User login
Click for Credit: Endometriosis surgery benefits; diabetes & aging; more
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Click for Credit: Missed HIV screening opps; aspirin & preeclampsia; more
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Click for Credit: STIs on the rise; psoriasis & cardiac risk; more
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Premenstrual Dysphoric Disorder: Diagnosis and Management in Primary Care
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
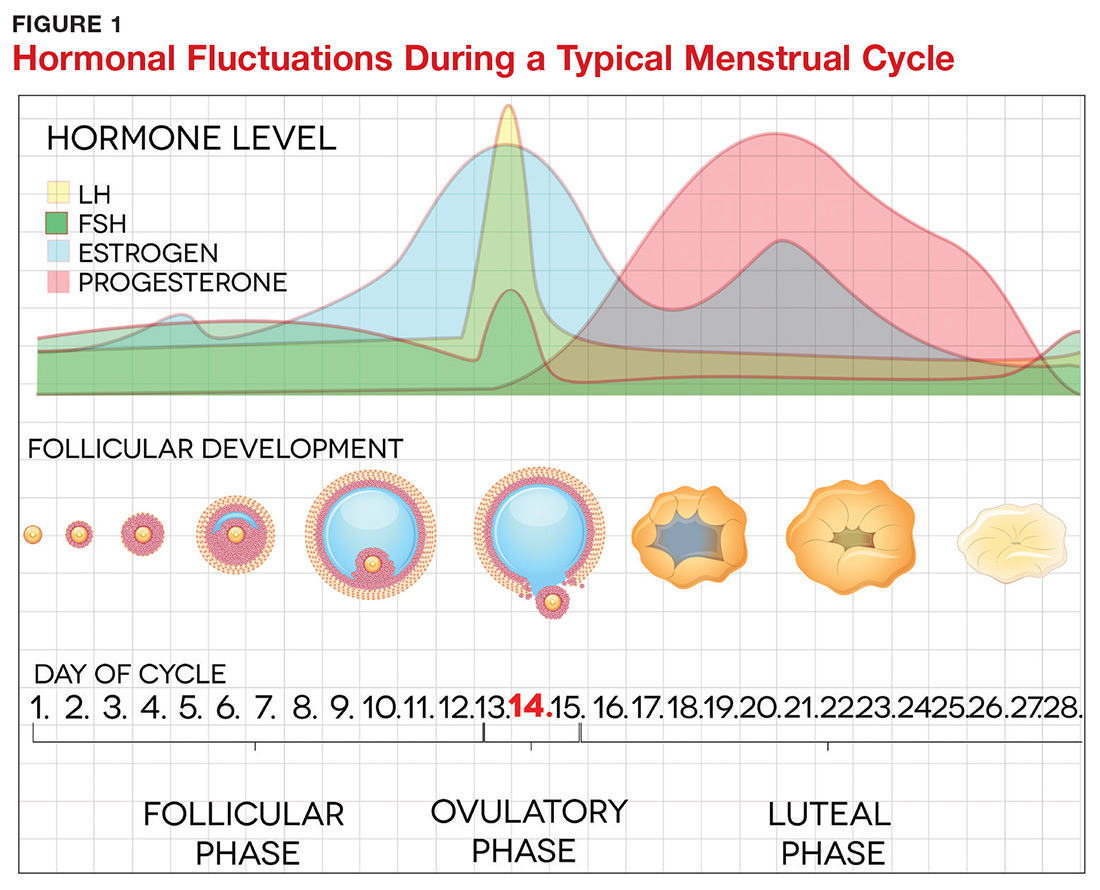
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6
This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
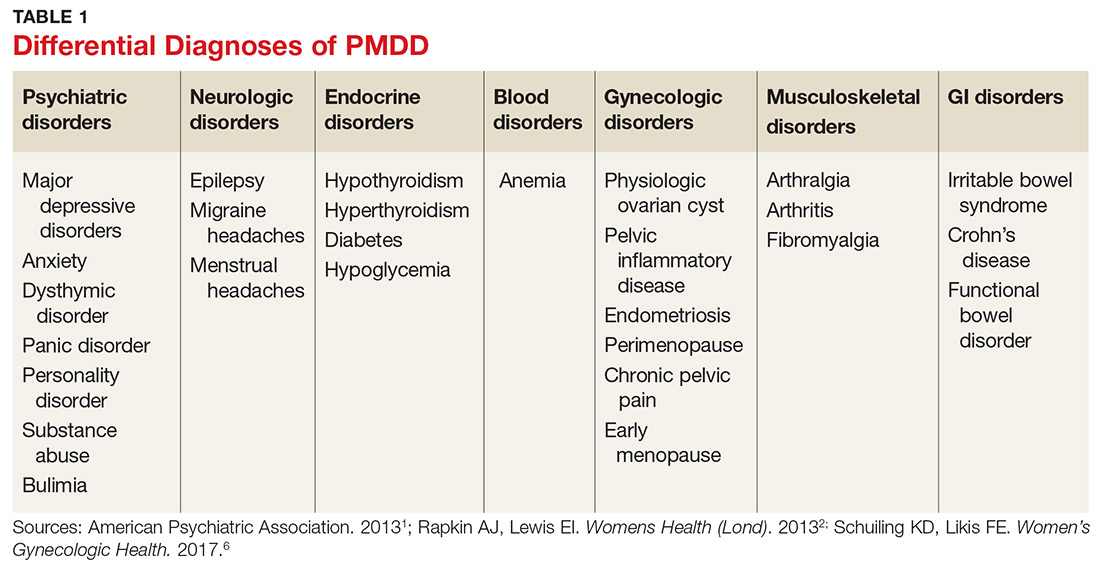
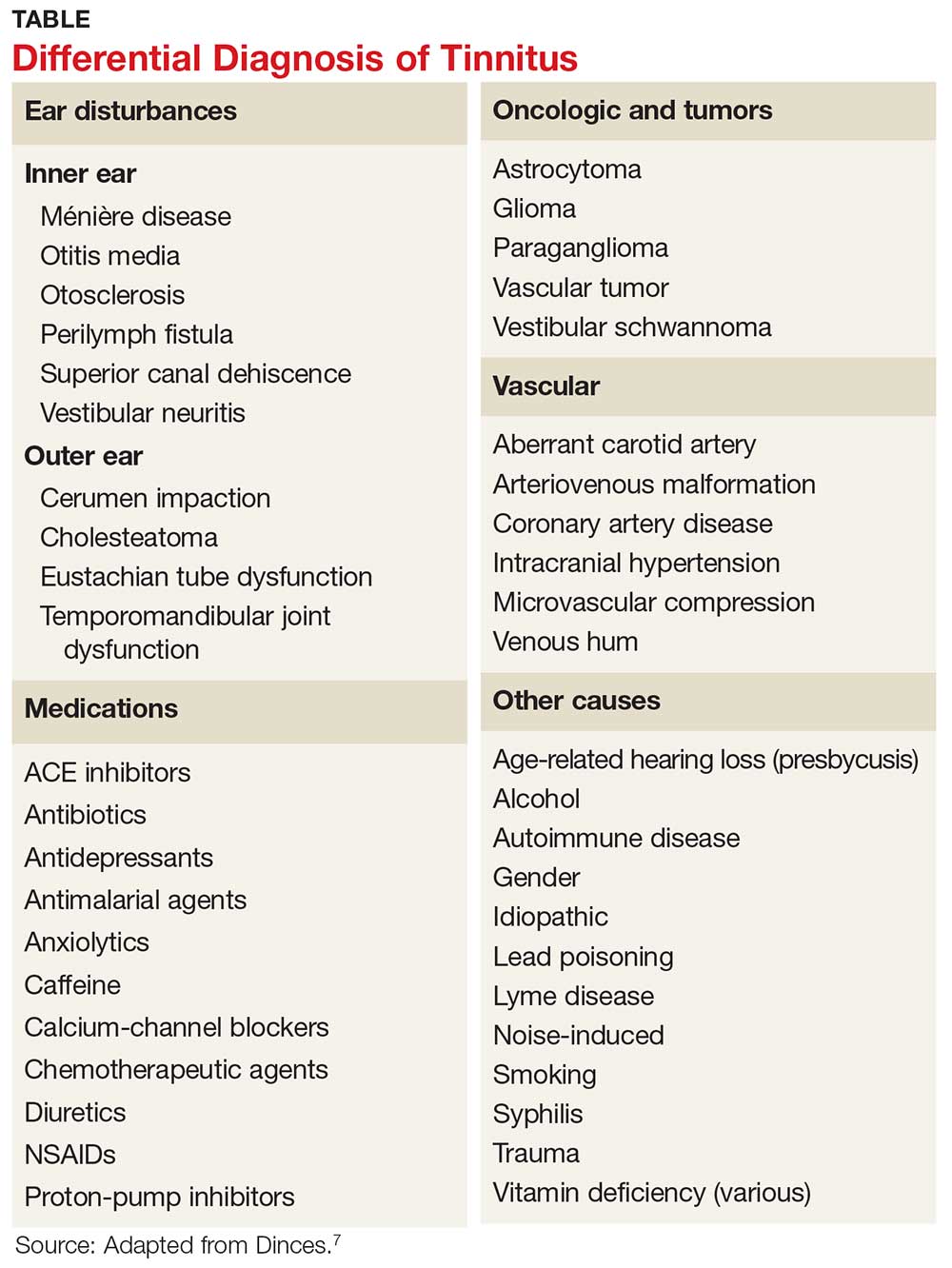
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5
It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
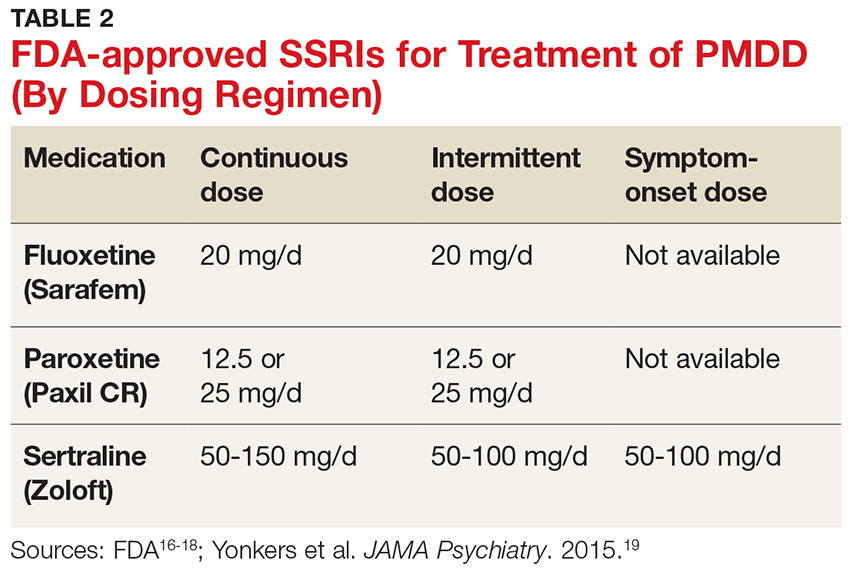
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19
Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6
This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5
It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19
Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6
This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5
It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19
Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
