User login
National Kidney Foundation (NKF): Spring Clinical Meetings
SLGT2 Inhibitors Show Promise in Renal Protection
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
EXPERT ANALYSIS FROM SCM 14
SLGT2 inhibitors show promise in renal protection
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
LAS VEGAS – In the opinion of Dr. David Cherney, it’s not uncommon for physicians to feel grim about the limited efficacy of available agents to treat chronic kidney disease in patients with type 1 and type 2 diabetes.
Over a 10-year period, about 20% of patients with type I diabetes treated with angiotensin-converting enzyme (ACE) monotherapy still develop progression of their kidney disease, according to Dr. Cherney of the department of medicine at the University of Toronto.
"The diabetic nephroprotection area has been incredibly disappointing in the last 5-8 years," he said at a meeting sponsored by the National Kidney Foundation. "Patients tend to progress despite therapy or have side effects, no matter what we do. Endothelial antagonists cause edema. Protein kinase C beta-antagonists have little therapeutic effect, and antioxidants such as bardoxolone caused serious side effects. None of the drugs that we think will work, based on animal studies, seem to work very well when translated into humans."
Neurohormonal pathway blockade with an ACE inhibitor or an angiotensin II receptor blocker corrects early hemodynamic abnormalities in type 1 diabetes, he noted. "But the effects are partial. Dual, renin-angiotensin-aldosterone system blockade is associated with risk. When we use dual blockade in patients with type 1 diabetes, we’re not able to reduce hyperfiltration. The development of novel and safe renal protective therapies is critical."
Enter sodium-glucose cotransporter 2 (SGLT2) inhibitors such as empagliflozin and canagliflozin, which over the past decade have demonstrated positive endocrine effects in type 2 diabetes. According to Dr. Cherney, they lower hemoglobin A1c by about 1% and lead to 2-4 kg of weight loss.
"That’s fantastic, compared with other agents such as insulin that cause weight gain," he said. "That weight loss is sustained for up to 2 years. These agents also seem to have a very low risk of hypoglycemia, and they also lower blood pressure."
Animal models of diabetes have used SGLT2 inhibitors such as empagliflozin (developed by Boehringer Ingelheim and Eli Lilly) to demonstrate a decline in hyperfiltration, a decrease in proteinuria, and a decrease in the structural evidence of nephropathy on histology. An SGLT2 inhibitor might offer renal protection in humans with diabetes by blocking sodium reabsorption proximally, restoring distal delivery, and normalizing afferent tone and glomerular filtration rate (GFR), Dr. Cherney said.
He and his associates conducted an open-label, pilot trial of empagliflozin in patients with type 1 diabetes, known as the ATIRMA study. The objectives were to assess the impact of empagliflozin on GFR under controlled conditions of euglycemia and hyperglycemia in type 1 diabetes patients with and without hyperfiltration, as well as to examine its safety and efficacy as an add-on therapy to insulin (Circulation 2014;129:587-97).
"We hypothesized that we would reduce hyperfiltration and improve glycemic parameters in type 1 diabetes," Dr. Cherney said. In all, 40 patients with a mean age of 25 years were screened and underwent a 2-week run-in period, then took empagliflozin 25 mg once daily for 8 weeks. There were 13 patients in the normofiltration group and 27 patients in the hyperfiltration group.
At the end of 8 weeks, patients in the hyperfiltration group had a dramatic increase in 24-hour urinary glucose excretion, to 140 g/day, "which is about twice what you see in the type 2 studies," he said. "They had very dramatic tubular flow response."
Patients in the hyperfiltration group also had a 20% reduction in their hyperfiltration, a fall in hyperperfusion, an increase in renal vascular resistance, and a small reduction in blood pressure. The researchers also observed a 0.4% reduction in HbA1c, "which was highly statistically significant," Dr. Cherney said. "We also saw that weight and waist circumference decreased, fasting capillary glucose improved significantly, and their insulin doses decreased. As a consequence, they had less hypoglycemia."
How does this compare to currently used agents? ACE inhibitors reduce hyperfiltration by about 19.7%, Dr. Cheney said, while empagliflozin decreased hyperfiltration by 19.2% – almost an identical effect.
"But remember, ACE inhibitors are associated with hyperkalemia, acute kidney injury, and other potential side effects," he noted. SGLT2 inhibitors "don’t cause hyperkalemia. They don’t cause acute kidney injury, as far as we know. And as far as we know, they don’t have any important interactions with other RAAS inhibitors."
Dr. Cherney characterized the findings from ATIRMA as "promising first steps.
"We’ve been tricked many times before with other drugs," he noted. "We can’t by any means say that this is conclusive, but we need to have long-term renal protection studies to prove their relevance. I think we can clearly say that renal studies are warranted in type 1 and type 2 diabetes."
Research is also needed to assess the interaction between SGLT2 inhibitors and other RAAS inhibitors, Dr. Cherney added, as well as how they provide primary and secondary preventive therapy in type 1 and type 2 diabetes.
Dr. Cherney disclosed that he has received research funding, consulting fees, and speaker honoraria from Boehringer Ingelheim. He has also received research funding from Astellas, consulting fees from Janssen and Astellas, and speaker honoraria from Janssen.
