User login
Pudendal Neuralgia
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
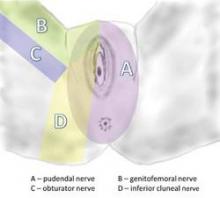
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
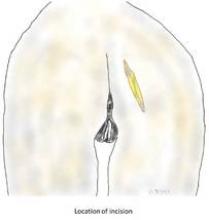
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
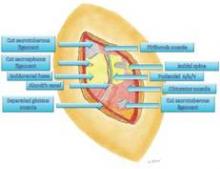
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
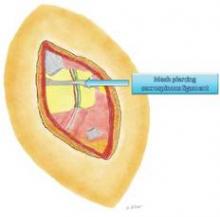
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
Pudendal neuralgia is an important but often unrecognized and undiagnosed cause of pelvic floor pain.
Its incidence is unknown, and there is relatively little data and scientific evidence in the literature on its diagnosis and treatment. However, I believe that a significant number of women who have burning pain in the vulva, clitoris, vagina, perineum, or rectum – including women who are diagnosed with interstitial cystitis, pelvic floor muscle spasms, vulvodynia, or other conditions – may in fact have pudendal neuralgia.
Indeed, pudendal neuralgia is largely a diagnosis of exclusion, and such conditions often must be ruled out. But the neuropathic condition should be suspected in women who have burning pain in any area along the distribution of the pudendal nerve. Awareness of the nerve’s anatomy and distribution, and of the hallmark characteristics and symptoms of pudendal neuralgia, is important, because earlier identification and treatment appears to provide better outcomes.
Pudendal neuralgia is but one type of pelvic neuralgia; neuropathic pain in the pelvic region also can stem from injury to the obturator, ilioinguinal, iliohypogastric, or genitofemoral nerves, for instance. Most of the patients in our practice, however, have pudendal neuralgia caused by mechanical compression – what is referred to as pudendal nerve entrapment – rather than disease of the nerve.
The condition is sometimes referred to as cyclist syndrome because, historically, the first documented group of patients with symptoms of pudendal neuralgia was competitive cyclists. There is a misconception, however, that the condition only occurs in cyclists. In fact, pudendal neuralgia and pudendal nerve entrapment specifically may be caused by various forms of pelvic trauma, from vaginal delivery (with or without instrumentation) and heavy lifting or falls on the back or pelvis, to previous gynecologic surgery, such as hysterectomy, cystocele repair, and mesh procedures for prolapse and incontinence.
Pudendal neuralgia is multifactorial, involving not only compression of the nerve, for instance, but also muscle spasm and peripheral and central sensitization of pain. Treatment involves a progression of conservative therapies followed by decompression surgery when these conservative treatments fail. We have made several modifications to the transgluteal approach as it was originally described, and believe this approach affords the best outcomes.
Anatomy and Symptoms
The pudendal nerve originates in the S2-S4 sacral foramina, and divides into three branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve. The nerve thus innervates the clitoris, vulva, labia, vagina, perineum, and rectum. Pain can be present along the entire nerve, or localized to the sites of nerve innervation. Symptoms can be unilateral or bilateral, although with bilateral pain there usually is a more affected side.
In most cases, patients will describe neuropathic pain – a burning, tingling, or numbing pain – that is worse with sitting, and less severe or absent when standing or lying down.
Initially, pain may be present only with sitting, but with time pain becomes more constant and severely aggravated by sitting. Many of my patients cannot tolerate sitting at all. Interestingly, patients usually report less pain when sitting on a toilet seat, a phenomenon that we believe is associated with pressure being applied to the ischial tuberosities rather than to the pelvic floor muscles. Pain usually gets progressively worse through the day.
Patients often will report the sensation of having a foreign body, frequently described as a golf ball or tennis ball, in the vagina, perineum, or rectum.
Pain with urination and/or bowel movements, and problems with frequency and urgency, also are often reported, as is pain with intercourse. Dyspareunia may be associated with penetration, sexual arousal, or orgasm, or any combination. Some patients report feeling persistent sexual arousal.
Occasionally, patients report having pain in regions outside the areas of innervation for the pudendal nerve, such as the lower back or posterior thigh. The presence of sciatica, or pain that radiates down the leg, for instance, should not rule out consideration of pudendal neuralgia.
Just as worsening pain with sitting is a defining characteristic, almost all patients also have an acute onset of discomfort or pain; their pain can be traced to some type of traumatic event.
One of my recent patients, for instance, was in a gym class doing a lunge with barbells on her shoulders when her legs gave out and she experienced the start of continuous pain in her vulvar area. Many of our patients trace the onset of their symptoms to immediately after gynecologic surgery, particularly vaginal procedures for prolapse or incontinence. (The pain in these cases is frequently attributed to normal postoperative pain.) Some patients report a more gradual onset of symptoms after surgery.
The pudendal nerve can be compressed in various locations along its course. The nerve runs between the sacrospinous and sacrotuberous ligaments, for instance, and entrapment between these two ligaments is probably the most common cause of pudendal neuralgia. This is where the nerve is compressed by the suturing of mesh placed during prolapse/incontinence surgery.
Another area of compression is Alcock’s canal; entrapment here is characteristic of pudendal neuralgia following vaginal childbirth. Compression also can occur where the clitoral nerve continues underneath the pubic ramus to the clitoris; this is typically where the nerve is compressed by a bicycle seat.
Diagnosis
The most important element of the diagnosis of pudendal neuralgia is the history, particularly regarding the onset of pain, the location of pain, and the nature of symptoms.
History and physical examination both are important for ruling out other reasons for pain, including vulvodynia, pelvic floor tension muscle spasm, and interstitial cystitis. A pelvic exam often will reveal significant tenderness in the pelvic floor muscles, especially in the area of the sacrospinous ligaments. Patients with pudendal neuralgia often have a trigger point – a place of maximal tenderness and pain – at the ischial spine. Palpation of this area to produce what’s known as a Tinel’s sign (with pain and symptoms) thus should be part of the exam.
Also key to diagnosis are computed tomography–guided blocks of the pudendal nerve. In our practice, we consider any degree of pain relief, for any duration of time after the block, as supportive of a diagnosis of pudendal neuralgia. Patients who do not experience immediate relief from a block are thought not to have the condition. These image-guided blocks must be performed by experienced interventional radiologists with a local anesthetic.
To date, there are no imaging studies that are reliable for diagnosis. Ongoing advances in magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) may make these modalities valuable in the future, but currently these techniques yield too many false negative results. Pudendal nerve motor terminal latency, which measures the conduction velocity of electrical impulses, is not useful given a high rate of intra- and interobserver variability and variations among patients who have had previous vaginal deliveries or pelvic surgery. Sensory threshold testing also has questionable reliability.
Initial Treatments
The initial approach to pudendal neuralgia should be conservative. Surgical decompression is the treatment of choice in patients with likely nerve entrapment, but determining the likelihood and extent of entrapment is a process. First, time must be spent in trying to identify and address the factors causing pain, and in trying to break the vicious cycle that occurs when neuropathic pain causes spasm of the pelvic floor muscles, which in turn leads to increased compression of the nerve and subsequent increases in pain levels.
While there are no official treatment algorithms, we have found – based on available data and our experience in treating more than 500 patients with pudendal neuralgia – that particular therapies can lead to marked improvements for many patients.
For some patients, especially those in whom bicycling or specific exercises initially caused the pain, avoidance of activities that worsen the pain, and other lifestyle modifications, can be helpful. Medical therapy with analgesics/pain management (such as oral pregabalin) and muscle relaxants also may be helpful for some patients. We have tried all kinds of muscle relaxants and have found that a vaginal suppository combining diazepam and baclofen is superior.
The most important treatment modality, however, is pelvic floor physical therapy. Such therapy is key because many patients have significant muscle spasm and subsequent muscle shortening. Therapists who are specially trained to work with pelvic floor muscle dysfunction can address these and other problems largely through various hands-on techniques, exercises, stretching, and education. Therapists can be identified on the International Pelvic Pain Society’s website, www.pelvicpain.org.
Botulinum toxin A (Botox) injections also are often a key part of therapy for patients with significant muscle spasm. In our practice, we administer approximately 200 units in 20 injections using a pudendal nerve block needle, under anesthesia. Not only does the treatment aid in muscle relaxation (thus increasing the patient’s tolerance to physical therapy), it also helps to differentiate between pain caused solely by muscle spasm, and pain caused by nerve injury and muscle spasm.
While patients who do not have neuralgia whose pain is caused solely or almost solely by muscle spasm will benefit significantly more from Botox injections, some patients with pudendal neuralgia will benefit from occasional, repeated Botox treatment in lieu of surgical decompression therapy. Many of our patients have been receiving Botox injections every 3-4 months, for instance.
Similarly, many other patients get significant pain relief from CT-guided injections of the nerve. While an initial CT-guided injection of anesthetic and steroid serves both diagnostic and therapeutic roles, a second and third injection can be performed to deliver more steroid and anesthetic into the pudendal nerve canal (Alcock’s canal) in a patient who responded to the first injection but whose pain has returned. Again, these injections must be performed by an experienced interventional radiologist in a CT scanner.
Injections are offered 6 weeks apart, but some patients have significant pain relief for 4-5 months, or even longer, after CT-guided nerve blocks. Patients who have long-term pain relief from CT-guided blocks will not be offered decompression surgery. One of our patients, for instance, is receiving nerve blocks every 8 months as part of her treatment.
Surgical Decompression
If patients do not have sufficient pain relief from conservative therapies (relief that enables them to return to normal daily function), surgical decompression of the nerve is indicated. An estimated 30%-40% of all patients with pudendal neuralgia will benefit from surgery.
Four different procedures have been described for decompressing an entrapped pudendal nerve: transgluteal, transischiorectal, transperineal, and endoscopic.
The transgluteal approach appears to be the most effective technique, allowing the best visualization of the pudendal nerve and the greatest extent of decompression along the length of the nerve. The main concern with this approach since it was originally described by Professor Roger Robert in Nantes, France, has been the required transection of the sacrotuberous ligament and the possible impact on stability of the sacroiliac joint. In our practice, however, we have made several modifications to the approach that minimize these concerns and, we believe, are improving recovery and outcomes.
The patient is placed in a prone jackknife position, and the electrodes of a NIMS monitor (Nerve Integrity Monitoring System; Medtronic, Minneapolis, Minn.) are placed in the anal sphincter.
An incision of approximately 7-10 cm in length is made across the gluteal region overlying the sacrotuberous ligament. The gluteus muscles are spread, with muscle fibers separated longitudinally, and once the ligament is reached, it is transected at its narrowest point.
The pudendal nerve then can be identified immediately below the ligament with use of a surgical microscope and the NIMS. When the surface of the nerve is touched, we are alerted by the NIMS monitor (part of the nerve runs to the anal center). In some patients, the pudendal nerve may actually be attached to the anterior surface of the sacrotuberous ligament.
The nerve is then decompressed along its entire length, from the piriformis muscle and as close as possible to the spinal cord, to the distal Alcock’s canal. Neurolysis is performed along each of the nerve’s branches – the inferior rectal nerve, the perineal nerve, and the dorsal clitoral nerve – until the nerve is completely free. In our practice, we most often find the nerve entrapped between the sacrospinous and sacrotuberous ligaments, which form a sort of "V" in the pelvis.
Because the sacrospinous ligament does not serve any anatomic purpose, I transect the ligament so that I can transpose the pudendal nerve anteriorly to give it more room.
Repair of the sacrotuberous ligament was not traditionally performed as part of the transgluteal approach, but we believe that repair is important for stability of the sacroiliac joint. Until recently, we used a graft of cadaver tendon to repair the ligament. Now, however, we transect the ligament with a z-shaped cut; this method allows us to repair the ligament without using any cadaver tissue.
In other modifications to the traditional approach, we wrap a piece of NeuraGen Nerve Guide (Integra LifeSciences, Plainsboro, N.J.), a nerve-protecting sheath made of collagen, around the nerve to prevent the formation or reformation of scar tissue. To promote nerve healing, we then cover the nerve with platelet-rich plasma that has been prepared from the patient’s own blood. The plasma contains growth factors that stimulate the production of myelin-producing cells.
Before closure, we also place a pain pump catheter along the course of the nerve. We believe that infusion of bupivacaine for 10-20 days postoperatively decreases the risk of central sensitization to pain and allows patients to be more mobile after surgery, which we encourage. It also may reduce the risk of scar formation. When neuropathic central pain is believed to be a significant problem, as it often is in patients whose nerves have been injured by surgical mesh, we also administer ketamine. An infusion of this old anesthetic can erase or reverse the troubling phenomena of central sensitization to pain.
Nerve entrapment involving mesh requires lengthy surgery. While other surgeons may trim the mesh, I firmly believe in removing all the mesh because we cannot determine which part of the mesh is causing pain.
Outcomes data from France show that approximately 30%-40% of patients are pain free after surgical decompression, with another 30% reporting improvement in pain and 30% reporting no change in their pain levels (Eur. Urol. 2005;47:403-8).
At our institution, using national scientific standards for the reporting of pain and extent of pain improvement, we have found that 70% of patients who undergo transgluteal surgical decompression have at least a 20% improvement in pain. Within this broad category are a significant number of patients who are pain free, and many who report improvements of 50% or more.
Interestingly, we have found that outcomes are similar among our much smaller number of "re-do" surgical patients. Thus far we have performed approximately 20 such transgluteal procedures – 17 on patients who had re-scarring of the nerve after surgery performed at other institutions, and 3 who had surgery many years ago in our practice, before we were able to optimally visualize the entire nerve and before we made modifications to improve the procedure. Just as with our first-time surgeries, approximately 70% of patients who underwent a second procedure had at least a 20% improvement in pain.
In all cases, the pudendal nerve recovers slowly, especially when it has been entrapped and injured for a long time, and improvements in pain often do not occur until about 4 months after surgery. Improvement typically continues for some time, up to 18 months after surgery. Patients may still have pain related to muscle spasms after surgery, so continued physical therapy and/or more Botox injections are often beneficial. Patients must also, of course, continue to avoid any offending factors or activities.
Dr. Hibner is a former fellow in advanced gynecologic surgery at Mayo Clinic, Scottsdale, Ariz., and is now professor of obstetrics and gynecology, Creighton University, Omaha, Neb., and associate clinical professor of obstetrics and gynecology, University of Arizona, Tucson. He also is director of the Arizona Center for Chronic Pelvic Pain, St. Joseph’s Hospital and Medical Center, Phoenix. To review his surgical procedure, visit SurgeryU at www.aagl.org/mastercourse. Dr. Hibner reported that he has no relevant financial disclosures.
The Unexpectedly Critically Ill Gravida
The process of labor and delivery is considered to be a joyous event in women’s lives, and most of the time it is. However, practitioners have to be aware of potential complications that can have dire adverse outcomes, causing maternal morbidity and mortality as well as severe consequences for the baby.
In considering such complications, physicians usually think of women who have serious underlying comorbidities. However, the three complications discussed here – amniotic fluid embolism, ruptured uterus, and peripartum cardiomyopathy – are conditions that can happen to otherwise young, healthy women. Fortunately these complications are rare. However, when they do happen, early recognition and prompt intervention are critical to optimizing the outcome. This means we must continually keep a high index of suspicion for all such complications so that we are ready in the event that labor and delivery does not proceed normally.
Amniotic Fluid Embolism
This complication is a leading cause of maternal morbidity and mortality in the United States and other developed countries. It should be considered in any patient who has sudden, unheralded cardiopulmonary collapse followed by profuse hemorrhage associated with disseminated intravascular coagulation (DIC).
While the hallmark presentation of amniotic fluid embolism (AFE) is this profound cardiopulmonary collapse with severe hemorrhage, it is important to note that published definitions of the condition state that coagulopathy may occur in isolation. In a 2011 review, Dr. Michael Benson points out that at least six case reports have described coagulopathy alone as the sole clinical sign of AFE (Clin. Dev. Immunol. 2012:946576 Epub 2011 Sept. 29 [doi:10.1155/2012/946576]).
AFE is a diagnosis of exclusion, and one that is based on symptoms and clinical presentation rather than on laboratory testing or histopathologic examination. There is broad consensus that a clinical diagnosis of AFE can be made based on one or more of four key signs/symptoms (in the absence of other medical conditions or explanations): cardiovascular collapse (hypotension and/or cardiac arrest); respiratory distress; DIC; and coma and/or seizures.
The condition can occur suddenly and unpredictably at any point during labor and delivery or in the immediate postpartum period. It also has been reported to occur as late as 48 hours after delivery.
Pulmonary thromboembolism often may be suspected, and indeed, it is part of the differential diagnosis. Patients with thrombotic pulmonary embolism do not usually develop the classic DIC type of coagulopathy, however, while patients with AFE are coagulopathic and often hemorrhage profusely.
The incidence of AFE has been difficult to determine. The authors of a 2009 evidence-based review of AFE reported that the estimated incidence based on large population-based studies is 1 in 15,200 deliveries in North America and 1 in 53,800 deliveries in Europe (Am. J. Obstet. Gynecol. 2009;201:445.e1-13). The incidence in an Australian population-based cohort was recently reported to be 3.3 per 100,000 deliveries (BJOG 2010;117:1417-21).
Other published reports and reviews have described an extremely broad range of estimated incidence. For instance, a report in the journal Anesthesia and Analgesia, published by the International Anesthesia Research Society, stated that AFE may occur between 1 in 8,000 and 1 in 80,000 deliveries (Anesth. Analg. 2009;108:1599-602).
The pathophysiology of AFE also is poorly understood, causing us great uncertainty as to why some apparently stable patients undergo such a profound, life-threatening collapse. When AFE was first described more than 70 years ago, it was thought to result from amniotic fluid entering the maternal circulation and obstructing the pulmonary blood flow – thus the name "amniotic fluid embolism." However, research over the decades has consistently discounted this view. As Dr. Benson states, current thinking has shifted away from embolism and toward a maternal immune response to the fetus.
Investigators have suggested possible immunologic mechanisms such as complement activation and reactions similar to anaphylaxis, but more research needs to be done. In the meantime, we must recognize that the name AFE probably does not accurately reflect what actually occurs in these patients.
Management is usually first directed at getting the patient through the initial cardiovascular insults – often hypotension and cardiac arrest – and at treating hypoxia and rapidly correcting maternal hemodynamic instability. Significant teamwork is required for the mother and baby to survive – and to survive neurologically intact. Anesthesia is needed for development and control of the airway, for instance, and critical care is essential for inotropic support. Cardiology also must be involved, as continuous cardiac, respiratory, and blood pressure monitoring – and aggressive respiratory and circulatory support – are key.
The nursing staff also can play a critical role in preventing subsequent pulmonary edema by keeping meticulous records of the intake and output of fluids. The overwhelming insult of AFE to the heart and lungs leaves patients at high risk of developing pulmonary edema, and meticulous record-keeping can help ensure that these patients are not overloaded.
Aggressive blood replacement also is required to reverse the coagulopathy associated with AFE. Transfusion of packed red blood cells is a priority, but fresh frozen plasma, platelets, and cryoprecipitate also should be available for prompt administration.
There have been promising reports of the use of recombinant factor VIIa (rVIIa) for treating hemorrhage in patients with AFE in recent years, but a recent review of case reports of AFE from 2003 to 2009 suggests that the procoagulant may actually worsen outcomes (Anesthesiology 2011;115:1201-8). Indeed, unlike patients with other types of postpartum hemorrhaging, women with AFE have high circulating tissue factor concentrations. Recombinant factor VIIa can combine with tissue factor and form intravascular clots, resulting in thrombosis of major organs.
If the patient is undelivered and has cardiac arrest, an emergency cesarean section is indicated. Prompt delivery during the resuscitation process not only increases the chances of perinatal survival without neurological sequelae, but also improves the maternal resuscitation effort. We have a 4-minute window for delivery from the time the code is called to avoid neurologic injury to the fetus and optimize outcomes for the mother. This 4-minute principle was adopted by the American Heart Association in 1986, and its clinical use has been supported by 20 years of published case reports since then (Am. J. Obstet. Gynecol. 2005;192:1916-21).
Although outcomes with AFE may be improving somewhat, AFE still causes significant morbidity and mortality. Investigators in the Australian AFE cohort study, for instance, recently reported maternal and perinatal fatality rates of 35% and 32%, respectively. These rates were similar to those from the U.K. Obstetric Surveillance System, according to the authors (BJOG 2010;117:1417-21).
Ruptured Uterus
In an effort to reduce rates of cesarean deliveries, obstetricians are swinging back once again to encouraging more women to attempt vaginal birth after cesarean delivery (VBAC). Because the rates of uterine rupture are higher in women who attempt VBAC, our index of suspicion should be acute for any woman who is laboring after having had a prior cesarean delivery. We also must do everything we can to assess a patient’s risks of failed VBAC resulting in emergency cesarean section and uterine rupture.
The American College of Obstetricians and Gynecologists (ACOG) now recommends that most women with one previous cesarean delivery and with a low transverse incision be counseled about VBAC and offered a trial of labor. ACOG points out in its 2010 practice bulletin on VBAC that in several large studies, the uterine rupture rate after a trial of labor in such women was approximately 0.5-0.9%.
The College also says that many women previously considered to be at high risk may now be considered candidates for a trial of labor after cesarean section (TOLAC). Among the conditions that are no longer necessarily contraindications for attempted VBAC: two previous low transverse cesarean deliveries; suspected fetal macrosomia; twin gestations; more than one previous cesarean delivery; a previous low vertical incision; gestation beyond 40 weeks; and even external cephalic version for breech presentation (Obstet. Gynecol. 2010;116:450-63).
The ACOG bulletin addresses the importance of counseling, and mentions the possible utility of a nomogram developed for predicting the chance of successful VBAC for individual patients. The tool incorporates six variables that are ascertainable at the first prenatal visit, including maternal age, body mass index, and history of vaginal delivery (Obstet. Gynecol. 2007;109:806-12). The tool, a calculator of sorts, was developed through research by the National Institute of Child Health and Development’s (NICHD’s) Maternal-Fetal Medicine Units Network, and is also available at http://www.bsc.gwu.edu/mfmu/vagbirth.html.
Such individualized risk assessment is critical. Another model for assessing risk and informing discussions with individual patients is one developed in the United Kingdom by Dr. Gordon C. S. Smith at Cambridge University and his associates (PLoS Med. 2005:2:e252). These investigators documented that women with a predicted cesarean section risk (an unsuccessful trial of labor) of less than 20% using their model had a minimal incidence of uterine rupture of 2.0 per 1,000, while those deemed to have a high risk of cesarean delivery – defined as greater than 40% – had an incidence of uterine rupture of 9.1 per 1,000.
However small it is in absolute terms, there is an inherent risk of the uterine incision rupturing during an attempt at labor after a previous cesarean section. Indicative of this inherent risk are recommendations by the authors of numerous studies, as well as ACOG, for VBAC to be attempted in facilities with staff available for emergency care. When the fetus is actually extruded through the incision and into the abdominal cavity, there is significant risk of severe maternal and perinatal morbidity and mortality secondary to blood loss and hypoxia.
Signs and symptoms of possible uterine rupture include the following: a change in fetal heart rate pattern from normal to a category 3 heart rate tracing; unexplained vaginal bleeding; frequent epidural dosing or pain that is not alleviated with epidural anesthesia already in place; and loss of uterine tone with an intrauterine pressure catheter (IUPC) in place. If an IUPC is flushed and the patient still has abnormal readings, a diagnosis of uterine scar disruption should be entertained.
In addition to prompt recognition, rapid delivery and blood replacement are key to improving outcomes. The coagulopathy in patients with a ruptured uterus is dilutional rather consumptive, so these patients require replacement not only of packed red blood cells but also of clotting factors and other blood products. As with other types of obstetric hemorrhage, blood loss is usually in excess of the amount perceived.
A recent population-based registry study of 94 identified uterine ruptures after previous cesarean section found that almost half of the mothers diagnosed with uterine rupture after TOLAC (versus during elective or emergency prelabor cesarean section) developed moderate postpartum hemorrhage, while 15% developed severe postpartum hemorrhage and 4% needed peripartum hysterectomy (BJOG 2010;117:809-20).
Perinatal complications occurred in 48 of the 81 (59%) ruptures that occurred after attempted VBAC. In nine (19%) cases, the outcomes were serious (three deaths, three cases of severe asphyxia, and three cases of posthypoxic encephalopathy). To reduce the risk of iatrogenic uterine scar disruption, care should be taken in choosing the appropriate method of induction.
Peripartum Cardiomyopathy
This complication is characterized by the development of heart failure due to significant left ventricular (LV) systolic dysfunction. It is a diagnosis of exclusion. Patients present with the same signs and symptoms characterizing other forms of heart failure secondary to LV dysfunction, and other causes of heart disease and forms of heart failure must be ruled out.
