User login
Can effective obesity counseling fit into the 20-minute appointment?
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
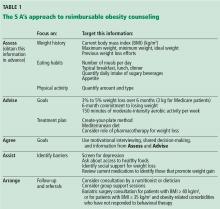
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
Is pregnancy safe after kidney transplant?
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
Which patients with respiratory disease need long-term azithromycin?
Azithromycin is prescribed for a variety of acute respiratory and nonrespiratory infections. However, it is also used in several chronic respiratory diseases.
MECHANISM OF ACTION
Macrolide antibiotics like azithromycin inhibit bacterial growth and replication by interrupting protein synthesis. But azithromycin also has immunomodulatory properties.1
In the acute phase of inflammation, azithromycin exerts an initial neutrophil degranulation effect and enhances the oxidative response that is primed by particulate stimulus, which could facilitate its antibacterial effects. In the late phase, it down-regulates the oxidative burst and increases apoptosis of neutrophils to promote healing without compromising immunity. Azithromycin also attenuates airway mucus hypersecretion, improves ciliary function, and promotes pulmonary epithelial cell healing.2,3
Collectively, these effects make the drug effective in many chronic inflammatory lung conditions (Table 1).
CYSTIC FIBROSIS
Cystic fibrosis is a genetic disease affecting many organs, but its effect on the upper and lower airways has the greatest impact on quality of life and survival. Impaired mucociliary clearance and repeated respiratory infections contribute to chronic inflammation and a progressive decline in lung function.4,5
A 2012 Cochrane review of 5 studies in 549 patients found that, compared with those taking placebo, patients taking azithromycin 250–500 mg 3 times a week had improvement in forced expiratory volume in 1 second (FEV1). The mean difference at 6 months was 3.97% (95% cofidence interval [CI] 1.74– 6.19). Patients on azithromycin were free from pulmonary exacerbation approximately twice as long as patients on placebo (odds ratio 1.96, 95% CI 1.15–3.33).6,7
The Cystic Fibrosis Foundation recommends long-term azithromycin therapy to improve lung function and reduce exacerbations in patients age 6 or older who have persistent Pseudomonas aeruginosa airway cultures (level of evidence: fair).8
DIFFUSE PANBRONCHIOLITIS
Diffuse panbronchiolitis, or diffuse chronic inflammatory bronchiolitis and sinusitis, is seen mainly in patients of Asian descent.9 In the past, the mortality rate was greater than 90%, but between 1970 and 1979 the 10-year survival rate increased by more than 40% with chronic macrolide therapy, ie, with erythromycin.10,11
Later retrospective studies of azithromycin 500 mg 3 times a week showed results comparable to those with erythromycin, with improvement in symptoms, lung function, arterial partial pressure of oxygen, and radiologic findings, as well as fewer adverse effects.12 These benefits justify the current recommendation for azithromycin as the mainstay of therapy in diffuse panbronchiolitis.
BRONCHIOLITIS OBLITERANS SYNDROME
Bronchiolitis obliterans syndrome is an airflow limitation that arises without infection or imaging evidence of bronchiolitis in patients who received allogeneic hematopoietic stem cell or lung transplant. It occurs in 50% of lung transplant recipients as a form of chronic graft rejection and in 6% to 20% of allogeneic stem cell transplant recipients as a manifestation of chronic graft-vs-host disease.13,14
Azithromycin has been used in its management. A meta-analysis of lung transplant recipients found a significant improvement in the survival rate and overall lung function after an average of 7 months of treatment with azithromycin, with a mean increase in FEV1 of 8.8% (95% CI 5.1–12.47, P < .001).14 The evidence currently supports long-term azithromycin 250 mg 3 times a week after lung transplant to reduce any decline in lung function and to lower the mortality rate.14,15
In allogeneic stem cell transplant recipients, the evidence for long-term azithromycin treatment is sparse. A recent prospective multicenter study evaluated the effect of an azithromycin-based regimen (fluticasone, azithromycin, and montelukast, plus a steroid pulse) in stem cell recipients with bronchiolitis obliterans syndrome during the first 3 months after diagnosis. In the treated group, 6% had a drop in FEV1 of more than 10% at 3-month follow-up compared with 40% of historical controls (95% CI 1%–19%, P < .001). Also, treatment resulted in a 50% reduction in the dose of systemic steroids and a substantial improvement in functional status.16
Given the limited options in the management of these patients and until further studies are available, azithromycin 3 times weekly is suggested.
NON-CYSTIC FIBROSIS BRONCHIECTASIS
Non-cystic fibrosis bronchiectasis is a chronic inflammatory lung condition characterized by irreversible dilation of the bronchi and bronchioles due to a variety of causes including recurrent or old infection, immunodeficiency, autoimmune conditions, and connective tissue disease; it can also be idiopathic.17
Altenburg et al,18 in a randomized, double-blind, placebo-controlled trial, found that azithromycin 250 mg 3 times a week for 12 months reduced the number of exacerbations from a median number of 2 per patient with placebo to 0 per patient with azithromycin (P < .001). At 3 months, the FEV1 as a percent of predicted had increased by 1.03% in the azithromycin group and decreased by 0.10% in the placebo group (P = .047). The number needed to treat with azithromycin to maintain clinical stability was 3.0.
Wong et al19 randomized patients to receive azithromycin 500 mg 3 times a week or placebo for 6 months. The rate of exacerbations was 0.59 per patient in the azithromycin group and 1.57 per patient in the placebo group (P < .0001). The FEV1 remained unchanged from baseline in the azithromycin group while decreasing in the placebo group, but the difference was not significant.
EXACERBATIONS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of death, poor quality of life, and healthcare expenditures.20 Prevention is therefore of the utmost importance.
Several studies have shown that azithromycin prophylaxis can reduce acute exacerbations of COPD. A recent meta-analysis showed that long-term macrolide prophylaxis significantly reduced exacerbations compared with rates in controls (risk ratio = 0.70, 95% CI 0.56–0.87, P < .01) and increased the median time to first COPD exacerbation by more than 90 days (P < .01).21 Long-term azithromycin therapy may be considered in selected patients who have frequent exacerbations despite optimal maintenance inhaler therapy.
PROPHYLAXIS IN IMMUNODEFICIENCY
Disseminated Mycobacterium avium complex (MAC) is an opportunistic infection most commonly occurring in patients with acquired immunodeficiency syndrome with CD4 counts below 50 cells/µL.22,23
In a double-blinded, randomized trial, patients who received azithromycin had a 47% reduction in the incidence of MAC infection.
Given the long half-life of azithromycin, it is effective with once-weekly dosing of 1,200 mg.23 Ideally, patients are placed on a prophylactic agent for disseminated MAC infection until the CD4 count reaches 100 cells/µL and remains at or above this level for 3 consecutive months.24
ADVERSE EFFECTS AND PRECAUTIONS
Long-term azithromycin therapy may produce bacterial resistance; the risk has been estimated at 2.7 times greater in patients who are on long-term azithromycin treatment.25 Also, patients at risk for MAC infection, such as those with cystic fibrosis, should be screened for it before starting treatment in order to prevent resistance to azithromycin.
The US Food and Drug Administration warns that azithromycin can lead to a prolonged corrected QT interval and potential fatal arrhythmias such as torsades de pointes. Major reviews have largely agreed that arrhythmias are more pronounced in patients with a coexisting cardiac risk factor such as existing QT-interval prolongation, low blood levels of potassium or magnesium, a slower than normal heart rate, or arrhythmias, or who are on class IA and III antiarrhythmic drugs.26–28
Other potential adverse effects of long-term azithromycin treatment are gastrointestinal symptoms and hearing impairment.29,30 A review of potential drug interactions is advised when patients are placed on long-term azithromycin therapy.