EXPERT ANALYSIS FROM SCM 14
Obese Teens Heading for Bariatric Surgery Already Show Kidney Damage
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
AT SCM 14
Obese teens heading for bariatric surgery already show kidney damage
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
LAS VEGAS – Seventeen percent of severely obese adolescents slated for bariatric surgery in the Teen-Longitudinal Assessment of Bariatric Surgery study already had micro- or macroalbuminuria.
At a meeting sponsored by the National Kidney Foundation, Dr. Nianzhou Xiao said that future reports from the ongoing Teen-LABS study will provide the answer to a critical question: Is this worrisome loss of kidney function so early in life reversible via surgical weight loss?
Dr. Xiao presented a cross-sectional baseline report on 242 severely obese adolescents with a median body mass index of 50.5 kg/m2. Fourteen percent had microalbuminuria and another 3.1% had macroalbuminuria. Although the group’s mean estimated glomerular filtration rate was 107.6 mL/min per 1.73 m2, 3% of the teens had an eGFR below 60 mL/min 1.73 m2, which is the definition of stage 3 chronic kidney disease.
In addition, 45% of the teens were hypertensive before surgery, 74% were dyslipidemic, and 13.6% had diabetes. The group’s median serum ferritin was 37 mcg/L, noted Dr. Xiao of Cincinnati Children’s Hospital Medical Center.
Multivariate analysis identified two independent risk factors for an elevated albumin to creatinine ratio: female gender, with an associated 2.34-fold increased risk, and elevated serum ferritin. For every 10 mcg/L of ferritin, the likelihood of an elevated albumin-to-creatinine ratio rose by 7%.
An estimated 4%-6% of U.S. children and adolescents are severely obese, defined as a body mass index of 35 kg/m2 or more or a body weight above the 120th percentile. The ongoing Teen-LABS study is the most comprehensive examination of kidney status in severely obese adolescents undergoing bariatric surgery.
Teen-LABS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Xiao reported having no financial conflicts.
AT SCM 14
Key clinical point: Follow-up will determine if baseline abnormalities in obese teens’ kidney function and other areas are reversible through weight-loss surgery.
Major finding: 14% of a large group of severely obese adolescents scheduled for bariatric surgery already had microalbuminuria and another 3.1% had macroalbuminuria. Three percent had stage 3 chronic kidney disease.
Data source: A cross-sectional analysis involving 242 severely obese adolescents in the ongoing Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study.
Disclosures: The ongoing study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
CKD guidelines elusive to many PCPs
LAS VEGAS – Slightly more than half of primary care physicians reported being unfamiliar with 14-year-old guidelines on nondiabetic chronic kidney disease, but 75% said they were willing to improve how they cared for patients with the condition, results from a Web-based survey showed.
Most non–dialysis-dependent chronic kidney disease (CKD) patients are cared for by their primary care physicians (PCPs). Studies suggest many CKD patients receive suboptimal care. Recently, CKD clinical practice guidelines were updated with additional emphasis on albuminuria, according to a report published in advance of its presentation by Dr. Khaled Abdel-Kader at a meeting sponsored by the National Kidney Foundation.
"Rigorous studies are needed to help identify systematic interventions that can overcome identified barriers and improve optimal CKD care delivery," Dr. Abdel-Kader said in an interview following his presentation. "Based on our findings, while overcoming knowledge deficits and attitudinal barriers remains important, many primary care physicians set appropriate care goals in CKD, but systems are needed to help them and their patients achieve these goals," (BMC Nephrol. 2014 April 22 [doi:10.1186/1471-2369-15-64]).
Dr. Neil Skolnik noted that CKD is an incredibly important topic, affecting more than 15% of the U.S. population. "This is an excellent study with a very good response rate for an e-mail survey, indicating that primary care physicians are interested in the care of patients with CKD.
"This interest is reflected in the answers provided on the survey. With half of primary care physicians unfamiliar with the CKD guidelines, and 75% expressing interest in improving their care of patients with CKD, the next step should focus on how to best disseminate information about the guidelines and provide tools for primary care physicians to best implement the guidelines," said Dr. Skolnik, who is professor of family and community medicine at Temple University, Philadelphia.
Dr. Abdel-Kader and his associates used data from the American Medical Association to conduct a cross-sectional, Web-based survey of PCPs in the United States in an effort to explore their understanding of CKD guidelines, self-reported practice behaviors, and barriers to implementing guideline recommendations, including albuminuria testing. The National Kidney Foundation issued its most recent update to its guidelines in 2000.
Of the 848 PCPs who opened an e-mail about the study, 165 (19.5%) responded. The majority of respondents (88%) spent at least half of their time in clinical care, and 46% were in private practice, said Dr. Abdel-Kader of the department of medicine at Vanderbilt University in Nashville, Tenn.
Nearly all respondents (96%) felt that glomerular filtration rate (GFR) values were helpful, while 75% and 91% reported testing for albuminuria in nondiabetic hypertensive patients with an estimated GFR (eGFR) of greater than 60 mL/min per 1.73 m2 or less than 60 mL/min per 1.73 m2, respectively. "However, frequent barriers cited included a lack of effect on management, limited time, and the perceived absence of guidelines recommending albuminuria testing," Dr. Abdel-Kader said.