This relatively uncommon myocardial complication can occur up to 5-6 months after delivery, but it usually occurs early in the postpartum period, with about 75% of cases presenting within the first month after delivery (Postgrad. Med. J. 2011;87:34-9). Most patients who are diagnosed during pregnancy present in the third trimester.
Various potential etiologies have been proposed – from viral myocarditis and abnormal hormonal regulation, to excessive prolactin production and an abnormal immune response to pregnancy – but its exact cause is still unknown.
Its incidence in the United States may be increasing. According to a recent review by Dr. Uri Elkayam, the incidence is estimated at approximately 1 in 3,200 deliveries, with a significantly higher incidence (up to 16-fold higher in one study) in African American women (J. Am. Coll. Cardiol. 2011;58:659-70).
Rates as high as 1 in 300 in Haiti and 1 in 100 in a small region of sub-Saharan Africa have also been reported in recent years, according to another review by Dr. Meredith Cruz and her associates (Obstet. Gynecol. Clin. N. Am. 2010;37:283-303).
Certainly, we must all be aware that certain ethnic groups and populations – most notably women of African descent – appear to be more at risk. Pregnancy-related hypertension and preeclampsia also are often cited as risk factors, as are multiparity, obesity, and older maternal age.
Diagnosis requires a high index of suspicion and vigilance, especially because many of the symptoms – shortness of breath, increased peripheral edema, and exhaustion, for instance – are similar to typical symptoms of a normal pregnancy. The diagnosis should be strongly considered in any woman who has nocturnal dyspnea. Chest pain, nocturnal cough, new regurgitant murmurs, pulmonary crackles, increased jugular venous pressure, or hepatomegaly also should raise suspicions, according to the review by Dr. Cruz and her associates.
The timing of delivery in patients diagnosed during pregnancy depends on the maternal status. If the mother is responding to medical management and is stable enough with regard to cardiovascular status to tolerate her heart failure, then induction of labor can be scheduled for or considered at 37 weeks’ gestation. If she is unstable or her LV function is poor or worsening, then early delivery should be considered.
Vaginal delivery often is preferable so that the potential risks associated with anesthesia and surgical delivery, such as clots or infection, can be avoided. Sometimes, however, cesarean delivery may be the only option. For a woman who is laboring, it is important to shorten the second stage of labor, with either low forceps or a vacuum device, in order to minimize pushing and ventricular work.
Management requires teamwork with cardiology, intensive care, anesthesiology, and nursing. After delivery, during a patient’s postpartum fluid shift, she should be managed in a critical care unit or another closely observed setting.
The management of peripartum cardiomyopathy – during pregnancy or afterward – is aimed at improving symptoms, slowing the progression of LV dysfunction and heart failure, and preventing arrhythmias and thromboembolism – both common complications.
Diuretics, nitrates, and hydralazine are often indicated and are safe in pregnancy, as is use of the beta-blocker metoprolol and either unfractionated heparin or low-molecular weight heparin for anticoagulation. (Anticoagulants are almost always indicated.) Nonpharmacologically, the focus is on reducing fluid and salt intake and on monitoring electrolyte levels and addressing any imbalances.
On the research front, animal studies and now preliminary data from a very small number of women with acute severe peripartum cardiomyopathy suggest that bromocriptine, an inhibitor of prolactin, may have a favorable effect on outcomes (Circulation 2010;121:1465-73).
Reported mortalities from the disease have ranged as high as 18%-56%, according to the Cruz review. On the other hand, many women will have a full recovery and a normalization of LV function. Dr. Elkayam concludes in his review that a normalization of LV function may occur in more than 50% of women with peripartum cardiomyopathy, mostly within 2-6 months after diagnosis.
Subsequent pregnancy is contraindicated in women who do not have a resolution of LV dysfunction, and even when LV function normalizes, there is a risk of recurrent and persistent dysfunction in a subsequent pregnancy.
Dr. Whiteman is associate professor and interim director of the division of maternal-fetal medicine at the University of South Florida, Tampa. She said she has no relevant financial disclosures.
The process of labor and delivery is considered to be a joyous event in women’s lives, and most of the time it is. However, practitioners have to be aware of potential complications that can have dire adverse outcomes, causing maternal morbidity and mortality as well as severe consequences for the baby.
In considering such complications, physicians usually think of women who have serious underlying comorbidities. However, the three complications discussed here – amniotic fluid embolism, ruptured uterus, and peripartum cardiomyopathy – are conditions that can happen to otherwise young, healthy women. Fortunately these complications are rare. However, when they do happen, early recognition and prompt intervention are critical to optimizing the outcome. This means we must continually keep a high index of suspicion for all such complications so that we are ready in the event that labor and delivery does not proceed normally.
Amniotic Fluid Embolism
This complication is a leading cause of maternal morbidity and mortality in the United States and other developed countries. It should be considered in any patient who has sudden, unheralded cardiopulmonary collapse followed by profuse hemorrhage associated with disseminated intravascular coagulation (DIC).
While the hallmark presentation of amniotic fluid embolism (AFE) is this profound cardiopulmonary collapse with severe hemorrhage, it is important to note that published definitions of the condition state that coagulopathy may occur in isolation. In a 2011 review, Dr. Michael Benson points out that at least six case reports have described coagulopathy alone as the sole clinical sign of AFE (Clin. Dev. Immunol. 2012:946576 Epub 2011 Sept. 29 [doi:10.1155/2012/946576]).
AFE is a diagnosis of exclusion, and one that is based on symptoms and clinical presentation rather than on laboratory testing or histopathologic examination. There is broad consensus that a clinical diagnosis of AFE can be made based on one or more of four key signs/symptoms (in the absence of other medical conditions or explanations): cardiovascular collapse (hypotension and/or cardiac arrest); respiratory distress; DIC; and coma and/or seizures.
The condition can occur suddenly and unpredictably at any point during labor and delivery or in the immediate postpartum period. It also has been reported to occur as late as 48 hours after delivery.
Pulmonary thromboembolism often may be suspected, and indeed, it is part of the differential diagnosis. Patients with thrombotic pulmonary embolism do not usually develop the classic DIC type of coagulopathy, however, while patients with AFE are coagulopathic and often hemorrhage profusely.
The incidence of AFE has been difficult to determine. The authors of a 2009 evidence-based review of AFE reported that the estimated incidence based on large population-based studies is 1 in 15,200 deliveries in North America and 1 in 53,800 deliveries in Europe (Am. J. Obstet. Gynecol. 2009;201:445.e1-13). The incidence in an Australian population-based cohort was recently reported to be 3.3 per 100,000 deliveries (BJOG 2010;117:1417-21).
Other published reports and reviews have described an extremely broad range of estimated incidence. For instance, a report in the journal Anesthesia and Analgesia, published by the International Anesthesia Research Society, stated that AFE may occur between 1 in 8,000 and 1 in 80,000 deliveries (Anesth. Analg. 2009;108:1599-602).
The pathophysiology of AFE also is poorly understood, causing us great uncertainty as to why some apparently stable patients undergo such a profound, life-threatening collapse. When AFE was first described more than 70 years ago, it was thought to result from amniotic fluid entering the maternal circulation and obstructing the pulmonary blood flow – thus the name "amniotic fluid embolism." However, research over the decades has consistently discounted this view. As Dr. Benson states, current thinking has shifted away from embolism and toward a maternal immune response to the fetus.
Investigators have suggested possible immunologic mechanisms such as complement activation and reactions similar to anaphylaxis, but more research needs to be done. In the meantime, we must recognize that the name AFE probably does not accurately reflect what actually occurs in these patients.
Management is usually first directed at getting the patient through the initial cardiovascular insults – often hypotension and cardiac arrest – and at treating hypoxia and rapidly correcting maternal hemodynamic instability. Significant teamwork is required for the mother and baby to survive – and to survive neurologically intact. Anesthesia is needed for development and control of the airway, for instance, and critical care is essential for inotropic support. Cardiology also must be involved, as continuous cardiac, respiratory, and blood pressure monitoring – and aggressive respiratory and circulatory support – are key.
The nursing staff also can play a critical role in preventing subsequent pulmonary edema by keeping meticulous records of the intake and output of fluids. The overwhelming insult of AFE to the heart and lungs leaves patients at high risk of developing pulmonary edema, and meticulous record-keeping can help ensure that these patients are not overloaded.
Aggressive blood replacement also is required to reverse the coagulopathy associated with AFE. Transfusion of packed red blood cells is a priority, but fresh frozen plasma, platelets, and cryoprecipitate also should be available for prompt administration.
There have been promising reports of the use of recombinant factor VIIa (rVIIa) for treating hemorrhage in patients with AFE in recent years, but a recent review of case reports of AFE from 2003 to 2009 suggests that the procoagulant may actually worsen outcomes (Anesthesiology 2011;115:1201-8). Indeed, unlike patients with other types of postpartum hemorrhaging, women with AFE have high circulating tissue factor concentrations. Recombinant factor VIIa can combine with tissue factor and form intravascular clots, resulting in thrombosis of major organs.
If the patient is undelivered and has cardiac arrest, an emergency cesarean section is indicated. Prompt delivery during the resuscitation process not only increases the chances of perinatal survival without neurological sequelae, but also improves the maternal resuscitation effort. We have a 4-minute window for delivery from the time the code is called to avoid neurologic injury to the fetus and optimize outcomes for the mother. This 4-minute principle was adopted by the American Heart Association in 1986, and its clinical use has been supported by 20 years of published case reports since then (Am. J. Obstet. Gynecol. 2005;192:1916-21).
Although outcomes with AFE may be improving somewhat, AFE still causes significant morbidity and mortality. Investigators in the Australian AFE cohort study, for instance, recently reported maternal and perinatal fatality rates of 35% and 32%, respectively. These rates were similar to those from the U.K. Obstetric Surveillance System, according to the authors (BJOG 2010;117:1417-21).
Ruptured Uterus
In an effort to reduce rates of cesarean deliveries, obstetricians are swinging back once again to encouraging more women to attempt vaginal birth after cesarean delivery (VBAC). Because the rates of uterine rupture are higher in women who attempt VBAC, our index of suspicion should be acute for any woman who is laboring after having had a prior cesarean delivery. We also must do everything we can to assess a patient’s risks of failed VBAC resulting in emergency cesarean section and uterine rupture.
The American College of Obstetricians and Gynecologists (ACOG) now recommends that most women with one previous cesarean delivery and with a low transverse incision be counseled about VBAC and offered a trial of labor. ACOG points out in its 2010 practice bulletin on VBAC that in several large studies, the uterine rupture rate after a trial of labor in such women was approximately 0.5-0.9%.
The College also says that many women previously considered to be at high risk may now be considered candidates for a trial of labor after cesarean section (TOLAC). Among the conditions that are no longer necessarily contraindications for attempted VBAC: two previous low transverse cesarean deliveries; suspected fetal macrosomia; twin gestations; more than one previous cesarean delivery; a previous low vertical incision; gestation beyond 40 weeks; and even external cephalic version for breech presentation (Obstet. Gynecol. 2010;116:450-63).
The ACOG bulletin addresses the importance of counseling, and mentions the possible utility of a nomogram developed for predicting the chance of successful VBAC for individual patients. The tool incorporates six variables that are ascertainable at the first prenatal visit, including maternal age, body mass index, and history of vaginal delivery (Obstet. Gynecol. 2007;109:806-12). The tool, a calculator of sorts, was developed through research by the National Institute of Child Health and Development’s (NICHD’s) Maternal-Fetal Medicine Units Network, and is also available at http://www.bsc.gwu.edu/mfmu/vagbirth.html.
Such individualized risk assessment is critical. Another model for assessing risk and informing discussions with individual patients is one developed in the United Kingdom by Dr. Gordon C. S. Smith at Cambridge University and his associates (PLoS Med. 2005:2:e252). These investigators documented that women with a predicted cesarean section risk (an unsuccessful trial of labor) of less than 20% using their model had a minimal incidence of uterine rupture of 2.0 per 1,000, while those deemed to have a high risk of cesarean delivery – defined as greater than 40% – had an incidence of uterine rupture of 9.1 per 1,000.
However small it is in absolute terms, there is an inherent risk of the uterine incision rupturing during an attempt at labor after a previous cesarean section. Indicative of this inherent risk are recommendations by the authors of numerous studies, as well as ACOG, for VBAC to be attempted in facilities with staff available for emergency care. When the fetus is actually extruded through the incision and into the abdominal cavity, there is significant risk of severe maternal and perinatal morbidity and mortality secondary to blood loss and hypoxia.
Signs and symptoms of possible uterine rupture include the following: a change in fetal heart rate pattern from normal to a category 3 heart rate tracing; unexplained vaginal bleeding; frequent epidural dosing or pain that is not alleviated with epidural anesthesia already in place; and loss of uterine tone with an intrauterine pressure catheter (IUPC) in place. If an IUPC is flushed and the patient still has abnormal readings, a diagnosis of uterine scar disruption should be entertained.
In addition to prompt recognition, rapid delivery and blood replacement are key to improving outcomes. The coagulopathy in patients with a ruptured uterus is dilutional rather consumptive, so these patients require replacement not only of packed red blood cells but also of clotting factors and other blood products. As with other types of obstetric hemorrhage, blood loss is usually in excess of the amount perceived.
A recent population-based registry study of 94 identified uterine ruptures after previous cesarean section found that almost half of the mothers diagnosed with uterine rupture after TOLAC (versus during elective or emergency prelabor cesarean section) developed moderate postpartum hemorrhage, while 15% developed severe postpartum hemorrhage and 4% needed peripartum hysterectomy (BJOG 2010;117:809-20).
Perinatal complications occurred in 48 of the 81 (59%) ruptures that occurred after attempted VBAC. In nine (19%) cases, the outcomes were serious (three deaths, three cases of severe asphyxia, and three cases of posthypoxic encephalopathy). To reduce the risk of iatrogenic uterine scar disruption, care should be taken in choosing the appropriate method of induction.
Peripartum Cardiomyopathy
This complication is characterized by the development of heart failure due to significant left ventricular (LV) systolic dysfunction. It is a diagnosis of exclusion. Patients present with the same signs and symptoms characterizing other forms of heart failure secondary to LV dysfunction, and other causes of heart disease and forms of heart failure must be ruled out.
This relatively uncommon myocardial complication can occur up to 5-6 months after delivery, but it usually occurs early in the postpartum period, with about 75% of cases presenting within the first month after delivery (Postgrad. Med. J. 2011;87:34-9). Most patients who are diagnosed during pregnancy present in the third trimester.
Various potential etiologies have been proposed – from viral myocarditis and abnormal hormonal regulation, to excessive prolactin production and an abnormal immune response to pregnancy – but its exact cause is still unknown.
Its incidence in the United States may be increasing. According to a recent review by Dr. Uri Elkayam, the incidence is estimated at approximately 1 in 3,200 deliveries, with a significantly higher incidence (up to 16-fold higher in one study) in African American women (J. Am. Coll. Cardiol. 2011;58:659-70).
Rates as high as 1 in 300 in Haiti and 1 in 100 in a small region of sub-Saharan Africa have also been reported in recent years, according to another review by Dr. Meredith Cruz and her associates (Obstet. Gynecol. Clin. N. Am. 2010;37:283-303).
Certainly, we must all be aware that certain ethnic groups and populations – most notably women of African descent – appear to be more at risk. Pregnancy-related hypertension and preeclampsia also are often cited as risk factors, as are multiparity, obesity, and older maternal age.
Diagnosis requires a high index of suspicion and vigilance, especially because many of the symptoms – shortness of breath, increased peripheral edema, and exhaustion, for instance – are similar to typical symptoms of a normal pregnancy. The diagnosis should be strongly considered in any woman who has nocturnal dyspnea. Chest pain, nocturnal cough, new regurgitant murmurs, pulmonary crackles, increased jugular venous pressure, or hepatomegaly also should raise suspicions, according to the review by Dr. Cruz and her associates.
The timing of delivery in patients diagnosed during pregnancy depends on the maternal status. If the mother is responding to medical management and is stable enough with regard to cardiovascular status to tolerate her heart failure, then induction of labor can be scheduled for or considered at 37 weeks’ gestation. If she is unstable or her LV function is poor or worsening, then early delivery should be considered.
Vaginal delivery often is preferable so that the potential risks associated with anesthesia and surgical delivery, such as clots or infection, can be avoided. Sometimes, however, cesarean delivery may be the only option. For a woman who is laboring, it is important to shorten the second stage of labor, with either low forceps or a vacuum device, in order to minimize pushing and ventricular work.
Management requires teamwork with cardiology, intensive care, anesthesiology, and nursing. After delivery, during a patient’s postpartum fluid shift, she should be managed in a critical care unit or another closely observed setting.
The management of peripartum cardiomyopathy – during pregnancy or afterward – is aimed at improving symptoms, slowing the progression of LV dysfunction and heart failure, and preventing arrhythmias and thromboembolism – both common complications.
Diuretics, nitrates, and hydralazine are often indicated and are safe in pregnancy, as is use of the beta-blocker metoprolol and either unfractionated heparin or low-molecular weight heparin for anticoagulation. (Anticoagulants are almost always indicated.) Nonpharmacologically, the focus is on reducing fluid and salt intake and on monitoring electrolyte levels and addressing any imbalances.
On the research front, animal studies and now preliminary data from a very small number of women with acute severe peripartum cardiomyopathy suggest that bromocriptine, an inhibitor of prolactin, may have a favorable effect on outcomes (Circulation 2010;121:1465-73).
Reported mortalities from the disease have ranged as high as 18%-56%, according to the Cruz review. On the other hand, many women will have a full recovery and a normalization of LV function. Dr. Elkayam concludes in his review that a normalization of LV function may occur in more than 50% of women with peripartum cardiomyopathy, mostly within 2-6 months after diagnosis.
Subsequent pregnancy is contraindicated in women who do not have a resolution of LV dysfunction, and even when LV function normalizes, there is a risk of recurrent and persistent dysfunction in a subsequent pregnancy.
Dr. Whiteman is associate professor and interim director of the division of maternal-fetal medicine at the University of South Florida, Tampa. She said she has no relevant financial disclosures.
The process of labor and delivery is considered to be a joyous event in women’s lives, and most of the time it is. However, practitioners have to be aware of potential complications that can have dire adverse outcomes, causing maternal morbidity and mortality as well as severe consequences for the baby.
In considering such complications, physicians usually think of women who have serious underlying comorbidities. However, the three complications discussed here – amniotic fluid embolism, ruptured uterus, and peripartum cardiomyopathy – are conditions that can happen to otherwise young, healthy women. Fortunately these complications are rare. However, when they do happen, early recognition and prompt intervention are critical to optimizing the outcome. This means we must continually keep a high index of suspicion for all such complications so that we are ready in the event that labor and delivery does not proceed normally.
Amniotic Fluid Embolism
This complication is a leading cause of maternal morbidity and mortality in the United States and other developed countries. It should be considered in any patient who has sudden, unheralded cardiopulmonary collapse followed by profuse hemorrhage associated with disseminated intravascular coagulation (DIC).
While the hallmark presentation of amniotic fluid embolism (AFE) is this profound cardiopulmonary collapse with severe hemorrhage, it is important to note that published definitions of the condition state that coagulopathy may occur in isolation. In a 2011 review, Dr. Michael Benson points out that at least six case reports have described coagulopathy alone as the sole clinical sign of AFE (Clin. Dev. Immunol. 2012:946576 Epub 2011 Sept. 29 [doi:10.1155/2012/946576]).
AFE is a diagnosis of exclusion, and one that is based on symptoms and clinical presentation rather than on laboratory testing or histopathologic examination. There is broad consensus that a clinical diagnosis of AFE can be made based on one or more of four key signs/symptoms (in the absence of other medical conditions or explanations): cardiovascular collapse (hypotension and/or cardiac arrest); respiratory distress; DIC; and coma and/or seizures.
The condition can occur suddenly and unpredictably at any point during labor and delivery or in the immediate postpartum period. It also has been reported to occur as late as 48 hours after delivery.
Pulmonary thromboembolism often may be suspected, and indeed, it is part of the differential diagnosis. Patients with thrombotic pulmonary embolism do not usually develop the classic DIC type of coagulopathy, however, while patients with AFE are coagulopathic and often hemorrhage profusely.
The incidence of AFE has been difficult to determine. The authors of a 2009 evidence-based review of AFE reported that the estimated incidence based on large population-based studies is 1 in 15,200 deliveries in North America and 1 in 53,800 deliveries in Europe (Am. J. Obstet. Gynecol. 2009;201:445.e1-13). The incidence in an Australian population-based cohort was recently reported to be 3.3 per 100,000 deliveries (BJOG 2010;117:1417-21).
Other published reports and reviews have described an extremely broad range of estimated incidence. For instance, a report in the journal Anesthesia and Analgesia, published by the International Anesthesia Research Society, stated that AFE may occur between 1 in 8,000 and 1 in 80,000 deliveries (Anesth. Analg. 2009;108:1599-602).
The pathophysiology of AFE also is poorly understood, causing us great uncertainty as to why some apparently stable patients undergo such a profound, life-threatening collapse. When AFE was first described more than 70 years ago, it was thought to result from amniotic fluid entering the maternal circulation and obstructing the pulmonary blood flow – thus the name "amniotic fluid embolism." However, research over the decades has consistently discounted this view. As Dr. Benson states, current thinking has shifted away from embolism and toward a maternal immune response to the fetus.
Investigators have suggested possible immunologic mechanisms such as complement activation and reactions similar to anaphylaxis, but more research needs to be done. In the meantime, we must recognize that the name AFE probably does not accurately reflect what actually occurs in these patients.
Management is usually first directed at getting the patient through the initial cardiovascular insults – often hypotension and cardiac arrest – and at treating hypoxia and rapidly correcting maternal hemodynamic instability. Significant teamwork is required for the mother and baby to survive – and to survive neurologically intact. Anesthesia is needed for development and control of the airway, for instance, and critical care is essential for inotropic support. Cardiology also must be involved, as continuous cardiac, respiratory, and blood pressure monitoring – and aggressive respiratory and circulatory support – are key.
The nursing staff also can play a critical role in preventing subsequent pulmonary edema by keeping meticulous records of the intake and output of fluids. The overwhelming insult of AFE to the heart and lungs leaves patients at high risk of developing pulmonary edema, and meticulous record-keeping can help ensure that these patients are not overloaded.
Aggressive blood replacement also is required to reverse the coagulopathy associated with AFE. Transfusion of packed red blood cells is a priority, but fresh frozen plasma, platelets, and cryoprecipitate also should be available for prompt administration.
There have been promising reports of the use of recombinant factor VIIa (rVIIa) for treating hemorrhage in patients with AFE in recent years, but a recent review of case reports of AFE from 2003 to 2009 suggests that the procoagulant may actually worsen outcomes (Anesthesiology 2011;115:1201-8). Indeed, unlike patients with other types of postpartum hemorrhaging, women with AFE have high circulating tissue factor concentrations. Recombinant factor VIIa can combine with tissue factor and form intravascular clots, resulting in thrombosis of major organs.
If the patient is undelivered and has cardiac arrest, an emergency cesarean section is indicated. Prompt delivery during the resuscitation process not only increases the chances of perinatal survival without neurological sequelae, but also improves the maternal resuscitation effort. We have a 4-minute window for delivery from the time the code is called to avoid neurologic injury to the fetus and optimize outcomes for the mother. This 4-minute principle was adopted by the American Heart Association in 1986, and its clinical use has been supported by 20 years of published case reports since then (Am. J. Obstet. Gynecol. 2005;192:1916-21).
Although outcomes with AFE may be improving somewhat, AFE still causes significant morbidity and mortality. Investigators in the Australian AFE cohort study, for instance, recently reported maternal and perinatal fatality rates of 35% and 32%, respectively. These rates were similar to those from the U.K. Obstetric Surveillance System, according to the authors (BJOG 2010;117:1417-21).
Ruptured Uterus
In an effort to reduce rates of cesarean deliveries, obstetricians are swinging back once again to encouraging more women to attempt vaginal birth after cesarean delivery (VBAC). Because the rates of uterine rupture are higher in women who attempt VBAC, our index of suspicion should be acute for any woman who is laboring after having had a prior cesarean delivery. We also must do everything we can to assess a patient’s risks of failed VBAC resulting in emergency cesarean section and uterine rupture.
The American College of Obstetricians and Gynecologists (ACOG) now recommends that most women with one previous cesarean delivery and with a low transverse incision be counseled about VBAC and offered a trial of labor. ACOG points out in its 2010 practice bulletin on VBAC that in several large studies, the uterine rupture rate after a trial of labor in such women was approximately 0.5-0.9%.
The College also says that many women previously considered to be at high risk may now be considered candidates for a trial of labor after cesarean section (TOLAC). Among the conditions that are no longer necessarily contraindications for attempted VBAC: two previous low transverse cesarean deliveries; suspected fetal macrosomia; twin gestations; more than one previous cesarean delivery; a previous low vertical incision; gestation beyond 40 weeks; and even external cephalic version for breech presentation (Obstet. Gynecol. 2010;116:450-63).
