding the registry study, which was conducted with university research funds.
Using transcatheter aortic valves for severe mitral annular calcification
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.
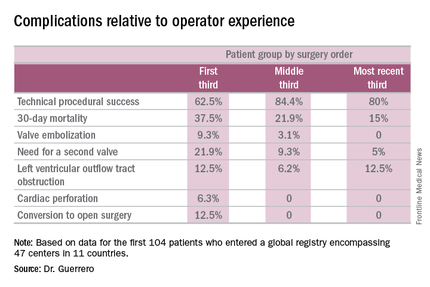
Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.

Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.

Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
AT EUROPCR 2016
Key clinical point: A report from a global registry of transcatheter aortic valve implantation in the mitral position shows the procedure is feasible.
Major finding: Thirty-day mortality fell from 37.5% in the first third of treated patients to 15% in the most recent tertile.
Data source: A real world registry that includes 104 patients at 47 centers in 11 countries to date.
Disclosures: The study presenter reported serving as a consultant to Edwards Lifesciences.
Bioabsorbable percutaneous device fully closes large femoral arteriotomies
PARIS – A new bioabsorbable sutureless device provides operators with a safe, simple, and dependable option for fully percutaneous closure of the large, 12-24 French femoral arteriotomies created for transcatheter aortic valve replacement or endovascular abdominal aortic aneurysm repair, Arne Schwindt, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented the 12-month follow-up results of the FRONTIER II study of the Vivasure PerQseal device. The device recently received European marketing approval on the strength of FRONTIER II but remains investigational in the United States.
The PerQseal device is deployed in less than 1 minute at the end of the primary procedure and achieves immediate hemostasis. It’s a simple three-step deployment process with essentially no learning curve, as evidenced by the fact that in FRONTIER II, the technical success rate starting from no experience was 97%, explained Dr. Schwindt of St. Franziskus Hospital in Muenster, Germany.
The large femoral arteriotomies created for TAVR or endovascular abdominal aortic aneurysm repair have typically required surgical cut down and sutured repair with a 3- to 5-cm incision. Vascular complications have been common. Indeed, the literature shows this method entails on average a 14.7% rate of major vascular complications up to 3 months post procedure, so that was the bar set in FRONTIER II: In order for the PerQseal to be deemed noninferior to surgical cut down and sutured repair, the rate of major complications directly related to the novel device at 3 months follow-up could be no greater than 14.7%. The current Valve Academic Research Consortium (VARC) 2 definition of major complications was used.
In fact, the vascular complication rate proved to be zero. Moreover, at 12 months of follow-up, no cases of groin fibrosis or scarring had been observed, Dr. Schwindt reported.
FRONTIER II was a single-arm, prospective, multicenter European study of 58 patients who received the PerQseal device for 66 closures. They were evaluated at discharge and 1, 3, and 12 months post procedure by Doppler ultrasound, with uniformly unremarkable findings.
PerQseal features a synthetic low-profile implant with over-the-wire delivery. The implant is loaded into a sheath, released by pulling back on the sheath, then pulled up against the arteriotomy from the inside. Blood pressure molds the device to the arterial wall and seals it. The device is fully absorbed in 180 days.
Session co-chair Dr. Ted E. Feldman was favorably impressed.
“This is really terrific. It’s very nice to see we’re finally making progress with large-bore closure devices,” commented Dr. Feldman, professor of medicine at Northwestern University, Chicago.
Dr. Schwindt reported receiving a research grant from Vivasure, which sponsored the FRONTIER II study. In addition, he serves as a consultant to a half dozen medical device companies.
PARIS – A new bioabsorbable sutureless device provides operators with a safe, simple, and dependable option for fully percutaneous closure of the large, 12-24 French femoral arteriotomies created for transcatheter aortic valve replacement or endovascular abdominal aortic aneurysm repair, Arne Schwindt, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented the 12-month follow-up results of the FRONTIER II study of the Vivasure PerQseal device. The device recently received European marketing approval on the strength of FRONTIER II but remains investigational in the United States.
The PerQseal device is deployed in less than 1 minute at the end of the primary procedure and achieves immediate hemostasis. It’s a simple three-step deployment process with essentially no learning curve, as evidenced by the fact that in FRONTIER II, the technical success rate starting from no experience was 97%, explained Dr. Schwindt of St. Franziskus Hospital in Muenster, Germany.
The large femoral arteriotomies created for TAVR or endovascular abdominal aortic aneurysm repair have typically required surgical cut down and sutured repair with a 3- to 5-cm incision. Vascular complications have been common. Indeed, the literature shows this method entails on average a 14.7% rate of major vascular complications up to 3 months post procedure, so that was the bar set in FRONTIER II: In order for the PerQseal to be deemed noninferior to surgical cut down and sutured repair, the rate of major complications directly related to the novel device at 3 months follow-up could be no greater than 14.7%. The current Valve Academic Research Consortium (VARC) 2 definition of major complications was used.
In fact, the vascular complication rate proved to be zero. Moreover, at 12 months of follow-up, no cases of groin fibrosis or scarring had been observed, Dr. Schwindt reported.
FRONTIER II was a single-arm, prospective, multicenter European study of 58 patients who received the PerQseal device for 66 closures. They were evaluated at discharge and 1, 3, and 12 months post procedure by Doppler ultrasound, with uniformly unremarkable findings.
PerQseal features a synthetic low-profile implant with over-the-wire delivery. The implant is loaded into a sheath, released by pulling back on the sheath, then pulled up against the arteriotomy from the inside. Blood pressure molds the device to the arterial wall and seals it. The device is fully absorbed in 180 days.
Session co-chair Dr. Ted E. Feldman was favorably impressed.
“This is really terrific. It’s very nice to see we’re finally making progress with large-bore closure devices,” commented Dr. Feldman, professor of medicine at Northwestern University, Chicago.
Dr. Schwindt reported receiving a research grant from Vivasure, which sponsored the FRONTIER II study. In addition, he serves as a consultant to a half dozen medical device companies.
PARIS – A new bioabsorbable sutureless device provides operators with a safe, simple, and dependable option for fully percutaneous closure of the large, 12-24 French femoral arteriotomies created for transcatheter aortic valve replacement or endovascular abdominal aortic aneurysm repair, Arne Schwindt, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He presented the 12-month follow-up results of the FRONTIER II study of the Vivasure PerQseal device. The device recently received European marketing approval on the strength of FRONTIER II but remains investigational in the United States.
The PerQseal device is deployed in less than 1 minute at the end of the primary procedure and achieves immediate hemostasis. It’s a simple three-step deployment process with essentially no learning curve, as evidenced by the fact that in FRONTIER II, the technical success rate starting from no experience was 97%, explained Dr. Schwindt of St. Franziskus Hospital in Muenster, Germany.