"While PCPs expressed very high agreement with the definition of CKD in patients with marked decrements in eGFR (eGFR less than 45 mL/min per 1.73 m2) or decrements in eGFR coupled with albuminuria, agreement was less robust when eGFR was greater than 60 mL/min per 1.73 m2 or in CKD stage 3a without albuminuria."
Most respondents (an average of 78%) felt that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers improved outcomes in patients with CKD, yet agreement was lower with severe vs. moderate albuminuria (78% vs. 85%, respectively; P = .03).
Slightly more than half of primary care physicians (51%) reported being unfamiliar with chronic kidney disease guidelines, yet 75% were receptive to systematic interventions in addition to CME to improve their care of CKD patients. "The importance of albuminuria in CKD has become a point of emphasis in guidelines relatively recently, and its value in CKD may not be clearly understood by many PCPs," Dr. Abdel-Kader said. "This is likely contributing to suboptimal targeting of CKD treatments in high-risk and low-risk patients. Working with PCPs to develop systematic interventions that help streamline and improve CKD care without disrupting work flow may have significant potential to improve CKD patient care."
The survey did have certain limitations, including its low response rate, he said. "Our respondents were younger and more likely to be internists than the PCPs we targeted," he noted. "Prior studies have shown that these characteristics tend to associate with greater familiarity with CKD guidelines and recommendations. Hence, recognition of CKD and guideline familiarity may be lower in the general PCP community than we document in our survey."
The study was supported by the National Institutes of Health. Dr. Abdel-Kader said he had no relevant financial conflicts to disclose.
LAS VEGAS – Slightly more than half of primary care physicians reported being unfamiliar with 14-year-old guidelines on nondiabetic chronic kidney disease, but 75% said they were willing to improve how they cared for patients with the condition, results from a Web-based survey showed.
Most non–dialysis-dependent chronic kidney disease (CKD) patients are cared for by their primary care physicians (PCPs). Studies suggest many CKD patients receive suboptimal care. Recently, CKD clinical practice guidelines were updated with additional emphasis on albuminuria, according to a report published in advance of its presentation by Dr. Khaled Abdel-Kader at a meeting sponsored by the National Kidney Foundation.
"Rigorous studies are needed to help identify systematic interventions that can overcome identified barriers and improve optimal CKD care delivery," Dr. Abdel-Kader said in an interview following his presentation. "Based on our findings, while overcoming knowledge deficits and attitudinal barriers remains important, many primary care physicians set appropriate care goals in CKD, but systems are needed to help them and their patients achieve these goals," (BMC Nephrol. 2014 April 22 [doi:10.1186/1471-2369-15-64]).
Dr. Neil Skolnik noted that CKD is an incredibly important topic, affecting more than 15% of the U.S. population. "This is an excellent study with a very good response rate for an e-mail survey, indicating that primary care physicians are interested in the care of patients with CKD.
"This interest is reflected in the answers provided on the survey. With half of primary care physicians unfamiliar with the CKD guidelines, and 75% expressing interest in improving their care of patients with CKD, the next step should focus on how to best disseminate information about the guidelines and provide tools for primary care physicians to best implement the guidelines," said Dr. Skolnik, who is professor of family and community medicine at Temple University, Philadelphia.
Dr. Abdel-Kader and his associates used data from the American Medical Association to conduct a cross-sectional, Web-based survey of PCPs in the United States in an effort to explore their understanding of CKD guidelines, self-reported practice behaviors, and barriers to implementing guideline recommendations, including albuminuria testing. The National Kidney Foundation issued its most recent update to its guidelines in 2000.
Of the 848 PCPs who opened an e-mail about the study, 165 (19.5%) responded. The majority of respondents (88%) spent at least half of their time in clinical care, and 46% were in private practice, said Dr. Abdel-Kader of the department of medicine at Vanderbilt University in Nashville, Tenn.
Nearly all respondents (96%) felt that glomerular filtration rate (GFR) values were helpful, while 75% and 91% reported testing for albuminuria in nondiabetic hypertensive patients with an estimated GFR (eGFR) of greater than 60 mL/min per 1.73 m2 or less than 60 mL/min per 1.73 m2, respectively. "However, frequent barriers cited included a lack of effect on management, limited time, and the perceived absence of guidelines recommending albuminuria testing," Dr. Abdel-Kader said.
"While PCPs expressed very high agreement with the definition of CKD in patients with marked decrements in eGFR (eGFR less than 45 mL/min per 1.73 m2) or decrements in eGFR coupled with albuminuria, agreement was less robust when eGFR was greater than 60 mL/min per 1.73 m2 or in CKD stage 3a without albuminuria."
Most respondents (an average of 78%) felt that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers improved outcomes in patients with CKD, yet agreement was lower with severe vs. moderate albuminuria (78% vs. 85%, respectively; P = .03).
Slightly more than half of primary care physicians (51%) reported being unfamiliar with chronic kidney disease guidelines, yet 75% were receptive to systematic interventions in addition to CME to improve their care of CKD patients. "The importance of albuminuria in CKD has become a point of emphasis in guidelines relatively recently, and its value in CKD may not be clearly understood by many PCPs," Dr. Abdel-Kader said. "This is likely contributing to suboptimal targeting of CKD treatments in high-risk and low-risk patients. Working with PCPs to develop systematic interventions that help streamline and improve CKD care without disrupting work flow may have significant potential to improve CKD patient care."