Although azithromycin is generally well tolerated, long-term treatment should be individualized and the benefits weighed against the risks. Patients should be monitored during treatment for any of the above adverse effects.
- Bailly S, Pocidalo JJ, Fay M, Gougerot-Pocidalo MA. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother 1991; 35:2016–2019.
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010; 23:590–615.
- Culić O, Eraković V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol 2002; 450:277–289.
- Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax 2013; 68:1157–1162.
- Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 2012; 186:857–865.
- Saiman L, Anstead M, Mayer-Hamblett N, et al; AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010; 303:1707–1715.
- Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2012;11:CD002203.
- Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176:957–969.
- Yanagihara K, Kadoto J, Kohno S. Diffuse panbronchiolitis—pathophysiology and treatment mechanisms. Int J Antimicrob Agents 2001; 18(suppl 1):S83–S87.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother 2004; 54:21–28.
- Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis 2013; 5:613–617.
- Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005; 25:490–493.
- Kingah PL, Muma G, Soubani A. Azithromycin improves lung function in patients with post-lung transplant bronchiolitis obliterans syndrome: a meta-analysis. Clin Transplant 2014; 28:906–910.
- Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax 2015; 70:442–450.
- Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22:710–716.
- Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med 2014; 108:1397–1408.
- Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309:1251–1259.
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380:660–667.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67:957–963.
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 2015; 10:e0121257.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Havlir DV, Dubé MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med 1996; 335:392–398.
- Uthman MM, Uthman OA, Yahaya I. Interventions for the prevention of Mycobacterium avium complex in adults and children with HIV. Cochrane Database Syst Rev 2013; 4:CD007191.
- Li H, Liu DH, Chen LL, et al. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob Agents Chemother 2014; 58:511–517.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Albert RK, Schuller JL; COPD Clinical Research Network. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 2014; 189:1173–1180.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Broad J, Sanger GJ. The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br J Pharmacol 2013; 168:1859–1867.
Azithromycin is prescribed for a variety of acute respiratory and nonrespiratory infections. However, it is also used in several chronic respiratory diseases.
MECHANISM OF ACTION
Macrolide antibiotics like azithromycin inhibit bacterial growth and replication by interrupting protein synthesis. But azithromycin also has immunomodulatory properties.1
In the acute phase of inflammation, azithromycin exerts an initial neutrophil degranulation effect and enhances the oxidative response that is primed by particulate stimulus, which could facilitate its antibacterial effects. In the late phase, it down-regulates the oxidative burst and increases apoptosis of neutrophils to promote healing without compromising immunity. Azithromycin also attenuates airway mucus hypersecretion, improves ciliary function, and promotes pulmonary epithelial cell healing.2,3
Collectively, these effects make the drug effective in many chronic inflammatory lung conditions (Table 1).
CYSTIC FIBROSIS
Cystic fibrosis is a genetic disease affecting many organs, but its effect on the upper and lower airways has the greatest impact on quality of life and survival. Impaired mucociliary clearance and repeated respiratory infections contribute to chronic inflammation and a progressive decline in lung function.4,5
A 2012 Cochrane review of 5 studies in 549 patients found that, compared with those taking placebo, patients taking azithromycin 250–500 mg 3 times a week had improvement in forced expiratory volume in 1 second (FEV1). The mean difference at 6 months was 3.97% (95% cofidence interval [CI] 1.74– 6.19). Patients on azithromycin were free from pulmonary exacerbation approximately twice as long as patients on placebo (odds ratio 1.96, 95% CI 1.15–3.33).6,7
The Cystic Fibrosis Foundation recommends long-term azithromycin therapy to improve lung function and reduce exacerbations in patients age 6 or older who have persistent Pseudomonas aeruginosa airway cultures (level of evidence: fair).8
DIFFUSE PANBRONCHIOLITIS
Diffuse panbronchiolitis, or diffuse chronic inflammatory bronchiolitis and sinusitis, is seen mainly in patients of Asian descent.9 In the past, the mortality rate was greater than 90%, but between 1970 and 1979 the 10-year survival rate increased by more than 40% with chronic macrolide therapy, ie, with erythromycin.10,11
Later retrospective studies of azithromycin 500 mg 3 times a week showed results comparable to those with erythromycin, with improvement in symptoms, lung function, arterial partial pressure of oxygen, and radiologic findings, as well as fewer adverse effects.12 These benefits justify the current recommendation for azithromycin as the mainstay of therapy in diffuse panbronchiolitis.
BRONCHIOLITIS OBLITERANS SYNDROME
Bronchiolitis obliterans syndrome is an airflow limitation that arises without infection or imaging evidence of bronchiolitis in patients who received allogeneic hematopoietic stem cell or lung transplant. It occurs in 50% of lung transplant recipients as a form of chronic graft rejection and in 6% to 20% of allogeneic stem cell transplant recipients as a manifestation of chronic graft-vs-host disease.13,14
Azithromycin has been used in its management. A meta-analysis of lung transplant recipients found a significant improvement in the survival rate and overall lung function after an average of 7 months of treatment with azithromycin, with a mean increase in FEV1 of 8.8% (95% CI 5.1–12.47, P < .001).14 The evidence currently supports long-term azithromycin 250 mg 3 times a week after lung transplant to reduce any decline in lung function and to lower the mortality rate.14,15
In allogeneic stem cell transplant recipients, the evidence for long-term azithromycin treatment is sparse. A recent prospective multicenter study evaluated the effect of an azithromycin-based regimen (fluticasone, azithromycin, and montelukast, plus a steroid pulse) in stem cell recipients with bronchiolitis obliterans syndrome during the first 3 months after diagnosis. In the treated group, 6% had a drop in FEV1 of more than 10% at 3-month follow-up compared with 40% of historical controls (95% CI 1%–19%, P < .001). Also, treatment resulted in a 50% reduction in the dose of systemic steroids and a substantial improvement in functional status.16
Given the limited options in the management of these patients and until further studies are available, azithromycin 3 times weekly is suggested.
NON-CYSTIC FIBROSIS BRONCHIECTASIS
Non-cystic fibrosis bronchiectasis is a chronic inflammatory lung condition characterized by irreversible dilation of the bronchi and bronchioles due to a variety of causes including recurrent or old infection, immunodeficiency, autoimmune conditions, and connective tissue disease; it can also be idiopathic.17
Altenburg et al,18 in a randomized, double-blind, placebo-controlled trial, found that azithromycin 250 mg 3 times a week for 12 months reduced the number of exacerbations from a median number of 2 per patient with placebo to 0 per patient with azithromycin (P < .001). At 3 months, the FEV1 as a percent of predicted had increased by 1.03% in the azithromycin group and decreased by 0.10% in the placebo group (P = .047). The number needed to treat with azithromycin to maintain clinical stability was 3.0.
Wong et al19 randomized patients to receive azithromycin 500 mg 3 times a week or placebo for 6 months. The rate of exacerbations was 0.59 per patient in the azithromycin group and 1.57 per patient in the placebo group (P < .0001). The FEV1 remained unchanged from baseline in the azithromycin group while decreasing in the placebo group, but the difference was not significant.
EXACERBATIONS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of death, poor quality of life, and healthcare expenditures.20 Prevention is therefore of the utmost importance.
Several studies have shown that azithromycin prophylaxis can reduce acute exacerbations of COPD. A recent meta-analysis showed that long-term macrolide prophylaxis significantly reduced exacerbations compared with rates in controls (risk ratio = 0.70, 95% CI 0.56–0.87, P < .01) and increased the median time to first COPD exacerbation by more than 90 days (P < .01).21 Long-term azithromycin therapy may be considered in selected patients who have frequent exacerbations despite optimal maintenance inhaler therapy.