The large femoral arteriotomies created for TAVR or endovascular abdominal aortic aneurysm repair have typically required surgical cut down and sutured repair with a 3- to 5-cm incision. Vascular complications have been common. Indeed, the literature shows this method entails on average a 14.7% rate of major vascular complications up to 3 months post procedure, so that was the bar set in FRONTIER II: In order for the PerQseal to be deemed noninferior to surgical cut down and sutured repair, the rate of major complications directly related to the novel device at 3 months follow-up could be no greater than 14.7%. The current Valve Academic Research Consortium (VARC) 2 definition of major complications was used.
In fact, the vascular complication rate proved to be zero. Moreover, at 12 months of follow-up, no cases of groin fibrosis or scarring had been observed, Dr. Schwindt reported.
FRONTIER II was a single-arm, prospective, multicenter European study of 58 patients who received the PerQseal device for 66 closures. They were evaluated at discharge and 1, 3, and 12 months post procedure by Doppler ultrasound, with uniformly unremarkable findings.
PerQseal features a synthetic low-profile implant with over-the-wire delivery. The implant is loaded into a sheath, released by pulling back on the sheath, then pulled up against the arteriotomy from the inside. Blood pressure molds the device to the arterial wall and seals it. The device is fully absorbed in 180 days.
Session co-chair Dr. Ted E. Feldman was favorably impressed.
“This is really terrific. It’s very nice to see we’re finally making progress with large-bore closure devices,” commented Dr. Feldman, professor of medicine at Northwestern University, Chicago.
Dr. Schwindt reported receiving a research grant from Vivasure, which sponsored the FRONTIER II study. In addition, he serves as a consultant to a half dozen medical device companies.
AT EUROPCR 2016
Key clinical point: The PerQseal device provides a simpler alternative to existing closure methods.
Major finding: No major vascular complications were seen during structured follow-up of recipients of the novel bioabsorbable fully percutaneous PerQseal device for closure of large-bore femoral arteriotomies.
Data source: FRONTIER II was a prospective, single-arm, 12-month multicenter study including 58 patients who underwent closure of 66 large femoral arteriotomies using the PerQseal device.
Disclosures: The presenter reported receiving a research grant from Vivasure, which sponsored the FRONTIER II study.
Most interventional cardiologists don’t fully grasp radiation risks
PARIS – What most interventional cardiologists and electrophysiologists do not know about their health risks due to occupational radiation exposure and how best to protect themselves could fill a book – or better still, make for an illuminating 2-hour expert panel discussion at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“There’s a problem of lack of awareness on the part of interventional cardiologists, and also of institutional insensitivity to the problem,” declared Emanuela Piccaluga, MD, an investigator in the eye-opening Healthy Cath Lab study. This Italian national study showed that cardiac catheterization laboratory staff had radiation exposure duration–dependent increased risks of cataracts, cancers, and skin lesions, as well as other radiogenic noncancer effects: anxiety and depression, hypertension, and hyperlipidemia.
Cardiologists at some European hospitals have to pay for their own lead aprons and other protective gear. And even if the hospital does pick up the bill, administrators often balk at authorizing replacement of a lead apron that has developed microfractures and cracks. They view these imperfections as cosmetic defects, unaware that the damage renders the apron less protective, according to Dr. Piccaluga of Niguarda Ca’ Granda Hospital in Milan.
Ariel Roguin, MD, head of interventional cardiology at Rambam Medical Center in Haifa, Israel, said every cardiologist working with radiation should understand the three principles of radiation reduction, which he refers to in shorthand as “TDS,” for Time, Distance, and Shielding. Radiation is here to stay in cardiology, he said, but interventionalists can maximize their safety by keeping the fluoroscopy time and number of acquired images down, standing as far away as possible from both the radiation source and patient while still getting the job done well, and using appropriate shielding routinely.
Dr. Roguin gained notoriety with his report that 26 of 30 interventional cardiologists with glioblastoma multiforme or other brain malignancies had left-hemisphere cancers and 1 had a midline malignancy; only 3 were right-sided (Eur Heart J. 2014 Mar;35[10]:599-600). That distribution is highly unlikely to be due to play of chance, given that an interventional cardiologist’s left side is the side that’s usually exposed to more radiation.
“We should form a wall against radiation. Apart from the leaded aprons, for every procedure we all should also use lead skirts going from the table to the floor to block backscatter, ceiling-mounted overhead radiation shields, special glasses to protect against cataracts, and thyroid collars. And it’s very important to wear a dosimeter with sound; it helps increase awareness of our exposure,” he said.
Dr. Roguin has been a pioneer in the use of a thin, 0.5-mm lead shield draped across the patient’s abdomen from the umbilicus down during radial-access angiography. In a 322-patient randomized trial, he and his coinvestigators showed that this practice results in a threefold reduction in radiation to the operator, albeit at the cost of doubling the patient’s radiation exposure (Catheter Cardiovasc Interv. 2015 Jun;85[7]:1164-70).
“We now routinely do our radials with the lead apron across the patient’s abdomen. We’ve reached the conclusion that we work with radiation in the cath lab every day for many years and the patient is there only once or twice in a lifetime, hopefully,” the cardiologist explained.
With the growing popularity of radial-access interventions, audience members wanted to know if there is an advantage in terms of radiation exposure to left versus right radial artery access. The answer is no, according to Dr. Roguin.
“There are several studies showing no difference in radiation exposure. Left radial artery access is faster, but you’re leaning on the patient and getting more radiation as a result, while with right radial access you have to do more catheter manipulation, which takes longer. Both approaches involve more radiation to the operator than the transfemoral approach,” he said.
Dr. Piccaluga presented highlights from the Healthy Cath Lab study, sponsored by the Italian Society of Invasive Cardiology and the Italian National Research Council’s Institute of Clinical Physiology. The study involved detailed self-administered questionnaires completed by 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians regularly exposed to ionizing radiation in the cardiac cath lab for a median of 10 years, along with 280 unexposed controls.
A variety of health problems were more frequent in the cath lab personnel regularly exposed to radiation. Rates were consistently highest in the cardiologists, followed next by the cath lab nurses, and then the radiation technicians.
Rates of health problems were highest in the 227 individuals with at least 13 years of cath lab radiation exposure. For example, their adjusted risks of cataracts, hypertension, hypercholesterolemia, and cancers were respectively 9-, 1.7-, 2.9-, and 4.5-fold fold greater than in unexposed controls, as detailed in a recent report (Circ Cardiovasc Interv. 2016 April. doi: 10.1161/CIRCINTERVENTIONS.115.003273).