The ACOG bulletin addresses the importance of counseling, and mentions the possible utility of a nomogram developed for predicting the chance of successful VBAC for individual patients. The tool incorporates six variables that are ascertainable at the first prenatal visit, including maternal age, body mass index, and history of vaginal delivery (Obstet. Gynecol. 2007;109:806-12). The tool, a calculator of sorts, was developed through research by the National Institute of Child Health and Development’s (NICHD’s) Maternal-Fetal Medicine Units Network, and is also available at http://www.bsc.gwu.edu/mfmu/vagbirth.html.
Such individualized risk assessment is critical. Another model for assessing risk and informing discussions with individual patients is one developed in the United Kingdom by Dr. Gordon C. S. Smith at Cambridge University and his associates (PLoS Med. 2005:2:e252). These investigators documented that women with a predicted cesarean section risk (an unsuccessful trial of labor) of less than 20% using their model had a minimal incidence of uterine rupture of 2.0 per 1,000, while those deemed to have a high risk of cesarean delivery – defined as greater than 40% – had an incidence of uterine rupture of 9.1 per 1,000.
However small it is in absolute terms, there is an inherent risk of the uterine incision rupturing during an attempt at labor after a previous cesarean section. Indicative of this inherent risk are recommendations by the authors of numerous studies, as well as ACOG, for VBAC to be attempted in facilities with staff available for emergency care. When the fetus is actually extruded through the incision and into the abdominal cavity, there is significant risk of severe maternal and perinatal morbidity and mortality secondary to blood loss and hypoxia.
Signs and symptoms of possible uterine rupture include the following: a change in fetal heart rate pattern from normal to a category 3 heart rate tracing; unexplained vaginal bleeding; frequent epidural dosing or pain that is not alleviated with epidural anesthesia already in place; and loss of uterine tone with an intrauterine pressure catheter (IUPC) in place. If an IUPC is flushed and the patient still has abnormal readings, a diagnosis of uterine scar disruption should be entertained.
In addition to prompt recognition, rapid delivery and blood replacement are key to improving outcomes. The coagulopathy in patients with a ruptured uterus is dilutional rather consumptive, so these patients require replacement not only of packed red blood cells but also of clotting factors and other blood products. As with other types of obstetric hemorrhage, blood loss is usually in excess of the amount perceived.
A recent population-based registry study of 94 identified uterine ruptures after previous cesarean section found that almost half of the mothers diagnosed with uterine rupture after TOLAC (versus during elective or emergency prelabor cesarean section) developed moderate postpartum hemorrhage, while 15% developed severe postpartum hemorrhage and 4% needed peripartum hysterectomy (BJOG 2010;117:809-20).
Perinatal complications occurred in 48 of the 81 (59%) ruptures that occurred after attempted VBAC. In nine (19%) cases, the outcomes were serious (three deaths, three cases of severe asphyxia, and three cases of posthypoxic encephalopathy). To reduce the risk of iatrogenic uterine scar disruption, care should be taken in choosing the appropriate method of induction.
Peripartum Cardiomyopathy
This complication is characterized by the development of heart failure due to significant left ventricular (LV) systolic dysfunction. It is a diagnosis of exclusion. Patients present with the same signs and symptoms characterizing other forms of heart failure secondary to LV dysfunction, and other causes of heart disease and forms of heart failure must be ruled out.
This relatively uncommon myocardial complication can occur up to 5-6 months after delivery, but it usually occurs early in the postpartum period, with about 75% of cases presenting within the first month after delivery (Postgrad. Med. J. 2011;87:34-9). Most patients who are diagnosed during pregnancy present in the third trimester.
Various potential etiologies have been proposed – from viral myocarditis and abnormal hormonal regulation, to excessive prolactin production and an abnormal immune response to pregnancy – but its exact cause is still unknown.
Its incidence in the United States may be increasing. According to a recent review by Dr. Uri Elkayam, the incidence is estimated at approximately 1 in 3,200 deliveries, with a significantly higher incidence (up to 16-fold higher in one study) in African American women (J. Am. Coll. Cardiol. 2011;58:659-70).
Rates as high as 1 in 300 in Haiti and 1 in 100 in a small region of sub-Saharan Africa have also been reported in recent years, according to another review by Dr. Meredith Cruz and her associates (Obstet. Gynecol. Clin. N. Am. 2010;37:283-303).
Certainly, we must all be aware that certain ethnic groups and populations – most notably women of African descent – appear to be more at risk. Pregnancy-related hypertension and preeclampsia also are often cited as risk factors, as are multiparity, obesity, and older maternal age.
Diagnosis requires a high index of suspicion and vigilance, especially because many of the symptoms – shortness of breath, increased peripheral edema, and exhaustion, for instance – are similar to typical symptoms of a normal pregnancy. The diagnosis should be strongly considered in any woman who has nocturnal dyspnea. Chest pain, nocturnal cough, new regurgitant murmurs, pulmonary crackles, increased jugular venous pressure, or hepatomegaly also should raise suspicions, according to the review by Dr. Cruz and her associates.
The timing of delivery in patients diagnosed during pregnancy depends on the maternal status. If the mother is responding to medical management and is stable enough with regard to cardiovascular status to tolerate her heart failure, then induction of labor can be scheduled for or considered at 37 weeks’ gestation. If she is unstable or her LV function is poor or worsening, then early delivery should be considered.
Vaginal delivery often is preferable so that the potential risks associated with anesthesia and surgical delivery, such as clots or infection, can be avoided. Sometimes, however, cesarean delivery may be the only option. For a woman who is laboring, it is important to shorten the second stage of labor, with either low forceps or a vacuum device, in order to minimize pushing and ventricular work.
Management requires teamwork with cardiology, intensive care, anesthesiology, and nursing. After delivery, during a patient’s postpartum fluid shift, she should be managed in a critical care unit or another closely observed setting.
The management of peripartum cardiomyopathy – during pregnancy or afterward – is aimed at improving symptoms, slowing the progression of LV dysfunction and heart failure, and preventing arrhythmias and thromboembolism – both common complications.
Diuretics, nitrates, and hydralazine are often indicated and are safe in pregnancy, as is use of the beta-blocker metoprolol and either unfractionated heparin or low-molecular weight heparin for anticoagulation. (Anticoagulants are almost always indicated.) Nonpharmacologically, the focus is on reducing fluid and salt intake and on monitoring electrolyte levels and addressing any imbalances.
On the research front, animal studies and now preliminary data from a very small number of women with acute severe peripartum cardiomyopathy suggest that bromocriptine, an inhibitor of prolactin, may have a favorable effect on outcomes (Circulation 2010;121:1465-73).
Reported mortalities from the disease have ranged as high as 18%-56%, according to the Cruz review. On the other hand, many women will have a full recovery and a normalization of LV function. Dr. Elkayam concludes in his review that a normalization of LV function may occur in more than 50% of women with peripartum cardiomyopathy, mostly within 2-6 months after diagnosis.
Subsequent pregnancy is contraindicated in women who do not have a resolution of LV dysfunction, and even when LV function normalizes, there is a risk of recurrent and persistent dysfunction in a subsequent pregnancy.
Dr. Whiteman is associate professor and interim director of the division of maternal-fetal medicine at the University of South Florida, Tampa. She said she has no relevant financial disclosures.
The Hoopla Over Mesh: What It Means for Practice
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
The Food and Drug Administration's warning last summer of the risks associated with transvaginal placement of mesh for repair of pelvic organ prolapse and stress urinary incontinence – and its overall, ongoing review of how mesh products are cleared for use–have changed the climate for ob.gyns. and patients. It has upped the ante for comprehensive patient counseling and brought to the fore the fact that pelvic floor repair is a combination of art, science, judgment, skill, training, and experience.
In July 2011, the FDA issued a “safety communication” to physicians and patients, which was based on an analysis of adverse event reports and a systematic literature review, warning that the transvaginal placement of mesh to treat pelvic organ prolapse (POP) appears to be riskier than traditional repairs without any evidence of greater effectiveness. While an earlier FDA notice issued in 2008 had said in essence that there may be a problem with transvaginal mesh, the most recent warning said there is a problem – that serious complications associated with surgical mesh used for transvaginal repair of POP are not rare.
The agency made a distinction between apical and posterior repair, and anterior repair, concluding that there is no evidence that either apical or posterior repair done with mesh provides any added benefit compared with traditional surgery without mesh.
With regard to anterior repair, the FDA concluded that mesh augmentation may provide an anatomic benefit compared with traditional nonmesh repair, although this anatomic benefit may not necessarily lead to better symptomatic results.
The FDA also reviewed all types of midurethral sling (MUS) devices used to treat stress urinary incontinence (SUI), grouping retropubic and transobturator slings as first-generation and mini-slings as second-generation devices.
Whereas these devices were deemed to be as effective as or better than traditional repairs, the FDA stated its concerns about the potential for long-term problems including mesh erosion and pelvic pain. Moreover, the agency stated the need for more data to better evaluate mini-slings for comparative efficacy and complications.
More broadly, the FDA is reevaluating how transvaginal mesh products should be regulated and brought to market. Unlike other devices that are widely used by ob.gyns., not one of the pelvic floor mesh kits for POP or midurethral slings for SUI has been evaluated by way of an independent, FDA-mandated randomized clinical trial. This is because transvaginal meshes are currently classified as class II devices and, as such, have been cleared for market by the less rigorous 510(k) notification process rather than a more rigorous premarket approval (PMA) process.
While the FDA considers the 510(k) pathway still suitable for MUS devices used to treat SUI, the agency is taking a harder look at transvaginal mesh used to repair POP and has recommended reclassification of these devices into class III. This switch would require the more onerous PMA process and allow the FDA to require clinical trials comparing procedures that involve mesh with those in which mesh is not used.
How the FDA Regulates Devices
That transvaginal mesh devices are embroiled in a broader and ongoing controversy over how best to regulate or approve medical devices is important to understand. Innovation and potential market share continue to drive a steady stream of new medical devices for gynecologic surgery.
Until 36 years ago there was no federal regulation of medical devices. The Medical Device Amendments of 1976 established three device classes, based on risk levels and the ability of postmarketing controls to manage those risks. The law then identified pathways, based largely on this classification system, for bringing devices to the market.
Class I devices are generally those for which general postmarketing controls such as good manufacturing processes and record keeping are deemed sufficient to provide reasonable assurance of safety and effectiveness. Devices in class II, which are “moderate risk,” need special controls such as performance standards and postmarketing surveillance to provide reasonable assurance of safety and effectiveness. In class III are life-sustaining or life-supporting “high-risk” devices that cannot be placed in class I or II because there is insufficient information to establish requisite assurance with postmarketing controls.
While FDA-approved randomized and controlled clinical trials are required for class III devices as part of the standard PMA process, class II devices are cleared for the market based on the substantially less rigorous 510(k) Premarket Notification Program process, which requires manufacturers to demonstrate safety and effectiveness by proving “substantial equivalence” to another device that is already cleared by the FDA based on intended use and product design.
Whereas clinical data are not required, this review of substantial equivalence requires labeling and performance data, including material safety, mechanical performance, and animal testing. Approval of the first surgical mesh for repair of POP was judged to be substantially equivalent to surgical mesh used for hernia repair.
In recent years there has been growing concern about this process of clearing medical devices based simply on substantial equivalence with a predicate. New products should not necessarily be assumed to have equal or improved safety and efficacy. The Institute of Medicine weighed in this past summer with a report on the 510(k) clearance process, calling it flawed in its ability to provide determinations about each device's safety and effectiveness.
The future of transvaginal mesh products is now entangled in these concerns. Unlike devices for endometrial ablation and transcervical hysteroscopic sterilization, which are justifiably classified as class III devices, all transvaginal mesh devices to date have been cleared as class II devices.
Since 2001, the FDA has cleared via the 510(k) approval process more than 100 synthetic mesh devices or kits indicated for POP repair, and more than 75 mesh devices to treat SUI (including 7 second-generation mini-slings), using the 510(k) notification process. None of the clearances were based on clinical data.
While there have indeed been some randomized clinical trials (in its recent review, FDA officials reported having looked at 22 randomized controlled trials and 38 observational studies on the use of mesh to treat POP), many of these trials have been designed and conducted with industry sponsorship.
The FDA typically calls upon its advisory panels to provide independent expert advice when specific issues or problems arise and when regulatory decisions need to be made both before and after approval of medical devices.
After issuing its “safety communication” last July, the FDA convened the Obstetrics and Gynecology Devices Advisory Panel in September to make recommendations regarding the safety and effectiveness of surgical mesh for repair of POP and SUI. Ironically, transvaginal mesh devices had previously been regulated by the FDA's Plastic Surgery Devices Panel.
The 2-day public hearing included presentations regarding adverse events and effectiveness of transvaginal mesh for POP and then SUI by FDA staff reviewers, key medical organizations, related industry as a consortium, and public advocacy groups as well as personal testimony by patients having undergone these procedures.
After hearing the testimony and an exhaustive discussion, the majority of panel members supported reclassifying mesh devices for POP from class II to class III. On the other hand, while the majority did not recommend the reclassification of devices for SUI, the panel concurred that more clinical data was warranted to establish the safety and efficacy of second-generation mini-slings.
The FDA's final regulatory decisions will slowly evolve as the issues of safety and effectiveness are balanced with reducing the burden for industry and continuing to foster a hospitable climate for medical innovation.
Adverse Event Reports
The FDA's safety communication released in July, which updated the 2008 FDA Public Health Notification, was generated by continuing concerns raised by rising reports of adverse events as well as concern voiced by the American Urogynecologic Society.
The adverse event reports have been compiled via the FDA's Manufacturer and User Facility Device Experience (MAUDE) database, which collects both mandated reporting by manufacturers and voluntary reports by physicians, patients, and any interested party. It is presumed that complications are generally underreported.
From 2008 to 2010, the FDA received 2,874 adverse event reports associated with urogynecologic mesh – about three times the number of reports filed from 2005 to 2007. Of these, 1,503 were associated with products for POP, and 1,371 were associated with products for SUI.
It is unclear, of course, how much of this increase reflects an increase in actual adverse events and how much stems from the increased use of mesh, an increased awareness of adverse events, possible duplication of reporting, and other factors that are inherent limitations of the reporting process. Moreover, the complication rate is not known because the total number of adverse events and the total number of implanted delivery systems are not known.
Erosion, exposure, and extrusion continue to be the most frequent and concerning adverse events associated with mesh used for POP and SUI. With its more recent review, the FDA has new concerns about the delayed appearances of erosion and mesh exposure. While there are few treatment cohorts to evaluate after 36 months, there have been a number of reports of long-term adverse outcomes – some at time points up to 60 months post procedure.
Moreover, the FDA is concerned about the risk for later development of dyspareunia and new pelvic pain from mesh contraction, retraction, vaginal shrinkage, and subsequent reoperation – problems not identified or flagged when the agency completed its last comprehensive review before issuing the 2008 notification.
Current State of Transvaginal Mesh
In the most recent safety communication, the FDA instructs patients to be aware of the risks associated with surgical mesh for transvaginal repair of POP and SUI. It warns patients that having transvaginal mesh surgery may increase their risk of needing additional surgery due to mesh-related complications, and it advises patients to ask their surgeons about all POP treatment options.
The alert also tells patients to notify their physicians regarding vaginal or pain symptoms after surgery with transvaginal mesh, and to let their health care providers know they have implanted mesh – advice that, in and of itself, can create fear. Any patient doing diligent research will see the statement and related discussion.
In issuing the communication, the FDA has set the bar at a higher level of expectation for patient counseling and informed consent.
While the FDA does not regulate the practice of medicine by regulating how or which physicians can use devices, the agency indirectly is regulating the use of transvaginal mesh devices through its alerts.
And without question, the probability for medical-legal conflict has been substantially heightened. Propelled by the FDA warnings, a cursory Internet search for “pelvic mesh lawyers” or “vaginal mesh lawsuit attorneys” yields a list of firms encouraging free case reviews.
Patients should be counseled that transvaginal mesh procedures are considered innovative techniques for pelvic floor repair that demonstrate high rates of anatomic cure in shorter-term series.
Preoperative counseling should cover the following principles and guidelines:
▸ There are potential adverse sequelae of transvaginal mesh repairs.
▸ There are limited data comparing transvaginal mesh systems with traditional vaginal prolapse repairs or with traditional use of graft material in the form of augmented colporrhaphy and sacrocolpopexy.
▸ The placement of surgical mesh for POP by sacrocolpopexy for apical prolapse is a well established clinical practice and may result in lower rates of mesh complications.
▸ Transvaginal apical or posterior repair with mesh does not appear to provide any added benefit compared with traditional surgery without mesh.
The main role for mesh with POP repair is in the anterior compartment, where a higher risk of recurrence with traditional repairs has been documented.
Overall, transvaginal mesh repair of POP is best suited to women who are high risk due to medical conditions and in those with recurrent prolapse, particularly of the anterior compartment.
▸ The effectiveness of retropubic and transobturator suburethral slings for SUI has been demonstrated, while the safety and effectiveness of single-incision mini-slings is less well established.
Rather than the fault of the device or method, the failure or success of transvaginal mesh repairs may rely far more on the skill and judgment of the surgeon.
All surgery incorporates an intricate blend of art and science. We must be realistic in evaluating our skills, experience, and expertise in performing transvaginal mesh procedures.
Even in the best of circumstances, factors such as obesity, hypoestrogenism, advanced age, poor nutrition, extreme life activity, multiparity, Northern European descent, smoking, prior reparative surgery, and diabetes may reduce the success of transvaginal mesh procedures and increase complications.
While patient concerns will be heightened, the decision to perform a particular type of restorative or reparative surgery for POP, with or without mesh, should always favor reduced risk along with optimal and durable outcome that is both anatomic and functional in nature. And clinical decision making, as always, must be guided by our Hippocratic vow “primum non nocere”!
Vitals
Source Elsevier Global Medical News
Source Elsevier Global Medical News
To Mesh or Not to Mesh?
On July 13, 2011, the Food and Drug Administration issued a safety communication, “Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse,” intended for health care providers and patients. Previously, on Oct. 20, 2008, the FDA issued a Public Health Notification and Additional Patient Information statement on serious complications associated with surgical mesh placed transvaginally to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
In the July 2011 bulletin, the FDA stated that “serious complications associated with surgical mesh for transvaginal repair of pelvic organ prolapse are not rare. … Furthermore, it is not clear that transvaginal pelvic organ prolapse repair with mesh is more effective than traditional nonmesh repair in all patients with pelvic organ prolapse and it may expose patients to greater risk.”
In its bulletin, the FDA noted a marked increase in reported adverse events related to surgical mesh devices used to repair POP and SUI in reporting years 2005-2007 vs. 2008-2010. The most frequent complications reported to the FDA regarding transvaginal mesh placement for POP were mesh erosion through the vagina, pain, infection, bleeding, dyspareunia, organ perforation, and urinary problems. Also noted were recurrent prolapse, neuromuscular problems, vaginal scarring/shrinkage, and emotional problems. Moreover, men may experience irritation and pain to the penis during intercourse secondary to exposed mesh.
The FDA also reported on its systematic review of literature from the period of 1996-2011 to evaluate transvaginal mesh safety and effectiveness. In particular, the FDA noted the following:
▸ Potential for additional risk when mesh is utilized in POP surgery.
▸ Greater rate of complications in POP surgery when mesh placed transvaginally, rather than transabdominally.
▸ No advantage of mesh for either apical or posterior repair, compared with traditional surgery without mesh.
▸ Although mesh may be beneficial anatomically for anterior repair, symptoms may not improve over conventional anterior repair.
The FDA then went on to make recommendations to both health care workers and patients.
Health care workers are advised to obtain specialized training for each mesh placement technique. Mesh should be considered only after weighing the risks and benefits, as well as considering other nonsurgical and surgical options including nonmesh and transabdominal mesh techniques.
Patients must be made aware that surgical mesh is a permanent implant, which may make future surgical repair more challenging.
Moreover, mesh may place the patient at greater risk for requiring additional surgery for the development of additional complications. Removal of mesh when complications arise may involve multiple surgeries and may negatively impact the patient's quality of life. Complete removal of the mesh may not be possible, and even if it is removed, symptoms may continue. Patients also must realize the lack of long-term data.
To understand how this latest FDA bulletin will impact the surgical treatment of POP and SUI, I have called upon Dr. Andrew I. Brill, director of minimally invasive surgery and reparative pelvic surgery at California Pacific Medical Center, San Francisco. He also is a voting member of the FDA Obstetrics and Gynecology Device Panel. Prior to moving to the Bay Area in 2006, Dr. Brill was professor of obstetrics and gynecology at the University of Illinois at Chicago, where he directed one of the first accredited fellowships in minimally invasive gynecology. Dr. Brill is a past president of both the AAGL and the board of directors of the AAGL/Society of Reproductive Surgeons Fellowship in Minimally Invasive Gynecology. Widely recognized in the United States and abroad as a leading educator in the field of minimally invasive gynecology, Dr. Brill is a frequent lecturer and telesurgeon, and he continues to be a regular contributor to peer literature and textbooks, having coauthored a leading textbook and more than 50 articles and book chapters.
Management of Shoulder Dystocia
Shoulder dystocia is not an uncommon obstetric complication, occurring in as many as 2 per 100 vaginal births. This obstetric emergency is associated with a number of adverse perinatal outcomes for both the mother and infant, the most serious of which remains neonatal brachial plexus injury. In a minority of cases in which there is prolonged impaction of the shoulders, birth asphyxia also may occur.
Obstetricians and other birth attendants must be fully prepared to effectively manage shoulder dystocia when it occurs. They also should understand the existing controversies regarding prevention and the pathogenesis of injuries associated with shoulder dystocia.
Shoulder dystocia generally is not a predictable event, which makes prevention extremely difficult. Because of the limited accuracy of ultrasound for estimating fetal size, the risk of shoulder dystocia and resulting injury must be fairly significant before prophylactic cesarean is considered as a preventive measure. There are, however, certain high-risk scenarios that call for consideration of prophylactic cesarean delivery.
Prevention
For the past several decades, clinical research has focused on whether shoulder dystocia can be predicted and/or prevented. Overall, most analyses have shown us that shoulder dystocia can be only minimally predicted, at best, and that prevention of this complication as well as associated injury is far from a simple undertaking.
The leading risk factor for shoulder dystocia is excessive birth weight, yet not all cases of shoulder dystocia involve infants who weigh more than 4,500 g, or even more than 4,000 g. In fact, most shoulder dystocia cases actually occur when birth weights are less than 4,000 g – especially in nondiabetic pregnancies. (In diabetic pregnancies, most shoulder dystocias and brachial plexus injuries do occur in infants with birth weights greater than 4,000 g.)
The possibility that birth weight estimates may help us to predict and/or prevent shoulder dystocia also is hindered by the fact that it remains difficult to identify large babies prior to delivery. Clinical estimation of size and the use of ultrasound are the two most commonly employed techniques for estimating birth size, but both have limited accuracy and may either underestimate or overestimate fetal size. Most large babies, moreover, can successfully undergo vaginal birth without the complication of shoulder dystocia, let alone brachial plexus injury.
All told, these realities limit our ability to use estimated birth weight in selecting those pregnancies that might benefit from prophylactic cesarean delivery.
To consider prophylactic cesarean delivery, the level of risk for shoulder dystocia and resultant injury must be fairly significant. The following are two clinical scenarios in which the risk of complications reaches a level at which the option of prophylactic cesarean section (including informed consent) should be discussed with the mother:
▸ A pregnancy complicated by diabetes in which the estimated fetal weight is greater than or equal to 4,500 g. Some experts have suggested that this threshold should, in fact, be lower in diabetic pregnancies. However, utilization of a lower threshold (such as 4,000 g or 4,250 g) must come with the recognition that it will spur the use of more cesarean deliveries to prevent injury.
▸ A patient with a history of shoulder dystocia birth, particularly when the fetus is believed to be of similar or greater weight than the previously affected fetus.
Determining the recurrence risk of shoulder dystocia has proved difficult because, in most clinical series, a large proportion of women with a history of the complication will undergo scheduled cesarean delivery in their subsequent pregnancies. This bias toward operative delivery may lead to an underestimation of the true recurrence risk. Regardless of this potential estimation bias, unless the estimated fetal weight in the woman's current pregnancy is significantly less than that of the prior pregnancy, we should counsel women with prior shoulder dystocia and offer them prophylactic cesarean delivery.
With respect to the predictive value of labor abnormalities, studies have yielded mixed results. The bottom line is that labor abnormalities are not particularly useful in predicting shoulder dystocia – except for cases of a prolonged second stage of labor when there is suspicion of a large infant. This combination of factors should alert the physician to the potential for shoulder dystocia. Operative vaginal delivery should generally be avoided in this scenario, because delivery above an outlet station may further increase the risk of shoulder dystocia and resultant injury.