PROPHYLAXIS IN IMMUNODEFICIENCY
Disseminated Mycobacterium avium complex (MAC) is an opportunistic infection most commonly occurring in patients with acquired immunodeficiency syndrome with CD4 counts below 50 cells/µL.22,23
In a double-blinded, randomized trial, patients who received azithromycin had a 47% reduction in the incidence of MAC infection.
Given the long half-life of azithromycin, it is effective with once-weekly dosing of 1,200 mg.23 Ideally, patients are placed on a prophylactic agent for disseminated MAC infection until the CD4 count reaches 100 cells/µL and remains at or above this level for 3 consecutive months.24
ADVERSE EFFECTS AND PRECAUTIONS
Long-term azithromycin therapy may produce bacterial resistance; the risk has been estimated at 2.7 times greater in patients who are on long-term azithromycin treatment.25 Also, patients at risk for MAC infection, such as those with cystic fibrosis, should be screened for it before starting treatment in order to prevent resistance to azithromycin.
The US Food and Drug Administration warns that azithromycin can lead to a prolonged corrected QT interval and potential fatal arrhythmias such as torsades de pointes. Major reviews have largely agreed that arrhythmias are more pronounced in patients with a coexisting cardiac risk factor such as existing QT-interval prolongation, low blood levels of potassium or magnesium, a slower than normal heart rate, or arrhythmias, or who are on class IA and III antiarrhythmic drugs.26–28
Other potential adverse effects of long-term azithromycin treatment are gastrointestinal symptoms and hearing impairment.29,30 A review of potential drug interactions is advised when patients are placed on long-term azithromycin therapy.
Although azithromycin is generally well tolerated, long-term treatment should be individualized and the benefits weighed against the risks. Patients should be monitored during treatment for any of the above adverse effects.
Azithromycin is prescribed for a variety of acute respiratory and nonrespiratory infections. However, it is also used in several chronic respiratory diseases.
MECHANISM OF ACTION
Macrolide antibiotics like azithromycin inhibit bacterial growth and replication by interrupting protein synthesis. But azithromycin also has immunomodulatory properties.1
In the acute phase of inflammation, azithromycin exerts an initial neutrophil degranulation effect and enhances the oxidative response that is primed by particulate stimulus, which could facilitate its antibacterial effects. In the late phase, it down-regulates the oxidative burst and increases apoptosis of neutrophils to promote healing without compromising immunity. Azithromycin also attenuates airway mucus hypersecretion, improves ciliary function, and promotes pulmonary epithelial cell healing.2,3
Collectively, these effects make the drug effective in many chronic inflammatory lung conditions (Table 1).
CYSTIC FIBROSIS
Cystic fibrosis is a genetic disease affecting many organs, but its effect on the upper and lower airways has the greatest impact on quality of life and survival. Impaired mucociliary clearance and repeated respiratory infections contribute to chronic inflammation and a progressive decline in lung function.4,5
A 2012 Cochrane review of 5 studies in 549 patients found that, compared with those taking placebo, patients taking azithromycin 250–500 mg 3 times a week had improvement in forced expiratory volume in 1 second (FEV1). The mean difference at 6 months was 3.97% (95% cofidence interval [CI] 1.74– 6.19). Patients on azithromycin were free from pulmonary exacerbation approximately twice as long as patients on placebo (odds ratio 1.96, 95% CI 1.15–3.33).6,7
The Cystic Fibrosis Foundation recommends long-term azithromycin therapy to improve lung function and reduce exacerbations in patients age 6 or older who have persistent Pseudomonas aeruginosa airway cultures (level of evidence: fair).8
DIFFUSE PANBRONCHIOLITIS
Diffuse panbronchiolitis, or diffuse chronic inflammatory bronchiolitis and sinusitis, is seen mainly in patients of Asian descent.9 In the past, the mortality rate was greater than 90%, but between 1970 and 1979 the 10-year survival rate increased by more than 40% with chronic macrolide therapy, ie, with erythromycin.10,11
Later retrospective studies of azithromycin 500 mg 3 times a week showed results comparable to those with erythromycin, with improvement in symptoms, lung function, arterial partial pressure of oxygen, and radiologic findings, as well as fewer adverse effects.12 These benefits justify the current recommendation for azithromycin as the mainstay of therapy in diffuse panbronchiolitis.
BRONCHIOLITIS OBLITERANS SYNDROME
Bronchiolitis obliterans syndrome is an airflow limitation that arises without infection or imaging evidence of bronchiolitis in patients who received allogeneic hematopoietic stem cell or lung transplant. It occurs in 50% of lung transplant recipients as a form of chronic graft rejection and in 6% to 20% of allogeneic stem cell transplant recipients as a manifestation of chronic graft-vs-host disease.13,14
Azithromycin has been used in its management. A meta-analysis of lung transplant recipients found a significant improvement in the survival rate and overall lung function after an average of 7 months of treatment with azithromycin, with a mean increase in FEV1 of 8.8% (95% CI 5.1–12.47, P < .001).14 The evidence currently supports long-term azithromycin 250 mg 3 times a week after lung transplant to reduce any decline in lung function and to lower the mortality rate.14,15
In allogeneic stem cell transplant recipients, the evidence for long-term azithromycin treatment is sparse. A recent prospective multicenter study evaluated the effect of an azithromycin-based regimen (fluticasone, azithromycin, and montelukast, plus a steroid pulse) in stem cell recipients with bronchiolitis obliterans syndrome during the first 3 months after diagnosis. In the treated group, 6% had a drop in FEV1 of more than 10% at 3-month follow-up compared with 40% of historical controls (95% CI 1%–19%, P < .001). Also, treatment resulted in a 50% reduction in the dose of systemic steroids and a substantial improvement in functional status.16
Given the limited options in the management of these patients and until further studies are available, azithromycin 3 times weekly is suggested.
NON-CYSTIC FIBROSIS BRONCHIECTASIS
Non-cystic fibrosis bronchiectasis is a chronic inflammatory lung condition characterized by irreversible dilation of the bronchi and bronchioles due to a variety of causes including recurrent or old infection, immunodeficiency, autoimmune conditions, and connective tissue disease; it can also be idiopathic.17
Altenburg et al,18 in a randomized, double-blind, placebo-controlled trial, found that azithromycin 250 mg 3 times a week for 12 months reduced the number of exacerbations from a median number of 2 per patient with placebo to 0 per patient with azithromycin (P < .001). At 3 months, the FEV1 as a percent of predicted had increased by 1.03% in the azithromycin group and decreased by 0.10% in the placebo group (P = .047). The number needed to treat with azithromycin to maintain clinical stability was 3.0.
Wong et al19 randomized patients to receive azithromycin 500 mg 3 times a week or placebo for 6 months. The rate of exacerbations was 0.59 per patient in the azithromycin group and 1.57 per patient in the placebo group (P < .0001). The FEV1 remained unchanged from baseline in the azithromycin group while decreasing in the placebo group, but the difference was not significant.
EXACERBATIONS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of death, poor quality of life, and healthcare expenditures.20 Prevention is therefore of the utmost importance.
Several studies have shown that azithromycin prophylaxis can reduce acute exacerbations of COPD. A recent meta-analysis showed that long-term macrolide prophylaxis significantly reduced exacerbations compared with rates in controls (risk ratio = 0.70, 95% CI 0.56–0.87, P < .01) and increased the median time to first COPD exacerbation by more than 90 days (P < .01).21 Long-term azithromycin therapy may be considered in selected patients who have frequent exacerbations despite optimal maintenance inhaler therapy.
PROPHYLAXIS IN IMMUNODEFICIENCY
Disseminated Mycobacterium avium complex (MAC) is an opportunistic infection most commonly occurring in patients with acquired immunodeficiency syndrome with CD4 counts below 50 cells/µL.22,23
In a double-blinded, randomized trial, patients who received azithromycin had a 47% reduction in the incidence of MAC infection.
