User login
FDA Advisers Endorse Subcutaneous ICD
GAITHERSBURG, MD. – The results of a clinical trial of a cardioverter defibrillator that is implanted subcutaneously and does not require transvenous leads support the approval of the device as a treatment for ventricular tachyarrhythmias, the majority of a Food and Drug Administration Advisory panel agreed at a meeting on April 26.
The FDA’s Circulatory System Devices Panel voted 7-1 that the benefits of the subcutaneous implantable cardioverter defibrillator (S-ICD) system outweighed its risks in patients who meet the criteria in the indication proposed by the manufacturer, Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
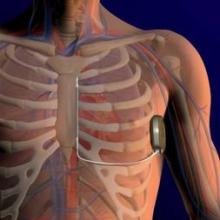
The components of the S-ICD system include a pulse generator, which is implanted subcutaneously in the lateral thoracic region and is connected to an electrode, with a defibrillation coil that is subcutaneously implanted along the rib margin to the sternum, without radiographic guidance, according to the company. The device has the capacity to deliver more than 100 shocks; with typical usage, it is expected to last a little more than 5 years.
Although they agreed that further follow-up of the safety and effectiveness of the device was needed and pointed out the importance of proper physician training and patient selection, the panel unanimously voted that there was "reasonable assurance" that the device was safe, and voted 7-1 that there was "reasonable assurance" that it was effective for this group of patients.
Panelists voting in favor of the risk-benefit profile said that the S-ICD would be a useful addition to the transvenous ICDs that are currently available. They agreed that the inappropriate shock rate in the clinical trial was comparable to the rates seen with currently available transvenous ICDs.
"This device is a substantial additional adjunctive tool for electrophysiologists to manage patients who are at high risk for sudden cardiac death," said Dr. Ralph Brindis, senior advisor for cardiovascular disease at Kaiser Permanente in Oakland, Calif. The availability of a device "in particular to manage patients who have infections of transvenous systems is a very important adjunct in the armamentarium." he added.
The dissenting panelist, Dr. David Milan, an electrophysiologist at Massachusetts General Hospital, Boston, said that it was important to balance enthusiasm for the device with "a cautious approach," noting that the standard for efficacy was quite high for the transvenous devices.
His concerns included whether the clinical effectiveness of the device was up to par with currently available devices, which he noted could not be assuaged by the number of patients and relatively short follow-up of patients in the pivotal trial.
The primary and safety effectiveness end points were met in the pivotal study, a prospective single arm study of 330 people with a class I, IIa, or IIb ICD indication, including patients who needed a replacement ICD, conducted in the United States, Europe, and New Zealand from January 2010 through May 2011, to evaluate the device in treating life-threatening ventricular arrhythmias. Their mean age was 52 years, which was younger than other contemporary ICD studies, but 60% were older than 50 years. In all, 74% were male; 65% were white and 24% were black; about 85% were on a beta-blocker; and about 8% were on antiarrhythmic drugs. More than half (61%) had heart failure, predominantly NYHA class II.
Among the 304 evaluable patients, the device was 98.8% effective in converting acute induced ventricular fibrillation, with 95% of the events treated in less than 21 seconds and 88% in less than 18 seconds. During the study, there were 109 spontaneous episodes of VT/VF. Of the 68 for which evaluable recorded data were available, all were successfully converted with the S-ICD, except for one case that terminated spontaneously and another case of VT/VF storm that required an external shock, according to the FDA. There were about 18 infections, of which 5 required surgical treatment, including four explants. The device was explanted in another seven patients for reasons that included repositioning, and a depleted battery. None of the eight deaths in the study was attributed to the device.
The S-ICD is distributed in 10 other countries, and so far, more than 1,200 patients have been implanted worldwide, according to Cameron Health.
The company has proposed a postmarketing study, a prospective multicenter observational registry to evaluate the long-term safety of the device.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
GAITHERSBURG, MD. – The results of a clinical trial of a cardioverter defibrillator that is implanted subcutaneously and does not require transvenous leads support the approval of the device as a treatment for ventricular tachyarrhythmias, the majority of a Food and Drug Administration Advisory panel agreed at a meeting on April 26.
The FDA’s Circulatory System Devices Panel voted 7-1 that the benefits of the subcutaneous implantable cardioverter defibrillator (S-ICD) system outweighed its risks in patients who meet the criteria in the indication proposed by the manufacturer, Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
The components of the S-ICD system include a pulse generator, which is implanted subcutaneously in the lateral thoracic region and is connected to an electrode, with a defibrillation coil that is subcutaneously implanted along the rib margin to the sternum, without radiographic guidance, according to the company. The device has the capacity to deliver more than 100 shocks; with typical usage, it is expected to last a little more than 5 years.
Although they agreed that further follow-up of the safety and effectiveness of the device was needed and pointed out the importance of proper physician training and patient selection, the panel unanimously voted that there was "reasonable assurance" that the device was safe, and voted 7-1 that there was "reasonable assurance" that it was effective for this group of patients.
Panelists voting in favor of the risk-benefit profile said that the S-ICD would be a useful addition to the transvenous ICDs that are currently available. They agreed that the inappropriate shock rate in the clinical trial was comparable to the rates seen with currently available transvenous ICDs.
"This device is a substantial additional adjunctive tool for electrophysiologists to manage patients who are at high risk for sudden cardiac death," said Dr. Ralph Brindis, senior advisor for cardiovascular disease at Kaiser Permanente in Oakland, Calif. The availability of a device "in particular to manage patients who have infections of transvenous systems is a very important adjunct in the armamentarium." he added.