(CME) Going Flat Out for Glycemic Control: The Role of New Basal Insulins in Patient-Centered T2DM Management
Based on material presented at the 2018 Metabolic & Endocrine Disease Summit (MEDS), this CME supplement to Clinician Reviews provides an overview of evidence and best practices for individualizing and intensifying antihyperglycemic therapy using current basal insulin options to achieve patient-centered goals in individuals with type 2 diabetes mellitus (T2DM). Physician assistants, nurse practitioners and nurses will have the opportunity to complete pre- and post-assessment questions to earn a maximum of 1.5 free CME/CE credits.
Dr. Vanita Aroda and Ms. Davida Kruger walk readers through the following learning objectives:
- Explain the role/usage of ultralong-acting basal insulins for addressing the underlying pathophysiology of T2DM
- Compare ultralong-acting and other basal insulins regarding therapeutic characteristics, including pharmacokinetic/pharmacodynamic profiles, efficacy, safety, and dosing
- Develop patient-centered treatment regimens that include ultralong-acting insulins to minimize barriers to successful use of basal insulin therapy
Based on material presented at the 2018 Metabolic & Endocrine Disease Summit (MEDS), this CME supplement to Clinician Reviews provides an overview of evidence and best practices for individualizing and intensifying antihyperglycemic therapy using current basal insulin options to achieve patient-centered goals in individuals with type 2 diabetes mellitus (T2DM). Physician assistants, nurse practitioners and nurses will have the opportunity to complete pre- and post-assessment questions to earn a maximum of 1.5 free CME/CE credits.
Dr. Vanita Aroda and Ms. Davida Kruger walk readers through the following learning objectives:
- Explain the role/usage of ultralong-acting basal insulins for addressing the underlying pathophysiology of T2DM
- Compare ultralong-acting and other basal insulins regarding therapeutic characteristics, including pharmacokinetic/pharmacodynamic profiles, efficacy, safety, and dosing
- Develop patient-centered treatment regimens that include ultralong-acting insulins to minimize barriers to successful use of basal insulin therapy
Based on material presented at the 2018 Metabolic & Endocrine Disease Summit (MEDS), this CME supplement to Clinician Reviews provides an overview of evidence and best practices for individualizing and intensifying antihyperglycemic therapy using current basal insulin options to achieve patient-centered goals in individuals with type 2 diabetes mellitus (T2DM). Physician assistants, nurse practitioners and nurses will have the opportunity to complete pre- and post-assessment questions to earn a maximum of 1.5 free CME/CE credits.