The survey did have certain limitations, including its low response rate, he said. "Our respondents were younger and more likely to be internists than the PCPs we targeted," he noted. "Prior studies have shown that these characteristics tend to associate with greater familiarity with CKD guidelines and recommendations. Hence, recognition of CKD and guideline familiarity may be lower in the general PCP community than we document in our survey."
The study was supported by the National Institutes of Health. Dr. Abdel-Kader said he had no relevant financial conflicts to disclose.
LAS VEGAS – Slightly more than half of primary care physicians reported being unfamiliar with 14-year-old guidelines on nondiabetic chronic kidney disease, but 75% said they were willing to improve how they cared for patients with the condition, results from a Web-based survey showed.
Most non–dialysis-dependent chronic kidney disease (CKD) patients are cared for by their primary care physicians (PCPs). Studies suggest many CKD patients receive suboptimal care. Recently, CKD clinical practice guidelines were updated with additional emphasis on albuminuria, according to a report published in advance of its presentation by Dr. Khaled Abdel-Kader at a meeting sponsored by the National Kidney Foundation.
"Rigorous studies are needed to help identify systematic interventions that can overcome identified barriers and improve optimal CKD care delivery," Dr. Abdel-Kader said in an interview following his presentation. "Based on our findings, while overcoming knowledge deficits and attitudinal barriers remains important, many primary care physicians set appropriate care goals in CKD, but systems are needed to help them and their patients achieve these goals," (BMC Nephrol. 2014 April 22 [doi:10.1186/1471-2369-15-64]).
Dr. Neil Skolnik noted that CKD is an incredibly important topic, affecting more than 15% of the U.S. population. "This is an excellent study with a very good response rate for an e-mail survey, indicating that primary care physicians are interested in the care of patients with CKD.
"This interest is reflected in the answers provided on the survey. With half of primary care physicians unfamiliar with the CKD guidelines, and 75% expressing interest in improving their care of patients with CKD, the next step should focus on how to best disseminate information about the guidelines and provide tools for primary care physicians to best implement the guidelines," said Dr. Skolnik, who is professor of family and community medicine at Temple University, Philadelphia.
Dr. Abdel-Kader and his associates used data from the American Medical Association to conduct a cross-sectional, Web-based survey of PCPs in the United States in an effort to explore their understanding of CKD guidelines, self-reported practice behaviors, and barriers to implementing guideline recommendations, including albuminuria testing. The National Kidney Foundation issued its most recent update to its guidelines in 2000.
Of the 848 PCPs who opened an e-mail about the study, 165 (19.5%) responded. The majority of respondents (88%) spent at least half of their time in clinical care, and 46% were in private practice, said Dr. Abdel-Kader of the department of medicine at Vanderbilt University in Nashville, Tenn.
Nearly all respondents (96%) felt that glomerular filtration rate (GFR) values were helpful, while 75% and 91% reported testing for albuminuria in nondiabetic hypertensive patients with an estimated GFR (eGFR) of greater than 60 mL/min per 1.73 m2 or less than 60 mL/min per 1.73 m2, respectively. "However, frequent barriers cited included a lack of effect on management, limited time, and the perceived absence of guidelines recommending albuminuria testing," Dr. Abdel-Kader said.
"While PCPs expressed very high agreement with the definition of CKD in patients with marked decrements in eGFR (eGFR less than 45 mL/min per 1.73 m2) or decrements in eGFR coupled with albuminuria, agreement was less robust when eGFR was greater than 60 mL/min per 1.73 m2 or in CKD stage 3a without albuminuria."
Most respondents (an average of 78%) felt that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers improved outcomes in patients with CKD, yet agreement was lower with severe vs. moderate albuminuria (78% vs. 85%, respectively; P = .03).
Slightly more than half of primary care physicians (51%) reported being unfamiliar with chronic kidney disease guidelines, yet 75% were receptive to systematic interventions in addition to CME to improve their care of CKD patients. "The importance of albuminuria in CKD has become a point of emphasis in guidelines relatively recently, and its value in CKD may not be clearly understood by many PCPs," Dr. Abdel-Kader said. "This is likely contributing to suboptimal targeting of CKD treatments in high-risk and low-risk patients. Working with PCPs to develop systematic interventions that help streamline and improve CKD care without disrupting work flow may have significant potential to improve CKD patient care."
The survey did have certain limitations, including its low response rate, he said. "Our respondents were younger and more likely to be internists than the PCPs we targeted," he noted. "Prior studies have shown that these characteristics tend to associate with greater familiarity with CKD guidelines and recommendations. Hence, recognition of CKD and guideline familiarity may be lower in the general PCP community than we document in our survey."
The study was supported by the National Institutes of Health. Dr. Abdel-Kader said he had no relevant financial conflicts to disclose.
AT SCM 14
Major Finding: About half of primary care physicians (51%) reported being unfamiliar with chronic kidney disease guidelines, yet 75% were receptive to systematic interventions in addition to CME to improve their care of CKD patients.