Dr. Piccaluga also shared data from several other pertinent recent studies in which she was a coinvestigator. In one, 83 cardiologists and nurses working in cardiac catheterization laboratories and 83 matched radiation-nonexposed controls completed a neuropsychological test battery. The radiation-exposed group scored significantly lower on measures of delayed recall, visual short-term memory, and verbal fluency, all of which are skills located in left hemisphere structures of the brain – the side with more exposure to ionizing radiation during interventional procedures (J Int Neuropsychol Soc. 2015 Oct;21[9]:670-6).
In another study, Dr. Piccaluga and her coinvestigators had participants perform an odor-sniffing test. Olfactory discrimination in the cardiac cath lab staffers was significantly diminished in a pattern that has been identified in other studies as an early signal of impending clinical onset of Alzheimer’s and Parkinson’s diseases (Int J Cardiol. 2014 Feb 15;171[3]:461-3).
And in yet another study, Dr. Piccaluga and her coworkers found that left and right carotid intima-media thickness as measured by ultrasound in cardiac cath lab personnel having high lifetime radiation exposure was significantly greater than in those with low exposure and in nonexposed controls. In the left carotid artery, but not the right, intimal-medial thickness was significantly correlated with a total occupational radiologic risk score.
Moreover, the Italian investigators found a significant reduction in leukocyte telomere length – a biomarker for accelerated vascular aging – in cardiac cath lab staff regularly exposed to ionizing radiation, compared with controls (JACC Cardiovasc Interv. 2015 Apr 20;8[4]:616-27).
All of these findings, she stressed, make a persuasive case for interventional cardiologists doing everything they can to protect themselves from unnecessary radiation exposure at all times.
How to best go about accomplishing this was the territory covered by Alaide Chieffo, MD, of San Raffaele Scientific Institute in Milan.
The patient-related factors germane to radiation dose – procedure complexity and body thickness – are outside physician control. But there are plenty of operator-dependent factors, including, for starters, procedural experience. In one classic study, Dr. Chieffo noted, Greek investigators showed that interventional cardiologists’ radiation exposure dose was 60% greater in their first year of practice than in their second year.
Distance from the patient is crucial, she observed, since the patient is the greatest source of radiation to the operator. If the operator is 35 cm from the patient, the radiation exposure is fourfold greater than at a distance of 70 cm. At a distance of 17.5 cm, the exposure intensity is 16-fold greater than at a 70-cm distance. And at 8.8 cm of distance, it’s 64 times greater.
Image acquisition is another key variable within the interventionalist’s control. Cine images entail 12- to 20-times greater radiation doses than those of fluoroscopy, so don’t resort to cine when fluoroscopy will do. Also, reducing the fluoroscopy frame rate from 15 to 7.5 frames per second significantly decreases the amount of radiation released while providing images of adequate quality for many procedures. Tight collimation, the use of manually inserted wedge filters, and thoughtful selection of tube angulations result in less radiation for both patient and physician. It has been shown that tube angulations that expose a patient to intense radiation levels increase the operator’s radiation exposure exponentially. The least-irradiating tube angulations are caudal posteroanterior 0°/30°– angulation for the left coronary main stem, cranial posteroanterior 0°/30°+ for the left anterior descending coronary artery bifurcation, and right anterior oblique views of 40° or more. Left anterior oblique projections are the most radiation intensive, according to a comprehensive study (J Am Coll Cardiol. 2004 Oct 6;44[7]:1420-8), Dr. Chieffo continued.
Panelist Ghada Mikhail, MD, of Imperial College London, said there is some relatively new operator protective gear available. She cited lightweight protective caps, for example, but an audience show of hands indicated almost no one uses them.
“Protectors for the breasts and gonads are available. You can wear them underneath the lead. The extra time to put them on is worthwhile,” she said.
“I think the risk of radiation is completely underestimated,” Dr. Mikhail added. “We have a responsibility to young trainees to teach them about radiation protection, which a lot of institutions and supervisors don’t do. That’s partly because a lot of them don’t know the details.”
All of the speakers indicated they had no financial conflicts regarding their presentations.
PARIS – What most interventional cardiologists and electrophysiologists do not know about their health risks due to occupational radiation exposure and how best to protect themselves could fill a book – or better still, make for an illuminating 2-hour expert panel discussion at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“There’s a problem of lack of awareness on the part of interventional cardiologists, and also of institutional insensitivity to the problem,” declared Emanuela Piccaluga, MD, an investigator in the eye-opening Healthy Cath Lab study. This Italian national study showed that cardiac catheterization laboratory staff had radiation exposure duration–dependent increased risks of cataracts, cancers, and skin lesions, as well as other radiogenic noncancer effects: anxiety and depression, hypertension, and hyperlipidemia.
Cardiologists at some European hospitals have to pay for their own lead aprons and other protective gear. And even if the hospital does pick up the bill, administrators often balk at authorizing replacement of a lead apron that has developed microfractures and cracks. They view these imperfections as cosmetic defects, unaware that the damage renders the apron less protective, according to Dr. Piccaluga of Niguarda Ca’ Granda Hospital in Milan.
Ariel Roguin, MD, head of interventional cardiology at Rambam Medical Center in Haifa, Israel, said every cardiologist working with radiation should understand the three principles of radiation reduction, which he refers to in shorthand as “TDS,” for Time, Distance, and Shielding. Radiation is here to stay in cardiology, he said, but interventionalists can maximize their safety by keeping the fluoroscopy time and number of acquired images down, standing as far away as possible from both the radiation source and patient while still getting the job done well, and using appropriate shielding routinely.
Dr. Roguin gained notoriety with his report that 26 of 30 interventional cardiologists with glioblastoma multiforme or other brain malignancies had left-hemisphere cancers and 1 had a midline malignancy; only 3 were right-sided (Eur Heart J. 2014 Mar;35[10]:599-600). That distribution is highly unlikely to be due to play of chance, given that an interventional cardiologist’s left side is the side that’s usually exposed to more radiation.
“We should form a wall against radiation. Apart from the leaded aprons, for every procedure we all should also use lead skirts going from the table to the floor to block backscatter, ceiling-mounted overhead radiation shields, special glasses to protect against cataracts, and thyroid collars. And it’s very important to wear a dosimeter with sound; it helps increase awareness of our exposure,” he said.