Given the long half-life of azithromycin, it is effective with once-weekly dosing of 1,200 mg.23 Ideally, patients are placed on a prophylactic agent for disseminated MAC infection until the CD4 count reaches 100 cells/µL and remains at or above this level for 3 consecutive months.24
ADVERSE EFFECTS AND PRECAUTIONS
Long-term azithromycin therapy may produce bacterial resistance; the risk has been estimated at 2.7 times greater in patients who are on long-term azithromycin treatment.25 Also, patients at risk for MAC infection, such as those with cystic fibrosis, should be screened for it before starting treatment in order to prevent resistance to azithromycin.
The US Food and Drug Administration warns that azithromycin can lead to a prolonged corrected QT interval and potential fatal arrhythmias such as torsades de pointes. Major reviews have largely agreed that arrhythmias are more pronounced in patients with a coexisting cardiac risk factor such as existing QT-interval prolongation, low blood levels of potassium or magnesium, a slower than normal heart rate, or arrhythmias, or who are on class IA and III antiarrhythmic drugs.26–28
Other potential adverse effects of long-term azithromycin treatment are gastrointestinal symptoms and hearing impairment.29,30 A review of potential drug interactions is advised when patients are placed on long-term azithromycin therapy.
Although azithromycin is generally well tolerated, long-term treatment should be individualized and the benefits weighed against the risks. Patients should be monitored during treatment for any of the above adverse effects.
- Bailly S, Pocidalo JJ, Fay M, Gougerot-Pocidalo MA. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother 1991; 35:2016–2019.
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010; 23:590–615.
- Culić O, Eraković V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol 2002; 450:277–289.
- Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax 2013; 68:1157–1162.
- Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 2012; 186:857–865.
- Saiman L, Anstead M, Mayer-Hamblett N, et al; AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010; 303:1707–1715.
- Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2012;11:CD002203.
- Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176:957–969.
- Yanagihara K, Kadoto J, Kohno S. Diffuse panbronchiolitis—pathophysiology and treatment mechanisms. Int J Antimicrob Agents 2001; 18(suppl 1):S83–S87.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother 2004; 54:21–28.
- Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis 2013; 5:613–617.
- Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005; 25:490–493.
- Kingah PL, Muma G, Soubani A. Azithromycin improves lung function in patients with post-lung transplant bronchiolitis obliterans syndrome: a meta-analysis. Clin Transplant 2014; 28:906–910.
- Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax 2015; 70:442–450.
- Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22:710–716.
- Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med 2014; 108:1397–1408.
- Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309:1251–1259.
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380:660–667.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67:957–963.
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 2015; 10:e0121257.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Havlir DV, Dubé MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med 1996; 335:392–398.
- Uthman MM, Uthman OA, Yahaya I. Interventions for the prevention of Mycobacterium avium complex in adults and children with HIV. Cochrane Database Syst Rev 2013; 4:CD007191.
- Li H, Liu DH, Chen LL, et al. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob Agents Chemother 2014; 58:511–517.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Albert RK, Schuller JL; COPD Clinical Research Network. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 2014; 189:1173–1180.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Broad J, Sanger GJ. The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br J Pharmacol 2013; 168:1859–1867.
- Bailly S, Pocidalo JJ, Fay M, Gougerot-Pocidalo MA. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother 1991; 35:2016–2019.
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010; 23:590–615.
- Culić O, Eraković V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol 2002; 450:277–289.
- Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax 2013; 68:1157–1162.
- Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 2012; 186:857–865.
- Saiman L, Anstead M, Mayer-Hamblett N, et al; AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010; 303:1707–1715.
- Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2012;11:CD002203.
- Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176:957–969.
- Yanagihara K, Kadoto J, Kohno S. Diffuse panbronchiolitis—pathophysiology and treatment mechanisms. Int J Antimicrob Agents 2001; 18(suppl 1):S83–S87.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother 2004; 54:21–28.
- Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis 2013; 5:613–617.
- Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J 2005; 25:490–493.
- Kingah PL, Muma G, Soubani A. Azithromycin improves lung function in patients with post-lung transplant bronchiolitis obliterans syndrome: a meta-analysis. Clin Transplant 2014; 28:906–910.
- Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax 2015; 70:442–450.
- Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22:710–716.
- Haworth CS, Bilton D, Elborn JS. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir Med 2014; 108:1397–1408.
- Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309:1251–1259.
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380:660–667.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67:957–963.
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 2015; 10:e0121257.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416.
- Havlir DV, Dubé MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med 1996; 335:392–398.
- Uthman MM, Uthman OA, Yahaya I. Interventions for the prevention of Mycobacterium avium complex in adults and children with HIV. Cochrane Database Syst Rev 2013; 4:CD007191.
- Li H, Liu DH, Chen LL, et al. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob Agents Chemother 2014; 58:511–517.
- Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368:1704–1712.
- Albert RK, Schuller JL; COPD Clinical Research Network. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 2014; 189:1173–1180.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366:1881–1890.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Broad J, Sanger GJ. The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br J Pharmacol 2013; 168:1859–1867.
Which bowel preparation should be used for colonoscopy in patients who have had bariatric surgery?
We routinely use low-volume (2-L) polyethylene glycol electrolyte preparations such as Moviprep and Miralax in split-dose regimens, in which patients drink half of the preparation the day before the procedure and the other half the day of the procedure. In our experience, these are well tolerated by patients with a history of bariatric surgery and provide adequate colon cleansing before colonoscopy.
RATIONALE
Adequate bowel preparation by the ingestion of a cleansing agent is extremely important before colonoscopy: the quality of colon preparation affects the diagnostic accuracy and safety of the procedure, as inadequate bowel preparation has been associated with failure to detect polyps and with a higher rate of adverse events during the procedure.1–3
The most commonly used bowel preparations can be divided into high-volume (which require drinking at least 4 L of a cathartic solution) and low-volume (which require drinking about 2 L).4 Polyethylene glycol electrolyte solutions are among the most commonly used and are available in both high-volume (eg, Golytely, Nulytely) and low-volume (eg, Moviprep, Miralax) forms.
Other low-volume preparations include sodium picosulfate (Prepopik), magnesium citrate, and sodium phosphate tablets. However, these should be avoided in patients with renal insufficiency.4
Prices for bowel preparations vary. For example, the average reported wholesale price of Golytely is $24.56, Moviprep $81.17, and Miralax $10.08.4 However, the final cost depends on the patient’s insurance coverage. Generic formulations are available for some preparations.
After bariatric surgery, patients have a smaller stomach
After bariatric surgery, patients have significantly reduced stomach volume, due either to resection of a part of the stomach (such as in partial gastrectomy) or to diversion of the gastrointestinal (GI) tract to bypass most of the stomach (such as in Roux-en-Y gastric bypass). This causes early satiety with smaller amounts of food and leads to weight loss. However, this restriction in stomach volume also makes it more difficult for the patient to tolerate the intake of large volumes of fluids for bowel cleansing before colonoscopy.
Bariatric surgery patients may require colonoscopy for indications such as colorectal cancer screening, chronic diarrhea, or GI bleeding, all of which are commonly encountered during routine clinical practice.
Guidelines are available
Currently, there are no published data available to support the use of one preparation over another in patients with a history of bariatric surgery. However, for patients who have had bariatric surgery, guidelines from the US Multi-Society Task Force on Colorectal Cancer—endorsed by the three major American gastroenterology societies, ie, the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy—recommend either a low-volume solution or, if a high-volume solution is used, extending the duration over which the preparation is consumed.5 In addition, it is recommended that patients consume sugar-free drinks and liquids to avoid dumping syndrome from high sugar content.6
The use of split-dose regimens is also strongly recommended for elective colonoscopy by the US Multi-Society Task Force on Colorectal Cancer.5
Our clinical experience has been in line with the above recommendations.