Data Source: A cross-sectional, Web-based survey of 165 PCPs in the United States.
Disclosures: The study was supported by the National Institutes of Health. Dr. Abdel-Kader said he had no relevant financial conflicts to disclose.
Proteinuria often undertreated in hospital settings
LAS VEGAS – The majority of patients with proteinuria who are admitted to hospitals affiliated with academic medical centers are not placed on life-prolonging therapy, results from a small study showed.
In addition, the 41% of patients with the condition were taking NSAIDS, which can worsen kidney function.
"Recognizing proteinuria and initiating the appropriate treatment are extremely important," Dr. Vishesh Kumar, a resident physician at Albany (N.Y.) Medical College, said in an interview following a meeting sponsored by the National Kidney Foundation, where the study was presented.
"If left untreated, proteinuria has been associated with cardiovascular mortality and progression of kidney disease. Both are very poor outcomes, and both can be either slowed in their onset, or in some cases prevented altogether. Our study has shed some light onto the scope of the problem."
Dr. Kumar, along with Dr. Arif Asif, professor of medicine at the medical college, and their associates conducted a multicenter retrospective study that set out to identify patients admitted to the hospital service with proteinuria and to establish whether they were optimally treated. "We know that it is easy to screen for proteinuria using a simple urine dipstick," he said. "We also have several antiproteinuric agents [to use] such as ACE [angiotensin-converting enzyme] inhibitors, angiotensin II receptor blockers, spironolactone, diltiazem, and verapamil. These medications have been shown in landmark trials in nephrology to help to reduce proteinuria, improve cardiovascular mortality, and slow the progression of kidney disease."
The study population included 298 patients (mean age, 60 years) who were admitted to two hospitals over a 6-month period. Of the 298 patients, 199 (66%) were confirmed to have proteinuria. Of these, 74 (37%) were treated with an antiproteinuric agent, and 81 (41%) were taking NSAIDs. Dr. Kumar said that he and his colleagues were very surprised "by the fact that many patients with proteinuria were taking NSAIDs, which can worsen proteinuria, and many of which are available over the counter. We have a huge opportunity for improvement of care in these patients."
He also reported that 63 of the patients (32%) had proteinuria and hypertension. Among this subset of patients, only 27 (43%) received an antiproteinuric agent.
Dr. Kumar acknowledged that certain limitations of the study, including its small sample size and the fact that "we did not collect any data involving these patients’ primary care. One could generalize that patients that come into hospital may not have as good a relationship with primary care providers and may not be screened with a urine analysis as an outpatient as frequently. Many of these things can be addressed in future studies."
The researchers reported having no financial conflicts of interest.
My question about this study is, "Why was the patient admitted to the hospital?" If proteinuria was an incidental finding because the patient presented with a fever and abdominal pain (so a urinalysis was performed to investigate a bladder infection), I am not sure it is so important to address the proteinuria during the index hospitalization.
 |
|
Of course, such findings must and should be reported in a discharge summary and referred to the PCP at follow-up for confirmation and treatment. But there is no immediate need to deal with the proteinuria (i.e., there are no adverse consequences if the treatment is delayed 1-2 weeks) if there are other more pressing issues for the hospitalist to address.
Many hospitalists are reluctant to begin new medications that are not directly tied to the primary reason for hospitalization. The transition of care at discharge already is a time-intensive process if done correctly. Adding multiple new medications (such as an ACE inhibitor plus an antibiotic, for example) will increase the time needed for education, raise the cost to the patient, etc. – all when the PCP may disagree with the ACE inhibitor chosen by the hospitalist (he or she may prefer an angiotensin II receptor blocker, for example).
Dr. Franklin A. Michota Jr. is director of academic affairs in the department of hospital medicine, and medical editor of Hospitalist News.
My question about this study is, "Why was the patient admitted to the hospital?" If proteinuria was an incidental finding because the patient presented with a fever and abdominal pain (so a urinalysis was performed to investigate a bladder infection), I am not sure it is so important to address the proteinuria during the index hospitalization.
 |
|
Of course, such findings must and should be reported in a discharge summary and referred to the PCP at follow-up for confirmation and treatment. But there is no immediate need to deal with the proteinuria (i.e., there are no adverse consequences if the treatment is delayed 1-2 weeks) if there are other more pressing issues for the hospitalist to address.
Many hospitalists are reluctant to begin new medications that are not directly tied to the primary reason for hospitalization. The transition of care at discharge already is a time-intensive process if done correctly. Adding multiple new medications (such as an ACE inhibitor plus an antibiotic, for example) will increase the time needed for education, raise the cost to the patient, etc. – all when the PCP may disagree with the ACE inhibitor chosen by the hospitalist (he or she may prefer an angiotensin II receptor blocker, for example).
Dr. Franklin A. Michota Jr. is director of academic affairs in the department of hospital medicine, and medical editor of Hospitalist News.
My question about this study is, "Why was the patient admitted to the hospital?" If proteinuria was an incidental finding because the patient presented with a fever and abdominal pain (so a urinalysis was performed to investigate a bladder infection), I am not sure it is so important to address the proteinuria during the index hospitalization.
 |
|
Of course, such findings must and should be reported in a discharge summary and referred to the PCP at follow-up for confirmation and treatment. But there is no immediate need to deal with the proteinuria (i.e., there are no adverse consequences if the treatment is delayed 1-2 weeks) if there are other more pressing issues for the hospitalist to address.