Dr. Roguin has been a pioneer in the use of a thin, 0.5-mm lead shield draped across the patient’s abdomen from the umbilicus down during radial-access angiography. In a 322-patient randomized trial, he and his coinvestigators showed that this practice results in a threefold reduction in radiation to the operator, albeit at the cost of doubling the patient’s radiation exposure (Catheter Cardiovasc Interv. 2015 Jun;85[7]:1164-70).
“We now routinely do our radials with the lead apron across the patient’s abdomen. We’ve reached the conclusion that we work with radiation in the cath lab every day for many years and the patient is there only once or twice in a lifetime, hopefully,” the cardiologist explained.
With the growing popularity of radial-access interventions, audience members wanted to know if there is an advantage in terms of radiation exposure to left versus right radial artery access. The answer is no, according to Dr. Roguin.
“There are several studies showing no difference in radiation exposure. Left radial artery access is faster, but you’re leaning on the patient and getting more radiation as a result, while with right radial access you have to do more catheter manipulation, which takes longer. Both approaches involve more radiation to the operator than the transfemoral approach,” he said.
Dr. Piccaluga presented highlights from the Healthy Cath Lab study, sponsored by the Italian Society of Invasive Cardiology and the Italian National Research Council’s Institute of Clinical Physiology. The study involved detailed self-administered questionnaires completed by 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians regularly exposed to ionizing radiation in the cardiac cath lab for a median of 10 years, along with 280 unexposed controls.
A variety of health problems were more frequent in the cath lab personnel regularly exposed to radiation. Rates were consistently highest in the cardiologists, followed next by the cath lab nurses, and then the radiation technicians.
Rates of health problems were highest in the 227 individuals with at least 13 years of cath lab radiation exposure. For example, their adjusted risks of cataracts, hypertension, hypercholesterolemia, and cancers were respectively 9-, 1.7-, 2.9-, and 4.5-fold fold greater than in unexposed controls, as detailed in a recent report (Circ Cardiovasc Interv. 2016 April. doi: 10.1161/CIRCINTERVENTIONS.115.003273).
Dr. Piccaluga also shared data from several other pertinent recent studies in which she was a coinvestigator. In one, 83 cardiologists and nurses working in cardiac catheterization laboratories and 83 matched radiation-nonexposed controls completed a neuropsychological test battery. The radiation-exposed group scored significantly lower on measures of delayed recall, visual short-term memory, and verbal fluency, all of which are skills located in left hemisphere structures of the brain – the side with more exposure to ionizing radiation during interventional procedures (J Int Neuropsychol Soc. 2015 Oct;21[9]:670-6).
In another study, Dr. Piccaluga and her coinvestigators had participants perform an odor-sniffing test. Olfactory discrimination in the cardiac cath lab staffers was significantly diminished in a pattern that has been identified in other studies as an early signal of impending clinical onset of Alzheimer’s and Parkinson’s diseases (Int J Cardiol. 2014 Feb 15;171[3]:461-3).
And in yet another study, Dr. Piccaluga and her coworkers found that left and right carotid intima-media thickness as measured by ultrasound in cardiac cath lab personnel having high lifetime radiation exposure was significantly greater than in those with low exposure and in nonexposed controls. In the left carotid artery, but not the right, intimal-medial thickness was significantly correlated with a total occupational radiologic risk score.
Moreover, the Italian investigators found a significant reduction in leukocyte telomere length – a biomarker for accelerated vascular aging – in cardiac cath lab staff regularly exposed to ionizing radiation, compared with controls (JACC Cardiovasc Interv. 2015 Apr 20;8[4]:616-27).
All of these findings, she stressed, make a persuasive case for interventional cardiologists doing everything they can to protect themselves from unnecessary radiation exposure at all times.
How to best go about accomplishing this was the territory covered by Alaide Chieffo, MD, of San Raffaele Scientific Institute in Milan.
The patient-related factors germane to radiation dose – procedure complexity and body thickness – are outside physician control. But there are plenty of operator-dependent factors, including, for starters, procedural experience. In one classic study, Dr. Chieffo noted, Greek investigators showed that interventional cardiologists’ radiation exposure dose was 60% greater in their first year of practice than in their second year.
Distance from the patient is crucial, she observed, since the patient is the greatest source of radiation to the operator. If the operator is 35 cm from the patient, the radiation exposure is fourfold greater than at a distance of 70 cm. At a distance of 17.5 cm, the exposure intensity is 16-fold greater than at a 70-cm distance. And at 8.8 cm of distance, it’s 64 times greater.
Image acquisition is another key variable within the interventionalist’s control. Cine images entail 12- to 20-times greater radiation doses than those of fluoroscopy, so don’t resort to cine when fluoroscopy will do. Also, reducing the fluoroscopy frame rate from 15 to 7.5 frames per second significantly decreases the amount of radiation released while providing images of adequate quality for many procedures. Tight collimation, the use of manually inserted wedge filters, and thoughtful selection of tube angulations result in less radiation for both patient and physician. It has been shown that tube angulations that expose a patient to intense radiation levels increase the operator’s radiation exposure exponentially. The least-irradiating tube angulations are caudal posteroanterior 0°/30°– angulation for the left coronary main stem, cranial posteroanterior 0°/30°+ for the left anterior descending coronary artery bifurcation, and right anterior oblique views of 40° or more. Left anterior oblique projections are the most radiation intensive, according to a comprehensive study (J Am Coll Cardiol. 2004 Oct 6;44[7]:1420-8), Dr. Chieffo continued.
Panelist Ghada Mikhail, MD, of Imperial College London, said there is some relatively new operator protective gear available. She cited lightweight protective caps, for example, but an audience show of hands indicated almost no one uses them.
“Protectors for the breasts and gonads are available. You can wear them underneath the lead. The extra time to put them on is worthwhile,” she said.
“I think the risk of radiation is completely underestimated,” Dr. Mikhail added. “We have a responsibility to young trainees to teach them about radiation protection, which a lot of institutions and supervisors don’t do. That’s partly because a lot of them don’t know the details.”
All of the speakers indicated they had no financial conflicts regarding their presentations.
PARIS – What most interventional cardiologists and electrophysiologists do not know about their health risks due to occupational radiation exposure and how best to protect themselves could fill a book – or better still, make for an illuminating 2-hour expert panel discussion at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“There’s a problem of lack of awareness on the part of interventional cardiologists, and also of institutional insensitivity to the problem,” declared Emanuela Piccaluga, MD, an investigator in the eye-opening Healthy Cath Lab study. This Italian national study showed that cardiac catheterization laboratory staff had radiation exposure duration–dependent increased risks of cataracts, cancers, and skin lesions, as well as other radiogenic noncancer effects: anxiety and depression, hypertension, and hyperlipidemia.