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012; 75:1197–1203.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81:31–53.
- Wexner SD, Beck DE, Baron TH, et al; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgerons (SAGES). Gastrointest Endosc 2006; 63:894–909.
- ASGE Standards of Practice Committee; Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc 2015; 81:781–794.
- Johnson DA, Barkun AN, Cohen LB, et al; US Multi-Society Task Force on Colorectal Cancer. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014; 147:903–924.
- Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C; Endocrine Society. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010; 95:4823–4843.
We routinely use low-volume (2-L) polyethylene glycol electrolyte preparations such as Moviprep and Miralax in split-dose regimens, in which patients drink half of the preparation the day before the procedure and the other half the day of the procedure. In our experience, these are well tolerated by patients with a history of bariatric surgery and provide adequate colon cleansing before colonoscopy.
RATIONALE
Adequate bowel preparation by the ingestion of a cleansing agent is extremely important before colonoscopy: the quality of colon preparation affects the diagnostic accuracy and safety of the procedure, as inadequate bowel preparation has been associated with failure to detect polyps and with a higher rate of adverse events during the procedure.1–3
The most commonly used bowel preparations can be divided into high-volume (which require drinking at least 4 L of a cathartic solution) and low-volume (which require drinking about 2 L).4 Polyethylene glycol electrolyte solutions are among the most commonly used and are available in both high-volume (eg, Golytely, Nulytely) and low-volume (eg, Moviprep, Miralax) forms.
Other low-volume preparations include sodium picosulfate (Prepopik), magnesium citrate, and sodium phosphate tablets. However, these should be avoided in patients with renal insufficiency.4
Prices for bowel preparations vary. For example, the average reported wholesale price of Golytely is $24.56, Moviprep $81.17, and Miralax $10.08.4 However, the final cost depends on the patient’s insurance coverage. Generic formulations are available for some preparations.
After bariatric surgery, patients have a smaller stomach
After bariatric surgery, patients have significantly reduced stomach volume, due either to resection of a part of the stomach (such as in partial gastrectomy) or to diversion of the gastrointestinal (GI) tract to bypass most of the stomach (such as in Roux-en-Y gastric bypass). This causes early satiety with smaller amounts of food and leads to weight loss. However, this restriction in stomach volume also makes it more difficult for the patient to tolerate the intake of large volumes of fluids for bowel cleansing before colonoscopy.
Bariatric surgery patients may require colonoscopy for indications such as colorectal cancer screening, chronic diarrhea, or GI bleeding, all of which are commonly encountered during routine clinical practice.
Guidelines are available
Currently, there are no published data available to support the use of one preparation over another in patients with a history of bariatric surgery. However, for patients who have had bariatric surgery, guidelines from the US Multi-Society Task Force on Colorectal Cancer—endorsed by the three major American gastroenterology societies, ie, the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy—recommend either a low-volume solution or, if a high-volume solution is used, extending the duration over which the preparation is consumed.5 In addition, it is recommended that patients consume sugar-free drinks and liquids to avoid dumping syndrome from high sugar content.6
The use of split-dose regimens is also strongly recommended for elective colonoscopy by the US Multi-Society Task Force on Colorectal Cancer.5
Our clinical experience has been in line with the above recommendations.
We routinely use low-volume (2-L) polyethylene glycol electrolyte preparations such as Moviprep and Miralax in split-dose regimens, in which patients drink half of the preparation the day before the procedure and the other half the day of the procedure. In our experience, these are well tolerated by patients with a history of bariatric surgery and provide adequate colon cleansing before colonoscopy.
RATIONALE
Adequate bowel preparation by the ingestion of a cleansing agent is extremely important before colonoscopy: the quality of colon preparation affects the diagnostic accuracy and safety of the procedure, as inadequate bowel preparation has been associated with failure to detect polyps and with a higher rate of adverse events during the procedure.1–3
The most commonly used bowel preparations can be divided into high-volume (which require drinking at least 4 L of a cathartic solution) and low-volume (which require drinking about 2 L).4 Polyethylene glycol electrolyte solutions are among the most commonly used and are available in both high-volume (eg, Golytely, Nulytely) and low-volume (eg, Moviprep, Miralax) forms.
Other low-volume preparations include sodium picosulfate (Prepopik), magnesium citrate, and sodium phosphate tablets. However, these should be avoided in patients with renal insufficiency.4
Prices for bowel preparations vary. For example, the average reported wholesale price of Golytely is $24.56, Moviprep $81.17, and Miralax $10.08.4 However, the final cost depends on the patient’s insurance coverage. Generic formulations are available for some preparations.
After bariatric surgery, patients have a smaller stomach
After bariatric surgery, patients have significantly reduced stomach volume, due either to resection of a part of the stomach (such as in partial gastrectomy) or to diversion of the gastrointestinal (GI) tract to bypass most of the stomach (such as in Roux-en-Y gastric bypass). This causes early satiety with smaller amounts of food and leads to weight loss. However, this restriction in stomach volume also makes it more difficult for the patient to tolerate the intake of large volumes of fluids for bowel cleansing before colonoscopy.
Bariatric surgery patients may require colonoscopy for indications such as colorectal cancer screening, chronic diarrhea, or GI bleeding, all of which are commonly encountered during routine clinical practice.
Guidelines are available
Currently, there are no published data available to support the use of one preparation over another in patients with a history of bariatric surgery. However, for patients who have had bariatric surgery, guidelines from the US Multi-Society Task Force on Colorectal Cancer—endorsed by the three major American gastroenterology societies, ie, the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy—recommend either a low-volume solution or, if a high-volume solution is used, extending the duration over which the preparation is consumed.5 In addition, it is recommended that patients consume sugar-free drinks and liquids to avoid dumping syndrome from high sugar content.6
The use of split-dose regimens is also strongly recommended for elective colonoscopy by the US Multi-Society Task Force on Colorectal Cancer.5
Our clinical experience has been in line with the above recommendations.
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012; 75:1197–1203.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81:31–53.
- Wexner SD, Beck DE, Baron TH, et al; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgerons (SAGES). Gastrointest Endosc 2006; 63:894–909.
- ASGE Standards of Practice Committee; Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc 2015; 81:781–794.
- Johnson DA, Barkun AN, Cohen LB, et al; US Multi-Society Task Force on Colorectal Cancer. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014; 147:903–924.
- Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C; Endocrine Society. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010; 95:4823–4843.
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012; 75:1197–1203.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81:31–53.
- Wexner SD, Beck DE, Baron TH, et al; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgerons (SAGES). Gastrointest Endosc 2006; 63:894–909.
- ASGE Standards of Practice Committee; Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc 2015; 81:781–794.
- Johnson DA, Barkun AN, Cohen LB, et al; US Multi-Society Task Force on Colorectal Cancer. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014; 147:903–924.
- Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C; Endocrine Society. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010; 95:4823–4843.
Which patients with nonalcoholic fatty liver disease should undergo liver biopsy?
Patients should undergo biopsy to guide management and prognosis if suspected of having steatohepatitis or fibrosis.
WHAT IS NAFLD?
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in the United States and is the second most common reason for liver transplant.1 It is thought to be the hepatic consequence of systemic insulin resistance and the metabolic syndrome characterized by obesity, dyslipidemia, and type 2 diabetes mellitus.
WHAT IS THE RELATIONSHIP BETWEEN NAFLD AND NASH?
NAFLD is defined by the accumulation of hepatic fat as evidenced by imaging or histologic study and without a coexisting cause of chronic liver disease or a secondary cause of hepatic steatosis, including significant alcohol use, medications, or an inherited or acquired metabolic state.