Dr. Vanita Aroda and Ms. Davida Kruger walk readers through the following learning objectives:
- Explain the role/usage of ultralong-acting basal insulins for addressing the underlying pathophysiology of T2DM
- Compare ultralong-acting and other basal insulins regarding therapeutic characteristics, including pharmacokinetic/pharmacodynamic profiles, efficacy, safety, and dosing
- Develop patient-centered treatment regimens that include ultralong-acting insulins to minimize barriers to successful use of basal insulin therapy
Click for Credit: Short-term NSAIDs; endometriosis; more
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term NSAIDs appear safe for high-risk patients
To take the posttest, go to: https://bit.ly/2PgXKGx
Expires October 8, 2019
2. Chronic liver disease raises death risk in pneumonia patients
To take the posttest, go to: https://bit.ly/2NPSXXA
Expires October 8, 2019
3. Half of outpatient antibiotics prescribed with no infectious disease code
To take the posttest, go to: https://bit.ly/2pWEWxU
Expires October 6, 2019
4. Secondary fractures in older men spike soon after first, but exercise may help
To take the posttest, go to: https://bit.ly/2OCNl8A
Expires October 3, 2019
5. Consider ART for younger endometriosis patients
To take the posttest, go to: https://bit.ly/2NO1Sc4
Expires October 5, 2019
What’s the Buzz? Treatment Strategies in Chronic Subjective Tinnitus
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7
Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17
Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7
Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17
Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
CE/CME No: CR-1810
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Distinguish primary tinnitus from secondary tinnitus.
• Understand and implement a full clinical evaluation of tinnitus, including imaging studies when appropriate.
• Discuss expectations regarding treatment options and realistic outcomes of currently recommended therapy.
• Direct patients to specialist care for cognitive behavioral therapy or tinnitus retraining therapy.
• Know when pharmacotherapeutic intervention is indicated.
FACULTY
Wendy Gillian Ross practices urgent care medicine in Lake Grove, New York, and primary care in Patchogue, New York. Randy Danielsen is Professor and Dean, Arizona School of Health Sciences, and Director, Center for the Future of the Health Professions, both at A.T. Still University, in Mesa, Arizona. He is Physician Assistant Editor-in-Chief of Clinician Reviews.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2019.
Article begins on next page >>
Tinnitus can be a debilitating condition that affects quality of life and is often not treated according to guidelines. Cognitive behavioral therapy and tinnitus retraining therapy have been successful in reducing tinnitus bother; pharmacotherapy is not widely accepted as successful, and can, in fact, be deleterious. This article describes pathophysiologic disturbances of hearing and how they relate to chronic subjective tinnitus, discusses the clinical evaluation of tinnitus as a presenting symptom, and reviews current treatments.
Primary chronic subjective tinnitus, often thought of more as a symptom than a diagnosis, affects millions of people worldwide. This troublesome condition has been chronicled as far back as the first century
It is estimated that only 20% of people who experience tinnitus actively seek treatment.2 In the United States, 2 to 3 million of the 12 million patients who do request treatment report lasting symptoms that they describe as debilitating.3 For patients who seek help, the treatment recommended by physicians is typically pharmacotherapeutic—which does not follow guidelines.4
The aim of this article is to reinforce a greater understanding of the mechanisms of tinnitus and integrate that knowledge into treatment guidelines. The article does not discuss surgical treatment of tinnitus.
DEFINITION AND CLASSIFICATION
A universal standard definition of chronic tinnitus does not exist; Trevis et al define it as a phantom sound that persists for more than three months.5 The quality and loudness of tinnitus is variable but is often described as a buzz, hiss, or ringing. Prevalence increases with age, smoking, male gender, and ethnicity, with the non-Latino white population statistically at greater risk.3 Comorbid conditions (eg, diabetes and other autoimmune diseases) are risk factors for tinnitus. A history of exposure to loud sound—occupational, environmental, or recreational—also can predispose a person to tinnitus.3
The American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) classifies tinnitus as primary (subjective) or secondary (objective). Primary tinnitus—representing the majority of cases—has no identifiable cause; there may be accompanying sensorineural hearing loss or hyperacusis. Secondary tinnitus can also be associated with sensorineural hearing loss but has an identifiable underlying cause.6 The differential diagnosis of tinnitus is listed in the Table.7
Tinnitus is further defined by its persistence. Persistent tinnitus is defined as tinnitus lasting more than six months, slightly longer than the duration offered by Trevis et al, who also define tinnitus as bothersome or non-bothersome, depending on its impact on quality of life.5,6 Causes of reduced quality of life include depression, anxiety, insomnia, and neurocognitive decline—all of which have been associated with chronic subjective tinnitus.8
Continue to: Researchers have discovered that...
Researchers have discovered that tinnitus is not simply a cochlear phenomenon. The pathology extends well beyond the auditory complex, having a deleterious effect on both the somatosensory and central nervous systems, providing some explanation for the prevalence of anxiety and depression associated with the disorder (see "Pathophysiology of tinnitus").9-17
Because of the insidious nature of tinnitus and lack of standard measures of severity, true prevalence is difficult to calculate.18
CLINICAL EVALUATION
Tinnitus can be a presenting complaint or elicited during history-taking. Symptomatic patients should receive full evaluation, including a complete physical exam, medication history, and laboratory workup.