Cardiologists at some European hospitals have to pay for their own lead aprons and other protective gear. And even if the hospital does pick up the bill, administrators often balk at authorizing replacement of a lead apron that has developed microfractures and cracks. They view these imperfections as cosmetic defects, unaware that the damage renders the apron less protective, according to Dr. Piccaluga of Niguarda Ca’ Granda Hospital in Milan.
Ariel Roguin, MD, head of interventional cardiology at Rambam Medical Center in Haifa, Israel, said every cardiologist working with radiation should understand the three principles of radiation reduction, which he refers to in shorthand as “TDS,” for Time, Distance, and Shielding. Radiation is here to stay in cardiology, he said, but interventionalists can maximize their safety by keeping the fluoroscopy time and number of acquired images down, standing as far away as possible from both the radiation source and patient while still getting the job done well, and using appropriate shielding routinely.
Dr. Roguin gained notoriety with his report that 26 of 30 interventional cardiologists with glioblastoma multiforme or other brain malignancies had left-hemisphere cancers and 1 had a midline malignancy; only 3 were right-sided (Eur Heart J. 2014 Mar;35[10]:599-600). That distribution is highly unlikely to be due to play of chance, given that an interventional cardiologist’s left side is the side that’s usually exposed to more radiation.
“We should form a wall against radiation. Apart from the leaded aprons, for every procedure we all should also use lead skirts going from the table to the floor to block backscatter, ceiling-mounted overhead radiation shields, special glasses to protect against cataracts, and thyroid collars. And it’s very important to wear a dosimeter with sound; it helps increase awareness of our exposure,” he said.
Dr. Roguin has been a pioneer in the use of a thin, 0.5-mm lead shield draped across the patient’s abdomen from the umbilicus down during radial-access angiography. In a 322-patient randomized trial, he and his coinvestigators showed that this practice results in a threefold reduction in radiation to the operator, albeit at the cost of doubling the patient’s radiation exposure (Catheter Cardiovasc Interv. 2015 Jun;85[7]:1164-70).
“We now routinely do our radials with the lead apron across the patient’s abdomen. We’ve reached the conclusion that we work with radiation in the cath lab every day for many years and the patient is there only once or twice in a lifetime, hopefully,” the cardiologist explained.
With the growing popularity of radial-access interventions, audience members wanted to know if there is an advantage in terms of radiation exposure to left versus right radial artery access. The answer is no, according to Dr. Roguin.
“There are several studies showing no difference in radiation exposure. Left radial artery access is faster, but you’re leaning on the patient and getting more radiation as a result, while with right radial access you have to do more catheter manipulation, which takes longer. Both approaches involve more radiation to the operator than the transfemoral approach,” he said.
Dr. Piccaluga presented highlights from the Healthy Cath Lab study, sponsored by the Italian Society of Invasive Cardiology and the Italian National Research Council’s Institute of Clinical Physiology. The study involved detailed self-administered questionnaires completed by 218 interventional cardiologists and electrophysiologists, 191 nurses, and 57 technicians regularly exposed to ionizing radiation in the cardiac cath lab for a median of 10 years, along with 280 unexposed controls.
A variety of health problems were more frequent in the cath lab personnel regularly exposed to radiation. Rates were consistently highest in the cardiologists, followed next by the cath lab nurses, and then the radiation technicians.
Rates of health problems were highest in the 227 individuals with at least 13 years of cath lab radiation exposure. For example, their adjusted risks of cataracts, hypertension, hypercholesterolemia, and cancers were respectively 9-, 1.7-, 2.9-, and 4.5-fold fold greater than in unexposed controls, as detailed in a recent report (Circ Cardiovasc Interv. 2016 April. doi: 10.1161/CIRCINTERVENTIONS.115.003273).
Dr. Piccaluga also shared data from several other pertinent recent studies in which she was a coinvestigator. In one, 83 cardiologists and nurses working in cardiac catheterization laboratories and 83 matched radiation-nonexposed controls completed a neuropsychological test battery. The radiation-exposed group scored significantly lower on measures of delayed recall, visual short-term memory, and verbal fluency, all of which are skills located in left hemisphere structures of the brain – the side with more exposure to ionizing radiation during interventional procedures (J Int Neuropsychol Soc. 2015 Oct;21[9]:670-6).
In another study, Dr. Piccaluga and her coinvestigators had participants perform an odor-sniffing test. Olfactory discrimination in the cardiac cath lab staffers was significantly diminished in a pattern that has been identified in other studies as an early signal of impending clinical onset of Alzheimer’s and Parkinson’s diseases (Int J Cardiol. 2014 Feb 15;171[3]:461-3).
And in yet another study, Dr. Piccaluga and her coworkers found that left and right carotid intima-media thickness as measured by ultrasound in cardiac cath lab personnel having high lifetime radiation exposure was significantly greater than in those with low exposure and in nonexposed controls. In the left carotid artery, but not the right, intimal-medial thickness was significantly correlated with a total occupational radiologic risk score.
Moreover, the Italian investigators found a significant reduction in leukocyte telomere length – a biomarker for accelerated vascular aging – in cardiac cath lab staff regularly exposed to ionizing radiation, compared with controls (JACC Cardiovasc Interv. 2015 Apr 20;8[4]:616-27).
All of these findings, she stressed, make a persuasive case for interventional cardiologists doing everything they can to protect themselves from unnecessary radiation exposure at all times.
How to best go about accomplishing this was the territory covered by Alaide Chieffo, MD, of San Raffaele Scientific Institute in Milan.
The patient-related factors germane to radiation dose – procedure complexity and body thickness – are outside physician control. But there are plenty of operator-dependent factors, including, for starters, procedural experience. In one classic study, Dr. Chieffo noted, Greek investigators showed that interventional cardiologists’ radiation exposure dose was 60% greater in their first year of practice than in their second year.
Distance from the patient is crucial, she observed, since the patient is the greatest source of radiation to the operator. If the operator is 35 cm from the patient, the radiation exposure is fourfold greater than at a distance of 70 cm. At a distance of 17.5 cm, the exposure intensity is 16-fold greater than at a 70-cm distance. And at 8.8 cm of distance, it’s 64 times greater.
Image acquisition is another key variable within the interventionalist’s control. Cine images entail 12- to 20-times greater radiation doses than those of fluoroscopy, so don’t resort to cine when fluoroscopy will do. Also, reducing the fluoroscopy frame rate from 15 to 7.5 frames per second significantly decreases the amount of radiation released while providing images of adequate quality for many procedures. Tight collimation, the use of manually inserted wedge filters, and thoughtful selection of tube angulations result in less radiation for both patient and physician. It has been shown that tube angulations that expose a patient to intense radiation levels increase the operator’s radiation exposure exponentially. The least-irradiating tube angulations are caudal posteroanterior 0°/30°– angulation for the left coronary main stem, cranial posteroanterior 0°/30°+ for the left anterior descending coronary artery bifurcation, and right anterior oblique views of 40° or more. Left anterior oblique projections are the most radiation intensive, according to a comprehensive study (J Am Coll Cardiol. 2004 Oct 6;44[7]:1420-8), Dr. Chieffo continued.