Management, Medicolegal Issues
As with any delivery, the goal of management should always be to deliver the infant as safely as possible, minimizing the risk of traumatic injury and birth asphyxia. In most cases of shoulder dystocia, the shoulders remain in an anterior-posterior position and fail to rotate. This creates the potential for brachial plexus injury as the nerves of the brachial plexus are stretched with the descent of the fetal head.
There is little objective study of the maneuvers employed for shoulder dystocia and their effectiveness in preventing neonatal injury, let alone prospective studies comparing the effectiveness of one maneuver vs. another. The choice of maneuvers thus remains provider specific. The maneuvers that are most commonly employed for shoulder dystocia, however, are utilized in order to disimpact the anterior shoulder from behind the symphysis pubis by effecting its rotation.
It is important to appreciate that the McRoberts maneuver, with or without suprapubic pressure, may be successful in only approximately 50% of shoulder dystocia cases.
Unfortunately, many young obstetricians have had limited exposure to shoulder dystocia and may have employed only this maneuver, and not others, in their clinical training. At some point, they will likely encounter a shoulder dystocia case that does not respond to the McRoberts and/or suprapubic pressure maneuvers. It is critical to be competent in performing a full repertoire of potentially effective maneuvers.
There is increasing evidence that obstetricians should have a low threshold for utilizing delivery of the posterior shoulder in the management of shoulder dystocia.
In one recently published, multicenter review of shoulder dystocia maneuvers, for instance, investigators identified women who had incurred a shoulder dystocia during delivery and compared cases involving neonatal injury with injury-free cases. Delivery of the posterior shoulder was associated with the highest rate of successful delivery, when compared with other maneuvers, and with similar rates of neonatal injury (Obstet. Gynecol. 2011;117:1272-8).
The value of posterior arm release lies in its ability to reduce the anterior-posterior diameter of the fetus more significantly than any other maneuver. It has been associated with a marked decrease in anterior nerve stretch and the force required to effect delivery (Obstet. Gynecol. 2003;101:1068-72; Am. J. Obstet. Gynecol. 2010;203:339.e1-5).
In many litigated cases involving shoulder dystocia and brachial plexus injury, it is asserted that unnecessary excess traction must have been employed for a permanent injury to have occurred. Such assertions imply that the obstetrician can perfectly gauge the amount of traction or force necessary to deliver the infant and yet avoid injury in the setting of shoulder dystocia, which is not the case.
Increasing evidence suggests that many cases of brachial plexus injury accompanying shoulder dystocia are multifactorial in origin, and are not simply a result of operator-induced traction and stretching of the nerves. Obstetricians are continually instructed early on in their careers that excess traction should be avoided, as should any fundal pressure that might further disimpact the shoulders.
I simply recommend abandoning any traction efforts once shoulder dystocia is clearly recognized. When the complication occurs, a team consisting of additional nursing personnel, anesthesia, and the most experienced obstetrician available should be immediately summoned, and expulsive efforts on behalf of the mother should be curtailed while maneuvers are being undertaken to disimpact the shoulders.
If two obstetricians are present, it often is helpful for the stronger of the two to deliver appropriate suprapubic pressure from above. The goal is to move the shoulders to an oblique position by exerting pressure from the back of the fetus. This maneuver cannot really be done effectively by a single operator or from below as has been depicted in some textbooks. Again, if this fails to work, a low threshold should exist for attempting a posterior arm release.
Maintaining accurate documentation in the medical record of all events preceding and surrounding the shoulder dystocia is important. This includes but is not limited to the following:
▸ Consideration of significant risk factors for macrosomia, including diabetic pregnancy management and results of gestational diabetes screening tests.
▸ Estimation of fetal size, either clinically or by ultrasound. Most experts believe that diabetic mothers should undergo ultrasound at term to assess fetal size.
▸ Description of instrumental delivery, including indication and station at application and duration of use.
▸ A detailed step-by-step description of the maneuvers used to disimpact the shoulders. The anterior shoulder should be identified as part of the documentation.
Training and Simulation
During the past few years, simulation and drills and other enhanced teaching methods have become an increasingly common part of the curriculum for training residents and nursing personnel in the management of shoulder dystocia. Because the complication occurs relatively infrequently but can have devastating consequences when it does, shoulder dystocia is one of only several obstetric emergencies to be targeted in efforts to improve patient safety.
As with the few other obstetric events that receive such attention, data on the impact of enhanced training on perinatal outcomes remain limited. There clearly is evidence that simulation and drills improve team performance, and it has been hoped that improved team performance will ultimately translate to better outcomes. At present, two studies have indicated that the incidence of brachial plexus injury may decline with the implementation of targeted training for maternity staff.
One of these studies retrospectively compared the management and neonatal outcomes of almost 20,000 births that were complicated by shoulder dystocia in the years before and after the introduction of shoulder dystocia training for all maternity staff in a hospital in the United Kingdom (Obstet. Gynecol. 2008;112:14-20). The rate of brachial plexus injury at birth was significantly reduced, from 7.4% to 2.3%, as was the rate of neonatal injury more broadly (from 9.3% to 2.3%).
In the other study – also a retrospective assessment – the rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 30% before a training protocol was implemented for maternity staff at Jamaica Hospital in New York, to 11% afterward (Am. J. Obstet. Gynecol. 2011;204:322.e1-6).
A recently published study from Ireland, however, failed to reveal any difference in the frequency of brachial plexus injury after the introduction of specific staff training in managing shoulder dystocia. In this single-hospital study, investigators assessed outcomes associated with more than 77,000 deliveries that occurred during two 5-year time periods, before and after training was instituted. The incidence of brachial plexus injury remained unchanged from 1.5 per 1,000 in 1994-1998 to 1.7 per 1,000 in 2004-2008 (Am. J. Obstet. Gynecol. 2011;204:324.e1-6).
Although the results of this latter study are disappointing, I believe they are unlikely to limit the enthusiasm for the simulation training and shoulder dystocia drills that have become fairly routine in many large maternity hospitals in the United States.
Regardless of the limited outcomes data we have available thus far, experience with simulation training has taught us that in order to retain necessary skills, repetitive participation in simulation training appears to be required. The relatively infrequent nature of severe shoulder dystocia cases makes the simulation model for learning very attractive.
The doctor inserts a hand (left), then he/she sweeps the arm across the baby's chest and over the mother's perineum.
Source Images: ©Elsevier, From Obstetrics: Normal and Problem Pregnancies, 5th Edition
Shoulder Dystocia
Routine vaginal deliveries can sometimes quickly become not-so-routine deliveries. When an otherwise normal labor process – and sometimes even a near-delivery – ends with a delayed or obstructed delivery of the fetal shoulder, the obstetrician and his or her team are challenged – physically and emotionally.
This complication is a nightmare for the family as well as the obstetrician who struggles to complete the process. What actually may be a matter of seconds or a minute can feel like an eternity.
We now know that diabetes and obesity are conditions that are increasing at a rapid pace in our society. With the rise in these two conditions (known collectively as diabesity), we can anticipate a rise in fetal macrosomia.
On the other hand, we know that not every macrosomic infant results in obstructed labor or shoulder dystocia. In addition, we currently do not have a very good biometric methodology by which we can precisely estimate fetal weight, or even the pelvic size. Thus, it is difficult to come to an objective conclusion regarding the probability of obstructed labor.
These are the variables that, together, create such a vexing and sometimes underappreciated conundrum.
To attempt to anticipate and to manage the problem, obstetrical specialists must rely on less-than-satisfactory biomedical parameters, historical experience, and their best judgment about medical condition.
Despite such imprecision and the lack of certainty we have for addressing the problem, there is some guidance that can be helpful in predicting the level of risk of shoulder dystocia, and in managing the complication should it occur. It is in this light that we have invited Dr. Mark B. Landon, a maternal-fetal medicine specialist, to discuss the problem of shoulder dystocia. Dr. Landon is the Richard L. Meiling Professor and chairman of the department of ob.gyn. at the Ohio State University, Columbus.
As Dr. Landon discusses, it is almost impossible to be absolutely perfect in preventing and managing shoulder dystocia. We can, however, improve our understanding of which scenarios call for the consideration of prophylactic cesarean section, and of how we can deliver affected infants as safely as possible. As Dr. Landon duly notes, it is critical for the obstetrician to be able to perform a repertoire of potentially effective maneuvers to manage shoulder dystocia.
Shoulder dystocia is not an uncommon obstetric complication, occurring in as many as 2 per 100 vaginal births. This obstetric emergency is associated with a number of adverse perinatal outcomes for both the mother and infant, the most serious of which remains neonatal brachial plexus injury. In a minority of cases in which there is prolonged impaction of the shoulders, birth asphyxia also may occur.
Obstetricians and other birth attendants must be fully prepared to effectively manage shoulder dystocia when it occurs. They also should understand the existing controversies regarding prevention and the pathogenesis of injuries associated with shoulder dystocia.
Shoulder dystocia generally is not a predictable event, which makes prevention extremely difficult. Because of the limited accuracy of ultrasound for estimating fetal size, the risk of shoulder dystocia and resulting injury must be fairly significant before prophylactic cesarean is considered as a preventive measure. There are, however, certain high-risk scenarios that call for consideration of prophylactic cesarean delivery.
Prevention
For the past several decades, clinical research has focused on whether shoulder dystocia can be predicted and/or prevented. Overall, most analyses have shown us that shoulder dystocia can be only minimally predicted, at best, and that prevention of this complication as well as associated injury is far from a simple undertaking.
The leading risk factor for shoulder dystocia is excessive birth weight, yet not all cases of shoulder dystocia involve infants who weigh more than 4,500 g, or even more than 4,000 g. In fact, most shoulder dystocia cases actually occur when birth weights are less than 4,000 g – especially in nondiabetic pregnancies. (In diabetic pregnancies, most shoulder dystocias and brachial plexus injuries do occur in infants with birth weights greater than 4,000 g.)
The possibility that birth weight estimates may help us to predict and/or prevent shoulder dystocia also is hindered by the fact that it remains difficult to identify large babies prior to delivery. Clinical estimation of size and the use of ultrasound are the two most commonly employed techniques for estimating birth size, but both have limited accuracy and may either underestimate or overestimate fetal size. Most large babies, moreover, can successfully undergo vaginal birth without the complication of shoulder dystocia, let alone brachial plexus injury.
All told, these realities limit our ability to use estimated birth weight in selecting those pregnancies that might benefit from prophylactic cesarean delivery.
To consider prophylactic cesarean delivery, the level of risk for shoulder dystocia and resultant injury must be fairly significant. The following are two clinical scenarios in which the risk of complications reaches a level at which the option of prophylactic cesarean section (including informed consent) should be discussed with the mother:
▸ A pregnancy complicated by diabetes in which the estimated fetal weight is greater than or equal to 4,500 g. Some experts have suggested that this threshold should, in fact, be lower in diabetic pregnancies. However, utilization of a lower threshold (such as 4,000 g or 4,250 g) must come with the recognition that it will spur the use of more cesarean deliveries to prevent injury.
▸ A patient with a history of shoulder dystocia birth, particularly when the fetus is believed to be of similar or greater weight than the previously affected fetus.
Determining the recurrence risk of shoulder dystocia has proved difficult because, in most clinical series, a large proportion of women with a history of the complication will undergo scheduled cesarean delivery in their subsequent pregnancies. This bias toward operative delivery may lead to an underestimation of the true recurrence risk. Regardless of this potential estimation bias, unless the estimated fetal weight in the woman's current pregnancy is significantly less than that of the prior pregnancy, we should counsel women with prior shoulder dystocia and offer them prophylactic cesarean delivery.
With respect to the predictive value of labor abnormalities, studies have yielded mixed results. The bottom line is that labor abnormalities are not particularly useful in predicting shoulder dystocia – except for cases of a prolonged second stage of labor when there is suspicion of a large infant. This combination of factors should alert the physician to the potential for shoulder dystocia. Operative vaginal delivery should generally be avoided in this scenario, because delivery above an outlet station may further increase the risk of shoulder dystocia and resultant injury.
Management, Medicolegal Issues
As with any delivery, the goal of management should always be to deliver the infant as safely as possible, minimizing the risk of traumatic injury and birth asphyxia. In most cases of shoulder dystocia, the shoulders remain in an anterior-posterior position and fail to rotate. This creates the potential for brachial plexus injury as the nerves of the brachial plexus are stretched with the descent of the fetal head.
There is little objective study of the maneuvers employed for shoulder dystocia and their effectiveness in preventing neonatal injury, let alone prospective studies comparing the effectiveness of one maneuver vs. another. The choice of maneuvers thus remains provider specific. The maneuvers that are most commonly employed for shoulder dystocia, however, are utilized in order to disimpact the anterior shoulder from behind the symphysis pubis by effecting its rotation.
It is important to appreciate that the McRoberts maneuver, with or without suprapubic pressure, may be successful in only approximately 50% of shoulder dystocia cases.
Unfortunately, many young obstetricians have had limited exposure to shoulder dystocia and may have employed only this maneuver, and not others, in their clinical training. At some point, they will likely encounter a shoulder dystocia case that does not respond to the McRoberts and/or suprapubic pressure maneuvers. It is critical to be competent in performing a full repertoire of potentially effective maneuvers.
There is increasing evidence that obstetricians should have a low threshold for utilizing delivery of the posterior shoulder in the management of shoulder dystocia.
In one recently published, multicenter review of shoulder dystocia maneuvers, for instance, investigators identified women who had incurred a shoulder dystocia during delivery and compared cases involving neonatal injury with injury-free cases. Delivery of the posterior shoulder was associated with the highest rate of successful delivery, when compared with other maneuvers, and with similar rates of neonatal injury (Obstet. Gynecol. 2011;117:1272-8).
The value of posterior arm release lies in its ability to reduce the anterior-posterior diameter of the fetus more significantly than any other maneuver. It has been associated with a marked decrease in anterior nerve stretch and the force required to effect delivery (Obstet. Gynecol. 2003;101:1068-72; Am. J. Obstet. Gynecol. 2010;203:339.e1-5).
In many litigated cases involving shoulder dystocia and brachial plexus injury, it is asserted that unnecessary excess traction must have been employed for a permanent injury to have occurred. Such assertions imply that the obstetrician can perfectly gauge the amount of traction or force necessary to deliver the infant and yet avoid injury in the setting of shoulder dystocia, which is not the case.
Increasing evidence suggests that many cases of brachial plexus injury accompanying shoulder dystocia are multifactorial in origin, and are not simply a result of operator-induced traction and stretching of the nerves. Obstetricians are continually instructed early on in their careers that excess traction should be avoided, as should any fundal pressure that might further disimpact the shoulders.
I simply recommend abandoning any traction efforts once shoulder dystocia is clearly recognized. When the complication occurs, a team consisting of additional nursing personnel, anesthesia, and the most experienced obstetrician available should be immediately summoned, and expulsive efforts on behalf of the mother should be curtailed while maneuvers are being undertaken to disimpact the shoulders.
If two obstetricians are present, it often is helpful for the stronger of the two to deliver appropriate suprapubic pressure from above. The goal is to move the shoulders to an oblique position by exerting pressure from the back of the fetus. This maneuver cannot really be done effectively by a single operator or from below as has been depicted in some textbooks. Again, if this fails to work, a low threshold should exist for attempting a posterior arm release.
Maintaining accurate documentation in the medical record of all events preceding and surrounding the shoulder dystocia is important. This includes but is not limited to the following:
▸ Consideration of significant risk factors for macrosomia, including diabetic pregnancy management and results of gestational diabetes screening tests.
▸ Estimation of fetal size, either clinically or by ultrasound. Most experts believe that diabetic mothers should undergo ultrasound at term to assess fetal size.
▸ Description of instrumental delivery, including indication and station at application and duration of use.
▸ A detailed step-by-step description of the maneuvers used to disimpact the shoulders. The anterior shoulder should be identified as part of the documentation.
Training and Simulation
During the past few years, simulation and drills and other enhanced teaching methods have become an increasingly common part of the curriculum for training residents and nursing personnel in the management of shoulder dystocia. Because the complication occurs relatively infrequently but can have devastating consequences when it does, shoulder dystocia is one of only several obstetric emergencies to be targeted in efforts to improve patient safety.
As with the few other obstetric events that receive such attention, data on the impact of enhanced training on perinatal outcomes remain limited. There clearly is evidence that simulation and drills improve team performance, and it has been hoped that improved team performance will ultimately translate to better outcomes. At present, two studies have indicated that the incidence of brachial plexus injury may decline with the implementation of targeted training for maternity staff.
One of these studies retrospectively compared the management and neonatal outcomes of almost 20,000 births that were complicated by shoulder dystocia in the years before and after the introduction of shoulder dystocia training for all maternity staff in a hospital in the United Kingdom (Obstet. Gynecol. 2008;112:14-20). The rate of brachial plexus injury at birth was significantly reduced, from 7.4% to 2.3%, as was the rate of neonatal injury more broadly (from 9.3% to 2.3%).
In the other study – also a retrospective assessment – the rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 30% before a training protocol was implemented for maternity staff at Jamaica Hospital in New York, to 11% afterward (Am. J. Obstet. Gynecol. 2011;204:322.e1-6).
A recently published study from Ireland, however, failed to reveal any difference in the frequency of brachial plexus injury after the introduction of specific staff training in managing shoulder dystocia. In this single-hospital study, investigators assessed outcomes associated with more than 77,000 deliveries that occurred during two 5-year time periods, before and after training was instituted. The incidence of brachial plexus injury remained unchanged from 1.5 per 1,000 in 1994-1998 to 1.7 per 1,000 in 2004-2008 (Am. J. Obstet. Gynecol. 2011;204:324.e1-6).
Although the results of this latter study are disappointing, I believe they are unlikely to limit the enthusiasm for the simulation training and shoulder dystocia drills that have become fairly routine in many large maternity hospitals in the United States.
Regardless of the limited outcomes data we have available thus far, experience with simulation training has taught us that in order to retain necessary skills, repetitive participation in simulation training appears to be required. The relatively infrequent nature of severe shoulder dystocia cases makes the simulation model for learning very attractive.
The doctor inserts a hand (left), then he/she sweeps the arm across the baby's chest and over the mother's perineum.
Source Images: ©Elsevier, From Obstetrics: Normal and Problem Pregnancies, 5th Edition
Shoulder Dystocia
Routine vaginal deliveries can sometimes quickly become not-so-routine deliveries. When an otherwise normal labor process – and sometimes even a near-delivery – ends with a delayed or obstructed delivery of the fetal shoulder, the obstetrician and his or her team are challenged – physically and emotionally.
This complication is a nightmare for the family as well as the obstetrician who struggles to complete the process. What actually may be a matter of seconds or a minute can feel like an eternity.
We now know that diabetes and obesity are conditions that are increasing at a rapid pace in our society. With the rise in these two conditions (known collectively as diabesity), we can anticipate a rise in fetal macrosomia.
On the other hand, we know that not every macrosomic infant results in obstructed labor or shoulder dystocia. In addition, we currently do not have a very good biometric methodology by which we can precisely estimate fetal weight, or even the pelvic size. Thus, it is difficult to come to an objective conclusion regarding the probability of obstructed labor.
These are the variables that, together, create such a vexing and sometimes underappreciated conundrum.
To attempt to anticipate and to manage the problem, obstetrical specialists must rely on less-than-satisfactory biomedical parameters, historical experience, and their best judgment about medical condition.
Despite such imprecision and the lack of certainty we have for addressing the problem, there is some guidance that can be helpful in predicting the level of risk of shoulder dystocia, and in managing the complication should it occur. It is in this light that we have invited Dr. Mark B. Landon, a maternal-fetal medicine specialist, to discuss the problem of shoulder dystocia. Dr. Landon is the Richard L. Meiling Professor and chairman of the department of ob.gyn. at the Ohio State University, Columbus.
As Dr. Landon discusses, it is almost impossible to be absolutely perfect in preventing and managing shoulder dystocia. We can, however, improve our understanding of which scenarios call for the consideration of prophylactic cesarean section, and of how we can deliver affected infants as safely as possible. As Dr. Landon duly notes, it is critical for the obstetrician to be able to perform a repertoire of potentially effective maneuvers to manage shoulder dystocia.
Shoulder dystocia is not an uncommon obstetric complication, occurring in as many as 2 per 100 vaginal births. This obstetric emergency is associated with a number of adverse perinatal outcomes for both the mother and infant, the most serious of which remains neonatal brachial plexus injury. In a minority of cases in which there is prolonged impaction of the shoulders, birth asphyxia also may occur.
Obstetricians and other birth attendants must be fully prepared to effectively manage shoulder dystocia when it occurs. They also should understand the existing controversies regarding prevention and the pathogenesis of injuries associated with shoulder dystocia.
Shoulder dystocia generally is not a predictable event, which makes prevention extremely difficult. Because of the limited accuracy of ultrasound for estimating fetal size, the risk of shoulder dystocia and resulting injury must be fairly significant before prophylactic cesarean is considered as a preventive measure. There are, however, certain high-risk scenarios that call for consideration of prophylactic cesarean delivery.
Prevention
For the past several decades, clinical research has focused on whether shoulder dystocia can be predicted and/or prevented. Overall, most analyses have shown us that shoulder dystocia can be only minimally predicted, at best, and that prevention of this complication as well as associated injury is far from a simple undertaking.
The leading risk factor for shoulder dystocia is excessive birth weight, yet not all cases of shoulder dystocia involve infants who weigh more than 4,500 g, or even more than 4,000 g. In fact, most shoulder dystocia cases actually occur when birth weights are less than 4,000 g – especially in nondiabetic pregnancies. (In diabetic pregnancies, most shoulder dystocias and brachial plexus injuries do occur in infants with birth weights greater than 4,000 g.)
The possibility that birth weight estimates may help us to predict and/or prevent shoulder dystocia also is hindered by the fact that it remains difficult to identify large babies prior to delivery. Clinical estimation of size and the use of ultrasound are the two most commonly employed techniques for estimating birth size, but both have limited accuracy and may either underestimate or overestimate fetal size. Most large babies, moreover, can successfully undergo vaginal birth without the complication of shoulder dystocia, let alone brachial plexus injury.
All told, these realities limit our ability to use estimated birth weight in selecting those pregnancies that might benefit from prophylactic cesarean delivery.
To consider prophylactic cesarean delivery, the level of risk for shoulder dystocia and resultant injury must be fairly significant. The following are two clinical scenarios in which the risk of complications reaches a level at which the option of prophylactic cesarean section (including informed consent) should be discussed with the mother:
▸ A pregnancy complicated by diabetes in which the estimated fetal weight is greater than or equal to 4,500 g. Some experts have suggested that this threshold should, in fact, be lower in diabetic pregnancies. However, utilization of a lower threshold (such as 4,000 g or 4,250 g) must come with the recognition that it will spur the use of more cesarean deliveries to prevent injury.
▸ A patient with a history of shoulder dystocia birth, particularly when the fetus is believed to be of similar or greater weight than the previously affected fetus.
Determining the recurrence risk of shoulder dystocia has proved difficult because, in most clinical series, a large proportion of women with a history of the complication will undergo scheduled cesarean delivery in their subsequent pregnancies. This bias toward operative delivery may lead to an underestimation of the true recurrence risk. Regardless of this potential estimation bias, unless the estimated fetal weight in the woman's current pregnancy is significantly less than that of the prior pregnancy, we should counsel women with prior shoulder dystocia and offer them prophylactic cesarean delivery.
With respect to the predictive value of labor abnormalities, studies have yielded mixed results. The bottom line is that labor abnormalities are not particularly useful in predicting shoulder dystocia – except for cases of a prolonged second stage of labor when there is suspicion of a large infant. This combination of factors should alert the physician to the potential for shoulder dystocia. Operative vaginal delivery should generally be avoided in this scenario, because delivery above an outlet station may further increase the risk of shoulder dystocia and resultant injury.
Management, Medicolegal Issues
As with any delivery, the goal of management should always be to deliver the infant as safely as possible, minimizing the risk of traumatic injury and birth asphyxia. In most cases of shoulder dystocia, the shoulders remain in an anterior-posterior position and fail to rotate. This creates the potential for brachial plexus injury as the nerves of the brachial plexus are stretched with the descent of the fetal head.
There is little objective study of the maneuvers employed for shoulder dystocia and their effectiveness in preventing neonatal injury, let alone prospective studies comparing the effectiveness of one maneuver vs. another. The choice of maneuvers thus remains provider specific. The maneuvers that are most commonly employed for shoulder dystocia, however, are utilized in order to disimpact the anterior shoulder from behind the symphysis pubis by effecting its rotation.
It is important to appreciate that the McRoberts maneuver, with or without suprapubic pressure, may be successful in only approximately 50% of shoulder dystocia cases.
