User login
Pigmented Lesion on the Left Shoulder in an Older Woman
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
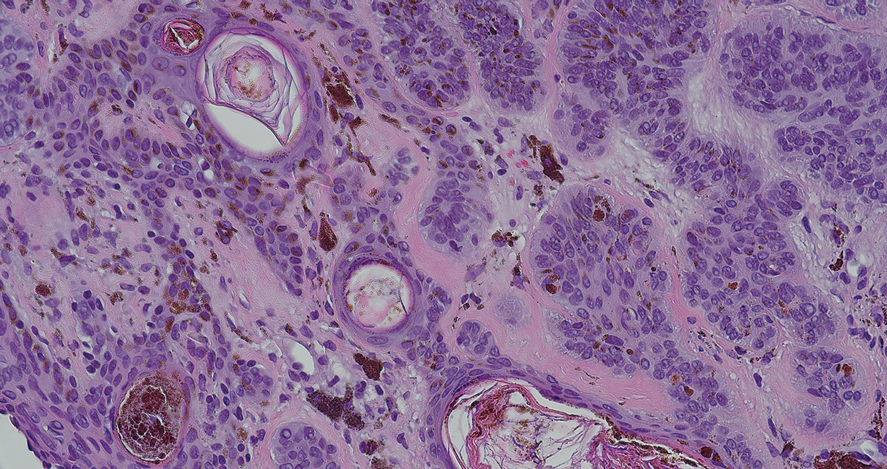
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.
Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
The Diagnosis: Pigmented Nodular Basal Cell Carcinoma
Dermoscopy of our patient’s irregular dark brown papule revealed large blue clustered clods and radial lines converging to a central dot (middle quiz image). Histopathology revealed nests of basaloid cells with peripheral palisading, small horn pseudocysts, and deposits of melanin extending into the dermis (Figure). These findings were consistent with a diagnosis of pigmented nodular basal cell carcinoma (BCC).
Nodular BCC represents 60% to 80% of all BCC cases; pigmented BCC represents 6% of BCC cases.1 Basal cell carcinomas frequently manifest as pearly papules with areas of pigment, surface telangiectases, and foci of ulceration. Dermoscopic features include fine arborizing vessels, blue-gray ovoid nests, spoke wheel–like structures, leaflike structures, and focal ulceration.1 Histopathology shows well-defined dermal nodules comprising basaloid epithelial cells with peripheral palisading, mucinous stroma, focal melanin deposits, and surrounding clefting.2 Arborizing vessels correspond to dilated vessels in the dermis.3 Blue-gray ovoid nests are wellcircumscribed ovoid or elongated structures that correspond histologically to well-defined large tumor nests with melanin aggregates invading the dermis. Spoke wheel–like structures are well-circumscribed radial projections connected to a pigmented central axis that correspond histologically to tumor nests near the epidermis and that appear as fingerlike projections with centrally located melanin deposits.3
The differential diagnosis of our patient’s lesion included nodular melanoma, lentigo maligna melanoma, deep penetrating nevus, and cellular blue nevus. Nodular melanoma is an invasive melanoma that lacks a radial growth phase. Dermoscopically, the more common features are a bluewhite veil, atypical vascular pattern, asymmetric pigmentation, atypical pigment network, and peripheral black globules.4 Histopathology reveals atypical melanocytes and architectural disorder.2 Pigmented nodular BCC also can display dark globules on dermoscopy but typically has smaller and more arborizing blood vessels and does not have a pigmented network. Furthermore, BCC would not have atypical melanocytes on histopathology.4,5
Dermoscopy of lentigo maligna melanoma displays hyperpigmented follicular openings, an annular-granular pattern, pigmented rhomboidal structures, and obliterated hair follicles.6 Histopathology demonstrates epidermal atrophy, increased pigmentation in basal keratinocytes, prominent solar elastosis, and an increased number of melanocytes that extend beyond the epidermis. 7 Pigmented nodular BCC can be distinguished from lentigo maligna melanoma dermoscopically by the presence of arborizing vessels, blue-gray ovoid nests, and lack of a pigment network.

