User login
Planned Home Births
In the last decade, there has been new and renewed support for planned home birth in the United States and in Europe. From 2004 to 2009, home births in the United States rose by 29%, increasing from 0.56% to 0.72% of all births, according to the Centers for Disease Control and Prevention. For non-Hispanic white women, planned home births rose by 36% from a rate of 0.80% in 2004 to 1.09% in 2009.
Although planned home birth for women with a prior cesarean delivery is still rare, there is CDC evidence that VBAC at home is increasing in the United States as well (Obstet. Gynecol. 2012;119:737-44).
Although these increases may be considered small, the changes are part of a congruence of events in the United States and other developed countries that demand our attention and professional response. One such event is a 2010 ruling by the European Court of Human Rights that states that the decision to become a parent includes the right of "choosing the circumstances of becoming a parent." This right includes the right to professional assistance at home birth, according to the ruling.
The full ramifications of this court decision, which originated in Hungary when a pregnant woman alleged that she was not able to give birth at home because health professionals were dissuaded by law from assisting her, remain to be seen. However, recent statements from professional associations favor the woman’s right to choose planned home birth.
The Royal College of Obstetricians and Gynecologists (RCOG) and the Royal College of Midwives (RCM) issued a statement in 2007 in support of planned home birth for women with uncomplicated pregnancies, saying there is "no reason why home birth should not be offered to women at low risk of complications." Home birth in such cases may confer "considerable benefits" for the mother and her family, increasing the likelihood of a birth that is "both satisfying and safe," the statement says.
In addition, the American College of Obstetricians and Gynecologists said in a 2011 committee opinion (#476) that while it believes hospitals and birthing centers are the safest setting for birth, "it respects the right of a woman to make a medically informed decision about delivery." In doing so, ACOG qualified its previous statement, which recommended against home birth (Obstet. Gynecol. 2011;117:425-8).
In the meantime, articles in the consumer press have focused on the benefits of planned home birth, indicating that home birth has become fashionable and that the midwife is increasingly regarded as a status symbol.
Planned home birth has been debated for decades, but this recent recrudescence of support-motivated ethicist Laurence B. McCullough, myself, and a team of physicians – a U.S. neonatologist and a pediatric neurologist and perinatologist from Europe – to review the change in the context of professional responsibility (Am. J. Obstet. Gynecol. 2013:208;31-8).
Advocates for planned home birth emphasize patient satisfaction, patient safety, cost effectiveness, and respect for women’s rights. Yet, as we have described in detail, none of these reasons or causes of support for home birth can or should stand unchallenged. Most importantly, planned home birth does not meet current obstetric standards for patient safety. One of the largest and most current studies, for instance, shows a two- to threefold increased risk of neonatal death with planned home birth, compared with hospital birth.
Some advocates of planned home birth accept this finding as well as other studies showing adverse outcomes and maintain that the level of risk is ethically acceptable. However, we feel that such views are antithetical to our professional responsibility. As obstetricians, our professional responsibility is to both the pregnant woman and the fetal patient. An overwhelming emphasis on maternal rights over fetal rights – a form of rights-based reductionism – is ethically incomplete, clinically inadequate, and therefore unprofessional.
Safety issues
A systematic review published in 2010 identified a doubling of the overall rate of neonatal mortality, and a tripling of the neonatal mortality rate among nonanomalous neonates, in planned home birth vs. planned hospital birth. Dr. Joseph R. Wax and his associates called these findings "especially striking" because women planning home births were "of similar and often lower obstetric risk than those planning hospital births."
The meta-analysis, which included 12 studies from the United States, Canada, Europe, and Australia, showed that women who chose home birth are "in large part successful in achieving their goal of delivering with less morbidity and medical intervention than experienced during hospital-based childbirth," but at a significant cost, the authors said (Obstet. Gynecol. 2010:203;243.e1-8).
A population-based study from South Australia on all births and perinatal deaths between 1991 and 2006 – one of the studies included in the review – reported that the overall perinatal mortality rate of nonhospital deliveries was similar to that for planned hospital births. However, there was a 7-fold higher risk of intrapartum death and a 27-fold higher risk of death from intrapartum asphyxia (Med. J. Austr. 2010:192;76-80).
A key complicating factor in planned home birth is the frequent need for transport to the hospital. Maternal and fetal reasons for transport during labor include failure for labor to progress, unbearable labor pain, fetal malpresentation, abrupt deterioration of fetal heart rate, uterine rupture, acute bleeding, placental abruption, acute sepsis, and cord prolapse.