Many hospitalists are reluctant to begin new medications that are not directly tied to the primary reason for hospitalization. The transition of care at discharge already is a time-intensive process if done correctly. Adding multiple new medications (such as an ACE inhibitor plus an antibiotic, for example) will increase the time needed for education, raise the cost to the patient, etc. – all when the PCP may disagree with the ACE inhibitor chosen by the hospitalist (he or she may prefer an angiotensin II receptor blocker, for example).
Dr. Franklin A. Michota Jr. is director of academic affairs in the department of hospital medicine, and medical editor of Hospitalist News.
LAS VEGAS – The majority of patients with proteinuria who are admitted to hospitals affiliated with academic medical centers are not placed on life-prolonging therapy, results from a small study showed.
In addition, the 41% of patients with the condition were taking NSAIDS, which can worsen kidney function.
"Recognizing proteinuria and initiating the appropriate treatment are extremely important," Dr. Vishesh Kumar, a resident physician at Albany (N.Y.) Medical College, said in an interview following a meeting sponsored by the National Kidney Foundation, where the study was presented.
"If left untreated, proteinuria has been associated with cardiovascular mortality and progression of kidney disease. Both are very poor outcomes, and both can be either slowed in their onset, or in some cases prevented altogether. Our study has shed some light onto the scope of the problem."
Dr. Kumar, along with Dr. Arif Asif, professor of medicine at the medical college, and their associates conducted a multicenter retrospective study that set out to identify patients admitted to the hospital service with proteinuria and to establish whether they were optimally treated. "We know that it is easy to screen for proteinuria using a simple urine dipstick," he said. "We also have several antiproteinuric agents [to use] such as ACE [angiotensin-converting enzyme] inhibitors, angiotensin II receptor blockers, spironolactone, diltiazem, and verapamil. These medications have been shown in landmark trials in nephrology to help to reduce proteinuria, improve cardiovascular mortality, and slow the progression of kidney disease."
The study population included 298 patients (mean age, 60 years) who were admitted to two hospitals over a 6-month period. Of the 298 patients, 199 (66%) were confirmed to have proteinuria. Of these, 74 (37%) were treated with an antiproteinuric agent, and 81 (41%) were taking NSAIDs. Dr. Kumar said that he and his colleagues were very surprised "by the fact that many patients with proteinuria were taking NSAIDs, which can worsen proteinuria, and many of which are available over the counter. We have a huge opportunity for improvement of care in these patients."
He also reported that 63 of the patients (32%) had proteinuria and hypertension. Among this subset of patients, only 27 (43%) received an antiproteinuric agent.
Dr. Kumar acknowledged that certain limitations of the study, including its small sample size and the fact that "we did not collect any data involving these patients’ primary care. One could generalize that patients that come into hospital may not have as good a relationship with primary care providers and may not be screened with a urine analysis as an outpatient as frequently. Many of these things can be addressed in future studies."
The researchers reported having no financial conflicts of interest.
LAS VEGAS – The majority of patients with proteinuria who are admitted to hospitals affiliated with academic medical centers are not placed on life-prolonging therapy, results from a small study showed.
In addition, the 41% of patients with the condition were taking NSAIDS, which can worsen kidney function.
"Recognizing proteinuria and initiating the appropriate treatment are extremely important," Dr. Vishesh Kumar, a resident physician at Albany (N.Y.) Medical College, said in an interview following a meeting sponsored by the National Kidney Foundation, where the study was presented.
"If left untreated, proteinuria has been associated with cardiovascular mortality and progression of kidney disease. Both are very poor outcomes, and both can be either slowed in their onset, or in some cases prevented altogether. Our study has shed some light onto the scope of the problem."
Dr. Kumar, along with Dr. Arif Asif, professor of medicine at the medical college, and their associates conducted a multicenter retrospective study that set out to identify patients admitted to the hospital service with proteinuria and to establish whether they were optimally treated. "We know that it is easy to screen for proteinuria using a simple urine dipstick," he said. "We also have several antiproteinuric agents [to use] such as ACE [angiotensin-converting enzyme] inhibitors, angiotensin II receptor blockers, spironolactone, diltiazem, and verapamil. These medications have been shown in landmark trials in nephrology to help to reduce proteinuria, improve cardiovascular mortality, and slow the progression of kidney disease."
The study population included 298 patients (mean age, 60 years) who were admitted to two hospitals over a 6-month period. Of the 298 patients, 199 (66%) were confirmed to have proteinuria. Of these, 74 (37%) were treated with an antiproteinuric agent, and 81 (41%) were taking NSAIDs. Dr. Kumar said that he and his colleagues were very surprised "by the fact that many patients with proteinuria were taking NSAIDs, which can worsen proteinuria, and many of which are available over the counter. We have a huge opportunity for improvement of care in these patients."
He also reported that 63 of the patients (32%) had proteinuria and hypertension. Among this subset of patients, only 27 (43%) received an antiproteinuric agent.