Deep penetrating nevus is a darkly pigmented melanocytic lesion that infiltrates deeply into the reticular dermis.8 Specific dermoscopic features have not been well established; however, a uniformly dark blue or black pattern is common. Histologically, this type of nevus is symmetric and wedge shaped with a broad base extending to the deep dermis and subcutaneous fat.8 Melanocytes do not exhibit atypia or bizarre mitoses. Although pigmented nodular BCC can appear similar to deep penetrating nevus, histologically there will be atypical basaloid epithelial cells in BCC.
Blue nevi clinically appear as a smooth blue-gray lesion with a steel blue ground-glass pattern on dermoscopy. Histopathology shows spindle-shaped melanocytes in the dermis, which distinguishes this lesion from BCC.9
Consider pigmented BCC when a patient presents with a pigmented lesion. Dermoscopy can help appreciate a pigmented BCC by looking for features such as a spoke wheel– like pattern, blue ovoid nests, arborizing blood vessels, and lack of a pigment network. Because pigmented BCC constitutes a small fraction of all BCCs, it is important to be familiar with its presentation and dermoscopic features.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
- Heath MS, Bar A. Basal cell carcinoma. Dermatol Clin. 2023;41:13-21. doi:10.1016/j.det.2022.07.005
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28:1005-1012.
- Wozniak-Rito A, Zalaudek I, Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-247. doi:10.1111/ced.13387
- Menzies SW, Moloney FJ, Byth K, et al. Dermoscopic valuation of nodular melanoma. JAMA Dermatol. 2013;149:699-709. doi:10.1001 /jamadermatol.2013.2466
- Pizzichetta MA, Kittler H, Stanganelli I, et al; Italian Melanoma Intergroup. Pigmented nodular melanoma: the predictive value of dermoscopic features using multivariate analysis. Br J Dermatol. 2015;173:106-114. doi:10.1111/bjd.13861
- Pralong P, Bathelier E, Dalle S, et al. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280-287. doi:10.1111/j.1365-2133.2012.10932.x
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838-841. doi:10.5858/2011-0051-RAIR.1
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240. doi:10.1016/j .jaad.2014.07.026
- Ferrera G, Argenziano G. Blue nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al, eds. Color Atlas of Melanocytic Lesions of the Skin. Springer; 2007:78-86.
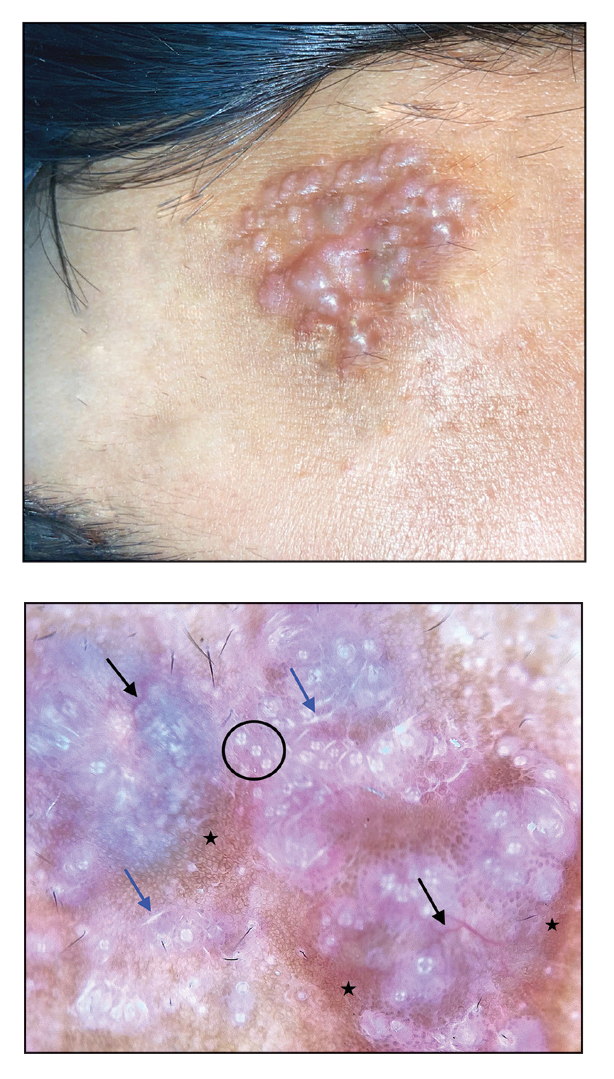
A 92-year-old woman presented to dermatology as a new patient for a full-body skin examination. She had a history of sarcoidosis and a liposarcoma that had been excised more than 20 years prior. She had no history of skin cancer; however, her granddaughter recently was diagnosed with melanoma. Physical examination revealed a 5-mm, irregular, dark brown papule on the left shoulder (top) that was evaluated by dermoscopy (middle). A tangential biopsy was performed for histopathologic analysis (bottom).