Panelist Ghada Mikhail, MD, of Imperial College London, said there is some relatively new operator protective gear available. She cited lightweight protective caps, for example, but an audience show of hands indicated almost no one uses them.
“Protectors for the breasts and gonads are available. You can wear them underneath the lead. The extra time to put them on is worthwhile,” she said.
“I think the risk of radiation is completely underestimated,” Dr. Mikhail added. “We have a responsibility to young trainees to teach them about radiation protection, which a lot of institutions and supervisors don’t do. That’s partly because a lot of them don’t know the details.”
All of the speakers indicated they had no financial conflicts regarding their presentations.
EXPERT ANALYSIS FROM EUROPCR 2016
New risk score predicts PCI outcomes in octogenarians
PARIS – A fast and simple clinically based scoring system enables physicians to determine the chance of a successful outcome for octogenarians undergoing percutaneous coronary intervention, James Cockburn, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
All six elements of the scoring system are readily available either at the time the very elderly patient presents or at diagnostic angiography, noted Dr. Cockburn of Brighton and Sussex University Hospital in Brighton, England.
He and his coworkers developed the risk score through analysis of a registry of 44,221 patients aged 80 or older who underwent percutaneous coronary intervention (PCI). The procedural success rate – defined as less than a 30% residual stenosis and TIMI (Thrombolysis in Myocardial Infarction) 3 antegrade blood flow – was 92.3%. The 30-day mortality rate was 3.9%. The investigators teased out a set of easily accessible clinical factors associated with 30-day mortality and came up with a novel risk scoring system using a 9-point scale.
The clinical factors and scoring system are as follows:
• Age. 1 point for being 80-89, and 2 points at age 90 or older.
• Indication for PCI. 1 point for unstable angina/non–ST segment elevation MI, 2 points for STEMI, 0 points for other indications.
• Ventilated preprocedure. 1 point if yes.
• Creatinine level above 200 umol/L. 1 point for yes.
• Preprocedural cardiogenic shock. 2 points for yes.
• Poor left ventricular ejection fraction. If less than 30%, 1 point.
Thus, scores can range from 1 to 9. Dr. Cockburn and his coworkers calculated the risk of 30-day mortality for each possible score. They validated the score by performing a receiver operator curve analysis that showed an area under the curve of 0.83, suggestive of relatively high sensitivity and specificity.
A score of 4 or less suggests a very good chance of survival at 30 days. In contrast, a score of 6 was associated with a two in three chance of death by 30 days. And it’s not hard to reach a 6: A patient who is 90 years old (2 points), presents with STEMI (2 points), and is in cardiogenic shock (2 points) is already there. But if a 90-year-old patient presents with unstable angina and none of the other risk factors, that’s a score of 3 points, with an estimated probability of death at 30 days of only 7%, he noted.
Dr. Cockburn stressed that this risk score should not be used to base decisions on whether to take very elderly patients to the cardiac catheterization laboratory. “It enables you to have a useful conversation with relatives in which you can explain that this is a very high-risk intervention, or perhaps a low-risk intervention,” according to the cardiologist.
Discussants were emphatic in their agreement with Dr. Cockburn that this risk score shouldn’t be utilized to decide who does or doesn’t get PCI. One panelist said that what’s really lacking now in clinical practice – and where that huge British registry database could be helpful – is a scoring system that would predict which patients who don’t present in cardiogenic shock are going to develop it post PCI.
Dr. Cockburn reported having no relevant financial conflicts.
PARIS – A fast and simple clinically based scoring system enables physicians to determine the chance of a successful outcome for octogenarians undergoing percutaneous coronary intervention, James Cockburn, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
All six elements of the scoring system are readily available either at the time the very elderly patient presents or at diagnostic angiography, noted Dr. Cockburn of Brighton and Sussex University Hospital in Brighton, England.
He and his coworkers developed the risk score through analysis of a registry of 44,221 patients aged 80 or older who underwent percutaneous coronary intervention (PCI). The procedural success rate – defined as less than a 30% residual stenosis and TIMI (Thrombolysis in Myocardial Infarction) 3 antegrade blood flow – was 92.3%. The 30-day mortality rate was 3.9%. The investigators teased out a set of easily accessible clinical factors associated with 30-day mortality and came up with a novel risk scoring system using a 9-point scale.
The clinical factors and scoring system are as follows:
• Age. 1 point for being 80-89, and 2 points at age 90 or older.
• Indication for PCI. 1 point for unstable angina/non–ST segment elevation MI, 2 points for STEMI, 0 points for other indications.
• Ventilated preprocedure. 1 point if yes.
• Creatinine level above 200 umol/L. 1 point for yes.
• Preprocedural cardiogenic shock. 2 points for yes.
• Poor left ventricular ejection fraction. If less than 30%, 1 point.

Thus, scores can range from 1 to 9. Dr. Cockburn and his coworkers calculated the risk of 30-day mortality for each possible score. They validated the score by performing a receiver operator curve analysis that showed an area under the curve of 0.83, suggestive of relatively high sensitivity and specificity.
A score of 4 or less suggests a very good chance of survival at 30 days. In contrast, a score of 6 was associated with a two in three chance of death by 30 days. And it’s not hard to reach a 6: A patient who is 90 years old (2 points), presents with STEMI (2 points), and is in cardiogenic shock (2 points) is already there. But if a 90-year-old patient presents with unstable angina and none of the other risk factors, that’s a score of 3 points, with an estimated probability of death at 30 days of only 7%, he noted.
Dr. Cockburn stressed that this risk score should not be used to base decisions on whether to take very elderly patients to the cardiac catheterization laboratory. “It enables you to have a useful conversation with relatives in which you can explain that this is a very high-risk intervention, or perhaps a low-risk intervention,” according to the cardiologist.
Discussants were emphatic in their agreement with Dr. Cockburn that this risk score shouldn’t be utilized to decide who does or doesn’t get PCI. One panelist said that what’s really lacking now in clinical practice – and where that huge British registry database could be helpful – is a scoring system that would predict which patients who don’t present in cardiogenic shock are going to develop it post PCI.
Dr. Cockburn reported having no relevant financial conflicts.