Neonatal reasons for transport include signs of respiratory distress, unexpected very low or very high birth weight, and acute sepsis. Indeed, in the 2010 meta-analysis, respiratory distress and failed resuscitation contributed disproportionately to neonatal deaths among planned home births.
The 2010 review concluded that more data are necessary before drawing any conclusions regarding maternal mortality in planned home vs. planned hospital delivery. Although rare, preventable maternal death may nevertheless sometimes occur. Just recently, an Australian midwife and home-birth advocate died from postpartum hemorrhage after attempting to deliver her second child at home.
These complications and high-risk conditions are often impossible to predict, even with the best possible prenatal screenings, risk assessments, and fetal surveillance during labor. Women need immediate access to in-hospital care and emergency cesarean delivery.
Even studies that generally support home birth have reported high rates of transport. For example, the recent Birthplace in England prospective cohort study reported transport rates from nonobstetric units to the hospital of 36%-45% for nulliparous women and 9%-13% for multiparous women (BMJ 2011:343;d7400).
Adverse outcomes were similarly much higher in this study in women having their first baby at home. For women "without any complicating factor at the start of care in labour," the adjusted odds ratio of a primary outcome event for births planned at home, compared with planned obstetric unit births, was 1.59. The primary outcome in this study was defined as a composite measure of perinatal mortality and intrapartum-related neonatal morbidities (which include early neonatal death, neonatal encephalopathy, meconium aspiration syndrome, and brachial plexus injury).
This adjusted odds ratio of an adverse event increased to 1.75 in a subgroup analysis of nulliparous women, and to 2.8 when the sample was restricted to nulliparous women with no complications at the start of labor. Although the authors did not elucidate on the issue of transport, the 59%-75% increase in a poor primary outcome may largely be attributed to the delay in access to hospital care from transport time.
In the Netherlands, where there is a long tradition of organized home birth with well-trained midwives, 49% of primiparous and 17% of multiparous women are transported during labor (BJOG 2008:115:570-8). Research done in the Netherlands also shows that women who are transferred to a hospital have a significantly higher rate of operative vaginal and secondary cesarean delivery.
In the United States, women tend to envision that any complications can be easily mitigated by a rapid and seamless transport and transition to the hospital, but in reality, even the best of transport systems experience unavoidable delays that can result in increased mortality and morbidity.
The standard of care in the United States is that "decision to incision" should take no more than 30 minutes, and ACOG has said in a recent practice bulletin that once a decision for operative delivery has been made in the context of a Category III EFM tracing, it should be accomplished as expeditiously as possible. The standards outside the United States are much the same, if not stricter. In Germany, for instance, 20 minutes is the standard used in the assessment of perinatal centers.
None of these standards of care can be consistently met when pregnant patients have started the labor process at home and then are transported to obstetric units, and the inherent problems with transport are largely irremediable even with significant investments of capital. Moreover, even if rates of emergency transport were low, there still should be considerable concern given the severity and frequency of the reasons for transfer.
Ethics, our response
The RCOG-RCM statement emphasizes the psychosocial importance of planned home birth and says that the focus should not be exclusively on the physical safety of planned home birth.
Other supporters of home birth, including some experts in the United States, focus on the absolute risk of planned home birth rather than the relative risk. According to these experts, in the broader context, the numbers of adverse outcomes are so small that it is ethically acceptable to support a patient’s desire for home birth. The ACOG, meanwhile, says that pregnant women should be informed of the risks of planned home birth, as summarized in the 2010 review.
It is antithetical to professional responsibility, however, to regard the risks of home birth, however small in the absolute sense, as ethically acceptable. Every life is important. The nature of a pregnant woman’s relationship to her soon-to-be-born child is primarily one of obligation to protect, not freedom. Hence, she does not have an unconditional, systematic right to control her body to the extent that her rights automatically override fetal rights. She does not have an unmitigated right to put her soon-to-be-born child at risk.
Supporting a woman’s autonomy-based rights at the expense of the rights of the fetal or neonatal patient is a form of "rights-based reductionism." Reductionism as an ethical model has an appealing simplicity, but it is ethically incomplete and unprofessional.