NAFLD has two subtypes: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is characterized by steatosis, including inflammation, in at least 5% of hepatocytes. NASH is defined by a constellation of features that include steatosis, lobular and portal inflammation, and liver cell injury in the form of hepatocyte ballooning.2
Clinically, it is especially important to distinguish patients with the NASH subtype, as most NAFLD patients have steatosis without necroinflammation or fibrosis and do not require medical therapy.
CLINICAL SIGNIFICANCE OF NAFLD: NASH IS WORSE THAN NAFL
NAFL carries an excellent prognosis in terms of histologic progression of liver disease, whereas NASH can histologically progress to fibrosis and, in up to 15% of patients, to cirrhosis.3
Progression of fibrosis poses secondary risks, including complications associated with portal hypertension (ascites, variceal hemorrhage, hepatic encephalopathy), end-stage liver disease, and hepatocellular carcinoma. In Western countries, 4% to 22% of cases of hepatocellular carcinoma are attributed to NAFLD.4
In a 2015 meta-analysis, patients with NAFL and stage 0 fibrosis at baseline progressed 1 stage of fibrosis over 14.3 years, whereas patients with NASH and stage 0 fibrosis experienced an accelerated rate of progression, advancing 1 stage of fibrosis over 7.1 years.5 A systematic review of patients with NASH identified age and inflammation on initial liver biopsy as independent predictors of progression to advanced fibrosis.6
Patients with NAFLD have a higher all-cause mortality rate than patients of the same age and sex without NAFLD.7
HOW SHOULD PATIENTS WITH NAFLD BE EVALUATED?
Initial evaluation of a patient with suspected NAFLD should include a thorough serologic evaluation to exclude coexisting causes of chronic liver disease. Tests include:
- A viral hepatitis panel
- Antinuclear antibody (ANA)
- Antismooth muscle antibody (ASMA)
- Antimitochondrial antibody (AMA)
- Iron studies
- Alpha-1 antitrypsin level
- Ceruloplasmin level.
Aminotransferase levels and imaging studies (ultrasonography, computed tomography, and magnetic resonance imaging) do not reliably convey the degree of NASH and fibrosis.
Biopsy. Whereas sensitive serologic tests have been introduced to detect and diagnose many causes of liver disease, liver biopsy (transjugular or percutaneous) with histologic examination remains the only way to accurately assess the degree of steatosis and, thus, to distinguish NAFL from NASH.2
The Pathology Committee of the NASH Clinical Research Network designed and validated a NAFLD scoring system,8,9 with points allocated for degrees of:
- Steatosis (0–3)
- Lobular inflammation (0–2)
- Hepatocellular ballooning (0–2)
- Fibrosis (0–4).
A NAFLD Activity Score of less than 3 is consistent with “not NASH,” a score of 3 or 4 with borderline NASH, and a score of 5 or more with NASH.8 However, the diagnosis of NASH is not based on the NAFLD scoring system, but rather on the pathologist’s overall evaluation of the liver biopsy.9
The metabolic syndrome is an established risk factor for steatohepatitis in patients with NAFLD, and its presence in patients with persistently elevated liver biochemical tests may help identify those who would benefit from further diagnostic and prognostic evaluation, including liver biopsy.2,10 In addition, a 2008 study that used a decision-tree modeling system demonstrated that early liver biopsy could provide a survival benefit.11
NONINVASIVE TESTING
Since liver biopsy is associated with procedure-related morbidity, mortality, and cost, researchers have been developing noninvasive markers of steatohepatitis and fibrosis.12
The NAFLD fibrosis score—based on patient age, body mass index, hyperglycemia, platelet count, albumin, and ratio of aspartate aminotransferase to alanine aminotransferase—has been shown to have an area under the receiver operating curve of 0.85 for predicting advanced fibrosis, with a negative predictive value of 88% to 93% and a positive predictive value of 82% to 90%.13 The NAFLD fibrosis score can be used to identify patients who may have fibrosis or cirrhosis and can help direct the use of liver biopsy in patients who would benefit from prognostication and potential treatment.
Of note, the NAFLD fibrosis score is only slightly less accurate than the imaging techniques of magnetic resonance elastography and transient elastography, particularly when the relative costs are considered.14
INDICATIONS FOR LIVER BIOPSY
There are two clear indications for liver biopsy in NAFLD.
Before starting any pharmacologic therapy for NAFLD. Most NAFLD patients have steatosis without NASH or fibrosis and do not require medical therapy. Importantly, the available treatments have significant adverse effects—prostate cancer with vitamin E, bladder cancer and weight gain with pioglitazone, and nausea with pentoxifylline.
Diagnosis. Up to 30% of patients have elevated serum ferritin and autoantibodies, including ANA, ASMA, and AMA. Liver biopsy is often needed to exclude hemochromatosis or autoimmune hepatitis.15 Occasionally, a possible confounding drug-induced liver injury may necessitate a liver biopsy.
LIFESTYLE MODIFICATION
The first step in managing patients who have NAFLD is to treat components of the metabolic syndrome, including obesity, dyslipidemia, and type 2 diabetes.
In a randomized controlled trial in 31 obese patients with biopsy-proven NASH,16 intensive lifestyle modification (consisting of diet, behavior modification, and 200 minutes of exercise weekly for 48 weeks) was shown to improve histologic NAFLD Activity Scores, including degrees of steatosis, necrosis, and inflammation. As a result, weight loss of 7% to 9% is generally recommended for patients with NAFLD.
DRUG THERAPIES
Much research has been directed toward identifying risk factors for progression of fibrosis and toward developing new therapies for patients with NAFLD. A 2015 meta-analysis concluded that pentoxifylline and obeticholic acid improve fibrosis, while vitamin E, thiazolidinediones, and obeticholic acid improve necroinflammation associated with NASH.17
Long-term studies are needed to determine the impact of these drugs on NASH-related morbidity, mortality, and need for liver transplant.
TAKE-AWAY POINTS
- NAFLD is the leading cause of chronic liver disease in the United States and is increasing as a reason for liver transplant.
- NASH is associated with the metabolic syndrome and can progress to fibrosis, cirrhosis, and end-stage liver disease. Noninvasive markers such as the NAFLD Activity Score can be useful in identifying patients who may have advanced fibrosis and can select patients who should be directed to liver biopsy for definitive diagnosis.8,9
- Liver biopsy is the gold standard for diagnosing steatohepatitis and fibrosis and is the only diagnostic tool used in clinical trials to direct pharmacotherapy for NASH.
- Liver biopsy should be reserved for patients suspected of having NASH or fibrosis and who might benefit from therapy.
- Liver biopsy is also indicated for those NAFLD patients who have confounding laboratory findings such as an elevated ferritin level and autoantibodies including ANA, ASMA, and AMA.
- Wong RJ, Aquilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148:547–555.
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609.
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873.
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013; 10:656–665.
- Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13:643–654.
- Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009; 51:371–379.
- Adams LA, Lymp JF, St. Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129:113–121.
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321.
- Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011; 53:810–820.
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37:917–923.
- Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int 2008; 28:650–658.
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011; 43:617–649.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854.
- Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637.e7.
- Neuschwander-Tetri BA, Clark JM, Bass NM, et al; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010; 52:913–924.
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51:121–129.
- Singh S, Khera R, Allen AM, Murad H, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 2015; 62:1417–1432.
Patients should undergo biopsy to guide management and prognosis if suspected of having steatohepatitis or fibrosis.
WHAT IS NAFLD?
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in the United States and is the second most common reason for liver transplant.1 It is thought to be the hepatic consequence of systemic insulin resistance and the metabolic syndrome characterized by obesity, dyslipidemia, and type 2 diabetes mellitus.
WHAT IS THE RELATIONSHIP BETWEEN NAFLD AND NASH?