Adverse effect of drugs
Medications that commonly cause tinnitus symptoms are NSAIDs, chemotherapeutic agents, and antibiotics (eg, macrolides and fluoroquinolones). Amiodarone, ACE inhibitors, proton-pump inhibitors, and calcium-channel blockers have also been implicated. Paradoxically, anxiolytics and tricyclic antidepressants, which are sometimes used to treat tinnitus, have been linked to causing the condition.7
Laboratory tests and imaging
Testing should include investigation for infectious disease, autoimmune disorders, and vitamin deficiency.7 According to the American College of Radiology, imaging is unnecessary in the workup of primary tinnitus. Any suspicion of a vascular cause noted on the physical exam (eg, an associated bruit or venous hum), however, should be explored with imaging. Furthermore, any case of tinnitus that lateralizes also requires additional investigation. Modalities of choice are MRI, CT, and CT angiography.19
Continue to: Referral for audiology evaluation
Referral for audiology evaluation
When no underlying pathology can be identified for tinnitus, the patient should be sent for a full audiology evaluation to screen for associated hearing loss. Discussion of audiology screening tests is beyond the scope of this article; however, testing includes otoscopy, audiography, tympanography, otoacoustic emission testing, auditory brainstem-response testing, and vestibular evoked myogenic potential testing.7
Probing nonphysical impacts
Quality of life and overall emotional wellness, including cognitive function, should be investigated in patients with tinnitus. Two questionnaires commonly used in the assessment of tinnitus bother are the Tinnitus Handicap Inventory and the Tinnitus Reaction Questionnaire.7 In a large, systematic review, Trevis et al report that “64% of studies investigating depression found an increase in depressive symptoms in people with chronic tinnitus compared to hearing control groups, and 62% of studies investigating anxiety reported significantly increased anxiety symptoms.”5
MANAGEMENT
Tinnitus management should be viewed two ways: treatment of perceived loudness and treatment of comorbid symptoms relating to tinnitus bother.6 In the same meta-analysis, Trevis and colleagues found that patients with tinnitus had higher rates of anxiety, depression, and overall decline in cognitive function, including processing speed, concentration, and sleep disorders.5 It is useful to keep this observation in mind when reviewing treatment options for tinnitus.
Five classic pharmacotherapeutic approaches to tinnitus management are
- Anticonvulsants
- Antidepressants
- Anesthetics
- Anxiolytics
- Lidocaine.
Newer medications that show some promise are N-methyl-D-aspartate (NMDA) receptor antagonists, notably neramexane. Alternative pharmaceuticals include vitamin-based treatments, cannabinoids, and herbal compounds.
Continue to: The AAOS-HNS supports...
The AAO–HNS supports nonpharmacotherapeutic treatment of tinnitus; its guidelines include a recommendation for cognitive behavioral therapy (CBT) as primary therapy.6 In addition, tinnitus-retraining therapy, tinnitus-masking therapy/sound therapy, meditation/mindfulness, and yoga all have been studied for their ability to alleviate tinnitus bother.
Pharmacotherapeutic management
Anticonvulsants have failed to provide strong evidence of usefulness in the treatment of tinnitus and are not supported by the AAO–HNS as such.6 This conclusion notwithstanding, the anticonvulsants carbamazepine and gabapentin have historically been two of the more common medications used to treat tinnitus.
Carbamazepine is a glutamate receptor antagonist that suppresses seizure activity. Based on prior research suggesting that spontaneous firing within the auditory complex is similar to seizure activity, Iranian researchers explored the hypothesis that carbamazepine might lessen tinnitus severity. Their study revealed, however, that carbamazepine did not statistically significantly reduce the severity of tinnitus, compared to placebo.20 While carbamazepine may be of limited use in the treatment of subjective tinnitus, recent literature confirms that it is not only useful, but also diagnostic, in typewriter tinnitus (ie, having a staccato quality, like the sound of typewriter keys being depressed). Typewriter tinnitus is a secondary cause of tinnitus related to disruption of the stapes in the middle ear.21
Gabapentin works by promoting gamma-aminobutyric acid (GABA) production in the brain. GABA is an inhibitory neurotransmitter, thus slowing down signals between neurons. Following on preliminary research that detected low levels of GABA in the inferior colliculus of rodents with salicylate-induced tinnitus, Aazh and colleagues conducted a double-blind study of gabapentin—and concluded that it yielded no improvement in symptoms, compared to placebo.22
Valproic acid has not been formally investigated but is commonly incorporated in the treatment of tinnitus.23 Lamotrigine has provided similarly disappointing results in the treatment of tinnitus.24
Continue to: Antidepressants and anxiolytics
Antidepressants and anxiolytics. Based on the results of their early clinical trials, Sullivan and colleagues concluded that tricyclic antidepressants produced significant improvement in tinnitus symptoms, due to the analgesic effects of these drugs. The researchers studied nortriptyline specifically; in severely depressed patients, the drug reduced the loudness of tinnitus and depressive symptoms. In non-depressed subjects, however, nortriptyline was not as efficacious.25
Selective serotonin reuptake inhibitors have not had the same success as nortriptyline. In a study of paroxetine conducted by Oishi and colleagues, there was little evidence that the drug reduced the loudness of tinnitus, although overall, it did reduce tinnitus bother and anxiety.26
Included in the category of anxiolytics, benzodiazepines have long been used to treat severe tinnitus-induced anxiety, with some success. However, as Elgoyhen and Langguth point out, studies of benzodiazepines for tinnitus have been limited in size.23
The AAO–HNS does not support routine use of antidepressants and anxiolytics for tinnitus bother.7
NMDA receptor antagonists. In a recent clinical trial, neramexane was studied for its efficacy in tinnitus. Neramexane acts at the cholinergic nicotinic and NMDA receptors in the efferent auditory system. Its complex reaction is thought to prevent transmission of unwanted sound not only to structures within the auditory system but beyond, to the medial geniculate body and lateral nucleus of the amygdala. The trial has proved some benefit concerning overall perception of tinnitus loudness; a phase 2 trial is being conducted.27
Continue to: Intra-tympanic anesthetics
Intra-tympanic anesthetics. Anesthetics, such as lidocaine, have had limited success and results have not been found to be sustained.