As professionals, obstetricians have the obligation, as a matter of professional integrity, to protect the pregnant, fetal, and neonatal patients. Under the ethical model that we call the "professional responsibility model of obstetric ethics," beneficence-based obligations must always be balanced against autonomy-based obligations to the pregnant patient. The obstetrician’s role is to identify and present medically reasonable alternatives for the management of pregnancy – that is, management for which there is evidence of a net clinical benefit. The patient has the right to select from among the medically reasonable alternatives.
Women’s questions about planned home birth should be respectfully addressed in an evidence-based manner. As obstetricians, we must inform women of the high transport rate and of the preventable risks of home birth to herself and the child. Women also should be made aware that emergency transport can be psychologically disruptive, even traumatizing. The risk of long-term harm was documented in a Dutch study in which 17% of all transported women reported having psychological difficulties up to 3 years after giving birth (Birth 2008:35;107-16).
Interestingly, the planned home-birth rate in the Netherlands has decreased from 38% to 23% in the last 20 years, largely because of an increased awareness of the media, patients, and physicians about the risks. This decline has occurred in spite of the fact that women have to pay additional fees for "nonindicated" hospital births.
Our professional response to women’s interest in planned home birth should be compassionate and understanding, taking into consideration some of the legitimate arguments supporting this method of delivery: The desire for empathetic caregivers and the comfort of home, greater control and undisrupted labor, and fewer interventions.
We must work to ensure that delivery in the hospital is safe, respectful and compassionate, as home-like as possible, and free of unnecessary operative deliveries, episiotomies, and other interventions. We need to scrutinize organizational policies and practices, and encourage and further develop collaborative models with nurse midwives, either within the hospital or at home-birth centers with access to full back-up. Simply put, we have to make hospital birth a more humane experience – without jeopardizing outcomes.
We have a clear professional obligation to provide excellent, nonjudgmental emergency care to women who are transported from planned home birth to the hospital. On the other hand, when a woman remains committed to planned home birth despite our communication, we must just say no to our participation, with the explanation that it is ethically unprofessional to participate in substandard care.
Dr. Chervenak is the Given Foundation Professor and chairman of the department of obstetrics and gynecology at Cornell University in New York. Dr. Chervenak reported that he has no disclosures relevant to this Master Class.
In the last decade, there has been new and renewed support for planned home birth in the United States and in Europe. From 2004 to 2009, home births in the United States rose by 29%, increasing from 0.56% to 0.72% of all births, according to the Centers for Disease Control and Prevention. For non-Hispanic white women, planned home births rose by 36% from a rate of 0.80% in 2004 to 1.09% in 2009.
Although planned home birth for women with a prior cesarean delivery is still rare, there is CDC evidence that VBAC at home is increasing in the United States as well (Obstet. Gynecol. 2012;119:737-44).
Although these increases may be considered small, the changes are part of a congruence of events in the United States and other developed countries that demand our attention and professional response. One such event is a 2010 ruling by the European Court of Human Rights that states that the decision to become a parent includes the right of "choosing the circumstances of becoming a parent." This right includes the right to professional assistance at home birth, according to the ruling.
The full ramifications of this court decision, which originated in Hungary when a pregnant woman alleged that she was not able to give birth at home because health professionals were dissuaded by law from assisting her, remain to be seen. However, recent statements from professional associations favor the woman’s right to choose planned home birth.
The Royal College of Obstetricians and Gynecologists (RCOG) and the Royal College of Midwives (RCM) issued a statement in 2007 in support of planned home birth for women with uncomplicated pregnancies, saying there is "no reason why home birth should not be offered to women at low risk of complications." Home birth in such cases may confer "considerable benefits" for the mother and her family, increasing the likelihood of a birth that is "both satisfying and safe," the statement says.
In addition, the American College of Obstetricians and Gynecologists said in a 2011 committee opinion (#476) that while it believes hospitals and birthing centers are the safest setting for birth, "it respects the right of a woman to make a medically informed decision about delivery." In doing so, ACOG qualified its previous statement, which recommended against home birth (Obstet. Gynecol. 2011;117:425-8).
In the meantime, articles in the consumer press have focused on the benefits of planned home birth, indicating that home birth has become fashionable and that the midwife is increasingly regarded as a status symbol.
Planned home birth has been debated for decades, but this recent recrudescence of support-motivated ethicist Laurence B. McCullough, myself, and a team of physicians – a U.S. neonatologist and a pediatric neurologist and perinatologist from Europe – to review the change in the context of professional responsibility (Am. J. Obstet. Gynecol. 2013:208;31-8).
