User login
Part 5: Screening for “Opathies” in Diabetes Patients
Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
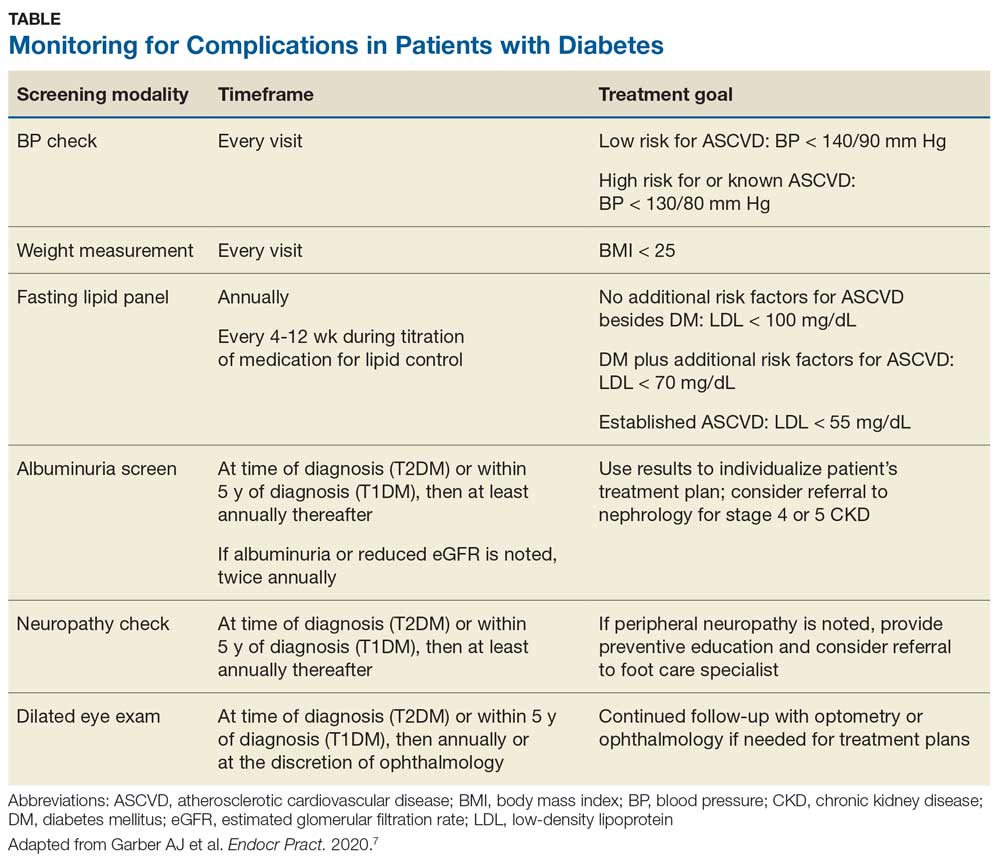
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8
Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Part 4: Monitoring for CKD in Diabetes Patients
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Part 3: Lipid Management in Diabetes Patients
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Part 2: Controlling BP in Diabetes Patients
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Part 1: Self-care for Diabetes Patients
Diabetes mellitus is prevalent in our society; 1 in 10 Americans has the condition and > 1 in 3 has prediabetes.1 Due to the widespread comorbidities and complications of this disease, the American Diabetes Association (ADA) recommends that diabetes management focus on evaluation and treatment of complications.2 Diabetes-related complications can be life-altering and challenging for patients because their quality of life suffers.
For providers, there are several evidence-based screening tools and preventive practices (in and beyond glycemic control) that reduce diabetes complications such as congestive heart failure, kidney failure, lower extremity amputation, and stroke.3 We as providers can treat patients by implementing appropriate goal-directed therapy.4-6
In this 5-part series, I will explore the evidence and recommendations for a multimodal approach in a patient with type 2 diabetes. Here—in Part 1—I explore the self-care behaviors our patients can adopt to improve their symptoms of diabetes.
Case Report
Mr. W is an overweight 64-year-old man with hypertension, hyperlipidemia, and type 2 diabetes mellitus. He visits the clinic for his yearly physical exam. He is concerned because his father, who had diabetes, developed renal failure and had multiple amputations near the end of his life. He is worried that he might face the same outcomes and asks you what he can do to avoid his father’s fate.