Alternative medical managements
Traditional Chinese herbal medications have been used for centuries and are increasingly popular in Western culture. Hilton and colleagues studied Ginkgo biloba, or maidenhair tree, a traditional Chinese herbal supplement available as an extract and as dried leaves. The main action of the extract is vasoregulatory; antiplatelet effects are also seen. Adverse effects include gastrointestinal upset and headache. In a systematic review, Hilton and colleagues concluded that Ginkgo did not reduce overall tinnitus loudness or severity; the review was limited, however, by the fact that only two studies met criteria for inclusion.28
Vitamins, lipoflavinoids, zinc, manganese, and melatonin are all supplements marketed to improve tinnitus symptoms. However, a cross-sectional study confirmed prior research that did not show any benefit from the use of these supplements.29
Cannabinoids are being studied for their proposed antiepileptic effects. There is a popular misconception of Cannabis as a singular chemical when in fact, it is a plant that contains hundreds of chemicals that each act differently on the brain. In a review, Smith and Zheng30 explain that two cannabinoid receptors, CB1 and CB2, are represented, and exert their effects, in different areas of the brain. CB1 receptors block calcium influx in presynaptic terminals, resulting in an inhibitory effect on neurotransmitter release.
CB1 receptors have been found in the dorsal cochlear nuclei, prompting research interest in how cannabinoids affect neurotransmission of unwanted sounds of tinnitus. To date, however, there are conflicting data concerning the benefit of cannabinoids and tinnitus. In fact, Smith and Zheng state that some data suggest that cannabinoids might make tinnitus worse.30
Continue to: Nonpharmacotherapeutic management
Nonpharmacotherapeutic management
Cognitive behavioral therapy. Conceptualized by Aaron T. Beck in the 1960s, cognitive behavioral therapy (CBT) is the leading recommendation made by the AAO–HNS in its tinnitus treatment guidelines.6 Beck’s work centered on the idea that behaviors are modifiable thoughts, through analysis of past experiences and assumptions based on those experiences. By understanding the core belief that a patient attaches to a feeling, Beck hypothesized that behaviors or responses to those feelings could be changed; this is accomplished through discussion to dispel unwarranted fears and by teaching coping mechanisms, such as relaxation. The idea behind CBT in the management of tinnitus is clear: The sound cannot be eliminated, but the patient’s response to the sound can be modified. Ultimately, through this modified response or habituation, the patient can relax and live with the sound.31
Since anxiety, depression, and insomnia are common comorbidities of tinnitus, a psychologic approach remains in the forefront of treatment recommendations. Hoare and colleagues reported that in “a meta-analysis of 10 randomized trials evaluating different forms of CBT (by the therapist and over the Internet), CBT improved tinnitus symptoms compared to non-CBT controls.”7
Tinnitus retraining therapy (TRT) is another form of habituation therapy, introduced by Jastreboff in the 1990s. His work furthered the idea that tinnitus could be reframed, as it is in CBT. Simply, he proposed that systems outside the auditory complex—namely the autonomic nervous system and the limbic system—respond to the signal produced by damaged hair cells in the cochlear nuclei. TRT retrains connections to block or ignore these signals.13 Unlike CBT, the aim of TRT is to eliminate the perception of sound.
By educating patients about the physiologic mechanisms of tinnitus, TRT reduces patient anxiety related to the sound. The process of habituation follows counseling. To accomplish this, the patient wears a sound generator, similar in appearance to hearing aids, using broadband noise. The sound does not mask the tinnitus but closes the gap between silence and the perception of tinnitus. The sound generator is worn for six hours daily for approximately 12 months.