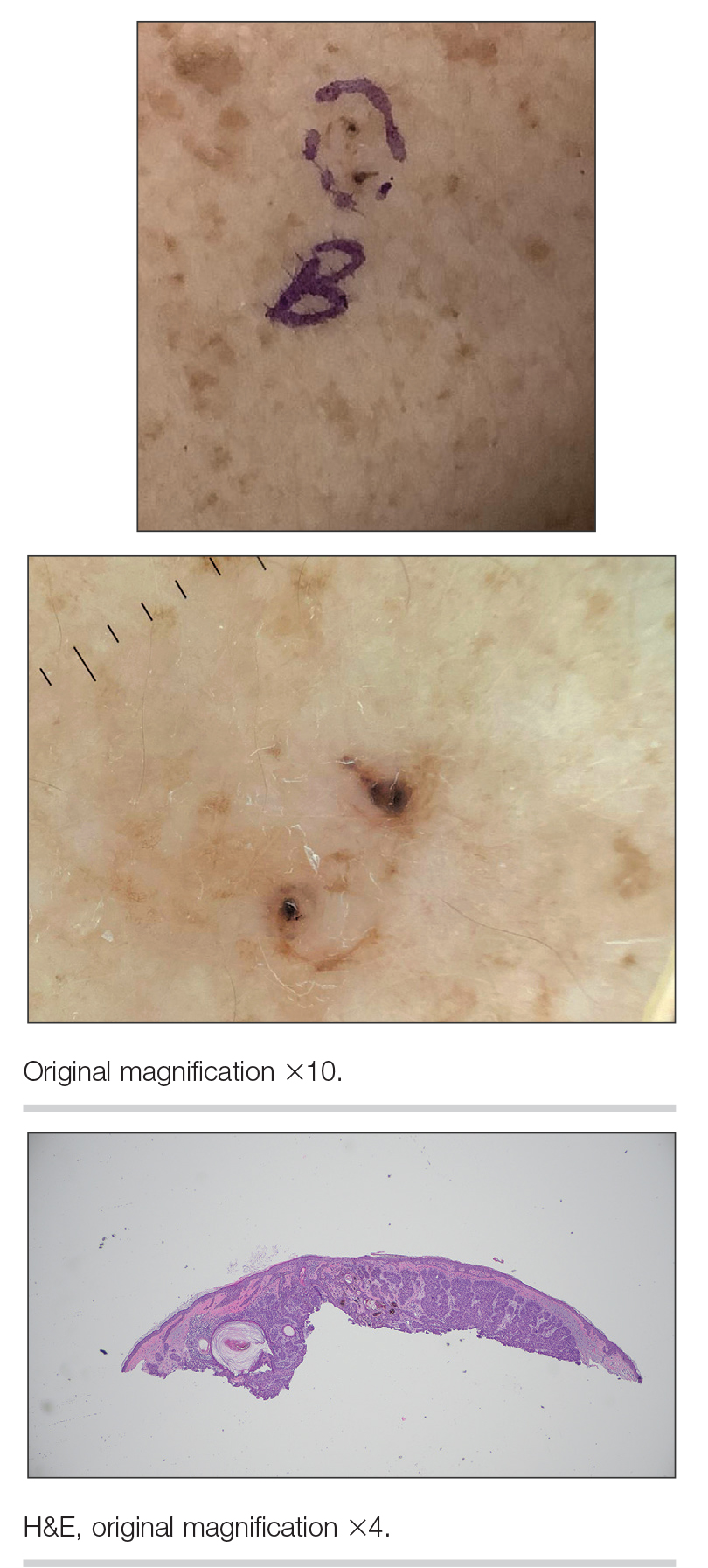
Noduloplaque on the Forehead
The Diagnosis: Giant Apocrine Hidrocystoma