NAFLD is defined by the accumulation of hepatic fat as evidenced by imaging or histologic study and without a coexisting cause of chronic liver disease or a secondary cause of hepatic steatosis, including significant alcohol use, medications, or an inherited or acquired metabolic state.
NAFLD has two subtypes: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is characterized by steatosis, including inflammation, in at least 5% of hepatocytes. NASH is defined by a constellation of features that include steatosis, lobular and portal inflammation, and liver cell injury in the form of hepatocyte ballooning.2
Clinically, it is especially important to distinguish patients with the NASH subtype, as most NAFLD patients have steatosis without necroinflammation or fibrosis and do not require medical therapy.
CLINICAL SIGNIFICANCE OF NAFLD: NASH IS WORSE THAN NAFL
NAFL carries an excellent prognosis in terms of histologic progression of liver disease, whereas NASH can histologically progress to fibrosis and, in up to 15% of patients, to cirrhosis.3
Progression of fibrosis poses secondary risks, including complications associated with portal hypertension (ascites, variceal hemorrhage, hepatic encephalopathy), end-stage liver disease, and hepatocellular carcinoma. In Western countries, 4% to 22% of cases of hepatocellular carcinoma are attributed to NAFLD.4
In a 2015 meta-analysis, patients with NAFL and stage 0 fibrosis at baseline progressed 1 stage of fibrosis over 14.3 years, whereas patients with NASH and stage 0 fibrosis experienced an accelerated rate of progression, advancing 1 stage of fibrosis over 7.1 years.5 A systematic review of patients with NASH identified age and inflammation on initial liver biopsy as independent predictors of progression to advanced fibrosis.6
Patients with NAFLD have a higher all-cause mortality rate than patients of the same age and sex without NAFLD.7
HOW SHOULD PATIENTS WITH NAFLD BE EVALUATED?
Initial evaluation of a patient with suspected NAFLD should include a thorough serologic evaluation to exclude coexisting causes of chronic liver disease. Tests include:
- A viral hepatitis panel
- Antinuclear antibody (ANA)
- Antismooth muscle antibody (ASMA)
- Antimitochondrial antibody (AMA)
- Iron studies
- Alpha-1 antitrypsin level
- Ceruloplasmin level.
Aminotransferase levels and imaging studies (ultrasonography, computed tomography, and magnetic resonance imaging) do not reliably convey the degree of NASH and fibrosis.
Biopsy. Whereas sensitive serologic tests have been introduced to detect and diagnose many causes of liver disease, liver biopsy (transjugular or percutaneous) with histologic examination remains the only way to accurately assess the degree of steatosis and, thus, to distinguish NAFL from NASH.2
The Pathology Committee of the NASH Clinical Research Network designed and validated a NAFLD scoring system,8,9 with points allocated for degrees of:
- Steatosis (0–3)
- Lobular inflammation (0–2)
- Hepatocellular ballooning (0–2)
- Fibrosis (0–4).
A NAFLD Activity Score of less than 3 is consistent with “not NASH,” a score of 3 or 4 with borderline NASH, and a score of 5 or more with NASH.8 However, the diagnosis of NASH is not based on the NAFLD scoring system, but rather on the pathologist’s overall evaluation of the liver biopsy.9
The metabolic syndrome is an established risk factor for steatohepatitis in patients with NAFLD, and its presence in patients with persistently elevated liver biochemical tests may help identify those who would benefit from further diagnostic and prognostic evaluation, including liver biopsy.2,10 In addition, a 2008 study that used a decision-tree modeling system demonstrated that early liver biopsy could provide a survival benefit.11
NONINVASIVE TESTING
Since liver biopsy is associated with procedure-related morbidity, mortality, and cost, researchers have been developing noninvasive markers of steatohepatitis and fibrosis.12
The NAFLD fibrosis score—based on patient age, body mass index, hyperglycemia, platelet count, albumin, and ratio of aspartate aminotransferase to alanine aminotransferase—has been shown to have an area under the receiver operating curve of 0.85 for predicting advanced fibrosis, with a negative predictive value of 88% to 93% and a positive predictive value of 82% to 90%.13 The NAFLD fibrosis score can be used to identify patients who may have fibrosis or cirrhosis and can help direct the use of liver biopsy in patients who would benefit from prognostication and potential treatment.
Of note, the NAFLD fibrosis score is only slightly less accurate than the imaging techniques of magnetic resonance elastography and transient elastography, particularly when the relative costs are considered.14
INDICATIONS FOR LIVER BIOPSY
There are two clear indications for liver biopsy in NAFLD.
Before starting any pharmacologic therapy for NAFLD. Most NAFLD patients have steatosis without NASH or fibrosis and do not require medical therapy. Importantly, the available treatments have significant adverse effects—prostate cancer with vitamin E, bladder cancer and weight gain with pioglitazone, and nausea with pentoxifylline.
Diagnosis. Up to 30% of patients have elevated serum ferritin and autoantibodies, including ANA, ASMA, and AMA. Liver biopsy is often needed to exclude hemochromatosis or autoimmune hepatitis.15 Occasionally, a possible confounding drug-induced liver injury may necessitate a liver biopsy.
LIFESTYLE MODIFICATION
The first step in managing patients who have NAFLD is to treat components of the metabolic syndrome, including obesity, dyslipidemia, and type 2 diabetes.
In a randomized controlled trial in 31 obese patients with biopsy-proven NASH,16 intensive lifestyle modification (consisting of diet, behavior modification, and 200 minutes of exercise weekly for 48 weeks) was shown to improve histologic NAFLD Activity Scores, including degrees of steatosis, necrosis, and inflammation. As a result, weight loss of 7% to 9% is generally recommended for patients with NAFLD.
DRUG THERAPIES
Much research has been directed toward identifying risk factors for progression of fibrosis and toward developing new therapies for patients with NAFLD. A 2015 meta-analysis concluded that pentoxifylline and obeticholic acid improve fibrosis, while vitamin E, thiazolidinediones, and obeticholic acid improve necroinflammation associated with NASH.17
Long-term studies are needed to determine the impact of these drugs on NASH-related morbidity, mortality, and need for liver transplant.
TAKE-AWAY POINTS
- NAFLD is the leading cause of chronic liver disease in the United States and is increasing as a reason for liver transplant.
- NASH is associated with the metabolic syndrome and can progress to fibrosis, cirrhosis, and end-stage liver disease. Noninvasive markers such as the NAFLD Activity Score can be useful in identifying patients who may have advanced fibrosis and can select patients who should be directed to liver biopsy for definitive diagnosis.8,9
- Liver biopsy is the gold standard for diagnosing steatohepatitis and fibrosis and is the only diagnostic tool used in clinical trials to direct pharmacotherapy for NASH.
- Liver biopsy should be reserved for patients suspected of having NASH or fibrosis and who might benefit from therapy.
- Liver biopsy is also indicated for those NAFLD patients who have confounding laboratory findings such as an elevated ferritin level and autoantibodies including ANA, ASMA, and AMA.
Patients should undergo biopsy to guide management and prognosis if suspected of having steatohepatitis or fibrosis.
WHAT IS NAFLD?
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in the United States and is the second most common reason for liver transplant.1 It is thought to be the hepatic consequence of systemic insulin resistance and the metabolic syndrome characterized by obesity, dyslipidemia, and type 2 diabetes mellitus.
WHAT IS THE RELATIONSHIP BETWEEN NAFLD AND NASH?
NAFLD is defined by the accumulation of hepatic fat as evidenced by imaging or histologic study and without a coexisting cause of chronic liver disease or a secondary cause of hepatic steatosis, including significant alcohol use, medications, or an inherited or acquired metabolic state.