Multiple studies have employed Jastreboff’s original technique, including a clinical trial by Bauer and colleagues. The published outcome of this study confirmed that patients experienced a positive and lasting effect with TRT.32 In addition, a small study of TRT conducted by Barozzi and colleagues, using different colors of sound (ie, how the frequency of a given sound corresponds to the light-wave frequency of a particular color), found statistically significant improvement. Allowing patients to pick a sound that they found more pleasant increased the effectiveness of the treatment.33 (Patients can learn more about TRT by visiting www.tinnitus-pjj.com, hosted by tinnitus researcher Pawel J. Jastreboff.)
Continue to: Alternative nonmedical therapies...
Alternative nonmedical therapies have become popular; they include meditation, yoga, physical therapy, mindfulness, and tinnitus-masking treatment with sound.
Results of a study of yoga and meditation showed that patients felt more relaxed, but that these interventions had no effect on the severity of tinnitus. The principle behind yoga practice, according to Köksoy and colleagues, is that the discipline is thought to affect the limbic system by deactivating the sympathetic response to stimulation from surrounding sounds. In addition, Köksoy states, other researchers have provided evidence that yoga increases circulating levels of antioxidants, which in turn reduce oxidative stress.34
Particularly among members of the millennial generation, mindfulness has become a buzzword. The practice refers to a “method for facing, exploring, and alleviating suffering by relating to present experiences.”35 Roland and colleagues conducted a clinical trial of mindfulness practiced by a cohort of patients with bothersome tinnitus; results were based on scores gleaned from standard rating scales (eg, Global Bothersome Scale, Cognitive and Affective Mindfulness Scale-Revised, Cognitive Failures Questionnaire, Tinnitus Handicap Inventory, and Tinnitus Functional Index). Evaluated before and four weeks after cessation of therapy, subjects reported that tinnitus bother was reduced, but none showed statistically significant improvement in depression, anxiety, or cognitive ability.35
Used for more than 40 years, sound-based therapy has been discussed in conjunction with TRT.36 It is recognized as an approved but optional treatment by the AAO–HNS. In response to a 2010 study by Hobson that used sound-based therapy alone for tinnitus, Tunkel and colleagues cautioned that the modality showed little benefit. The major downside to acoustic therapy, according to the AAO–HNS clinical guidelines, is cost and patients’ excessive expectation of effectiveness.6
According to the AAO–HNS, repetitive-transcranial magnetic stimulation is not supported as a valid treatment for tinnitus because it can lead to seizures in patients who are taking medication that lowers the seizure threshold or who have a secondary cause of tinnitus, such as a tumor—therefore creating risk that outweighs any benefit.6
Continue to: CONCLUSION
CONCLUSION
For a large percentage of the population, chronic subjective tinnitus is a significant variable in the evaluation of quality of life. The condition is not completely understood and often displays features unique to the individual. Much of the initial response to research linking tinnitus with shared pathways typical for chronic pain, anxiety, and depression has resulted in pharmacotherapeutic management that is not always warranted—or successful.
Clinical research into the pathophysiology of tinnitus is providing a better understanding of the neurophysiologic mechanisms that underpin the science of chronic tinnitus. With this information, researchers can one day design medical management that targets specific receptors, resulting in greater management success.
The psychologic impact of tinnitus cannot be underestimated. When almost one-third of patients complain of debilitating symptoms that can also result in neurocognitive decline, tinnitus becomes a condition that cannot be ignored. Guidelines set forth by the AAO–HNS state that CBT and TRT offer some reprieve from symptoms and teach patients habituation without further damage to hearing. The use of broad-based sound generators has been well established as a useful management tool, although it is not curative.
The limitations of some studies that reviewed alternative medicines include small sample size and difficulty comparing research analysis because of disparities in tinnitus rating scales. Also, age bias, comorbid conditions, and study drop-out rates affected overall statistical significance of some studies. Additional, high-quality research is warranted in this area.
Continue to: Prevention of tinnitus...
Prevention of tinnitus through education on hearing loss and its causes should be regarded as implicit; occupational noise and recreational use of music devices put people at heightened risk for hearing loss and tinnitus. Information and open discussion that include the discovery of tinnitus symptoms during routine physical examination are recommended.
Last, providers who adhere to recognized guidelines will aid patients in coping with the challenges that tinnitus presents. As research continues to unravel the complex interaction between neurons, medical science is hopeful that curative treatments will become available.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
1. Maltby MT. Ancient voices on tinnitus: the pathology and treatment of tinnitus in Celsus and the Hippocratic Corpus compared and contrasted. Int Tinnitus J. 2012;17(2):140-145.