PARIS – A fast and simple clinically based scoring system enables physicians to determine the chance of a successful outcome for octogenarians undergoing percutaneous coronary intervention, James Cockburn, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
All six elements of the scoring system are readily available either at the time the very elderly patient presents or at diagnostic angiography, noted Dr. Cockburn of Brighton and Sussex University Hospital in Brighton, England.
He and his coworkers developed the risk score through analysis of a registry of 44,221 patients aged 80 or older who underwent percutaneous coronary intervention (PCI). The procedural success rate – defined as less than a 30% residual stenosis and TIMI (Thrombolysis in Myocardial Infarction) 3 antegrade blood flow – was 92.3%. The 30-day mortality rate was 3.9%. The investigators teased out a set of easily accessible clinical factors associated with 30-day mortality and came up with a novel risk scoring system using a 9-point scale.
The clinical factors and scoring system are as follows:
• Age. 1 point for being 80-89, and 2 points at age 90 or older.
• Indication for PCI. 1 point for unstable angina/non–ST segment elevation MI, 2 points for STEMI, 0 points for other indications.
• Ventilated preprocedure. 1 point if yes.
• Creatinine level above 200 umol/L. 1 point for yes.
• Preprocedural cardiogenic shock. 2 points for yes.
• Poor left ventricular ejection fraction. If less than 30%, 1 point.

Thus, scores can range from 1 to 9. Dr. Cockburn and his coworkers calculated the risk of 30-day mortality for each possible score. They validated the score by performing a receiver operator curve analysis that showed an area under the curve of 0.83, suggestive of relatively high sensitivity and specificity.
A score of 4 or less suggests a very good chance of survival at 30 days. In contrast, a score of 6 was associated with a two in three chance of death by 30 days. And it’s not hard to reach a 6: A patient who is 90 years old (2 points), presents with STEMI (2 points), and is in cardiogenic shock (2 points) is already there. But if a 90-year-old patient presents with unstable angina and none of the other risk factors, that’s a score of 3 points, with an estimated probability of death at 30 days of only 7%, he noted.
Dr. Cockburn stressed that this risk score should not be used to base decisions on whether to take very elderly patients to the cardiac catheterization laboratory. “It enables you to have a useful conversation with relatives in which you can explain that this is a very high-risk intervention, or perhaps a low-risk intervention,” according to the cardiologist.
Discussants were emphatic in their agreement with Dr. Cockburn that this risk score shouldn’t be utilized to decide who does or doesn’t get PCI. One panelist said that what’s really lacking now in clinical practice – and where that huge British registry database could be helpful – is a scoring system that would predict which patients who don’t present in cardiogenic shock are going to develop it post PCI.
Dr. Cockburn reported having no relevant financial conflicts.
AT EUROPCR 2016
Key clinical point: Six readily obtainable clinical factors can be added up to allow accurate estimates of 30-day mortality risk after PCI.
Major finding: Very elderly patients with a score of 3 out of a possible 9 had an estimated 30-day mortality risk of 7%, while at a score of 5, the risk jumped to 40%, and at 6 to 66%.
Data source: This novel 30-day risk scoring system for octogenarians undergoing PCI was derived from a registry of 44,221 such patients.
Disclosures: The presenter reported having no relevant financial conflicts.
Below-knee angioplasty for limb salvage: Keep it simple
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
AT EUROPCR 2016
Key clinical point: Experts agree that plain balloon angioplasty is the way to go for limb salvage in patients with critical limb ischemia.
Major finding: The amputation-free survival rate in a consecutive series of patients who underwent below-the-knee angioplasty for critical limb ischemia was 88% at a mean of 15.1 months of follow-up.
Data source: A retrospective case series comprising 82 consecutive patients.
Disclosures: The presenter reported having no financial conflicts.
Permanent pacemaker in TAVR: Earlier implantation costs much less
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
AT EUROPCR 2016
Key clinical point: The incremental cost of permanent pacemaker implantation more than 24 hours after transcatheter aortic valve replacement is almost twice as great as if the pacemaker goes in within 24 hours.
Major finding: The mean cost of hospitalization for transcatheter aortic valve replacement without permanent pacemaker implication in Medicare patients in 2014 was $63,136, compared with $80,441 if they needed a pacemaker.
Data source: This was a retrospective study of the health care costs and lengths of stay for all 12,148 hospitalizations for transcatheter aortic valve replacement in the Medicare inpatient database for 2014.
Disclosures: The presenter is an employee of Edwards Lifesciences, which funded the study.
Minimalist TAVR without on-site surgery under study
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.
The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.

The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
PARIS – The buzzword in transcatheter aortic valve replacement today is “minimalist.” The search is on for ways in which to safely simplify the procedure to reduce the current unsustainably high cost and improve the patient experience.
Among the elements typically involved in minimalist TAVR are performance of the procedure in the cardiac catheterization laboratory via transfemoral access rather than in the costlier operating room, use of conscious sedation rather than general anesthesia, transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients. But these are tepid measures compared with what’s under study in Germany.
The German health care system is engaged in a study of what has to be the ultimate in minimalist TAVR: doing it in hospitals without on-site cardiac surgery. And the short-term results in more than 1,300 German patients treated in such a setting look every bit as good as in patients whose procedure took place in hospitals more conventionally equipped with both cardiology and cardiothoracic surgery departments, Holger Eggebrecht, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Lack of a cardiac surgery department on site should not be regarded as a contraindication for TAVR,” concluded Dr. Eggebrecht of Agaplesion Bethanien Hospital in Frankfurt, Germany.
Of course, it is deemed an absolute contraindication to TAVR both in the current European Society of Cardiology and U.S. guidelines. But that position was developed in an earlier era when procedural safety had not yet been established. It was based on the expert consensus opinion of physicians drawn mostly from large tertiary centers with both cardiology and cardiac surgery on site. And this absolute contraindication was never supported by any data, he said.
In the United States, arguably the most litigious nation in the world, it’s virtually unthinkable – at least for now – to perform TAVR without a cardiothoracic surgeon on site in the event bailout emergency cardiac surgery should become necessary. But Germany, which boasts universal health care coverage at an affordable cost, operates differently. Indeed, in Dr. Eggebrecht’s study of all 17,919 transfemoral TAVR procedures performed in Germany during 2013 and 2014, fully 22 of the 77 hospitals where the procedures took place had no on-site cardiac surgery department.
He presented a comparison of in-hospital outcomes in the 1,332 German patients (7.4%) whose TAVR took place in hospitals without a cardiac surgery department and the 16,587 patients treated in hospitals with both cardiology and cardiac surgery departments. The data came from the prospective German Quality Assurance Registry on Aortic Valve Replacement, which records in extensive detail all TAVR and surgical replacements in the country. Participation in the registry is mandatory.