Unfortunately, many young obstetricians have had limited exposure to shoulder dystocia and may have employed only this maneuver, and not others, in their clinical training. At some point, they will likely encounter a shoulder dystocia case that does not respond to the McRoberts and/or suprapubic pressure maneuvers. It is critical to be competent in performing a full repertoire of potentially effective maneuvers.
There is increasing evidence that obstetricians should have a low threshold for utilizing delivery of the posterior shoulder in the management of shoulder dystocia.
In one recently published, multicenter review of shoulder dystocia maneuvers, for instance, investigators identified women who had incurred a shoulder dystocia during delivery and compared cases involving neonatal injury with injury-free cases. Delivery of the posterior shoulder was associated with the highest rate of successful delivery, when compared with other maneuvers, and with similar rates of neonatal injury (Obstet. Gynecol. 2011;117:1272-8).
The value of posterior arm release lies in its ability to reduce the anterior-posterior diameter of the fetus more significantly than any other maneuver. It has been associated with a marked decrease in anterior nerve stretch and the force required to effect delivery (Obstet. Gynecol. 2003;101:1068-72; Am. J. Obstet. Gynecol. 2010;203:339.e1-5).
In many litigated cases involving shoulder dystocia and brachial plexus injury, it is asserted that unnecessary excess traction must have been employed for a permanent injury to have occurred. Such assertions imply that the obstetrician can perfectly gauge the amount of traction or force necessary to deliver the infant and yet avoid injury in the setting of shoulder dystocia, which is not the case.
Increasing evidence suggests that many cases of brachial plexus injury accompanying shoulder dystocia are multifactorial in origin, and are not simply a result of operator-induced traction and stretching of the nerves. Obstetricians are continually instructed early on in their careers that excess traction should be avoided, as should any fundal pressure that might further disimpact the shoulders.
I simply recommend abandoning any traction efforts once shoulder dystocia is clearly recognized. When the complication occurs, a team consisting of additional nursing personnel, anesthesia, and the most experienced obstetrician available should be immediately summoned, and expulsive efforts on behalf of the mother should be curtailed while maneuvers are being undertaken to disimpact the shoulders.
If two obstetricians are present, it often is helpful for the stronger of the two to deliver appropriate suprapubic pressure from above. The goal is to move the shoulders to an oblique position by exerting pressure from the back of the fetus. This maneuver cannot really be done effectively by a single operator or from below as has been depicted in some textbooks. Again, if this fails to work, a low threshold should exist for attempting a posterior arm release.
Maintaining accurate documentation in the medical record of all events preceding and surrounding the shoulder dystocia is important. This includes but is not limited to the following:
▸ Consideration of significant risk factors for macrosomia, including diabetic pregnancy management and results of gestational diabetes screening tests.
▸ Estimation of fetal size, either clinically or by ultrasound. Most experts believe that diabetic mothers should undergo ultrasound at term to assess fetal size.
▸ Description of instrumental delivery, including indication and station at application and duration of use.
▸ A detailed step-by-step description of the maneuvers used to disimpact the shoulders. The anterior shoulder should be identified as part of the documentation.
Training and Simulation
During the past few years, simulation and drills and other enhanced teaching methods have become an increasingly common part of the curriculum for training residents and nursing personnel in the management of shoulder dystocia. Because the complication occurs relatively infrequently but can have devastating consequences when it does, shoulder dystocia is one of only several obstetric emergencies to be targeted in efforts to improve patient safety.
As with the few other obstetric events that receive such attention, data on the impact of enhanced training on perinatal outcomes remain limited. There clearly is evidence that simulation and drills improve team performance, and it has been hoped that improved team performance will ultimately translate to better outcomes. At present, two studies have indicated that the incidence of brachial plexus injury may decline with the implementation of targeted training for maternity staff.
One of these studies retrospectively compared the management and neonatal outcomes of almost 20,000 births that were complicated by shoulder dystocia in the years before and after the introduction of shoulder dystocia training for all maternity staff in a hospital in the United Kingdom (Obstet. Gynecol. 2008;112:14-20). The rate of brachial plexus injury at birth was significantly reduced, from 7.4% to 2.3%, as was the rate of neonatal injury more broadly (from 9.3% to 2.3%).
In the other study – also a retrospective assessment – the rate of obstetric brachial plexus injury in cases of shoulder dystocia fell from 30% before a training protocol was implemented for maternity staff at Jamaica Hospital in New York, to 11% afterward (Am. J. Obstet. Gynecol. 2011;204:322.e1-6).
A recently published study from Ireland, however, failed to reveal any difference in the frequency of brachial plexus injury after the introduction of specific staff training in managing shoulder dystocia. In this single-hospital study, investigators assessed outcomes associated with more than 77,000 deliveries that occurred during two 5-year time periods, before and after training was instituted. The incidence of brachial plexus injury remained unchanged from 1.5 per 1,000 in 1994-1998 to 1.7 per 1,000 in 2004-2008 (Am. J. Obstet. Gynecol. 2011;204:324.e1-6).
Although the results of this latter study are disappointing, I believe they are unlikely to limit the enthusiasm for the simulation training and shoulder dystocia drills that have become fairly routine in many large maternity hospitals in the United States.
Regardless of the limited outcomes data we have available thus far, experience with simulation training has taught us that in order to retain necessary skills, repetitive participation in simulation training appears to be required. The relatively infrequent nature of severe shoulder dystocia cases makes the simulation model for learning very attractive.
The doctor inserts a hand (left), then he/she sweeps the arm across the baby's chest and over the mother's perineum.
Source Images: ©Elsevier, From Obstetrics: Normal and Problem Pregnancies, 5th Edition
Shoulder Dystocia
Routine vaginal deliveries can sometimes quickly become not-so-routine deliveries. When an otherwise normal labor process – and sometimes even a near-delivery – ends with a delayed or obstructed delivery of the fetal shoulder, the obstetrician and his or her team are challenged – physically and emotionally.
This complication is a nightmare for the family as well as the obstetrician who struggles to complete the process. What actually may be a matter of seconds or a minute can feel like an eternity.
We now know that diabetes and obesity are conditions that are increasing at a rapid pace in our society. With the rise in these two conditions (known collectively as diabesity), we can anticipate a rise in fetal macrosomia.
On the other hand, we know that not every macrosomic infant results in obstructed labor or shoulder dystocia. In addition, we currently do not have a very good biometric methodology by which we can precisely estimate fetal weight, or even the pelvic size. Thus, it is difficult to come to an objective conclusion regarding the probability of obstructed labor.
These are the variables that, together, create such a vexing and sometimes underappreciated conundrum.
To attempt to anticipate and to manage the problem, obstetrical specialists must rely on less-than-satisfactory biomedical parameters, historical experience, and their best judgment about medical condition.
Despite such imprecision and the lack of certainty we have for addressing the problem, there is some guidance that can be helpful in predicting the level of risk of shoulder dystocia, and in managing the complication should it occur. It is in this light that we have invited Dr. Mark B. Landon, a maternal-fetal medicine specialist, to discuss the problem of shoulder dystocia. Dr. Landon is the Richard L. Meiling Professor and chairman of the department of ob.gyn. at the Ohio State University, Columbus.
As Dr. Landon discusses, it is almost impossible to be absolutely perfect in preventing and managing shoulder dystocia. We can, however, improve our understanding of which scenarios call for the consideration of prophylactic cesarean section, and of how we can deliver affected infants as safely as possible. As Dr. Landon duly notes, it is critical for the obstetrician to be able to perform a repertoire of potentially effective maneuvers to manage shoulder dystocia.
The Where and Why of Postsurgical Adhesions
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
The prevention of postsurgical adhesions is one of the greatest unmet needs in medicine today. Surgical series have shown that adhesions are present after 80%-90% of abdominal and pelvic surgeries, and that these abnormal fibrous connections have a tremendous propensity to reform after adhesiolysis. (We will define adhesions here as “attachments between surfaces at nonanatomical locations.”)
In gynecologic surgery, postoperative adhesions are a frequent cause of infertility, pain, bowel obstruction, and difficulty in later procedures. Adhesions can occur after minimally invasive procedures, which have the potential for trocar injury to structures adherent to the anterior abdominal wall. Other intraoperative injuries can occur due to obscured normal anatomy or restricted access. A significant number of patients also undergo second surgeries to treat sequelae that are directly related to adhesions.
The literature is replete with studies of adhesion development and reports of its incidence and its consequences. Still, the problem of postoperative adhesion development often goes underestimated or unrecognized. This is because we don't routinely perform early second-look operations to assess adhesion development, and because there are no serum markers or sensitive imaging techniques to allow their identification. In addition, we do not follow our patients who seek care from other providers as insurance coverage changes or as other health problems arise, such as bowel obstruction being treated by a general surgeon.
As gynecologic surgeons, we must appreciate that while infections, endometriosis, and other peritoneal insults may contribute to adhesion development, surgery is the most common cause. We also must appreciate how tissue injury leads to the development of adhesions, and why adhesion reformation so commonly occurs.
This understanding is critical to our consideration and use of the “barrier” products currently available for reducing postsurgical adhesions — and critical to our efforts to employ the tenets of gynecologic microsurgery and to achieve as optimal a surgical outcome as possible. At this point in time, use of approved surgical adjuvants in combination with good surgical technique offers the best chance at adhesion reduction and prevention.
Incidence of Adhesions
A series of reports published in the early to mid-1980s documented how commonly adhesions develop after various types of reproductive pelvic surgery. Through early second-look laparoscopy, postoperative adhesions were found to occur, in these studies, in 55%–100% of patients after their primary gynecologic surgery.
In a multicenter study published in 1987, my colleagues and I also showed that gynecologic surgeries performed at the time of laparotomy are frequently complicated by both adhesion reformation and de novo adhesion formation. More than half of the 161 women (51%) who had a second-look laparoscopy 1–12 weeks after reproductive pelvic surgery were found to have de novo adhesion formation (adhesions in at least one new location). Adhesion reformation was also widespread: At the initial laparotomy, 121 of the patients (all of whom were treated for infertility) were noted to have some form of adhesion, and adhesion reformation subsequently occurred at the site of adhesiolysis in 85% of these women, with no differences with respect to adhesion type (Fertil. Steril. 1987;47;864–6).
It was hoped, and largely expected, that the growth of laparoscopy and minimally invasive surgery approaches in more recent years would reduce postoperative adhesion development — that minimally invasive techniques would prove to be less adhesiogenic than laparotomy. Questions remain, but thus far, such hopes have diminished and our expectations for significant improvement have gone unsubstantiated.
One multicenter study on adhesion development after initial laparoscopic procedures found that the incidence of adhesions at an early second-look procedure was 97% — no lower than in prior reports of second-look laparoscopy after laparotomy.
In this study, 68 women underwent operative laparoscopic procedures, including adhesiolysis, and had second-look procedures within 90 days. The good news was that de novo adhesion formation between the two laparoscopic procedures occurred in only 8 of the women (12%) and at 11 of 47 possible sites — much less frequently than after laparotomy. Adhesion scores also decreased at the second look compared with the status of the pelvis at the initial procedure. Still, with the high rate of adhesion reformation, almost all of the women developed postoperative adhesions.
Thus, even when the initial procedure was performed laparoscopically, adhesion development was an all-too-common occurrence, and appeared to be independent of the character of the initial adhesion (Fertil. Steril. 1991;55:700–4).
More recently, data from randomized studies of various adhesion barriers and potential anti-adhesion adjuvants have further dashed hopes that laparoscopy per se can reduce adhesion development.
For instance, in a recent small pilot study of a fibrin-based product called Adhexil, “control” ovaries that were not treated had a 27% increase in the mean adhesion score between an initial laparoscopic procedure and second-look laparoscopy. The women in the study had undergone bilateral ovarian surgery, with ovaries randomized for application of the product or no treatment (Fertil. Steril. 2011;95:1086–90). Clearly, a laparoscopic approach to their procedures did not prevent the development of adhesions.
Many of the initial studies on adhesion development were comprised of patients with infertility, but more recent observations have been extended to women without infertility and to men. Studies have covered patients undergoing colectomies, for instance, as well as neonates undergoing cardiothoracic procedures.
In a recent review article on adhesion prevention and reduction, members of an interdisciplinary consensus conference stated that adhesions develop after “nearly all” abdominal and pelvic procedures performed through either standard laparotomy or laparoscopic approaches. With respect to gynecologic surgery, they point out, research has shown that the most common site for postsurgical adhesion development is the ovary (Surg. Innov. 2010;17:183–8).
Consequences
Pelvic adhesions are a well-recognized cause of infertility, contributing to up to an estimated 40% of the cases of infertility in women. Adhesions are also a leading cause of bowel obstruction and a significant cause of chronic or recurrent pelvic pain.
The contribution of pelvic adhesions to chronic pelvic pain is not completely understood. Adhesions may be the cause of pain in some women, and in other women, an incidental finding that is not contributing to pain. In patients who have endometriosis as well, the question remains as to the contribution of endometriosis per se, or adhesions, to the pain. Endometriosis can cause adhesions and chronic pelvic pain, presumably through the cyclic generation of inflammatory molecules.
The relationship between chronic pain and adhesions is further complicated by ensuing questions about the efficacy of adhesiolysis. The two randomized trials that have thus far examined the role of adhesiolysis in the reduction of chronic pelvic pain failed to demonstrate a significant improvement in pain after adhesiolysis; however, the high failure rates after follow-up may be due to adhesion reformation and de novo adhesion formation (Fertil. Steril. 2004;82:1483–91). Performance of more randomized comparisons in the future may yield improved outcomes when adhesiolysis is paired with postprocedure use of anti-adhesion adjuvants.
Despite the uncertainties, multiple studies support the current estimation that adhesions cause or significantly contribute to chronic pelvic pain in up to 30% of women with the problem. As the Ovarian Adhesion Study Group noted in one of its reports, adhesions have been reported as a primary cause of chronic pelvic pain in 13%-36% of women, depending on the study (Obstet. Gynecol. 1995;86:335–40). Economic analyses also have quantified the impact of adhesion-related hospital readmissions. A study done in the United Kingdom, for instance, concluded that 6% of all hospital readmissions in patients who had undergone abdominal or pelvic surgery were directly related to adhesions (Lancet 1999;353:1476–80).httother report on hospitalizations for lower abdominal adhesiolysis in the United States estimated that in 1988, the cost of adhesions stemming from gynecologic procedures alone was almost $1.2 billion. This estimate did not include outpatient and indirect costs (Surg. Gynecol. Obstet. 1993;176:271–6).
Why, How Adhesions Develop
Our current understanding is that adhesions develop as a result of injury to and devascularization of the peritoneum, and the subsequent inflammatory response and peritoneal wound healing process. Tissue hypoxia triggers a cascade of intracellular responses that, in combination with the fibrinous collection of blood and serosanguinous fluid at the tissue surface, may result in adhesion development.
In the initial postsurgical period, either overt bleeding or oozing may occur at the site(s) of tissue injury, forming clots. In combination with serosanguinous fluid, which may leak from damaged peritoneal surfaces, a fibrinous mass thus develops at the surgical sites and sites of tissue injury. This represents an initial step in peritoneal repair.
When surrounding tissue is normal and there is a sufficient amount of plasminogen activator present in the peritoneum — and when numerous other events and conditions are optimal — the resulting fibrinous mass can be degraded. As that occurs, and tissue healing continues, fibroblasts are recruited to the surface of the injury site from underlying tissues.
If the fibrinous mass is no longer present, fibroblasts “stop” at the tissue surfaces, and become covered by mesothelial cells which line the peritoneal surface as the process of remesothelialization occurs. This process appears to be initiated within hours after surgery and is generally believed to be completed in 3–5 days. (In such instances, healing would have occurred without adhesions, although subperitoneal fibrosis may have occurred.)
Various hypoxia-driven responses, however, such as a reduction in plasminogen activator activity, can cause the fibrinous mass to persist during the healing process, before remesothelialization occurs. In this case, fibroblasts migrate not only to, but through, the injury site, and into the persisting fibrinous mass. This is subsequently followed by deposition of collagen, fibronectin, and other extracellular matrix materials — creating the beginnings of a true adhesion.
In such cases, remesothelialization still occurs, but the mesothelial cells cover the adhesion as well as the normal tissue surfaces, forming adhesive bands and other types of connections between opposing serosal tissue surfaces. Angiogenesis then occurs as the hypoxic tissue in the adhesion sends signals (such as vascular endothelial growth factor) in an attempt to reestablish a supply of oxygen and nutrients to the injured and devascularized tissues. Subsequently, as the tissue remodels, there is a propensity for the adhesion to become more vascular and denser.
Understanding this process is important because the products currently available for reducing adhesions act as barriers during this critical period of remesothelialization, keeping peritoneal surfaces apart and minimizing the potential development of a fibrinous mass that bridges tissue surfaces. If an adhesion does not form during the 3–5-day period of remesothelialization, it is theorized that there will not be any adhesion development — unless there's new injury to the tissue surfaces.
Once an adhesion forms, however, it has acquired a particular “adhesion phenotype” — different from that of normal peritoneum — that appears to be irreversible. This is likely why it is so difficult to prevent adhesion reformation after adhesiolysis. Rates of adhesion reformation — even in the best of surgical hands — run between 80% and 90%, compared with a 50% chance of de novo adhesion development after surgery (at new sites of injury).
The identification of an adhesion phenotype came originally from comparisons of normal peritoneal and adhesion tissues harvested from the same patient, and were later confirmed in cell culture studies in which normal peritoneal fibroblasts were subjected to hypoxia (2% O2 conditions). Fibroblasts cultured under hypoxic conditions were subsequently found to have developed particular molecular biologic characterizations that are different from those of normal peritoneal fibroblasts.
When exposed to normal amounts of oxygen again, the fibroblasts did not go back to being normal fibroblasts — they continued to manifest the adhesion phenotype (J. Am Assoc. Gynecol. Laparosc. 2004;11:307–14). These findings have been confirmed in animal and human studies, and such relationships have also been identified in other peritoneal tissue types such as mesothelial cells and macrophages.
Further research on the pathogenesis of adhesions and the molecular biologic differences between normal peritoneum and adhesions may allow identification of which patients, and which sites within a patient, are most at risk for adhesion development, as well as the discovery of new ways to reduce the development of postoperative adhesions and their clinical sequelae.
It is possible that a future generation of barrier products not only will work as a barrier separating surfaces prior to remesothelialization, but will also have local biologic effects — delivering adhesion-reducing drugs or biologics, for instance, to specific localized tissue sites. A personalized approach to adhesion prevention also might be possible, with particular factors deemed to increase adhesion risk in individual patients (a deficiency of plasminogen activator, for instance) being corrected.
In the meantime, as we've learned more about the pathophysiological state under which adhesions develop, we have found that adhesion development may occur faster than we had thought. In one recent rodent study, we identified postoperative tissue attachments as early as 2 hours after cecal abrasion. We noted considerable local edema and vessel dilatation within 2 hours of injury, angiogenesis and fibrin deposition at 8 hours, and cell proliferation at 24 hours (Fertil. Steril. 2010;93:2734–7). And interestingly, recent studies in mice have shown that laparoscopic insufflation per se can induce peritoneal adhesions, with the adhesions increasing proportionally with both increasing duration of insufflation and an increase in intraperitoneal pressure.
Prevention in Practice
During the past decade a variety of surgical adjuvants — from procoagulants and fibrinolytic agents to anti-inflammatory drugs and antibiotics — have been investigated for use in reducing the occurrence, extent, and severity of adhesions. Unfortunately, most approaches seemingly have been futile. Some products have shown trends toward efficacy in animal or early human studies and need further investigation.
The three synthetic products that are approved by the Food and Drug Administration—Gynecare Interceed, Seprafilm, and Adept — can help reduce postoperative adhesions after gynecologic procedures, and should be considered as potentially useful surgical adjuvants. A meta-analysis of studies of Gynecare Interceed, for instance, found that approximately twice as many operative sites were adhesion free after use of the barrier than after surgery alone (J. Reprod. Med. 1999;44:325–31).
Gynecare Interceed (Johnson & Johnson) and Seprafilm (Genzyme) are approved for use only at laparotomy, while Adept (Baxter International), an icodextrin solution that disperses throughout the abdominopelvic cavity, is approved for use only in laparoscopic surgery.
The key consideration to make when using Interceed — a biodegradable woven fabric composed of oxidized, regenerated cellulose — is the importance of achieving meticulous hemostasis. Its efficacy is reduced, or can even be lost, in the presence of blood. Care also must be taken not to stretch the material or alter its shape and the spacing between the weaves. Otherwise, the material, once gelated, will have a greater potential for spaces in which the tissue surfaces would not be separated and thus a greater potential for blood coagulation and fibroblast in-growth. Multiple pieces of the material may be overlapped, but there have been no benefits demonstrated (at least in animal studies) to using double layers.
Care in application also is critically important for Seprafilm, a film composed of modified hyaluronic acid and carboxymethylcellulose. Seprafilm is brittle and is difficult to apply through small incisions. While it's not impossible to deliver the product laparoscopically, many surgeons have found this very difficult. And in the United States, it is approved for use with laparotomy only.
In applying Seprafilm, it is critical to first get good exposure of the field and then position the film very carefully. Attempts to reposition the product will often result in tears or breaks. Of course, just as with Interceed, this device is believed to work primarily by separating tissue surfaces, and thus it has little to no chance of success if it does not completely separate the surgically injured tissue from other tissue surfaces in the initial postoperative period while remesothelialization is occurring.
The use of adjuvants, moreover, is no substitute for good surgical technique that aims to minimize tissue injury, tissue devascularization, and inflammation. This is easier to achieve, of course, during a microsurgical procedure such as a tubal anastomosis than in a patient with severe endometriosis or many large fibroids. Still, to the extent possible for the procedure being conducted, the tenets of gynecologic microsurgery should always be considered:
▸ Handle as little tissue as possible, as minimally as possible. To the extent possible, handle only those portions that will subsequently be excised.
▸ Keep tissues moist. Tissue drying leads to injury and loss of mesothelial cells. Raw surfaces are more prone to develop adhesions.
▸ Take special care in the use of suture. Consider whether clinical situations will allow use of less reactive and smaller-caliber suture. When using suture to tie off blood vessels, skeletonize the vessels so as to minimize the amount of tissue distal to the suture that will become hypoxic and serve as a nidus to adhesion development.
▸ Target the use of electrosurgery and other energy sources. Use it in specific localized sites where it's needed, such as to stop bleeding, but avoid widely dispersed use, when possible, again to minimize the amount of residual devitalized tissue remaining in the pelvis at the conclusion of the surgical procedure.
Pelvic Adhesions — An Update
“A number of human interventional trials and animal studies have evaluated techniques and materials designed to prevent and reduce postsurgical adhesions. The results have been inconclusive and sometimes contradictory. Thus, preventing postsurgical adhesions remains an art, rather than a science.” So I began my introduction to the program on adhesion prevention at the 2010 Congress of the Society of Laparoscopic Surgeons in New York City.
Patients with adhesions can present with small bowel obstruction or with complaints of infertility, chronic pain, or dyspareunia. Unfortunately, adhesive disease is problematic. Four percent of the patients undergoing abdominal and pelvic surgery will be readmitted due to adhesion-related complications. In excess of 400,000 surgical procedures are performed annually in the United States for lysis of adhesions. In a Scottish National Health Service Study of nearly 9,000 women who previously underwent open gynecologic surgery, just less than 3% were readmitted secondary to adhesions; the highest readmit rate was ovarian surgery (BJOG 2000;107:855–62).
While one would expect a reduction in the number of patients undergoing laparoscopic surgery, in reality, the verdict is not yet clear. A 1998 meta-analysis showed a decrease in both reformed (26.6% vs. 14.3%) and de novo adhesions (45.2% vs. 37.2%) in the laparoscopic group, compared with laparotomy (Fertil. Steril. 1998;70:702–11).
Despite this, other authors cite pneumoperitoneum, prolonged surgery, high insufflation pressure, and overzealous use of energy to cut and coagulate as reasons why laparoscopic surgery increases risk of adhesions.
The economic impact of adhesions is staggering, in excess of $1.3 billion in the United States per year.
For this current excerpt of the Master Class in Gynecologic Surgery, I have solicited the wisdom of Dr. Michael Diamond. He is the Kamran S. Moghissi Professor of Obstetrics and Gynecology and associate chairman of the department of obstetrics and gynecology at Wayne State University, Detroit. Dr. Diamond also is director of the division of reproductive endocrinology and infertility and assistant dean for clinical and translational research at the university. Dr. Diamond has spent much of his academic career involved in the pathogenesis, prevention, and treatment of pelvic adhesions. He is truly considered the world's leader in this area, and we are honored to have Dr. Diamond as guest author of this important area of our surgical arena.