Advising Your Patient on Self-care
The cornerstone of diabetes management is appropriate self-care. Both the ADA and the American Association of Clinical Endocrinologists (AACE) recommend that treatment plans should encourage the patient to adopt healthy lifestyle behaviors, including a healthy diet, regular exercise, weight control, and avoidance of tobacco.2,7,8 These interventions have positive effects on blood pressure, glucose control, and lipid levels. They can also reduce the risk for diabetic complications, including atherosclerotic cardiovascular disease (ASCVD), which is the foremost cause of death among patients with diabetes. During a patient visit, clinicians can suggest the following self-care interventions for improving long-term outcomes.
Education sessions. The ADA recommends that individuals with diabetes participate in diabetes self-management education and support (DSMES) sessions.2 In these sessions, patients with diabetes are instructed on a variety of self-care behaviors, including lifestyle interventions, medication management, self-monitoring, and problem-solving.9 These programs—often paid for in part by health insurance—are taught by health care professionals such as registered dieticians, nutritionists, or certified diabetes educators.9,10 Evidence suggests DSMES increases patients’ sense of self-efficacy and may improve blood sugar management.10 Clinicians can help guide their patients through the Association of Diabetes Care & Education Specialists’ online database to identify a DSMES program near them (see www.diabeteseducator.org/living-with-diabetes/find-an-education-program).11
Diet. The AACE recommends a plant-based diet high in polyunsaturated and monounsaturated fatty acids and limited in trans fatty acids and saturated fats.7 Evidence strongly suggests that a Mediterranean diet with high vegetable intake and decreased saturated fats helps to reduce the risk for major cardiovascular events (myocardial infarction and stroke).12
Continue to: Exercise
Exercise. Both the ADA and AACE recommend that most adults with diabetes engage in at least 150 min/week of moderate-to-vigorous aerobic and strength-training exercises.2,7 Clinicians should evaluate patients with sedentary lifestyles prior to them engaging in vigorous physical activity beyond simple walking.2 The ADA also recommends that patients should avoid sitting for long periods of time by engaging in physical activity at least every 30 minutes.2 For adults who may not be able to participate in moderate-to-vigorous exercise, recommend alternative flexibility and balance-training activities, such as yoga or tai chi, 2 to 3 times per week.2
Weight management—a combined effort of diet, exercise, and behavioral therapy—is pivotal in the management of type 2 diabetes due to the potential benefits in insulin resistance, blood pressure, hyperlipidemia, and other factors.2 Weight loss may also improve glycemic control and reduce the need for glucose-lowering medications.2 For patients who struggle with weight loss, consider prescribing FDA-approved weight-loss medications (phentermine, orlistat, lorcaserin, naltrexone/bupropion, liraglutide) or, in some cases, referring for bariatric surgery.2,7
Sleep hygiene is an important element in any preventive treatment plan. This includes interventions as simple as going to bed at the same time every night, sleeping in a dark room, sleeping for at least 7 hours, and removing electronic devices from the bedroom.13 Patients should avoid alcohol, caffeine, and large meals before bedtime.13
Additionally, obstructive sleep apnea (OSA) is often underdiagnosed in patients with diabetes and contributes to insulin resistance, inflammation, and elevated blood pressure.7,14 For early identification of OSA, order a sleep study when appropriate and refer patients to sleep specialists if needed. Patients who are recommended for treatment should be monitored for increasing compliance with care and to ensure benefit from treatment.
In Part 2, we’ll check in with Mr. W as I discuss the role of blood pressure monitoring and antihypertensive medications in reducing cardiovascular risks in patients with diabetes.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Diabetes mellitus is prevalent in our society; 1 in 10 Americans has the condition and > 1 in 3 has prediabetes.1 Due to the widespread comorbidities and complications of this disease, the American Diabetes Association (ADA) recommends that diabetes management focus on evaluation and treatment of complications.2 Diabetes-related complications can be life-altering and challenging for patients because their quality of life suffers.
For providers, there are several evidence-based screening tools and preventive practices (in and beyond glycemic control) that reduce diabetes complications such as congestive heart failure, kidney failure, lower extremity amputation, and stroke.3 We as providers can treat patients by implementing appropriate goal-directed therapy.4-6
In this 5-part series, I will explore the evidence and recommendations for a multimodal approach in a patient with type 2 diabetes. Here—in Part 1—I explore the self-care behaviors our patients can adopt to improve their symptoms of diabetes.