The main study finding: Even though patients at no–cardiac surgery hospitals were older, had more comorbid conditions, and were at higher predicted perioperative risk of mortality, the rates of intraprocedural complications, in-hospital strokes, and mortality were similar in the two groups.
Moreover, when Dr. Eggebrecht and coinvestigators performed a case-control substudy involving 555 patient pairs extensively matched on more than a dozen variables, including a requirement for identical scores on validated risk prediction tools for estimating in-hospital mortality, the results were the same as in the full study population.

The key to the high-quality TAVR outcomes documented at hospitals without a cardiac surgery department, Dr. Eggebrecht emphasized, is that in Germany TAVR can be performed only at hospitals where a contractually obligated heart team has signed off on the procedure. At hospitals without a cardiac surgery department, this heart team is composed of in-house cardiologists and visiting cardiac surgeons from collaborating hospitals. In TAVRs at some of these hospitals, a collaborating cardiac surgeon is present for the procedure and brings along a heart-lung machine which is primed and ready to go, if needed, as was the case in just 0.4% of the 1,332 TAVRs. At others, the surgeon is off site.
“I would think our data show that close cooperation within the heart team is the key to successful outcomes, not having a cardiac surgeon on site,” he concluded.
Audience member Volkmar Falk, MD, strode briskly to a microphone and made no effort to hide his incredulity at this project.
“What is the real advantage in not having a surgeon present? I don’t get it,” declared Dr. Falk, professor and director of the department of cardiovascular and vascular surgery at Charité University Hospital in Berlin.
“For a surgeon, this is all quite difficult to understand,” he continued. “If a surgeon has to come to a TAVR rescue from 10 km away, I don’t know how well that works. And if surgeons have to travel with all their equipment in order to be on site, I think this is a logistical nightmare and shouldn’t be done at all.
“All of the studies, all the clinical trials we always discuss, have been done in the setting of hospitals where the procedure was done together with a surgeon on site. That’s why we have these excellent results,” Dr. Falk added.
Dr. Eggebrecht replied, “We’re having a scientific discussion here, and I think our data clearly show that you can construct a successful heart team even though you don’t have a cardiac surgery department on site.”
An Israeli cardiologist in the audience said a similar effort is underway in his country to open up TAVR to hospitals without an on-site cardiac surgeon. His objection is that, at least in Israel, a hospital with no on-site cardiac surgery is a marker for a TAVR center that is low volume, is late to embrace TAVR, and has cardiologists who are probably still early on the procedural learning curve.
Dr. Eggebrecht said that this is not the case in Germany, where some of the most experienced TAVR operators work at sites without a cardiac surgery department.
He reported that his study was partially funded by the German Cardiac Society, and he had no financial conflicts.
Simultaneously with his presentation, the study was published online (Eur Heart J. 2016 May 17. pii: ehw190).
AT EUROPCR 2016
Key clinical point: TAVR can be performed in hospitals safely without a cardiac surgery department.
Major finding: In-hospital mortality occurred in 3.8% of patients who underwent TAVR at hospitals without on-site cardiac surgery and in 4.2% of patients whose procedure was done at hospitals with a cardiac surgery department.
Data source: An analysis of in-hospital outcomes of all 17,919 patients who underwent TAVR in Germany during 2013 and 2014, including 1,332 whose procedures took place at one of the 22 hospitals with no on-site cardiac surgery department.
Disclosures: The study was partially funded by the German Cardiac Society. The presenter reported having no financial conflicts.
IVUS has role for annular sizing in TAVR
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
PARIS – Intravascular ultrasound can reliably be used in lieu of multidetector computerized tomography for the key task of annular sizing in patients undergoing transcatheter aortic valve replacement, Dr. Diaa Hakim declared at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Multidetector CT (MDCT) is considered the standard imaging method for this purpose. But the requirement for contrast media makes MDCT problematic for patients with chronic kidney disease, who can easily be driven into acute kidney injury through exposure to this material.
Moreover, renal failure is common among patients with a failing native aortic valve. Interventionalists who perform transaortic valve replacement (TAVR) are encountering renal failure more and more frequently as the nonsurgical treatment takes off in popularity. An alternative imaging method is sorely needed, observed Dr. Hakim of the University of Alabama at Birmingham.
Unlike MDCT, intravascular ultrasound (IVUS) doesn’t require contrast. And in Dr. Hakim’s head-to-head comparative trial conducted in 50 consecutive TAVR patients who underwent annular sizing by both methods, there were no significant differences between the two in measurements of maximum and minimum annular diameter, mean annular diameter, or annular area.
The decision as to the size of the replacement aortic valve was based upon MDCT, which was performed first. Then came IVUS carried out with a Boston Science Atlantis PV Peripheral IVUS catheter at 8-French and 15 Hz. The catheter was advanced over the guidewire, then pullback imaging was obtained automatically from the left ventricular outflow tract to the aortic root. The IVUS measurements were made at the level of basal attachment of the aortic valve cusps, which was quite close to the same point as the MDCT measurements.
Post TAVR, 37 of the 50 patients had no or trivial paravalvular regurgitation. Six patients developed acute kidney injury.
Asked if he believes IVUS now enables operators to routinely skip MDCT for TAVR patients, Dr. Hakim replied, “Not for the moment.” In patients with chronic kidney disease, yes, but in order for IVUS for annular sizing to expand beyond that population it will be necessary for device makers to develop an IVUS catheter with better visualization, a device designed specifically to see all the details of the aortic valve and annulus. He noted that the Atlantis PV Peripheral IVUS catheter employed in his study was designed for the aorta, not the aortic valve. It doesn’t provide optimal imaging of the valve cusps, nor can it measure paravalvular regurgitation after valve implantation.
How much time does IVUS for annular sizing add to the TAVR procedure? “Five minutes, no more,” according to Dr. Hakim.
He reported having no financial conflicts regarding this study, conducted free of commercial support.
AT EUROPCR 2016
Key clinical point: Intravascular ultrasound is a reliable alternative to multidetector CT for annular sizing in TAVR patients with chronic kidney disease for whom contrast media could be a problem.
Major finding: Measurements of aortic annulus maximum and minimum diameter, mean annular diameter, and annular area didn’t differ significantly whether measured by multidetector CT or contrast-free intravascular ultrasound.
Data source: This head-to-head study included 50 consecutive TAVR patients who underwent annular sizing by both CT and intravascular ultrasound.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.






