2. Wolever RQ, Price R, Hazelton GA, et al. Complementary therapies for significant dysfunction from tinnitus: treatment review and potential for integrative medicine. Evid Based Complement Alternat Med. 2015;15:931418.
3. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718.
4. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-965.
5. Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62-86.
6. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
7. Dinces EA. Treatment of tinnitus. UpToDate. April 12, 2018. www.uptodate.com/contents/treatment-of-tinnitus. Accessed September 17, 2018.
8. Gudwani S, Munjal SK, Panda NK, Kohli A. Association of chronic subjective tinnitus with neuro-cognitive performance. Int Tinnitus J. 2017;21:90-97.
9. Jastreboff PJ. 25 years of tinnitus retraining therapy. HNO. 2015;63:307-311.
10. Pujol R. Journey into the world of hearing. 2016. www.cochlea.eu/en. Accessed September 17, 2018.
11. Adjamian P, Hall DA, Palmer AR, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain.Neurosci Biobehav Rev. 2014;45:119-133.
12. Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-160.
13. Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17(2):236-240.
14. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52-60.
15. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819-826.
16. Møller AR. Sensorineural tinnitus: its pathology and probable therapies. Int J Otolaryngol. 2016;2016:2830157.
17. Chen YC, Xia W, Chen H, et al. Tinnitus distress is linked to enhanced resting‐state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp. 2017;38(5):2384-2397.
18. McCormack A, Edmonson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
19. Kessler MM, Moussa M, Bykowski J, et al; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Tinnitus. J Am Coll Radiol. 2017;14:S584-S591.
20. Gerami H, Saberi A, Nemati, S, et al. Effects of oxcarbazepine versus carbamazepine on tinnitus: a randomized double-blind placebo-controlled clinical trial. Iran J Neurol. 2012;11(3):106-110.
21. Sunwoo W, Jeon YJ, Bae YJ, et al. Typewriter tinnitus revisited: the typical symptoms and the initial response to carbamazepine are the most reliable diagnostic clues. Sci Rep. 2017;7:10615.
22. Aazh H, El Refaie A, Humphriss R. Gabapentin for tinnitus: a systematic review. Am J Audiol. 2011;20:151-158.
23. Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300-305.
24. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
25. Sullivan M, Katon W, Russo J, et al. A randomized trial of nortriptyline for severe chronic tinnitus. Effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251-2259.
26. Oishi N, Kanzaki S, Shinden S, et al. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol Neurootol. 2010;15(3):187-193.
27. Suckfüll M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1.
28. Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;CD003852. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003852.pub3/full. Accessed September 17, 2018.
29. Coelho C, Tyler R, Ji H, et al. Survey on the effectiveness of dietary supplements to treat tinnitus. Am J Audiol. 2016;25:184-205.
30. Smith PF, Zheng Y. Cannabinoids, cannabinoid receptors and tinnitus. Hear Res. 2015;332:210-216.
31. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005233.pub3/full. Accessed September 17, 2018.
32. Bauer CA, Berry JL, Brozoski TJ. The effect of tinnitus retraining therapy on chronic tinnitus: a controlled trial. Laryngoscope Investig Otolaryngol. 2017;2(4):166-177.
33. Barozzi S, Ambrosetti U, Callaway SL, et al. Effects of tinnitus retraining therapy with different colours of sound. Int Tinnitus J. 2017;21:139-143.
34. Köksoy S, Eti CM, Karatas¸ M, Vayisoglu Y. The effects of yoga in patients suffering from subjective tinnitus. Int Arch Otorhinolaryngol. 2018;22(1):9-13.
35. Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926.
36. Ibarra D, Tavira-Sanchez F, Recuero-Lopez M, Anthony BW. In-ear medical devices for acoustic therapies in tinnitus treatments, state of the art. Auris Nasus Larynx. 2018;45:6-12.
Clone of Hot Topics in Primary Care 2018
Click here to read the full supplement.
This supplement offers the opportunity to earn a total of 1.0 CME credits.
Credit is awarded for successful completion of the online evaluations at the link below. This link may also be found within the supplement on the first page of the article.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
Click here to read the full supplement.
This supplement offers the opportunity to earn a total of 1.0 CME credits.
Credit is awarded for successful completion of the online evaluations at the link below. This link may also be found within the supplement on the first page of the article.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
Click here to read the full supplement.
This supplement offers the opportunity to earn a total of 1.0 CME credits.
Credit is awarded for successful completion of the online evaluations at the link below. This link may also be found within the supplement on the first page of the article.
- Long-term Treatment of Gout: New Opportunities for Improved Outcomes
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to www.pceconsortium.org/gout.
Click for Credit: Alcohol use while breastfeeding; cigarette smoking in HCV; more
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Diverticulitis: A Primer for Primary Care Providers
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7
Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.
RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17
PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7
Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.
RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17
PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7
Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.
RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17
PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.