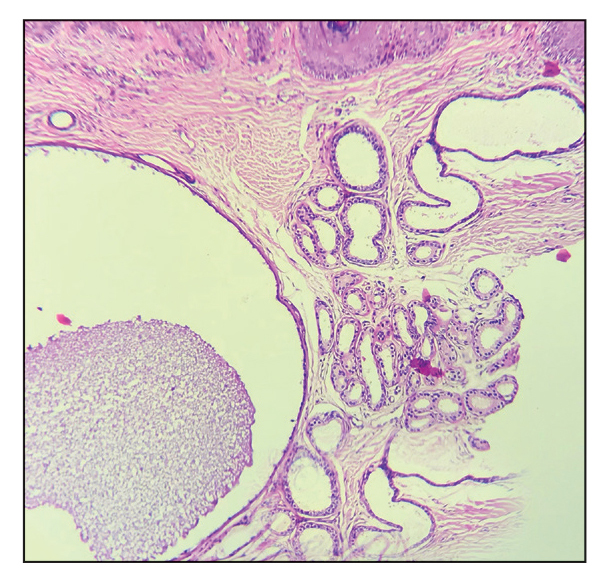
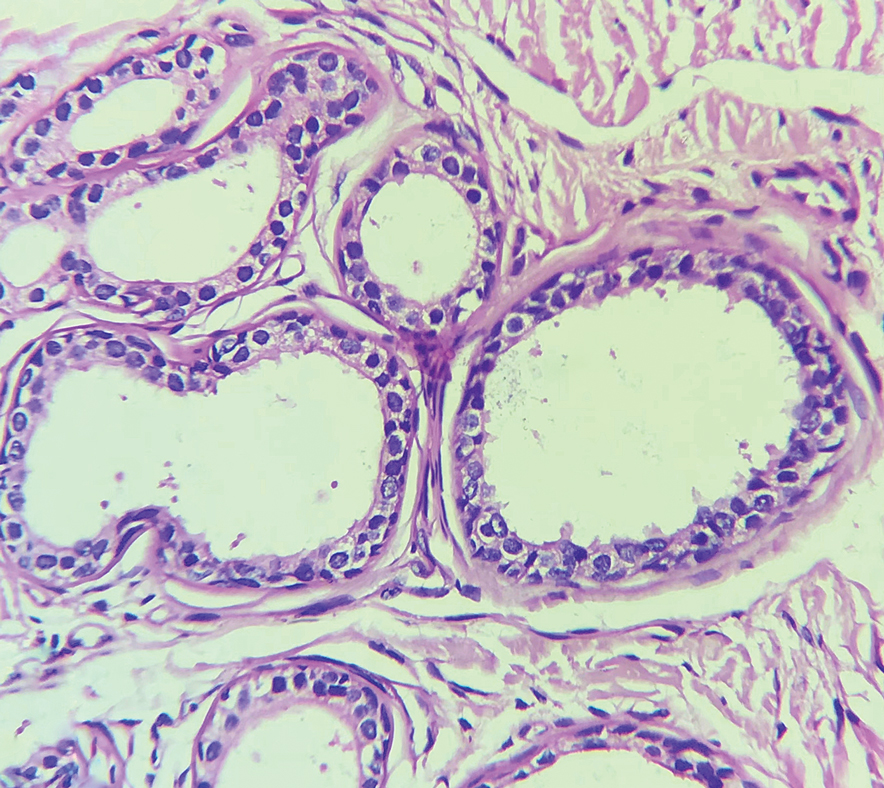
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.
Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
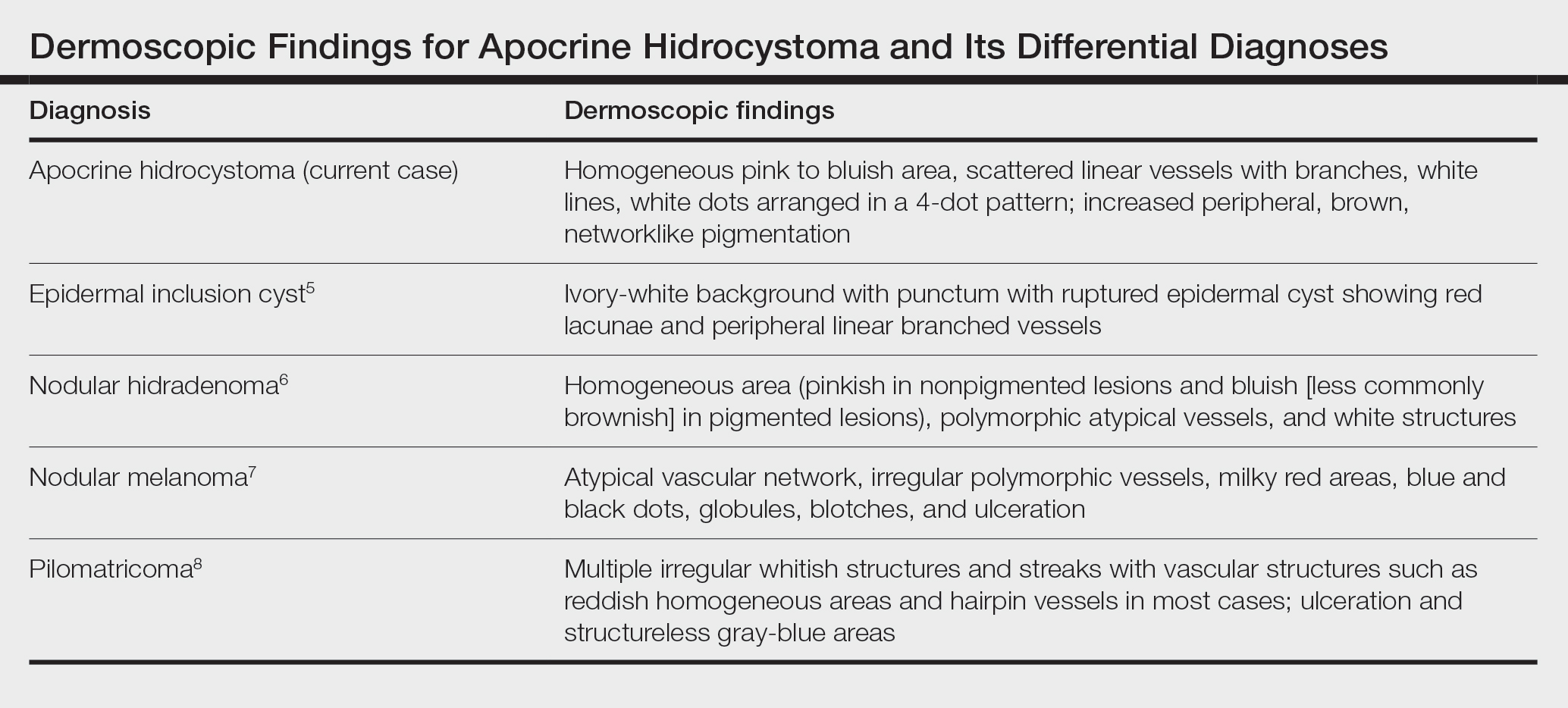
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.
The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
A 21-year-old man presented with a raised lesion on the forehead that had started as a single papule 16 years prior and gradually increased in number and size. There were no associated symptoms and no history of seasonal variation in the size of the lesions. Physical examination revealed multiple erythematous to slightly bluish translucent papules that coalesced to form a 3×3-cm noduloplaque with cystic consistency on the right side of the forehead (top). Dermoscopic examination (middle) (polarized noncontact mode) revealed a homogeneous pink to bluish background, scattered linear vessels with branches (black arrows), multiple chrysalislike shiny white lines (blue arrows), and dots arranged in a 4-dot pattern (black circle) resembling a four-leaf clover. Increased peripheral, brown, networklike pigmentation (black stars) also was noted on dermoscopy. Histopathologic examination of the noduloplaque was performed (bottom).