Optimal Management of Gestational Diabetes Mellitus
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
We've learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother's diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24–25), we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon's landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks' gestation. GDM is typically diagnosed at about 28 weeks' gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks' gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks' gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon's study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by “resting” pancreatic islet cells.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug's half-life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer's trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer's trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007; 30:S251-60).hWe also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote “pancreatic burnout,” thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it's taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a “useful” life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose. Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer's trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%. On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies' birth weights were not appreciably different.
Source Elsevier Global Medical News
GDM and the Developing Fetus
A growing body of research has convincingly demonstrated that even periods of mild hyperglycemia during pregnancy can have long-term adverse consequences on the developing fetus. Therefore, there is a growing sentiment in the ob.gyn. and diabetes communities for an aggressive approach to the detection, treatment, and monitoring of the most frequent causes of hyperglycemic events during pregnancy. Significant controversies remain on how best to implement this approach.
In the area of gestational diabetes mellitus (GDM) treatment, multiple controversies exist regarding whether to manage GDM very aggressively (i.e., with insulin as the first line of therapy) or with less aggressive approaches first, followed by insulin as a last resort. The former approach, while likely to be effective in controlling hyperglycemia, is viewed by many physicians – and their patients – as not acceptable given that GDM is a relatively mild form of diabetes and most cases will resolve spontaneously after pregnancy.
In this month's Master Class, Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, returns to provide us with a superbly written essay on the state of the evidence in managing GDM. Dr. Moore's Master Class briefly discusses the growing prevalence of GDM in the United States and worldwide, as well as the scientific evidence linking intrauterine hyperglycemia with adverse pregnancy outcomes. He then provides a detailed analysis of the best available science on trials of dietary approaches to GDM as well as trials on oral antihyperglycemic drugs and how they compare with one another and with insulin.
Dr. Moore also demonstrates how this knowledge is being applied to his own patients as well as how they've been able to adapt, accept, and comply with this relatively new approach to managing GDM. Once again, we are honored that Dr. Moore has agreed serve as the Master Class guest professor, providing important insights into how GDM might be managed optimally.
Key Points
▸ Prenatal exposure to hyperglycemia programs the fetus for a higher risk of being born overweight, of becoming obese in adolescence or adulthood, and of developing diabetes later in life. Two randomized trials have demonstrated the positive impact of treating even mild forms of GDM.
▸ Many patients can be managed with diet alone, but the effectiveness of dietary treatment must be carefully monitored, with insulin or oral antihyperglycemic agents added early – before significant body fat is accumulated by the fetus.
▸ Glyburide is just as effective as insulin in achieving optimal glycemic control and is significantly less likely to cause hypoglycemia in mothers, with no adverse neonatal or fetal effects, numerous studies have shown. Glyburide is not a 12-hour medication in pregnant women as it is in nonpregnant women, however. Ob.gyns must appreciate the dosing implications of the agent's different pharmacodynamics in pregnancy.
▸ Metformin also has equivalent efficacy to insulin, and several small recent studies have shown no significant difference with glyburide. Metformin has a theoretical advantage over glyburide in that it's an insulin sensitizer, but the downside is a higher chance of needing supplementary insulin later in pregnancy. Patients can be counseled accordingly.
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
We've learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother's diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24–25), we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon's landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks' gestation. GDM is typically diagnosed at about 28 weeks' gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks' gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks' gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon's study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by “resting” pancreatic islet cells.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug's half-life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer's trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer's trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007; 30:S251-60).hWe also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote “pancreatic burnout,” thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it's taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a “useful” life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose. Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer's trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%. On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies' birth weights were not appreciably different.
Source Elsevier Global Medical News
GDM and the Developing Fetus
A growing body of research has convincingly demonstrated that even periods of mild hyperglycemia during pregnancy can have long-term adverse consequences on the developing fetus. Therefore, there is a growing sentiment in the ob.gyn. and diabetes communities for an aggressive approach to the detection, treatment, and monitoring of the most frequent causes of hyperglycemic events during pregnancy. Significant controversies remain on how best to implement this approach.
In the area of gestational diabetes mellitus (GDM) treatment, multiple controversies exist regarding whether to manage GDM very aggressively (i.e., with insulin as the first line of therapy) or with less aggressive approaches first, followed by insulin as a last resort. The former approach, while likely to be effective in controlling hyperglycemia, is viewed by many physicians – and their patients – as not acceptable given that GDM is a relatively mild form of diabetes and most cases will resolve spontaneously after pregnancy.
In this month's Master Class, Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, returns to provide us with a superbly written essay on the state of the evidence in managing GDM. Dr. Moore's Master Class briefly discusses the growing prevalence of GDM in the United States and worldwide, as well as the scientific evidence linking intrauterine hyperglycemia with adverse pregnancy outcomes. He then provides a detailed analysis of the best available science on trials of dietary approaches to GDM as well as trials on oral antihyperglycemic drugs and how they compare with one another and with insulin.
Dr. Moore also demonstrates how this knowledge is being applied to his own patients as well as how they've been able to adapt, accept, and comply with this relatively new approach to managing GDM. Once again, we are honored that Dr. Moore has agreed serve as the Master Class guest professor, providing important insights into how GDM might be managed optimally.
Key Points
▸ Prenatal exposure to hyperglycemia programs the fetus for a higher risk of being born overweight, of becoming obese in adolescence or adulthood, and of developing diabetes later in life. Two randomized trials have demonstrated the positive impact of treating even mild forms of GDM.
▸ Many patients can be managed with diet alone, but the effectiveness of dietary treatment must be carefully monitored, with insulin or oral antihyperglycemic agents added early – before significant body fat is accumulated by the fetus.
▸ Glyburide is just as effective as insulin in achieving optimal glycemic control and is significantly less likely to cause hypoglycemia in mothers, with no adverse neonatal or fetal effects, numerous studies have shown. Glyburide is not a 12-hour medication in pregnant women as it is in nonpregnant women, however. Ob.gyns must appreciate the dosing implications of the agent's different pharmacodynamics in pregnancy.
▸ Metformin also has equivalent efficacy to insulin, and several small recent studies have shown no significant difference with glyburide. Metformin has a theoretical advantage over glyburide in that it's an insulin sensitizer, but the downside is a higher chance of needing supplementary insulin later in pregnancy. Patients can be counseled accordingly.
We now know that gestational diabetes mellitus is a serious condition that, if not properly diagnosed and managed, can have cyclic, intergenerational consequences. Newborns exposed to maternal hyperglycemia during pregnancy have a high risk of being born overweight and of eventually becoming obese children and adults. These newborns also are at a high risk of developing diabetes themselves later in life.
The prevalence of gestational diabetes mellitus (GDM) is increasing in every ethnic group. In the Kaiser Permanente system in Colorado, a state which has traditionally had the lowest obesity rate of any state in the United States, the prevalence of GDM doubled from 1994 to 2002, with significant increases in all racial/ethnic groups (Diabetes Care 2005;28:579-84). Such increases in GDM prevalence are happening worldwide – one part of a worldwide epidemic of obesity and diabetes that is overtaking our youth.
We've learned that GDM is one sign post on the way to the development of overt type 2 diabetes. Indeed, a majority of women with GDM will acquire diabetes within 5 years.
In the last decade or so, our clinical research focus has centered on the in utero risks to the fetus. In a striking study of the potential impact of intrauterine hyperglycemia exposure on later development, Dr. D. Dabelea and coinvestigators compared siblings in the Pima Indian population who were born before and after their mothers were diagnosed with diabetes. The children who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents than their siblings who were born before their mother's diagnosis of diabetes. Even though these siblings ate the same diet and came from the same gene pools (with the same fathers), they experienced dramatically different health outcomes in adolescence as a result of the differing intrauterine environments (Diabetes 2000;49:2208-11).
This and other studies have given us a body of supplementary science showing that exposure to high blood glucose in utero causes accumulation of fat in the fetus. Even though that baby fat might be lost in early childhood, prenatal exposure nevertheless genetically programs the fetus for a higher risk of developing fatness as an adult.
As I detailed in the last Master Class in obstetrics (see Ob.Gyn News, July 2011, pp. 24–25), we now also have evidence from two randomized controlled trials that interventions to control blood glucose are effective in reducing rates of newborn obesity and therefore should improve adolescent and adult health downstream.
The two randomized trials – the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86) and a study published several years later by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009; 361:1339-48) – demonstrated the positive impact of treating even mild forms of GDM, with the largest effects being on reducing newborn obesity. Although the offspring of mothers who were treated and not treated in those studies have not yet been followed into adulthood, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles downstream.
Treating GDM, and learning how to maximize glucose control, has thus moved to center stage in obstetric practice.
Trials of Dietary Change
In Dr. Landon's landmark study, more than 90% of the women randomized to the treatment group (versus usual prenatal care) needed only dietary counseling and education about blood glucose control for effective treatment of abnormal blood glucose levels. Surprisingly, fewer than 10% needed insulin as well.
That we can manage many of our patients with diet alone is welcome good news. To be successful with this approach, however, we must be vigilant in monitoring the effectiveness of dietary counseling and identifying early on those patients for whom dietary treatment is not enough.
We also must be more vigilant in detecting GDM, because the maximal time of fetal fat accretion is at about 32-34 weeks' gestation. GDM is typically diagnosed at about 28 weeks' gestation, and patients usually are not engaged in a regime of blood sugar testing and dietary change until about 30-31 weeks. If we wait until 34-35 weeks' gestation to change course with treatment – adding insulin or oral hypoglycemic agents – significant body fat accumulation by the fetus already will have occurred.
Screening for GDM even earlier than currently recommended, at 26 weeks' gestation if possible, and providing dietary counseling as early as possible are worthwhile goals. Our advice is that patients be moved on to a medication regimen if more than one-third of their blood glucose measurements are still abnormal after 2 weeks of dietary change. A more stringent standard may be more prudent, but for now we believe there is enough evidence to warrant this modest change in practice, and we find that it is a rule that most patients can understand.
We also must caution that the effectiveness of dietary change may be significantly less in many populations than it was in Dr. Landon's study because his study focused on a subset of women who had only mild glucose intolerance. In our patient population, for example, we can achieve good glucose control with diet alone in about 60%-70% of cases.
The Science on Glyburide
Pharmacologic therapy for patients in whom dietary measures fail is no longer limited to insulin. Insulin is certainly still an option as a first-line therapy, and is necessary as an adjunct therapy in patients who are not achieving glucose targets with another agent. It has proven efficacy and well-studied pharmacokinetics. It does not cross the placenta, and research has shown that it may be beneficial by “resting” pancreatic islet cells.
Insulin is not an optimal therapy for GDM for several reasons, however. Many patients find it cumbersome to use, and most offices are not equipped for, or used to, teaching women how to give themselves the insulin injections. Insulin itself is also unfamiliar to many patients and can even be scary; some of the families we care for see insulin as a stigma, believing that a person who takes insulin has diabetes while a person who takes a pill does not truly have the condition.
In our practice, we have found that women who take oral hypoglycemics are more likely to have better glycemic control, probably because their drug compliance is better. With insulin, our patients tend to be suboptimally compliant.
Glyburide, one of the oral anti-hyperglycemic drugs that we have been able to transfer from use in the nonpregnant diabetic population to use during pregnancy, has been well used and studied by this point in time.
When Dr. Oded Langer and his colleagues led the first and only randomized trial comparing glyburide and insulin more than a decade ago, women with GDM were rarely treated with a sulfonylurea drug largely because of reports of prolonged severe hypoglycemia in neonates born to mothers who were receiving the drug at the time of delivery. There were also questions about whether glyburide, a second-generation sulfonylurea, could effectively control postprandial peaks in blood glucose while avoiding periods of hypoglycemia in the mother.
In the nonpregnant population, glyburide has been used for decades as a twice-daily oral medication. After months of use, patients develop active metabolites that prolong the drug's half-life and enable it to last for 12 hours, at least.
Glyburide use in pregnancy is a slightly different story, however. Patients take the medication for a relatively short time and consequently may not build up the active metabolites that nonpregnant patients acquire. The metabolic changes in pregnancy also make women vulnerable to hypoglycemia at certain times of the day, typically in the late morning, the late afternoon, and between 3 a.m. and 4 a.m.
Dr. Langer's trial, which randomized 404 women with GDM to receive glyburide or insulin, demonstrated similar outcomes in the insulin and glyburide groups. There were no differences in mean birth weight, the percentage of large for gestational age newborns, macrosomia, fetal anomalies, or newborn hypoglycemia. The rate of maternal hypoglycemia, however, was much higher in the insulin-treated group; 20% of the women receiving insulin experienced symptomatic hypoglycemia, compared with only 2% of the women taking glyburide.
In short, glyburide was just as effective as insulin in achieving desired levels of glycemic control (a fasting blood glucose less than 90 mg/dL and 2-hour postprandial glucose of 120 mg/dL) and controlling fetal obesity, while being significantly less likely to cause hypoglycemia in the mothers. (N. Engl. J. Med. 2000;343:1134-8).
Glyburide dosing in Dr. Langer's trial was increased weekly, as needed, to a maximum of 20 mg per day; women took the drug twice a day. Insulin was administered per a standard intensified schedule of regular NPH (intermediate-acting, lasting 6-12 hours) and regular TID (lasting 2-4 hours).
Despite the impressive findings from the trial, some have contended that the results of one randomized trial are insufficient for adopting glyburide as a first-line therapy. However, numerous retrospective or case-controlled studies also have since shown glyburide to be a clinically effective alternative to insulin therapy, with no adverse neonatal or fetal effects. These studies have shown, moreover, that it can be easier to avoid hypoglycemia and achieve optimal glycemic control with glyburide than with insulin.
One of the best large retrospective studies looked at 584 women at Kaiser Permanente Northern California and found that glyburide was at least as effective as insulin in achieving glycemic control and resulted in similar birth weights in women with GDM who had failed diet therapy alone (Am. J. Obstet. Gynecol. 2005;193:118-24).
Several recent reviews of glyburide studies, such as one that looked at nine glyburide studies covering 745 patients taking glyburide and 645 patients taking insulin, also have been published (Ann. Pharmacother. 2008;42:483-90). In 2007, moreover, the 5th International Workshop-Conference on GDM concluded that glyburide is a legitimate alternative to insulin for GDM (Diabetes Care 2007; 30:S251-60).hWe also now know that unlike other, first-generation sulfonylureas that tend to cross the placenta freely, glyburide is 99.8% protein-bound and thus crosses the placenta only minimally.
Theoretically, there is one potential problem with glyburide. Because the drug acts by stimulating maternal pancreatic insulin production, it could potentially promote “pancreatic burnout,” thus shortening the time to development of overt diabetes in women whose pancreas is struggling to begin with. Women who are obese and have significant insulin resistance at the start of their pregnancies thus might be susceptible to pancreatic burnout. Although this potential effect has not been demonstrated in any trials, it must be kept in mind.
It would be informative to conduct long-term follow-up studies that track the children of mothers who used glyburide during their pregnancies, but at this point it is unclear if such studies will be designed and carried out. The likelihood of additional randomized trials being conducted is practically nil, given the extent to which women already are choosing the oral hypoglycemics over insulin.
Glyburide in Practice
As clinicians, we must appreciate that the pharmacodynamics of glyburide are quite different in pregnant women, with important dosing implications for our patients. Indeed, for pregnant women, glyburide is not the 12-hour medication that it is in nonpregnant women.
During pregnancy, glyburide action peaks about 2.5 hours after it's taken, and the increased renal clearance and metabolism of pregnancy (in addition to the short duration of therapy in this patient population) leave the drug with a “useful” life of only about 6-8 hours.
Because blood glucose peaks 60-90 minutes after a meal, we instruct our patients to take a glyburide dose a full hour before a planned meal. Otherwise, postprandial glucose peaks will not be controlled. Usually, a dose taken an hour before breakfast will help control postprandial peaks after breakfast and lunch but will not last for dinner. Another dose 1 hour before an evening meal can be given.
To effectively control fasting blood glucose, we instruct patients to take a glyburide dose between 10 p.m. and midnight so that the drug will still be active in the early morning when it is needed. If the dose is taken too early at night – at 8-9 p.m., for instance – it will peak between 10 p.m. and midnight, and will not be working at 6 a.m.
As it is with insulin, careful glucose monitoring is critical for determining optimal administration of glyburide and for balancing glyburide action with meals and snacks. Individual glycemic profiles should be analyzed each week, with the goal of keeping fasting blood glucose below 90 mg/dL, and postprandial levels below 130 mg/dL, while preventing maternal hypoglycemia.
Attention must be paid not only to times of consistent elevation in blood glucose levels, but also to the potential for dosage overlap – for instance, a prelunch dosage administered to correct consistently high postprandial glucose levels after the mid-day meal could lead to low blood glucose levels at about 4-5 p.m. as its action overlaps with the end duration of a morning dose. Patients should always be prepared for vulnerable times and have a glucose tablet, juice box, or food with them to correct any periods of hypoglycemia.
Insulin should be added if more than 30% of blood glucose readings are above target with administration of 15-20 mg/day of glyburide.
Metformin as an Option
As ob.gyns, our experience with metformin, the other oral anti-hyperglycemic agent now available for treating GDM, came originally from its use as an infertility treatment in women with polycystic ovary syndrome (PCOS).
Metformin is frequently prescribed for women with PCOS to improve ovulation. These women have significant insulin resistance and are at high risk for developing GDM during their pregnancies. The main concern in this population, however, has been infertility, and studies have shown that metformin induces ovulation in women with PCOS.
Although metformin crosses the placenta, numerous studies have shown no increase in birth anomalies in women who conceive while taking the agent.
A study published a decade ago in women who chose whether or not to continue metformin treatment throughout their pregnancies showed that of those who discontinued metformin, 31% developed GDM, compared with only 3% of those who continued their metformin treatment (Fertil. Steril. 2002;77:520-5). These results helped fuel the idea that the agent may be a logical treatment for women with GDM.
Metformin also has a theoretical advantage over glyburide since its mechanism of action gets directly to the root of the problem of GDM. Metformin is an insulin sensitizer, and the root cause of GDM is resistance to insulin, or insulin insensitivity, at the tissue level.
In a study by Dr. J.A. Rowan published in 2008 that randomized more than 700 patients to either insulin or metformin, there were no appreciable differences in neonatal and maternal outcomes – from birth weight and neonatal morbidity to maternal hypoglycemia and glycemic control (N. Engl J. Med. 2008;358:2003-15). However, whereas 4% of the glyburide group in Dr. Langer's trial had to eventually add insulin (and up to 10%-20% in other studies), 47% of the patients taking metformin in this trial had to add insulin to maintain glycemic control.
Indeed, the downside to metformin, this and other studies have shown, is a high so-called failure rate – the need for supplementary insulin, which in this case typically occurs later in the pregnancy – of between 30% and 50%. On the other hand, patients generally will be more satisfied starting treatment with metformin than insulin. In weighing glyburide and metformin, patients should be counseled about their chances of needing insulin later in the pregnancy: about 10% with glyburide and closer to 50% with metformin.
In terms of glycemic control and other outcomes, several smaller, recent studies comparing the two agents have shown no statistical difference between them. Interestingly, most studies have shown less maternal weight gain in patients taking metformin than glyburide – about 6 pounds – but the significance of this difference is unclear since the babies' birth weights were not appreciably different.
Source Elsevier Global Medical News
GDM and the Developing Fetus
A growing body of research has convincingly demonstrated that even periods of mild hyperglycemia during pregnancy can have long-term adverse consequences on the developing fetus. Therefore, there is a growing sentiment in the ob.gyn. and diabetes communities for an aggressive approach to the detection, treatment, and monitoring of the most frequent causes of hyperglycemic events during pregnancy. Significant controversies remain on how best to implement this approach.
In the area of gestational diabetes mellitus (GDM) treatment, multiple controversies exist regarding whether to manage GDM very aggressively (i.e., with insulin as the first line of therapy) or with less aggressive approaches first, followed by insulin as a last resort. The former approach, while likely to be effective in controlling hyperglycemia, is viewed by many physicians – and their patients – as not acceptable given that GDM is a relatively mild form of diabetes and most cases will resolve spontaneously after pregnancy.
In this month's Master Class, Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, returns to provide us with a superbly written essay on the state of the evidence in managing GDM. Dr. Moore's Master Class briefly discusses the growing prevalence of GDM in the United States and worldwide, as well as the scientific evidence linking intrauterine hyperglycemia with adverse pregnancy outcomes. He then provides a detailed analysis of the best available science on trials of dietary approaches to GDM as well as trials on oral antihyperglycemic drugs and how they compare with one another and with insulin.
Dr. Moore also demonstrates how this knowledge is being applied to his own patients as well as how they've been able to adapt, accept, and comply with this relatively new approach to managing GDM. Once again, we are honored that Dr. Moore has agreed serve as the Master Class guest professor, providing important insights into how GDM might be managed optimally.
Key Points
▸ Prenatal exposure to hyperglycemia programs the fetus for a higher risk of being born overweight, of becoming obese in adolescence or adulthood, and of developing diabetes later in life. Two randomized trials have demonstrated the positive impact of treating even mild forms of GDM.
▸ Many patients can be managed with diet alone, but the effectiveness of dietary treatment must be carefully monitored, with insulin or oral antihyperglycemic agents added early – before significant body fat is accumulated by the fetus.
▸ Glyburide is just as effective as insulin in achieving optimal glycemic control and is significantly less likely to cause hypoglycemia in mothers, with no adverse neonatal or fetal effects, numerous studies have shown. Glyburide is not a 12-hour medication in pregnant women as it is in nonpregnant women, however. Ob.gyns must appreciate the dosing implications of the agent's different pharmacodynamics in pregnancy.
▸ Metformin also has equivalent efficacy to insulin, and several small recent studies have shown no significant difference with glyburide. Metformin has a theoretical advantage over glyburide in that it's an insulin sensitizer, but the downside is a higher chance of needing supplementary insulin later in pregnancy. Patients can be counseled accordingly.
Endometriosis: Current Diagnosis and Treatment
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
A disease that affects 10%-15% of women of reproductive age, endometriosis is quite prevalent. In 1990, investigators in Belgium first described deep endometriosis to highlight the diagnostic and therapeutic aspects of the disease (Fertil. Steril. 1990;53:978–83). In contrast to superficial disease, deep endometriosis constitutes the most severe form of endometriosis and includes nodules affecting the pouch of Douglas, retrocervical area, bladder, ureter, or the intestinal wall. Less frequently, the rectovaginal septum is involved (Arq. Gastroenterol. 2003;40:192–7). The treatment of bowel endometriosis is challenging, as it is a benign disease that may infiltrate the bowel, requiring a surgical treatment with increased risks.
Preoperative Diagnosis Using Imaging
The definitive diagnosis of deep endometriosis with bowel involvement is reached principally at the time of surgery. However, some clinical characteristics identified by history and physical examination, laboratory tests, and diagnostic imaging may raise suspicion for this form of endometriosis. A surgical approach is still recommended for confirmation and treatment.
Transvaginal ultrasonography (TVUS) still appears to be the superior imaging technique, providing the best cost-benefit ratio for cases of ovarian or deep endometriosis. The presence of a hypoechoic lesion located in the posterior pelvic compartment (see
When performed after complete bowel preparation and during the perimenstrual phase, TVUS carried out by a trained professional provides useful information for therapeutic management.
MRI can be performed to identify deep lesions. (See
Excretory urography or uro-MRI also is useful for evaluating whether the ureters are involved. When urinary tract involvement is suspected, one of these types of imaging should be performed to fully document the state of the urinary tract before surgery.
If we have doubts about the bowel involvement even after TVUS with bowel preparation, we recommend rectal echoendoscopy. (See
Rectal echoendoscopy also permits identification of the distance between the lesion and the rectal lumen, as well as identification of extrinsic compression and lesions of the rectal submucosa. This information can be critical in the preoperative planning of the type of surgery required and the need to have the help of a colorectal surgeon. The chart on page 19 shows the algorithm for preoperative work-up depending on clinical and TVUS findings.
Treatment: Clinical or Surgical?
Medical treatment of deep endometriosis, as opposed to surgical treatment, remains controversial. Dr. Luigi Fedele and his associates in Italy reported a substantial improvement in pain during 6 months of treatment with GnRH analogs (Am. J. Obstet. Gynecol. 2000;183:1462–7). Similar improvements in pain were also observed by our group with both an intrauterine device medicated with levonorgestrel and with a GnRH analog (Hum. Reprod. 2005;20:1993–8). In Dr. Fedele's study, however, an early relapse occurred following discontinuation of treatment. In addition, the endometriotic lesions underwent a discrete but significant reduction in size as detected by TVUS during treatment, but returned to their original size 6 months after suspension of GnRH treatment.
In cases of intractable pain (measured by scores greater than 7 in the visual analog scale) and/or two previously failed IVF cycles, surgical treatment is required. Access for surgical treatment may be by laparotomy or laparoscopy, depending on the surgeon's experience; however, laparoscopy can provide a better visualization of the lesions, allowing a more precise excision.