The dissenting panelist, Dr. David Milan, an electrophysiologist at Massachusetts General Hospital, Boston, said that it was important to balance enthusiasm for the device with "a cautious approach," noting that the standard for efficacy was quite high for the transvenous devices.
His concerns included whether the clinical effectiveness of the device was up to par with currently available devices, which he noted could not be assuaged by the number of patients and relatively short follow-up of patients in the pivotal trial.
The primary and safety effectiveness end points were met in the pivotal study, a prospective single arm study of 330 people with a class I, IIa, or IIb ICD indication, including patients who needed a replacement ICD, conducted in the United States, Europe, and New Zealand from January 2010 through May 2011, to evaluate the device in treating life-threatening ventricular arrhythmias. Their mean age was 52 years, which was younger than other contemporary ICD studies, but 60% were older than 50 years. In all, 74% were male; 65% were white and 24% were black; about 85% were on a beta-blocker; and about 8% were on antiarrhythmic drugs. More than half (61%) had heart failure, predominantly NYHA class II.
Among the 304 evaluable patients, the device was 98.8% effective in converting acute induced ventricular fibrillation, with 95% of the events treated in less than 21 seconds and 88% in less than 18 seconds. During the study, there were 109 spontaneous episodes of VT/VF. Of the 68 for which evaluable recorded data were available, all were successfully converted with the S-ICD, except for one case that terminated spontaneously and another case of VT/VF storm that required an external shock, according to the FDA. There were about 18 infections, of which 5 required surgical treatment, including four explants. The device was explanted in another seven patients for reasons that included repositioning, and a depleted battery. None of the eight deaths in the study was attributed to the device.
The S-ICD is distributed in 10 other countries, and so far, more than 1,200 patients have been implanted worldwide, according to Cameron Health.
The company has proposed a postmarketing study, a prospective multicenter observational registry to evaluate the long-term safety of the device.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
GAITHERSBURG, MD. – The results of a clinical trial of a cardioverter defibrillator that is implanted subcutaneously and does not require transvenous leads support the approval of the device as a treatment for ventricular tachyarrhythmias, the majority of a Food and Drug Administration Advisory panel agreed at a meeting on April 26.
The FDA’s Circulatory System Devices Panel voted 7-1 that the benefits of the subcutaneous implantable cardioverter defibrillator (S-ICD) system outweighed its risks in patients who meet the criteria in the indication proposed by the manufacturer, Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
The components of the S-ICD system include a pulse generator, which is implanted subcutaneously in the lateral thoracic region and is connected to an electrode, with a defibrillation coil that is subcutaneously implanted along the rib margin to the sternum, without radiographic guidance, according to the company. The device has the capacity to deliver more than 100 shocks; with typical usage, it is expected to last a little more than 5 years.
Although they agreed that further follow-up of the safety and effectiveness of the device was needed and pointed out the importance of proper physician training and patient selection, the panel unanimously voted that there was "reasonable assurance" that the device was safe, and voted 7-1 that there was "reasonable assurance" that it was effective for this group of patients.
Panelists voting in favor of the risk-benefit profile said that the S-ICD would be a useful addition to the transvenous ICDs that are currently available. They agreed that the inappropriate shock rate in the clinical trial was comparable to the rates seen with currently available transvenous ICDs.
"This device is a substantial additional adjunctive tool for electrophysiologists to manage patients who are at high risk for sudden cardiac death," said Dr. Ralph Brindis, senior advisor for cardiovascular disease at Kaiser Permanente in Oakland, Calif. The availability of a device "in particular to manage patients who have infections of transvenous systems is a very important adjunct in the armamentarium." he added.
The dissenting panelist, Dr. David Milan, an electrophysiologist at Massachusetts General Hospital, Boston, said that it was important to balance enthusiasm for the device with "a cautious approach," noting that the standard for efficacy was quite high for the transvenous devices.
His concerns included whether the clinical effectiveness of the device was up to par with currently available devices, which he noted could not be assuaged by the number of patients and relatively short follow-up of patients in the pivotal trial.
The primary and safety effectiveness end points were met in the pivotal study, a prospective single arm study of 330 people with a class I, IIa, or IIb ICD indication, including patients who needed a replacement ICD, conducted in the United States, Europe, and New Zealand from January 2010 through May 2011, to evaluate the device in treating life-threatening ventricular arrhythmias. Their mean age was 52 years, which was younger than other contemporary ICD studies, but 60% were older than 50 years. In all, 74% were male; 65% were white and 24% were black; about 85% were on a beta-blocker; and about 8% were on antiarrhythmic drugs. More than half (61%) had heart failure, predominantly NYHA class II.
Among the 304 evaluable patients, the device was 98.8% effective in converting acute induced ventricular fibrillation, with 95% of the events treated in less than 21 seconds and 88% in less than 18 seconds. During the study, there were 109 spontaneous episodes of VT/VF. Of the 68 for which evaluable recorded data were available, all were successfully converted with the S-ICD, except for one case that terminated spontaneously and another case of VT/VF storm that required an external shock, according to the FDA. There were about 18 infections, of which 5 required surgical treatment, including four explants. The device was explanted in another seven patients for reasons that included repositioning, and a depleted battery. None of the eight deaths in the study was attributed to the device.
The S-ICD is distributed in 10 other countries, and so far, more than 1,200 patients have been implanted worldwide, according to Cameron Health.
The company has proposed a postmarketing study, a prospective multicenter observational registry to evaluate the long-term safety of the device.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
FROM A MEETING OF THE FDA'S CIRCULATORY SYSTEM DEVICES PANEL