Dr. Kumar acknowledged that certain limitations of the study, including its small sample size and the fact that "we did not collect any data involving these patients’ primary care. One could generalize that patients that come into hospital may not have as good a relationship with primary care providers and may not be screened with a urine analysis as an outpatient as frequently. Many of these things can be addressed in future studies."
The researchers reported having no financial conflicts of interest.
AT SCM 14
Moderate wine intake may benefit kidneys, curb heart disease risk
LAS VEGAS – Moderate intake of wine was associated with a 37% lower prevalence of chronic kidney disease in healthy adults, compared with no wine consumption at all, according to an analysis of data from the National Health and Nutrition Examination Survey.
In addition, survey respondents with chronic kidney disease (which increases the risk of cardiovascular disease) who drank less than one glass of wine a day had 29% lower odds of cardiovascular disease, compared with those who drank no wine at all.
"Since this is an observational study and we looked at the data as a cross-sectional analysis, we can’t say that there’s a cause and effect relationship," lead author Dr. Tapan Mehta emphasized in an interview at a meeting sponsored by the National Kidney Foundation. "But this is the first time that [the link between] wine intake and cardiovascular disease risk has been studied in patients who have kidney disease."
Dr. Mehta, of the division of renal disease and hypertension at the University of Colorado Anschutz Medical Center, Aurora, and his associates performed a cross-sectional analysis of 5,852 men and women aged 21 years and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2006. The researchers analyzed wine intake in three categories: none, less than one glass per day, and one or more glasses per day. They examined the prevalence of chronic kidney disease (defined as MDRD [Modification of Diet in Renal Disease Study Group] estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 or albumin/creatinine ratio of 30 mg/g or greater) according to wine intake. Next, they examined the relationship between wine intake and cardiovascular disease (CVD), defined as history of angina, myocardial infarction, or stroke.
The mean age of study participants was 48 years, and 52% were female. Of the 5,852 individuals, 2,455 drank less than one glass of wine per day, and 1,031 had kidney disease.
In unadjusted analysis, those who drank less than one glass of wine per day had a 0.63 reduced odds of chronic kidney disease (CKD), compared with those who did not drink wine (P less than .0001). This association remained significant after adjustment for demographics, waist circumference, diabetes, hypertension, HDL cholesterol, and triglycerides (odds ratio, 0.75; P = .004).
The researchers observed that the relationship between wine intake and kidney disease appeared to be driven by a significant association between consumption of less than one glass of wine per day and microalbuminuria, which was defined as an albumin/creatinine ratio of 30 mg/g or greater (OR, 0.62; P = .006). No association between wine intake and an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 was seen. "We didn’t expect that," Dr. Mehta said.
When the analysis was limited to the 1,031 individuals with CKD, the odds of CVD was 0.71 (P =.02) for those drinking less than one glass of wine per day, compared with nondrinkers after adjustment for demographics and CVD risk factors.
Dr. Mehta acknowledged certain limitations of the study, including its observational design and the potential for recall bias in the food intake component of NHANES. "Also, there is no specification as to red wine or white wine consumption," he said. "We also did not look at other alcohol, such as beer or liquor."
Dr. Mehta had no relevant financial conflicts to disclose.
LAS VEGAS – Moderate intake of wine was associated with a 37% lower prevalence of chronic kidney disease in healthy adults, compared with no wine consumption at all, according to an analysis of data from the National Health and Nutrition Examination Survey.
In addition, survey respondents with chronic kidney disease (which increases the risk of cardiovascular disease) who drank less than one glass of wine a day had 29% lower odds of cardiovascular disease, compared with those who drank no wine at all.
"Since this is an observational study and we looked at the data as a cross-sectional analysis, we can’t say that there’s a cause and effect relationship," lead author Dr. Tapan Mehta emphasized in an interview at a meeting sponsored by the National Kidney Foundation. "But this is the first time that [the link between] wine intake and cardiovascular disease risk has been studied in patients who have kidney disease."
Dr. Mehta, of the division of renal disease and hypertension at the University of Colorado Anschutz Medical Center, Aurora, and his associates performed a cross-sectional analysis of 5,852 men and women aged 21 years and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2006. The researchers analyzed wine intake in three categories: none, less than one glass per day, and one or more glasses per day. They examined the prevalence of chronic kidney disease (defined as MDRD [Modification of Diet in Renal Disease Study Group] estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 or albumin/creatinine ratio of 30 mg/g or greater) according to wine intake. Next, they examined the relationship between wine intake and cardiovascular disease (CVD), defined as history of angina, myocardial infarction, or stroke.
The mean age of study participants was 48 years, and 52% were female. Of the 5,852 individuals, 2,455 drank less than one glass of wine per day, and 1,031 had kidney disease.
In unadjusted analysis, those who drank less than one glass of wine per day had a 0.63 reduced odds of chronic kidney disease (CKD), compared with those who did not drink wine (P less than .0001). This association remained significant after adjustment for demographics, waist circumference, diabetes, hypertension, HDL cholesterol, and triglycerides (odds ratio, 0.75; P = .004).
The researchers observed that the relationship between wine intake and kidney disease appeared to be driven by a significant association between consumption of less than one glass of wine per day and microalbuminuria, which was defined as an albumin/creatinine ratio of 30 mg/g or greater (OR, 0.62; P = .006). No association between wine intake and an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 was seen. "We didn’t expect that," Dr. Mehta said.