Surgical Preparation and Technique
Whenever there is clinical suspicion of deep endometriosis, adequate presurgical bowel preparation is indicated. We recommend the use of 3–4 liters of an oral solution of polyethylene glycol (PEG) the day before surgery, followed by one or two Fleet enemas or a mannitol preparation.
Administration of antibiotics should be carried out during anesthetic induction, preferably using a second-generation cephalosporin (2 g intravenously).
When the preoperative rectal ultrasound permits identification of the depth of the lesion, this information can be used to define the type of surgery that will be performed. In the case of unifocal lesions less than 3 cm in size (major diameter) and affecting the serous and external muscular layers of the rectum or sigmoid, resection of the nodule alone may be indicated. This procedure may be done manually or with the help of a circular stapler. (
Our technique approached laparoscopically is as follows:
▸ The lesion on the rectosigmoid is delineated, and adhesions are lysed from contiguous organs such as adnexae, the uterus, or other loops of bowel. We prefer to use scissors or a hook.
▸ To resect the lesion manually (without the use of a disposable stapler), the endometriotic nodule is excised, taking care not to leave any residual disease behind. The defect is then repaired in a double-layer fashion. On the mucosal layer, 3–0 absorbable suture is used in a running and transverse manner to avoid bowel constriction. On the seromuscular layer, 3–0 permanent suture is used in a running manner to imbricate over the first layer.
▸ If a circular stapler is used, the following steps are followed: A stitch is placed in the lesion in order to invaginate it into the stapler. (See
▸ The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
If, on the other hand, the lesion is deeper, affecting the deep muscle or the submucosal or mucosal layers, then segmental resection of the bowel is recommended. Complete surgical resection of endometrial foci has been shown to result in improved quality of life and decreased rates of recurrence (Fertil. Steril. 2004;82:878–84).
Segmental resection of the rectosigmoid can be performed laparoscopically (J. Minim. Invasive Gynecol. 2008;15:280–5). Our technique involves the following steps:
▸ Both ureters are identified (see
▸ The mesosigmoid is divided with an ultrasonic device.
▸ A linear stapler is utilized on the rectosigmoid distal to the lesion.
▸ After excision of all endometriotic implants, the right-lower trocar site is extended to 4 cm in order to remove the surgical specimen(s) and to prepare the proximal stump. (See
▸ An incision is made on the proximal stump in order to insert the anvil of the circular stapler.
▸ A purse-string suture holding the anvil in place is performed prior to replacement of the sigmoid into the abdominal cavity.
▸ The 4-cm fascial incision is closed in order to finish the procedure laparoscopically.
▸ The circular stapler is inserted through the anus in order to complete the end-to-end reanastomosis. The anastomosis is tested by gently injecting air and/or methylene blue through the rectum (with an Asepto, or large bulb syringe) while the surgeon occludes the proximal sigmoid with an atraumatic instrument. Absence of air bubbles and/or methylene blue while the anastomotic site is submerged in sterile water in the pelvis confirms a tight anastomosis.
▸ A large drain is left adjacent to the anastomosis prior to closure of trocar sites. The drain is generally removed 4 days postoperatively.
Deep endometriosis is associated with more severe pain and significantly greater rates of infertility, compared with superficial endometriosis. Because of the high risks of surgical intervention, preoperative diagnosis using imaging modalities can be helpful in planning surgical strategy. Improved outcomes are achieved with complete surgical resection, which can be performed through minimally invasive techniques.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Abrão, or visit
Vitals
Rectal Endometriosis
Deep endometriosis compromising the rectum continues to be a diagnostic and therapeutic challenge. The resultant pelvic pain, dyspareunia, dysmenorrhea, and infertility risk are well documented in literature. Despite the fact that there are numerous studies to evaluate deep endometriosis, including colonoscopy, MRI, vaginal and rectal ultrasound, and barium enema, there continues to be no standard road map for evaluation. In addition, there continues to be debate in the literature when patients should undergo shaving of the endometrioma, discoid resection of the endometrioma, or complete bowel resection.
Since the inception of the Master Class in Gynecologic Surgery, as Editor, I have used only experts who practice within the confines of the United States. However, given the internationally recognized expertise in both the diagnosis and treatment of deep and extensive endometriosis, I believed it was imperative to invite Dr. Mauricio S. Abrão to discuss the diagnosis and treatment of deep endometriosis compromising the rectum.
Dr. Abrão was born in São Paulo, Brazil in 1962, where he went on to complete medical school, and in 1988, his residency in obstetrics and gynecology. In 1989, Dr. Abrão founded the endometriosis division within the department of the teaching hospital of the University of São Paulo School of Medicine, where he currently is Docent Professor.
Since 2007, Dr. Abrão has been president of the Brazilian Society of Endometriosis and Minimally Invasive Endoscopy, and has been a board member of the World Endometriosis Society since 1998. He currently is on the board of trustees of the AAGL and is the chairman of the society's special interest group on endometriosis. Dr. Abrão is leading the AAGL initiative on producing a new classification on endometriosis. A prolific author, Dr. Abrão has nearly 100 papers published in peer-reviewed journals, the majority dealing with endometriosis.
It is with great admiration and respect that I introduce my friend, Dr. Abrão, to this edition of the Master Class in gynecologic surgery.
A Sea Change in the Understanding of GDM Management
The tide has turned in our understanding of both the effects of maternal hyperglycemia and the effectiveness of current treatment approaches. Consequently, we are facing an impending sea change in the way in which gestational diabetes is diagnosed and managed.
Recent research has detailed the risks posed to a fetus exposed to hyperglycemia during pregnancy – even at levels that in the past have been considered mild and, thus, largely inconsequential. We also now have evidence that we can offer therapies for gestational diabetes mellitus (GDM) with confidence that we can use them to change the outcome for the fetus, the newborn, the child, and possibly the adult.
This impending change comes after decades of diagnosing gestational diabetes based largely on relatively arbitrary thresholds. Dr. John B. O'sullivan and statistician Claire Mahan developed the diagnostic criteria more than 40 years ago based on certain statistical phenomena associated with the development of adult-onset diabetes after pregnancy. Before then, during the 1940s, 1950s, and 1960s, 1%-2% of all pregnant women were diagnosed with GDM.
In recent years, many of us have had the experience as clinicians of delivering larger, more obese babies whose mothers had been found to have “normal” blood glucose levels. Many of us also have delivered babies with significant adiposity, sometimes perilously low blood glucose, shoulder dystocia, nerve injuries, and other complications that typically occur as a consequence of fetal overgrowth.
We often attribute these complications to a diagnostic method we have known for some time wasn't perfect, but until recently, we did not have the clinical research findings to guide us in our efforts to fine-tune the diagnosis of GDM and turn the tide.
Insights on Fetal Risk
The landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, led by Dr. Boyd E. Metzger, was an attempt to clarify what level of maternal glucose intolerance is associated with an excess risk of an adverse pregnancy outcome.
The HAPO study, which involved 15 centers in nine countries, examined the outcomes of more than 25,000 pregnancies. In designing the HAPO study, Dr. Metzger and his colleagues did something that had never formally been done before: They administered a 75-g oral glucose tolerance test (OGTT) to the mothers between 24 and 32 weeks' gestation (as close to 28 weeks as possible), and defined GDM as an abnormal 2-hour 75-g OGTT result. They then followed the births of women identified as having GDM, and compared them with the births of mothers who did not have gestational diabetes as defined by traditional measures.
Outside the United States, the 75-g, one-step OGTT has been the standard for GDM diagnosis for some time. In the United States, many of us still use an awkward two-step system in which women initially are given a 50-g oral challenge. Only if they register an excessive value on the 50-g challenge do they come back for a definitive 3-hour, 100-g OGTT.
Quite a few outcomes were measured in the HAPO study, but the major outcomes were birth weight greater than the 90th percentile, the level of cord-blood serum-C-peptide (an index of fetal beta-cell function and fetal hyperinsulinemia) above the 90th percentile, and percent body fat greater than the 90th percentile.
The glucose results of the majority of women remained blinded (data were not blinded if the 2-hour plasma glucose level was greater than 200 mg/dL, or diagnostic of diabetes, or if the fasting plasma glucose level exceeded 105 mg/dL or the random plasma glucose level was 160 mg/dL). After birth and the assessment of fetal outcomes, these outcomes were arrayed against earlier results of the mothers' 2-hour 75-g glucose challenge tests and the fasting blood glucose levels, both of which were measured at the same time during pregnancy. (Fasting plasma glucose levels varied from as little as 75 mg/dL all the way up to the predefined threshold of 100 mg/dL.)
Considering percent of body fat greater than the 90th percentile, one would expect no more than 10% of babies without diagnosed GDM in the mothers to have hyperinsulinemia and large amounts of body fat.
Dr. Metzger found otherwise: 17% of babies whose mothers had a fasting blood glucose of 90 mg/dL, for instance – a level most clinicians have viewed as normal – had large levels of body fat, and many of these babies also had hyperinsulinemia. Overall, there was no “golden” level of maternal glucose that predicted a fat baby. However, neonatal adiposity increased progressively as fasting blood glucose levels rose above 80 mg/dL.
In the case of 1-hour 75-g OGTT results, fatness increased progressively at levels greater than 105 mg/dL, and with 2-hour results, fatness rose progressively at levels over 90 mg/dL (Diabetes 2009;58:453–9).
Such continuous linear relationships between maternal glucose and adverse fetal outcomes were seen studywide for birth weight and other outcomes (N. Engl. J. Med. 2008;358:1991–2002).
Among the most striking findings was that a significant number of fat babies were born to women whose blood glucose levels were considered “normal.”
The question at this point became, What should we do about it? Should we allow these obese babies to be born without any intervention, or can we treat them before birth?
Insights on Treatment
Many experts have been doubtful that treatment of mothers with GDM would be effective in altering newborn outcomes. However, the Australian Carbohydrate Intolerance Study in Pregnant Women, published in 2005, concluded that early treatment of GDM reduces serious perinatal morbidity and may improve health-related quality of life. In this study, women with GDM were randomized to receive dietary advice, blood glucose monitoring, and insulin therapy as needed (the treatment group), or routine care (N. Engl. J. Med. 2005;352:2477–86).
In another randomized study published several years later, Dr. Mark B. Landon and his colleagues finally convinced many experts of the value of aggressive screening and early intervention for GDM. Dr. Landon focused on a subset of women who had an abnormal result on a 3-hour 100-g OGTT but a fasting glucose level below 95 mg/L. These women thus had only mild glucose intolerance. (An abnormal result was defined as two or three timed glucose measurements that exceeded certain thresholds: 1-hour, 180 mg/dL; 2-hour, 155 mg/dL; and 3-hour, 140 mg/dL.)
In 14 centers across the United States, 958 patients were randomized to receive treatment of their diabetes or nothing but usual prenatal care. Treatment included formal nutritional counseling and diet therapy, along with insulin if needed. The majority of the women (93%) needed only dietary counseling and education about blood glucose control, while the other 7% needed insulin as well.
Women receiving dietary counseling checked their blood glucose levels before they got up in the morning, and 2 hours after each major meal. In essence, they planned and adjusted their diet based on their blood glucose readings.
What did we learn from this trial? We learned that the incidence of large-for-gestational-age births (greater than the 90th percentile) was cut in half from approximately 14% in the untreated group to 7% in the treated group. There also was a 10%-14% reduction in fat mass in the babies born to the women who received treatment, as well as significant reductions in mean birth weight and birth weight greater than 4,000 g. Most importantly, treatment also reduced the number of injuries that occurred during birth, while the number of small-for-gestational-age infants did not increase (N. Engl. J. Med. 2009;361:1339–48).
With these two randomized studies demonstrating significantly reduced risks with early GDM treatment, the question shifted from the broader issue of whether it is worthwhile to treat women with GDM to the more specific question of who needs treatment the most.
A New Approach
Today, in most demographic and ethnic groups in the United States, the incidence of gestational diabetes is between 4% and 12%, with a national incidence of about 8%. These are the patients we are already treating.
The HAPO trial, however, has shown us that there are a significant number of babies whose mothers have mild hyperglycemia and who are not being treated for this condition. These babies have neonatal adiposity and subsequently are being injured during the birth process.
In addition, we now have multiple epidemiologic studies demonstrating that adiposity at birth markedly increases – by as much as 30%–40% – the risk of being fat as a child and as an adolescent. Studies also have shown that the risk of developing childhood and adolescent type 2 diabetes proportionately increases with increasing neonatal adiposity.
Thus, the goal is no longer just to prevent neonatal adiposity so that babies will not be injured during birth; it now includes helping mothers control their glucose profiles so that their babies will have better health during their childhood and adult years.
However, the answer to the current, pressing question of whether we should offer treatment to women who are not now defined as having gestational diabetes is not yet clearly answered.
In 2008, after the initial release of HAPO study findings, a group called the International Association of Diabetes and Pregnancy Study Groups (IADPSG) was created to discuss the definition of gestational diabetes in light of the new HAPO findings and other research demonstrating improved outcomes with treatment.
In 2010, the consensus group released revised recommendations for glucose tolerance testing, suggesting that everyone convert to the 2-hour 75-g OGTT and that we lower the cutoff points used for diagnosis to protect as many babies as possible from becoming obese.
The group deliberated how much risk to address, or cover, with new cut points. Is a 150% increase in risk, for example, too much? Or a doubling of newborn fatness? In looking at a possible lifetime of obesity, type 2 diabetes, and heart disease, how much testing and treatment is just right? In the end, the group chose cutoff points for the fasting, 1-hour, and 2-hour plasma glucose measurements that conveyed an odds ratio for adverse outcomes of at least 1.75.
This means that a fasting plasma glucose of 92 mg/dL or more almost doubles the adverse fetal outcome risk; so does a 1-hour value after the 75-g OGTT of at least 180 mg/dL, and a 2-hour value of at least 153 mg/dL. If any one of these values is elevated, according to the IADPSG, a fetus is at risk and the mother should be treated for hyperglycemia (Diabetes Care 2010;33:676–82).
The Near Future
With the new criteria proposed by the IADPSG, the number of women who will be defined as having GDM using the 75-g OGTT will double to approximately 16%, compared with about 8% today. This doubling of incidence obviously will require additional resources and intervention.
The question now is, Are we going to adopt these new criteria? The practice approach for GDM in the United States normally follows guidelines for diabetes put forth by the American Diabetes Association and/or guidelines for pregnancy developed by the American College of Obstetricians and Gynecologists. Although the ADA has revised its recommendations for diagnosis of GDM to embrace the criteria of IADPSG, neither body has issued a directive or a formal set of guidelines for clinicians.
The National Institute of Child Health and Human Development is planning a workshop on GDM for next year, and it is quite possible that the proceedings from this NICHD workshop will inform future statements or guidelines from these organizations. In all likelihood, new screening criteria will be widely adopted within several years.
In the meantime, providers must decide what to do. There is nothing wrong with continuing two-stage testing. However, those who do should realistically consider its disadvantages: For one, this process identifies only 80%–90% of the women who actually have abnormal glucose levels, so many at-risk newborns will be missed even though their mothers were tested.
Secondly, the timing of the two-step process is problematic. Most women are given lab orders for the OGTT at about 28 weeks' gestation. By 29 or 30 weeks, they'll have results. If abnormal, the office staff must call and tell the patient to schedule the second OGTT test. Our own studies have shown that each step takes about 7–12 days to complete. In our system, it can then take up to 10 days for a woman diagnosed with GDM to receive care. She will be instructed in glucose monitoring and her care team will check with her every week.
In the end, it may be 6–8 weeks after initial testing before the woman's glucose intolerance is effectively addressed. The maximal time of fetal fat accretion is at about 34 weeks. If we do not have a diagnosis made and treatment plan underway by 32 weeks, we will have significantly decreased our chance of preventing obesity in her newborn.
Aggressive efforts to get screening done at about 26 weeks would be worthwhile, especially if you are working within a system that can accommodate a greater number of women with identified glucose intolerance. To ensure the outcomes that we're seeking, we must ensure that our patients receive adequate dietary and other interventions.
There also are questions about whether the identification of more women at risk of an adverse pregnancy outcome could itself create risk, particularly since it is well documented that women with GDM are more likely to be delivered earlier or through cesarean section, regardless of the level of achieved glucose control. (In the Landon study, interestingly, the rate of cesarean delivery was reduced in the intervention group.)
On the other hand, wider identification offers such hope for reducing fetal adiposity, and its many adverse consequences, that it should be immediately considered.
'Old' (Current) vs. 'New' (Upcoming)
Two-Step Approach to GDM Dx:
▸ Initial screening with a 50-g glucose challenge test at 24–28 weeks' gestation in women at greater than low risk of GDM. Women at very high risk should be screened as soon as possible after confirmation of pregnancy.
▸ Diagnostic 100-g oral glucose tolerance test (on separate day, after overnight fast) in women who meet or exceed chosen threshold on 50-g screening (140 mg/dL or more, or 130 mg/dL or more for higher sensitivity).
▸ GDM diagnosis made if at least two of these plasma glucose values are met or exceeded after the 100-g OGTT: fasting, 95 mg/dL; 1 hour, 180 mg/dL; 2 hour, 155 mg/dL; 3 hour, 140 mg/dL (if a 3-hour test is done).
One-Step Approach to GDM Dx:
▸ Screening of all women at 24–28 weeks' gestation not known to have type 2 diabetes with 75-g oral glucose tolerance test (after overnight fast).
▸ GDM diagnosis made if any one of these fasting plasma glucose values are met or exceeded: fasting, 92 mg/dL; 1 hour, 180 mg/dL; 2 hour, 153 mg/dL.
Sources: American Diabetes Association's Standards of Medical Care in Diabetes 2010 (Diabetes Care 2010;33:S11–61); American College of Obstetricians and Gynecologists Practice Bulletin, September 2001; ADA's Standards of Medical Care in Diabetes 2011 (Diabetes Care 2011;34:S11–61).
The Consequences of GDM
The thresholds for deciding when to begin treating hyperglycemia were established almost 50 years ago at a time when we had significantly less knowledge about the risk factors for and consequences of hyperglycemia in pregnancy. Because of this lack of understanding about the causes and consequences of hyperglycemia and our sometimes rigid adherence to these cutoffs, many women were not treated who should have been.
There is a growing recognition in the research and clinical communities that gestational diabetes mellitus (GDM) is a much more serious condition than had been previously believed even a decade ago. We now know that GDM, if not properly diagnosed and managed, can have intergenerational consequences in terms of propagating risks for obesity, diabetes, heart disease, and other disorders. Furthermore, there is a new and growing realization that even mild hyperglycemia significantly below what has traditionally been defined as diabetes can have significant adverse consequences for both mother and infant.
Perhaps the most significant complication of maternal hyperglycemia faced by ob.gyns. is the growing number of large-for-gestational-age (LGA) infants being born. For obvious reasons, LGA infants are more difficult to deliver and significantly more prone to experiencing shoulder dystocia and other injuries during normal or cesarean delivery, and cesarean delivery has its own set of complications for both baby and mother.
The large, multicenter Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study recently documented that by managing hyperglycemia – even among women who previously had not been considered to have any glucose control problems – the incidence of LGA-related problems and other adverse birth outcomes could be significantly reduced.
To discuss in detail the findings of the HAPO study and its potential clinical implications, we have invited Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, to write this Master Class.
Dr. Moore's essay discusses both the unique design and findings of the HAPO study, and also explores the quandary faced by members of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) in their attempts to translate HAPO's findings into clinically useful recommendations and guidelines.
In a sign of how complex and time consuming it can be to translate clinical research findings into clinical practice, the recommendations of the IADPSG are now being debated among research and medical societies, with some suggesting that the thresholds introduced by the HAPO study and advanced by the IADPSG are not significantly different from the current levels.
We greatly appreciate Dr. Moore's insights into these complicated but exciting developments. His Master Class installment will help all of us to better understand this complex issue so that we can potentially play a role in speeding up the process of changing the way we manage GDM.
The tide has turned in our understanding of both the effects of maternal hyperglycemia and the effectiveness of current treatment approaches. Consequently, we are facing an impending sea change in the way in which gestational diabetes is diagnosed and managed.
Recent research has detailed the risks posed to a fetus exposed to hyperglycemia during pregnancy – even at levels that in the past have been considered mild and, thus, largely inconsequential. We also now have evidence that we can offer therapies for gestational diabetes mellitus (GDM) with confidence that we can use them to change the outcome for the fetus, the newborn, the child, and possibly the adult.
This impending change comes after decades of diagnosing gestational diabetes based largely on relatively arbitrary thresholds. Dr. John B. O'sullivan and statistician Claire Mahan developed the diagnostic criteria more than 40 years ago based on certain statistical phenomena associated with the development of adult-onset diabetes after pregnancy. Before then, during the 1940s, 1950s, and 1960s, 1%-2% of all pregnant women were diagnosed with GDM.
In recent years, many of us have had the experience as clinicians of delivering larger, more obese babies whose mothers had been found to have “normal” blood glucose levels. Many of us also have delivered babies with significant adiposity, sometimes perilously low blood glucose, shoulder dystocia, nerve injuries, and other complications that typically occur as a consequence of fetal overgrowth.
We often attribute these complications to a diagnostic method we have known for some time wasn't perfect, but until recently, we did not have the clinical research findings to guide us in our efforts to fine-tune the diagnosis of GDM and turn the tide.
Insights on Fetal Risk
The landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, led by Dr. Boyd E. Metzger, was an attempt to clarify what level of maternal glucose intolerance is associated with an excess risk of an adverse pregnancy outcome.
The HAPO study, which involved 15 centers in nine countries, examined the outcomes of more than 25,000 pregnancies. In designing the HAPO study, Dr. Metzger and his colleagues did something that had never formally been done before: They administered a 75-g oral glucose tolerance test (OGTT) to the mothers between 24 and 32 weeks' gestation (as close to 28 weeks as possible), and defined GDM as an abnormal 2-hour 75-g OGTT result. They then followed the births of women identified as having GDM, and compared them with the births of mothers who did not have gestational diabetes as defined by traditional measures.
Outside the United States, the 75-g, one-step OGTT has been the standard for GDM diagnosis for some time. In the United States, many of us still use an awkward two-step system in which women initially are given a 50-g oral challenge. Only if they register an excessive value on the 50-g challenge do they come back for a definitive 3-hour, 100-g OGTT.
Quite a few outcomes were measured in the HAPO study, but the major outcomes were birth weight greater than the 90th percentile, the level of cord-blood serum-C-peptide (an index of fetal beta-cell function and fetal hyperinsulinemia) above the 90th percentile, and percent body fat greater than the 90th percentile.
The glucose results of the majority of women remained blinded (data were not blinded if the 2-hour plasma glucose level was greater than 200 mg/dL, or diagnostic of diabetes, or if the fasting plasma glucose level exceeded 105 mg/dL or the random plasma glucose level was 160 mg/dL). After birth and the assessment of fetal outcomes, these outcomes were arrayed against earlier results of the mothers' 2-hour 75-g glucose challenge tests and the fasting blood glucose levels, both of which were measured at the same time during pregnancy. (Fasting plasma glucose levels varied from as little as 75 mg/dL all the way up to the predefined threshold of 100 mg/dL.)
Considering percent of body fat greater than the 90th percentile, one would expect no more than 10% of babies without diagnosed GDM in the mothers to have hyperinsulinemia and large amounts of body fat.
Dr. Metzger found otherwise: 17% of babies whose mothers had a fasting blood glucose of 90 mg/dL, for instance – a level most clinicians have viewed as normal – had large levels of body fat, and many of these babies also had hyperinsulinemia. Overall, there was no “golden” level of maternal glucose that predicted a fat baby. However, neonatal adiposity increased progressively as fasting blood glucose levels rose above 80 mg/dL.
In the case of 1-hour 75-g OGTT results, fatness increased progressively at levels greater than 105 mg/dL, and with 2-hour results, fatness rose progressively at levels over 90 mg/dL (Diabetes 2009;58:453–9).
Such continuous linear relationships between maternal glucose and adverse fetal outcomes were seen studywide for birth weight and other outcomes (N. Engl. J. Med. 2008;358:1991–2002).
Among the most striking findings was that a significant number of fat babies were born to women whose blood glucose levels were considered “normal.”
The question at this point became, What should we do about it? Should we allow these obese babies to be born without any intervention, or can we treat them before birth?
Insights on Treatment
Many experts have been doubtful that treatment of mothers with GDM would be effective in altering newborn outcomes. However, the Australian Carbohydrate Intolerance Study in Pregnant Women, published in 2005, concluded that early treatment of GDM reduces serious perinatal morbidity and may improve health-related quality of life. In this study, women with GDM were randomized to receive dietary advice, blood glucose monitoring, and insulin therapy as needed (the treatment group), or routine care (N. Engl. J. Med. 2005;352:2477–86).