Advocates for planned home birth emphasize patient satisfaction, patient safety, cost effectiveness, and respect for women’s rights. Yet, as we have described in detail, none of these reasons or causes of support for home birth can or should stand unchallenged. Most importantly, planned home birth does not meet current obstetric standards for patient safety. One of the largest and most current studies, for instance, shows a two- to threefold increased risk of neonatal death with planned home birth, compared with hospital birth.
Some advocates of planned home birth accept this finding as well as other studies showing adverse outcomes and maintain that the level of risk is ethically acceptable. However, we feel that such views are antithetical to our professional responsibility. As obstetricians, our professional responsibility is to both the pregnant woman and the fetal patient. An overwhelming emphasis on maternal rights over fetal rights – a form of rights-based reductionism – is ethically incomplete, clinically inadequate, and therefore unprofessional.
Safety issues
A systematic review published in 2010 identified a doubling of the overall rate of neonatal mortality, and a tripling of the neonatal mortality rate among nonanomalous neonates, in planned home birth vs. planned hospital birth. Dr. Joseph R. Wax and his associates called these findings "especially striking" because women planning home births were "of similar and often lower obstetric risk than those planning hospital births."
The meta-analysis, which included 12 studies from the United States, Canada, Europe, and Australia, showed that women who chose home birth are "in large part successful in achieving their goal of delivering with less morbidity and medical intervention than experienced during hospital-based childbirth," but at a significant cost, the authors said (Obstet. Gynecol. 2010:203;243.e1-8).
A population-based study from South Australia on all births and perinatal deaths between 1991 and 2006 – one of the studies included in the review – reported that the overall perinatal mortality rate of nonhospital deliveries was similar to that for planned hospital births. However, there was a 7-fold higher risk of intrapartum death and a 27-fold higher risk of death from intrapartum asphyxia (Med. J. Austr. 2010:192;76-80).
A key complicating factor in planned home birth is the frequent need for transport to the hospital. Maternal and fetal reasons for transport during labor include failure for labor to progress, unbearable labor pain, fetal malpresentation, abrupt deterioration of fetal heart rate, uterine rupture, acute bleeding, placental abruption, acute sepsis, and cord prolapse.
Neonatal reasons for transport include signs of respiratory distress, unexpected very low or very high birth weight, and acute sepsis. Indeed, in the 2010 meta-analysis, respiratory distress and failed resuscitation contributed disproportionately to neonatal deaths among planned home births.
The 2010 review concluded that more data are necessary before drawing any conclusions regarding maternal mortality in planned home vs. planned hospital delivery. Although rare, preventable maternal death may nevertheless sometimes occur. Just recently, an Australian midwife and home-birth advocate died from postpartum hemorrhage after attempting to deliver her second child at home.
These complications and high-risk conditions are often impossible to predict, even with the best possible prenatal screenings, risk assessments, and fetal surveillance during labor. Women need immediate access to in-hospital care and emergency cesarean delivery.
Even studies that generally support home birth have reported high rates of transport. For example, the recent Birthplace in England prospective cohort study reported transport rates from nonobstetric units to the hospital of 36%-45% for nulliparous women and 9%-13% for multiparous women (BMJ 2011:343;d7400).
Adverse outcomes were similarly much higher in this study in women having their first baby at home. For women "without any complicating factor at the start of care in labour," the adjusted odds ratio of a primary outcome event for births planned at home, compared with planned obstetric unit births, was 1.59. The primary outcome in this study was defined as a composite measure of perinatal mortality and intrapartum-related neonatal morbidities (which include early neonatal death, neonatal encephalopathy, meconium aspiration syndrome, and brachial plexus injury).
This adjusted odds ratio of an adverse event increased to 1.75 in a subgroup analysis of nulliparous women, and to 2.8 when the sample was restricted to nulliparous women with no complications at the start of labor. Although the authors did not elucidate on the issue of transport, the 59%-75% increase in a poor primary outcome may largely be attributed to the delay in access to hospital care from transport time.
In the Netherlands, where there is a long tradition of organized home birth with well-trained midwives, 49% of primiparous and 17% of multiparous women are transported during labor (BJOG 2008:115:570-8). Research done in the Netherlands also shows that women who are transferred to a hospital have a significantly higher rate of operative vaginal and secondary cesarean delivery.
In the United States, women tend to envision that any complications can be easily mitigated by a rapid and seamless transport and transition to the hospital, but in reality, even the best of transport systems experience unavoidable delays that can result in increased mortality and morbidity.