When the analysis was limited to the 1,031 individuals with CKD, the odds of CVD was 0.71 (P =.02) for those drinking less than one glass of wine per day, compared with nondrinkers after adjustment for demographics and CVD risk factors.
Dr. Mehta acknowledged certain limitations of the study, including its observational design and the potential for recall bias in the food intake component of NHANES. "Also, there is no specification as to red wine or white wine consumption," he said. "We also did not look at other alcohol, such as beer or liquor."
Dr. Mehta had no relevant financial conflicts to disclose.
LAS VEGAS – Moderate intake of wine was associated with a 37% lower prevalence of chronic kidney disease in healthy adults, compared with no wine consumption at all, according to an analysis of data from the National Health and Nutrition Examination Survey.
In addition, survey respondents with chronic kidney disease (which increases the risk of cardiovascular disease) who drank less than one glass of wine a day had 29% lower odds of cardiovascular disease, compared with those who drank no wine at all.
"Since this is an observational study and we looked at the data as a cross-sectional analysis, we can’t say that there’s a cause and effect relationship," lead author Dr. Tapan Mehta emphasized in an interview at a meeting sponsored by the National Kidney Foundation. "But this is the first time that [the link between] wine intake and cardiovascular disease risk has been studied in patients who have kidney disease."
Dr. Mehta, of the division of renal disease and hypertension at the University of Colorado Anschutz Medical Center, Aurora, and his associates performed a cross-sectional analysis of 5,852 men and women aged 21 years and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2006. The researchers analyzed wine intake in three categories: none, less than one glass per day, and one or more glasses per day. They examined the prevalence of chronic kidney disease (defined as MDRD [Modification of Diet in Renal Disease Study Group] estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 or albumin/creatinine ratio of 30 mg/g or greater) according to wine intake. Next, they examined the relationship between wine intake and cardiovascular disease (CVD), defined as history of angina, myocardial infarction, or stroke.
The mean age of study participants was 48 years, and 52% were female. Of the 5,852 individuals, 2,455 drank less than one glass of wine per day, and 1,031 had kidney disease.
In unadjusted analysis, those who drank less than one glass of wine per day had a 0.63 reduced odds of chronic kidney disease (CKD), compared with those who did not drink wine (P less than .0001). This association remained significant after adjustment for demographics, waist circumference, diabetes, hypertension, HDL cholesterol, and triglycerides (odds ratio, 0.75; P = .004).
The researchers observed that the relationship between wine intake and kidney disease appeared to be driven by a significant association between consumption of less than one glass of wine per day and microalbuminuria, which was defined as an albumin/creatinine ratio of 30 mg/g or greater (OR, 0.62; P = .006). No association between wine intake and an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2 was seen. "We didn’t expect that," Dr. Mehta said.
When the analysis was limited to the 1,031 individuals with CKD, the odds of CVD was 0.71 (P =.02) for those drinking less than one glass of wine per day, compared with nondrinkers after adjustment for demographics and CVD risk factors.
Dr. Mehta acknowledged certain limitations of the study, including its observational design and the potential for recall bias in the food intake component of NHANES. "Also, there is no specification as to red wine or white wine consumption," he said. "We also did not look at other alcohol, such as beer or liquor."
Dr. Mehta had no relevant financial conflicts to disclose.
AT SCM 14
Major finding: In unadjusted analysis, study participants who drank less than one glass of wine per day had a 0.63 reduced odds of CKD compared with those who did not drink wine (P less than .0001). This association remained significant after adjustment for demographics, waist circumference, diabetes, hypertension, HDL cholesterol, and triglycerides (OR, 0.75; P = .004).
Data source: A cross-sectional analysis of 5,852 men and women aged 21 years of age and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2006.
Disclosures: Dr. Mehta had no relevant financial conflicts to disclose.
VIDEO: Is wine good for the kidneys?
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
LAS VEGAS – Consuming less than one glass of wine per day may help keep the kidneys healthy and may protect the heart in patients who already have chronic kidney disease, according to an analysis of data from the National Health and Nutrition Examination Survey.
In this video interview from a meeting sponsored by the National Kidney Foundation, Dr. Tapan Mehta, a renal fellow at the University of Colorado, Aurora, highlights the findings and discusses the potential clinical implications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
AT SCM 2014
Novel complex shows unique benefits in chronic kidney disease
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.
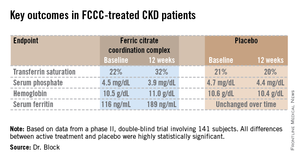
"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
AT SCM 14
Major finding: FCCC safely repleted low iron stores, reduced elevated serum phosphate, raised hemoglobin, and slashed FGF23 levels, compared with placebo.
Data source: A prospective, double-blind, placebo-controlled, 12-week trial in which 141 patients with non–dialysis-dependent CKD, iron-deficiency anemia, and elevated serum phosphate were assigned to FCCC or placebo.
Disclosures: The study was sponsored by Keryx Biopharmaceuticals. Dr. Block is a consultant to the company and was principal investigator in the trial.