Case Report
Mr. W is an overweight 64-year-old man with hypertension, hyperlipidemia, and type 2 diabetes mellitus. He visits the clinic for his yearly physical exam. He is concerned because his father, who had diabetes, developed renal failure and had multiple amputations near the end of his life. He is worried that he might face the same outcomes and asks you what he can do to avoid his father’s fate.
Advising Your Patient on Self-care
The cornerstone of diabetes management is appropriate self-care. Both the ADA and the American Association of Clinical Endocrinologists (AACE) recommend that treatment plans should encourage the patient to adopt healthy lifestyle behaviors, including a healthy diet, regular exercise, weight control, and avoidance of tobacco.2,7,8 These interventions have positive effects on blood pressure, glucose control, and lipid levels. They can also reduce the risk for diabetic complications, including atherosclerotic cardiovascular disease (ASCVD), which is the foremost cause of death among patients with diabetes. During a patient visit, clinicians can suggest the following self-care interventions for improving long-term outcomes.
Education sessions. The ADA recommends that individuals with diabetes participate in diabetes self-management education and support (DSMES) sessions.2 In these sessions, patients with diabetes are instructed on a variety of self-care behaviors, including lifestyle interventions, medication management, self-monitoring, and problem-solving.9 These programs—often paid for in part by health insurance—are taught by health care professionals such as registered dieticians, nutritionists, or certified diabetes educators.9,10 Evidence suggests DSMES increases patients’ sense of self-efficacy and may improve blood sugar management.10 Clinicians can help guide their patients through the Association of Diabetes Care & Education Specialists’ online database to identify a DSMES program near them (see www.diabeteseducator.org/living-with-diabetes/find-an-education-program).11
Diet. The AACE recommends a plant-based diet high in polyunsaturated and monounsaturated fatty acids and limited in trans fatty acids and saturated fats.7 Evidence strongly suggests that a Mediterranean diet with high vegetable intake and decreased saturated fats helps to reduce the risk for major cardiovascular events (myocardial infarction and stroke).12
Continue to: Exercise
Exercise. Both the ADA and AACE recommend that most adults with diabetes engage in at least 150 min/week of moderate-to-vigorous aerobic and strength-training exercises.2,7 Clinicians should evaluate patients with sedentary lifestyles prior to them engaging in vigorous physical activity beyond simple walking.2 The ADA also recommends that patients should avoid sitting for long periods of time by engaging in physical activity at least every 30 minutes.2 For adults who may not be able to participate in moderate-to-vigorous exercise, recommend alternative flexibility and balance-training activities, such as yoga or tai chi, 2 to 3 times per week.2
Weight management—a combined effort of diet, exercise, and behavioral therapy—is pivotal in the management of type 2 diabetes due to the potential benefits in insulin resistance, blood pressure, hyperlipidemia, and other factors.2 Weight loss may also improve glycemic control and reduce the need for glucose-lowering medications.2 For patients who struggle with weight loss, consider prescribing FDA-approved weight-loss medications (phentermine, orlistat, lorcaserin, naltrexone/bupropion, liraglutide) or, in some cases, referring for bariatric surgery.2,7
Sleep hygiene is an important element in any preventive treatment plan. This includes interventions as simple as going to bed at the same time every night, sleeping in a dark room, sleeping for at least 7 hours, and removing electronic devices from the bedroom.13 Patients should avoid alcohol, caffeine, and large meals before bedtime.13
Additionally, obstructive sleep apnea (OSA) is often underdiagnosed in patients with diabetes and contributes to insulin resistance, inflammation, and elevated blood pressure.7,14 For early identification of OSA, order a sleep study when appropriate and refer patients to sleep specialists if needed. Patients who are recommended for treatment should be monitored for increasing compliance with care and to ensure benefit from treatment.
In Part 2, we’ll check in with Mr. W as I discuss the role of blood pressure monitoring and antihypertensive medications in reducing cardiovascular risks in patients with diabetes.
Diabetes mellitus is prevalent in our society; 1 in 10 Americans has the condition and > 1 in 3 has prediabetes.1 Due to the widespread comorbidities and complications of this disease, the American Diabetes Association (ADA) recommends that diabetes management focus on evaluation and treatment of complications.2 Diabetes-related complications can be life-altering and challenging for patients because their quality of life suffers.
For providers, there are several evidence-based screening tools and preventive practices (in and beyond glycemic control) that reduce diabetes complications such as congestive heart failure, kidney failure, lower extremity amputation, and stroke.3 We as providers can treat patients by implementing appropriate goal-directed therapy.4-6
In this 5-part series, I will explore the evidence and recommendations for a multimodal approach in a patient with type 2 diabetes. Here—in Part 1—I explore the self-care behaviors our patients can adopt to improve their symptoms of diabetes.