In another randomized study published several years later, Dr. Mark B. Landon and his colleagues finally convinced many experts of the value of aggressive screening and early intervention for GDM. Dr. Landon focused on a subset of women who had an abnormal result on a 3-hour 100-g OGTT but a fasting glucose level below 95 mg/L. These women thus had only mild glucose intolerance. (An abnormal result was defined as two or three timed glucose measurements that exceeded certain thresholds: 1-hour, 180 mg/dL; 2-hour, 155 mg/dL; and 3-hour, 140 mg/dL.)
In 14 centers across the United States, 958 patients were randomized to receive treatment of their diabetes or nothing but usual prenatal care. Treatment included formal nutritional counseling and diet therapy, along with insulin if needed. The majority of the women (93%) needed only dietary counseling and education about blood glucose control, while the other 7% needed insulin as well.
Women receiving dietary counseling checked their blood glucose levels before they got up in the morning, and 2 hours after each major meal. In essence, they planned and adjusted their diet based on their blood glucose readings.
What did we learn from this trial? We learned that the incidence of large-for-gestational-age births (greater than the 90th percentile) was cut in half from approximately 14% in the untreated group to 7% in the treated group. There also was a 10%-14% reduction in fat mass in the babies born to the women who received treatment, as well as significant reductions in mean birth weight and birth weight greater than 4,000 g. Most importantly, treatment also reduced the number of injuries that occurred during birth, while the number of small-for-gestational-age infants did not increase (N. Engl. J. Med. 2009;361:1339–48).
With these two randomized studies demonstrating significantly reduced risks with early GDM treatment, the question shifted from the broader issue of whether it is worthwhile to treat women with GDM to the more specific question of who needs treatment the most.
A New Approach
Today, in most demographic and ethnic groups in the United States, the incidence of gestational diabetes is between 4% and 12%, with a national incidence of about 8%. These are the patients we are already treating.
The HAPO trial, however, has shown us that there are a significant number of babies whose mothers have mild hyperglycemia and who are not being treated for this condition. These babies have neonatal adiposity and subsequently are being injured during the birth process.
In addition, we now have multiple epidemiologic studies demonstrating that adiposity at birth markedly increases – by as much as 30%–40% – the risk of being fat as a child and as an adolescent. Studies also have shown that the risk of developing childhood and adolescent type 2 diabetes proportionately increases with increasing neonatal adiposity.
Thus, the goal is no longer just to prevent neonatal adiposity so that babies will not be injured during birth; it now includes helping mothers control their glucose profiles so that their babies will have better health during their childhood and adult years.
However, the answer to the current, pressing question of whether we should offer treatment to women who are not now defined as having gestational diabetes is not yet clearly answered.
In 2008, after the initial release of HAPO study findings, a group called the International Association of Diabetes and Pregnancy Study Groups (IADPSG) was created to discuss the definition of gestational diabetes in light of the new HAPO findings and other research demonstrating improved outcomes with treatment.
In 2010, the consensus group released revised recommendations for glucose tolerance testing, suggesting that everyone convert to the 2-hour 75-g OGTT and that we lower the cutoff points used for diagnosis to protect as many babies as possible from becoming obese.
The group deliberated how much risk to address, or cover, with new cut points. Is a 150% increase in risk, for example, too much? Or a doubling of newborn fatness? In looking at a possible lifetime of obesity, type 2 diabetes, and heart disease, how much testing and treatment is just right? In the end, the group chose cutoff points for the fasting, 1-hour, and 2-hour plasma glucose measurements that conveyed an odds ratio for adverse outcomes of at least 1.75.
This means that a fasting plasma glucose of 92 mg/dL or more almost doubles the adverse fetal outcome risk; so does a 1-hour value after the 75-g OGTT of at least 180 mg/dL, and a 2-hour value of at least 153 mg/dL. If any one of these values is elevated, according to the IADPSG, a fetus is at risk and the mother should be treated for hyperglycemia (Diabetes Care 2010;33:676–82).
The Near Future
With the new criteria proposed by the IADPSG, the number of women who will be defined as having GDM using the 75-g OGTT will double to approximately 16%, compared with about 8% today. This doubling of incidence obviously will require additional resources and intervention.
The question now is, Are we going to adopt these new criteria? The practice approach for GDM in the United States normally follows guidelines for diabetes put forth by the American Diabetes Association and/or guidelines for pregnancy developed by the American College of Obstetricians and Gynecologists. Although the ADA has revised its recommendations for diagnosis of GDM to embrace the criteria of IADPSG, neither body has issued a directive or a formal set of guidelines for clinicians.
The National Institute of Child Health and Human Development is planning a workshop on GDM for next year, and it is quite possible that the proceedings from this NICHD workshop will inform future statements or guidelines from these organizations. In all likelihood, new screening criteria will be widely adopted within several years.
In the meantime, providers must decide what to do. There is nothing wrong with continuing two-stage testing. However, those who do should realistically consider its disadvantages: For one, this process identifies only 80%–90% of the women who actually have abnormal glucose levels, so many at-risk newborns will be missed even though their mothers were tested.
Secondly, the timing of the two-step process is problematic. Most women are given lab orders for the OGTT at about 28 weeks' gestation. By 29 or 30 weeks, they'll have results. If abnormal, the office staff must call and tell the patient to schedule the second OGTT test. Our own studies have shown that each step takes about 7–12 days to complete. In our system, it can then take up to 10 days for a woman diagnosed with GDM to receive care. She will be instructed in glucose monitoring and her care team will check with her every week.
In the end, it may be 6–8 weeks after initial testing before the woman's glucose intolerance is effectively addressed. The maximal time of fetal fat accretion is at about 34 weeks. If we do not have a diagnosis made and treatment plan underway by 32 weeks, we will have significantly decreased our chance of preventing obesity in her newborn.
Aggressive efforts to get screening done at about 26 weeks would be worthwhile, especially if you are working within a system that can accommodate a greater number of women with identified glucose intolerance. To ensure the outcomes that we're seeking, we must ensure that our patients receive adequate dietary and other interventions.
There also are questions about whether the identification of more women at risk of an adverse pregnancy outcome could itself create risk, particularly since it is well documented that women with GDM are more likely to be delivered earlier or through cesarean section, regardless of the level of achieved glucose control. (In the Landon study, interestingly, the rate of cesarean delivery was reduced in the intervention group.)
On the other hand, wider identification offers such hope for reducing fetal adiposity, and its many adverse consequences, that it should be immediately considered.
'Old' (Current) vs. 'New' (Upcoming)
Two-Step Approach to GDM Dx:
▸ Initial screening with a 50-g glucose challenge test at 24–28 weeks' gestation in women at greater than low risk of GDM. Women at very high risk should be screened as soon as possible after confirmation of pregnancy.
▸ Diagnostic 100-g oral glucose tolerance test (on separate day, after overnight fast) in women who meet or exceed chosen threshold on 50-g screening (140 mg/dL or more, or 130 mg/dL or more for higher sensitivity).
▸ GDM diagnosis made if at least two of these plasma glucose values are met or exceeded after the 100-g OGTT: fasting, 95 mg/dL; 1 hour, 180 mg/dL; 2 hour, 155 mg/dL; 3 hour, 140 mg/dL (if a 3-hour test is done).
One-Step Approach to GDM Dx:
▸ Screening of all women at 24–28 weeks' gestation not known to have type 2 diabetes with 75-g oral glucose tolerance test (after overnight fast).
▸ GDM diagnosis made if any one of these fasting plasma glucose values are met or exceeded: fasting, 92 mg/dL; 1 hour, 180 mg/dL; 2 hour, 153 mg/dL.
Sources: American Diabetes Association's Standards of Medical Care in Diabetes 2010 (Diabetes Care 2010;33:S11–61); American College of Obstetricians and Gynecologists Practice Bulletin, September 2001; ADA's Standards of Medical Care in Diabetes 2011 (Diabetes Care 2011;34:S11–61).
The Consequences of GDM
The thresholds for deciding when to begin treating hyperglycemia were established almost 50 years ago at a time when we had significantly less knowledge about the risk factors for and consequences of hyperglycemia in pregnancy. Because of this lack of understanding about the causes and consequences of hyperglycemia and our sometimes rigid adherence to these cutoffs, many women were not treated who should have been.
There is a growing recognition in the research and clinical communities that gestational diabetes mellitus (GDM) is a much more serious condition than had been previously believed even a decade ago. We now know that GDM, if not properly diagnosed and managed, can have intergenerational consequences in terms of propagating risks for obesity, diabetes, heart disease, and other disorders. Furthermore, there is a new and growing realization that even mild hyperglycemia significantly below what has traditionally been defined as diabetes can have significant adverse consequences for both mother and infant.
Perhaps the most significant complication of maternal hyperglycemia faced by ob.gyns. is the growing number of large-for-gestational-age (LGA) infants being born. For obvious reasons, LGA infants are more difficult to deliver and significantly more prone to experiencing shoulder dystocia and other injuries during normal or cesarean delivery, and cesarean delivery has its own set of complications for both baby and mother.
The large, multicenter Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study recently documented that by managing hyperglycemia – even among women who previously had not been considered to have any glucose control problems – the incidence of LGA-related problems and other adverse birth outcomes could be significantly reduced.
To discuss in detail the findings of the HAPO study and its potential clinical implications, we have invited Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, to write this Master Class.
Dr. Moore's essay discusses both the unique design and findings of the HAPO study, and also explores the quandary faced by members of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) in their attempts to translate HAPO's findings into clinically useful recommendations and guidelines.
In a sign of how complex and time consuming it can be to translate clinical research findings into clinical practice, the recommendations of the IADPSG are now being debated among research and medical societies, with some suggesting that the thresholds introduced by the HAPO study and advanced by the IADPSG are not significantly different from the current levels.
We greatly appreciate Dr. Moore's insights into these complicated but exciting developments. His Master Class installment will help all of us to better understand this complex issue so that we can potentially play a role in speeding up the process of changing the way we manage GDM.
The tide has turned in our understanding of both the effects of maternal hyperglycemia and the effectiveness of current treatment approaches. Consequently, we are facing an impending sea change in the way in which gestational diabetes is diagnosed and managed.
Recent research has detailed the risks posed to a fetus exposed to hyperglycemia during pregnancy – even at levels that in the past have been considered mild and, thus, largely inconsequential. We also now have evidence that we can offer therapies for gestational diabetes mellitus (GDM) with confidence that we can use them to change the outcome for the fetus, the newborn, the child, and possibly the adult.
This impending change comes after decades of diagnosing gestational diabetes based largely on relatively arbitrary thresholds. Dr. John B. O'sullivan and statistician Claire Mahan developed the diagnostic criteria more than 40 years ago based on certain statistical phenomena associated with the development of adult-onset diabetes after pregnancy. Before then, during the 1940s, 1950s, and 1960s, 1%-2% of all pregnant women were diagnosed with GDM.
In recent years, many of us have had the experience as clinicians of delivering larger, more obese babies whose mothers had been found to have “normal” blood glucose levels. Many of us also have delivered babies with significant adiposity, sometimes perilously low blood glucose, shoulder dystocia, nerve injuries, and other complications that typically occur as a consequence of fetal overgrowth.
We often attribute these complications to a diagnostic method we have known for some time wasn't perfect, but until recently, we did not have the clinical research findings to guide us in our efforts to fine-tune the diagnosis of GDM and turn the tide.
Insights on Fetal Risk
The landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, led by Dr. Boyd E. Metzger, was an attempt to clarify what level of maternal glucose intolerance is associated with an excess risk of an adverse pregnancy outcome.
The HAPO study, which involved 15 centers in nine countries, examined the outcomes of more than 25,000 pregnancies. In designing the HAPO study, Dr. Metzger and his colleagues did something that had never formally been done before: They administered a 75-g oral glucose tolerance test (OGTT) to the mothers between 24 and 32 weeks' gestation (as close to 28 weeks as possible), and defined GDM as an abnormal 2-hour 75-g OGTT result. They then followed the births of women identified as having GDM, and compared them with the births of mothers who did not have gestational diabetes as defined by traditional measures.
Outside the United States, the 75-g, one-step OGTT has been the standard for GDM diagnosis for some time. In the United States, many of us still use an awkward two-step system in which women initially are given a 50-g oral challenge. Only if they register an excessive value on the 50-g challenge do they come back for a definitive 3-hour, 100-g OGTT.
Quite a few outcomes were measured in the HAPO study, but the major outcomes were birth weight greater than the 90th percentile, the level of cord-blood serum-C-peptide (an index of fetal beta-cell function and fetal hyperinsulinemia) above the 90th percentile, and percent body fat greater than the 90th percentile.
The glucose results of the majority of women remained blinded (data were not blinded if the 2-hour plasma glucose level was greater than 200 mg/dL, or diagnostic of diabetes, or if the fasting plasma glucose level exceeded 105 mg/dL or the random plasma glucose level was 160 mg/dL). After birth and the assessment of fetal outcomes, these outcomes were arrayed against earlier results of the mothers' 2-hour 75-g glucose challenge tests and the fasting blood glucose levels, both of which were measured at the same time during pregnancy. (Fasting plasma glucose levels varied from as little as 75 mg/dL all the way up to the predefined threshold of 100 mg/dL.)
Considering percent of body fat greater than the 90th percentile, one would expect no more than 10% of babies without diagnosed GDM in the mothers to have hyperinsulinemia and large amounts of body fat.
Dr. Metzger found otherwise: 17% of babies whose mothers had a fasting blood glucose of 90 mg/dL, for instance – a level most clinicians have viewed as normal – had large levels of body fat, and many of these babies also had hyperinsulinemia. Overall, there was no “golden” level of maternal glucose that predicted a fat baby. However, neonatal adiposity increased progressively as fasting blood glucose levels rose above 80 mg/dL.
In the case of 1-hour 75-g OGTT results, fatness increased progressively at levels greater than 105 mg/dL, and with 2-hour results, fatness rose progressively at levels over 90 mg/dL (Diabetes 2009;58:453–9).
Such continuous linear relationships between maternal glucose and adverse fetal outcomes were seen studywide for birth weight and other outcomes (N. Engl. J. Med. 2008;358:1991–2002).
Among the most striking findings was that a significant number of fat babies were born to women whose blood glucose levels were considered “normal.”
The question at this point became, What should we do about it? Should we allow these obese babies to be born without any intervention, or can we treat them before birth?
Insights on Treatment
Many experts have been doubtful that treatment of mothers with GDM would be effective in altering newborn outcomes. However, the Australian Carbohydrate Intolerance Study in Pregnant Women, published in 2005, concluded that early treatment of GDM reduces serious perinatal morbidity and may improve health-related quality of life. In this study, women with GDM were randomized to receive dietary advice, blood glucose monitoring, and insulin therapy as needed (the treatment group), or routine care (N. Engl. J. Med. 2005;352:2477–86).
In another randomized study published several years later, Dr. Mark B. Landon and his colleagues finally convinced many experts of the value of aggressive screening and early intervention for GDM. Dr. Landon focused on a subset of women who had an abnormal result on a 3-hour 100-g OGTT but a fasting glucose level below 95 mg/L. These women thus had only mild glucose intolerance. (An abnormal result was defined as two or three timed glucose measurements that exceeded certain thresholds: 1-hour, 180 mg/dL; 2-hour, 155 mg/dL; and 3-hour, 140 mg/dL.)
In 14 centers across the United States, 958 patients were randomized to receive treatment of their diabetes or nothing but usual prenatal care. Treatment included formal nutritional counseling and diet therapy, along with insulin if needed. The majority of the women (93%) needed only dietary counseling and education about blood glucose control, while the other 7% needed insulin as well.
Women receiving dietary counseling checked their blood glucose levels before they got up in the morning, and 2 hours after each major meal. In essence, they planned and adjusted their diet based on their blood glucose readings.
What did we learn from this trial? We learned that the incidence of large-for-gestational-age births (greater than the 90th percentile) was cut in half from approximately 14% in the untreated group to 7% in the treated group. There also was a 10%-14% reduction in fat mass in the babies born to the women who received treatment, as well as significant reductions in mean birth weight and birth weight greater than 4,000 g. Most importantly, treatment also reduced the number of injuries that occurred during birth, while the number of small-for-gestational-age infants did not increase (N. Engl. J. Med. 2009;361:1339–48).
With these two randomized studies demonstrating significantly reduced risks with early GDM treatment, the question shifted from the broader issue of whether it is worthwhile to treat women with GDM to the more specific question of who needs treatment the most.
A New Approach
Today, in most demographic and ethnic groups in the United States, the incidence of gestational diabetes is between 4% and 12%, with a national incidence of about 8%. These are the patients we are already treating.
The HAPO trial, however, has shown us that there are a significant number of babies whose mothers have mild hyperglycemia and who are not being treated for this condition. These babies have neonatal adiposity and subsequently are being injured during the birth process.
In addition, we now have multiple epidemiologic studies demonstrating that adiposity at birth markedly increases – by as much as 30%–40% – the risk of being fat as a child and as an adolescent. Studies also have shown that the risk of developing childhood and adolescent type 2 diabetes proportionately increases with increasing neonatal adiposity.
Thus, the goal is no longer just to prevent neonatal adiposity so that babies will not be injured during birth; it now includes helping mothers control their glucose profiles so that their babies will have better health during their childhood and adult years.
However, the answer to the current, pressing question of whether we should offer treatment to women who are not now defined as having gestational diabetes is not yet clearly answered.
In 2008, after the initial release of HAPO study findings, a group called the International Association of Diabetes and Pregnancy Study Groups (IADPSG) was created to discuss the definition of gestational diabetes in light of the new HAPO findings and other research demonstrating improved outcomes with treatment.
In 2010, the consensus group released revised recommendations for glucose tolerance testing, suggesting that everyone convert to the 2-hour 75-g OGTT and that we lower the cutoff points used for diagnosis to protect as many babies as possible from becoming obese.
The group deliberated how much risk to address, or cover, with new cut points. Is a 150% increase in risk, for example, too much? Or a doubling of newborn fatness? In looking at a possible lifetime of obesity, type 2 diabetes, and heart disease, how much testing and treatment is just right? In the end, the group chose cutoff points for the fasting, 1-hour, and 2-hour plasma glucose measurements that conveyed an odds ratio for adverse outcomes of at least 1.75.
This means that a fasting plasma glucose of 92 mg/dL or more almost doubles the adverse fetal outcome risk; so does a 1-hour value after the 75-g OGTT of at least 180 mg/dL, and a 2-hour value of at least 153 mg/dL. If any one of these values is elevated, according to the IADPSG, a fetus is at risk and the mother should be treated for hyperglycemia (Diabetes Care 2010;33:676–82).
The Near Future
With the new criteria proposed by the IADPSG, the number of women who will be defined as having GDM using the 75-g OGTT will double to approximately 16%, compared with about 8% today. This doubling of incidence obviously will require additional resources and intervention.
The question now is, Are we going to adopt these new criteria? The practice approach for GDM in the United States normally follows guidelines for diabetes put forth by the American Diabetes Association and/or guidelines for pregnancy developed by the American College of Obstetricians and Gynecologists. Although the ADA has revised its recommendations for diagnosis of GDM to embrace the criteria of IADPSG, neither body has issued a directive or a formal set of guidelines for clinicians.
The National Institute of Child Health and Human Development is planning a workshop on GDM for next year, and it is quite possible that the proceedings from this NICHD workshop will inform future statements or guidelines from these organizations. In all likelihood, new screening criteria will be widely adopted within several years.
In the meantime, providers must decide what to do. There is nothing wrong with continuing two-stage testing. However, those who do should realistically consider its disadvantages: For one, this process identifies only 80%–90% of the women who actually have abnormal glucose levels, so many at-risk newborns will be missed even though their mothers were tested.
Secondly, the timing of the two-step process is problematic. Most women are given lab orders for the OGTT at about 28 weeks' gestation. By 29 or 30 weeks, they'll have results. If abnormal, the office staff must call and tell the patient to schedule the second OGTT test. Our own studies have shown that each step takes about 7–12 days to complete. In our system, it can then take up to 10 days for a woman diagnosed with GDM to receive care. She will be instructed in glucose monitoring and her care team will check with her every week.
In the end, it may be 6–8 weeks after initial testing before the woman's glucose intolerance is effectively addressed. The maximal time of fetal fat accretion is at about 34 weeks. If we do not have a diagnosis made and treatment plan underway by 32 weeks, we will have significantly decreased our chance of preventing obesity in her newborn.
Aggressive efforts to get screening done at about 26 weeks would be worthwhile, especially if you are working within a system that can accommodate a greater number of women with identified glucose intolerance. To ensure the outcomes that we're seeking, we must ensure that our patients receive adequate dietary and other interventions.
There also are questions about whether the identification of more women at risk of an adverse pregnancy outcome could itself create risk, particularly since it is well documented that women with GDM are more likely to be delivered earlier or through cesarean section, regardless of the level of achieved glucose control. (In the Landon study, interestingly, the rate of cesarean delivery was reduced in the intervention group.)
On the other hand, wider identification offers such hope for reducing fetal adiposity, and its many adverse consequences, that it should be immediately considered.
'Old' (Current) vs. 'New' (Upcoming)
Two-Step Approach to GDM Dx:
▸ Initial screening with a 50-g glucose challenge test at 24–28 weeks' gestation in women at greater than low risk of GDM. Women at very high risk should be screened as soon as possible after confirmation of pregnancy.
▸ Diagnostic 100-g oral glucose tolerance test (on separate day, after overnight fast) in women who meet or exceed chosen threshold on 50-g screening (140 mg/dL or more, or 130 mg/dL or more for higher sensitivity).
▸ GDM diagnosis made if at least two of these plasma glucose values are met or exceeded after the 100-g OGTT: fasting, 95 mg/dL; 1 hour, 180 mg/dL; 2 hour, 155 mg/dL; 3 hour, 140 mg/dL (if a 3-hour test is done).
One-Step Approach to GDM Dx:
▸ Screening of all women at 24–28 weeks' gestation not known to have type 2 diabetes with 75-g oral glucose tolerance test (after overnight fast).
▸ GDM diagnosis made if any one of these fasting plasma glucose values are met or exceeded: fasting, 92 mg/dL; 1 hour, 180 mg/dL; 2 hour, 153 mg/dL.
Sources: American Diabetes Association's Standards of Medical Care in Diabetes 2010 (Diabetes Care 2010;33:S11–61); American College of Obstetricians and Gynecologists Practice Bulletin, September 2001; ADA's Standards of Medical Care in Diabetes 2011 (Diabetes Care 2011;34:S11–61).
The Consequences of GDM
The thresholds for deciding when to begin treating hyperglycemia were established almost 50 years ago at a time when we had significantly less knowledge about the risk factors for and consequences of hyperglycemia in pregnancy. Because of this lack of understanding about the causes and consequences of hyperglycemia and our sometimes rigid adherence to these cutoffs, many women were not treated who should have been.
There is a growing recognition in the research and clinical communities that gestational diabetes mellitus (GDM) is a much more serious condition than had been previously believed even a decade ago. We now know that GDM, if not properly diagnosed and managed, can have intergenerational consequences in terms of propagating risks for obesity, diabetes, heart disease, and other disorders. Furthermore, there is a new and growing realization that even mild hyperglycemia significantly below what has traditionally been defined as diabetes can have significant adverse consequences for both mother and infant.
Perhaps the most significant complication of maternal hyperglycemia faced by ob.gyns. is the growing number of large-for-gestational-age (LGA) infants being born. For obvious reasons, LGA infants are more difficult to deliver and significantly more prone to experiencing shoulder dystocia and other injuries during normal or cesarean delivery, and cesarean delivery has its own set of complications for both baby and mother.
The large, multicenter Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study recently documented that by managing hyperglycemia – even among women who previously had not been considered to have any glucose control problems – the incidence of LGA-related problems and other adverse birth outcomes could be significantly reduced.
To discuss in detail the findings of the HAPO study and its potential clinical implications, we have invited Dr. Thomas R. Moore, professor and chairman of the department of reproductive medicine at the University of California, San Diego, to write this Master Class.
Dr. Moore's essay discusses both the unique design and findings of the HAPO study, and also explores the quandary faced by members of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) in their attempts to translate HAPO's findings into clinically useful recommendations and guidelines.
In a sign of how complex and time consuming it can be to translate clinical research findings into clinical practice, the recommendations of the IADPSG are now being debated among research and medical societies, with some suggesting that the thresholds introduced by the HAPO study and advanced by the IADPSG are not significantly different from the current levels.
We greatly appreciate Dr. Moore's insights into these complicated but exciting developments. His Master Class installment will help all of us to better understand this complex issue so that we can potentially play a role in speeding up the process of changing the way we manage GDM.
The Benefits of Robot-Assisted Myomectomy
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
The Ever-Changing Laparoscopic Myomectomy
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.