NAFLD has two subtypes: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is characterized by steatosis, including inflammation, in at least 5% of hepatocytes. NASH is defined by a constellation of features that include steatosis, lobular and portal inflammation, and liver cell injury in the form of hepatocyte ballooning.2
Clinically, it is especially important to distinguish patients with the NASH subtype, as most NAFLD patients have steatosis without necroinflammation or fibrosis and do not require medical therapy.
CLINICAL SIGNIFICANCE OF NAFLD: NASH IS WORSE THAN NAFL
NAFL carries an excellent prognosis in terms of histologic progression of liver disease, whereas NASH can histologically progress to fibrosis and, in up to 15% of patients, to cirrhosis.3
Progression of fibrosis poses secondary risks, including complications associated with portal hypertension (ascites, variceal hemorrhage, hepatic encephalopathy), end-stage liver disease, and hepatocellular carcinoma. In Western countries, 4% to 22% of cases of hepatocellular carcinoma are attributed to NAFLD.4
In a 2015 meta-analysis, patients with NAFL and stage 0 fibrosis at baseline progressed 1 stage of fibrosis over 14.3 years, whereas patients with NASH and stage 0 fibrosis experienced an accelerated rate of progression, advancing 1 stage of fibrosis over 7.1 years.5 A systematic review of patients with NASH identified age and inflammation on initial liver biopsy as independent predictors of progression to advanced fibrosis.6
Patients with NAFLD have a higher all-cause mortality rate than patients of the same age and sex without NAFLD.7
HOW SHOULD PATIENTS WITH NAFLD BE EVALUATED?
Initial evaluation of a patient with suspected NAFLD should include a thorough serologic evaluation to exclude coexisting causes of chronic liver disease. Tests include:
- A viral hepatitis panel
- Antinuclear antibody (ANA)
- Antismooth muscle antibody (ASMA)
- Antimitochondrial antibody (AMA)
- Iron studies
- Alpha-1 antitrypsin level
- Ceruloplasmin level.
Aminotransferase levels and imaging studies (ultrasonography, computed tomography, and magnetic resonance imaging) do not reliably convey the degree of NASH and fibrosis.
Biopsy. Whereas sensitive serologic tests have been introduced to detect and diagnose many causes of liver disease, liver biopsy (transjugular or percutaneous) with histologic examination remains the only way to accurately assess the degree of steatosis and, thus, to distinguish NAFL from NASH.2
The Pathology Committee of the NASH Clinical Research Network designed and validated a NAFLD scoring system,8,9 with points allocated for degrees of:
- Steatosis (0–3)
- Lobular inflammation (0–2)
- Hepatocellular ballooning (0–2)
- Fibrosis (0–4).
A NAFLD Activity Score of less than 3 is consistent with “not NASH,” a score of 3 or 4 with borderline NASH, and a score of 5 or more with NASH.8 However, the diagnosis of NASH is not based on the NAFLD scoring system, but rather on the pathologist’s overall evaluation of the liver biopsy.9
The metabolic syndrome is an established risk factor for steatohepatitis in patients with NAFLD, and its presence in patients with persistently elevated liver biochemical tests may help identify those who would benefit from further diagnostic and prognostic evaluation, including liver biopsy.2,10 In addition, a 2008 study that used a decision-tree modeling system demonstrated that early liver biopsy could provide a survival benefit.11
NONINVASIVE TESTING
Since liver biopsy is associated with procedure-related morbidity, mortality, and cost, researchers have been developing noninvasive markers of steatohepatitis and fibrosis.12
The NAFLD fibrosis score—based on patient age, body mass index, hyperglycemia, platelet count, albumin, and ratio of aspartate aminotransferase to alanine aminotransferase—has been shown to have an area under the receiver operating curve of 0.85 for predicting advanced fibrosis, with a negative predictive value of 88% to 93% and a positive predictive value of 82% to 90%.13 The NAFLD fibrosis score can be used to identify patients who may have fibrosis or cirrhosis and can help direct the use of liver biopsy in patients who would benefit from prognostication and potential treatment.
Of note, the NAFLD fibrosis score is only slightly less accurate than the imaging techniques of magnetic resonance elastography and transient elastography, particularly when the relative costs are considered.14
INDICATIONS FOR LIVER BIOPSY
There are two clear indications for liver biopsy in NAFLD.
Before starting any pharmacologic therapy for NAFLD. Most NAFLD patients have steatosis without NASH or fibrosis and do not require medical therapy. Importantly, the available treatments have significant adverse effects—prostate cancer with vitamin E, bladder cancer and weight gain with pioglitazone, and nausea with pentoxifylline.
Diagnosis. Up to 30% of patients have elevated serum ferritin and autoantibodies, including ANA, ASMA, and AMA. Liver biopsy is often needed to exclude hemochromatosis or autoimmune hepatitis.15 Occasionally, a possible confounding drug-induced liver injury may necessitate a liver biopsy.
LIFESTYLE MODIFICATION
The first step in managing patients who have NAFLD is to treat components of the metabolic syndrome, including obesity, dyslipidemia, and type 2 diabetes.
In a randomized controlled trial in 31 obese patients with biopsy-proven NASH,16 intensive lifestyle modification (consisting of diet, behavior modification, and 200 minutes of exercise weekly for 48 weeks) was shown to improve histologic NAFLD Activity Scores, including degrees of steatosis, necrosis, and inflammation. As a result, weight loss of 7% to 9% is generally recommended for patients with NAFLD.
DRUG THERAPIES
Much research has been directed toward identifying risk factors for progression of fibrosis and toward developing new therapies for patients with NAFLD. A 2015 meta-analysis concluded that pentoxifylline and obeticholic acid improve fibrosis, while vitamin E, thiazolidinediones, and obeticholic acid improve necroinflammation associated with NASH.17
Long-term studies are needed to determine the impact of these drugs on NASH-related morbidity, mortality, and need for liver transplant.
TAKE-AWAY POINTS
- NAFLD is the leading cause of chronic liver disease in the United States and is increasing as a reason for liver transplant.
- NASH is associated with the metabolic syndrome and can progress to fibrosis, cirrhosis, and end-stage liver disease. Noninvasive markers such as the NAFLD Activity Score can be useful in identifying patients who may have advanced fibrosis and can select patients who should be directed to liver biopsy for definitive diagnosis.8,9
- Liver biopsy is the gold standard for diagnosing steatohepatitis and fibrosis and is the only diagnostic tool used in clinical trials to direct pharmacotherapy for NASH.
- Liver biopsy should be reserved for patients suspected of having NASH or fibrosis and who might benefit from therapy.
- Liver biopsy is also indicated for those NAFLD patients who have confounding laboratory findings such as an elevated ferritin level and autoantibodies including ANA, ASMA, and AMA.
- Wong RJ, Aquilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148:547–555.
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609.
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873.
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013; 10:656–665.
- Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13:643–654.
- Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009; 51:371–379.
- Adams LA, Lymp JF, St. Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129:113–121.
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321.
- Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011; 53:810–820.
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37:917–923.
- Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int 2008; 28:650–658.
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011; 43:617–649.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854.
- Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637.e7.
- Neuschwander-Tetri BA, Clark JM, Bass NM, et al; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010; 52:913–924.
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51:121–129.
- Singh S, Khera R, Allen AM, Murad H, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 2015; 62:1417–1432.
- Wong RJ, Aquilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148:547–555.
- Chalasani N, Younossi Z, Lavine JE, et al; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609.
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873.
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013; 10:656–665.
- Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13:643–654.
- Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009; 51:371–379.
- Adams LA, Lymp JF, St. Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129:113–121.
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321.
- Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011; 53:810–820.
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37:917–923.
- Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int 2008; 28:650–658.
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011; 43:617–649.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854.
- Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637.e7.
- Neuschwander-Tetri BA, Clark JM, Bass NM, et al; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010; 52:913–924.
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51:121–129.
- Singh S, Khera R, Allen AM, Murad H, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 2015; 62:1417–1432.