Case Report
Mr. W is an overweight 64-year-old man with hypertension, hyperlipidemia, and type 2 diabetes mellitus. He visits the clinic for his yearly physical exam. He is concerned because his father, who had diabetes, developed renal failure and had multiple amputations near the end of his life. He is worried that he might face the same outcomes and asks you what he can do to avoid his father’s fate.
Advising Your Patient on Self-care
The cornerstone of diabetes management is appropriate self-care. Both the ADA and the American Association of Clinical Endocrinologists (AACE) recommend that treatment plans should encourage the patient to adopt healthy lifestyle behaviors, including a healthy diet, regular exercise, weight control, and avoidance of tobacco.2,7,8 These interventions have positive effects on blood pressure, glucose control, and lipid levels. They can also reduce the risk for diabetic complications, including atherosclerotic cardiovascular disease (ASCVD), which is the foremost cause of death among patients with diabetes. During a patient visit, clinicians can suggest the following self-care interventions for improving long-term outcomes.
Education sessions. The ADA recommends that individuals with diabetes participate in diabetes self-management education and support (DSMES) sessions.2 In these sessions, patients with diabetes are instructed on a variety of self-care behaviors, including lifestyle interventions, medication management, self-monitoring, and problem-solving.9 These programs—often paid for in part by health insurance—are taught by health care professionals such as registered dieticians, nutritionists, or certified diabetes educators.9,10 Evidence suggests DSMES increases patients’ sense of self-efficacy and may improve blood sugar management.10 Clinicians can help guide their patients through the Association of Diabetes Care & Education Specialists’ online database to identify a DSMES program near them (see www.diabeteseducator.org/living-with-diabetes/find-an-education-program).11
Diet. The AACE recommends a plant-based diet high in polyunsaturated and monounsaturated fatty acids and limited in trans fatty acids and saturated fats.7 Evidence strongly suggests that a Mediterranean diet with high vegetable intake and decreased saturated fats helps to reduce the risk for major cardiovascular events (myocardial infarction and stroke).12
Continue to: Exercise
Exercise. Both the ADA and AACE recommend that most adults with diabetes engage in at least 150 min/week of moderate-to-vigorous aerobic and strength-training exercises.2,7 Clinicians should evaluate patients with sedentary lifestyles prior to them engaging in vigorous physical activity beyond simple walking.2 The ADA also recommends that patients should avoid sitting for long periods of time by engaging in physical activity at least every 30 minutes.2 For adults who may not be able to participate in moderate-to-vigorous exercise, recommend alternative flexibility and balance-training activities, such as yoga or tai chi, 2 to 3 times per week.2
Weight management—a combined effort of diet, exercise, and behavioral therapy—is pivotal in the management of type 2 diabetes due to the potential benefits in insulin resistance, blood pressure, hyperlipidemia, and other factors.2 Weight loss may also improve glycemic control and reduce the need for glucose-lowering medications.2 For patients who struggle with weight loss, consider prescribing FDA-approved weight-loss medications (phentermine, orlistat, lorcaserin, naltrexone/bupropion, liraglutide) or, in some cases, referring for bariatric surgery.2,7
Sleep hygiene is an important element in any preventive treatment plan. This includes interventions as simple as going to bed at the same time every night, sleeping in a dark room, sleeping for at least 7 hours, and removing electronic devices from the bedroom.13 Patients should avoid alcohol, caffeine, and large meals before bedtime.13
Additionally, obstructive sleep apnea (OSA) is often underdiagnosed in patients with diabetes and contributes to insulin resistance, inflammation, and elevated blood pressure.7,14 For early identification of OSA, order a sleep study when appropriate and refer patients to sleep specialists if needed. Patients who are recommended for treatment should be monitored for increasing compliance with care and to ensure benefit from treatment.
In Part 2, we’ll check in with Mr. W as I discuss the role of blood pressure monitoring and antihypertensive medications in reducing cardiovascular risks in patients with diabetes.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
How Motivational Interviewing Helps Patients with Diabetes
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
10 (Safe) Ways to Reduce Patients’ Insulin Costs
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Screening for Endocrine Hypertension
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
Diabetes and the Commercial Motor Vehicle Driver
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
Osteoporosis: Breaking Down the Treatment Options
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.