The standard of care in the United States is that "decision to incision" should take no more than 30 minutes, and ACOG has said in a recent practice bulletin that once a decision for operative delivery has been made in the context of a Category III EFM tracing, it should be accomplished as expeditiously as possible. The standards outside the United States are much the same, if not stricter. In Germany, for instance, 20 minutes is the standard used in the assessment of perinatal centers.
None of these standards of care can be consistently met when pregnant patients have started the labor process at home and then are transported to obstetric units, and the inherent problems with transport are largely irremediable even with significant investments of capital. Moreover, even if rates of emergency transport were low, there still should be considerable concern given the severity and frequency of the reasons for transfer.
Ethics, our response
The RCOG-RCM statement emphasizes the psychosocial importance of planned home birth and says that the focus should not be exclusively on the physical safety of planned home birth.
Other supporters of home birth, including some experts in the United States, focus on the absolute risk of planned home birth rather than the relative risk. According to these experts, in the broader context, the numbers of adverse outcomes are so small that it is ethically acceptable to support a patient’s desire for home birth. The ACOG, meanwhile, says that pregnant women should be informed of the risks of planned home birth, as summarized in the 2010 review.
It is antithetical to professional responsibility, however, to regard the risks of home birth, however small in the absolute sense, as ethically acceptable. Every life is important. The nature of a pregnant woman’s relationship to her soon-to-be-born child is primarily one of obligation to protect, not freedom. Hence, she does not have an unconditional, systematic right to control her body to the extent that her rights automatically override fetal rights. She does not have an unmitigated right to put her soon-to-be-born child at risk.
Supporting a woman’s autonomy-based rights at the expense of the rights of the fetal or neonatal patient is a form of "rights-based reductionism." Reductionism as an ethical model has an appealing simplicity, but it is ethically incomplete and unprofessional.
As professionals, obstetricians have the obligation, as a matter of professional integrity, to protect the pregnant, fetal, and neonatal patients. Under the ethical model that we call the "professional responsibility model of obstetric ethics," beneficence-based obligations must always be balanced against autonomy-based obligations to the pregnant patient. The obstetrician’s role is to identify and present medically reasonable alternatives for the management of pregnancy – that is, management for which there is evidence of a net clinical benefit. The patient has the right to select from among the medically reasonable alternatives.
Women’s questions about planned home birth should be respectfully addressed in an evidence-based manner. As obstetricians, we must inform women of the high transport rate and of the preventable risks of home birth to herself and the child. Women also should be made aware that emergency transport can be psychologically disruptive, even traumatizing. The risk of long-term harm was documented in a Dutch study in which 17% of all transported women reported having psychological difficulties up to 3 years after giving birth (Birth 2008:35;107-16).
Interestingly, the planned home-birth rate in the Netherlands has decreased from 38% to 23% in the last 20 years, largely because of an increased awareness of the media, patients, and physicians about the risks. This decline has occurred in spite of the fact that women have to pay additional fees for "nonindicated" hospital births.
Our professional response to women’s interest in planned home birth should be compassionate and understanding, taking into consideration some of the legitimate arguments supporting this method of delivery: The desire for empathetic caregivers and the comfort of home, greater control and undisrupted labor, and fewer interventions.
We must work to ensure that delivery in the hospital is safe, respectful and compassionate, as home-like as possible, and free of unnecessary operative deliveries, episiotomies, and other interventions. We need to scrutinize organizational policies and practices, and encourage and further develop collaborative models with nurse midwives, either within the hospital or at home-birth centers with access to full back-up. Simply put, we have to make hospital birth a more humane experience – without jeopardizing outcomes.
We have a clear professional obligation to provide excellent, nonjudgmental emergency care to women who are transported from planned home birth to the hospital. On the other hand, when a woman remains committed to planned home birth despite our communication, we must just say no to our participation, with the explanation that it is ethically unprofessional to participate in substandard care.
Dr. Chervenak is the Given Foundation Professor and chairman of the department of obstetrics and gynecology at Cornell University in New York. Dr. Chervenak reported that he has no disclosures relevant to this Master Class.
In the last decade, there has been new and renewed support for planned home birth in the United States and in Europe. From 2004 to 2009, home births in the United States rose by 29%, increasing from 0.56% to 0.72% of all births, according to the Centers for Disease Control and Prevention. For non-Hispanic white women, planned home births rose by 36% from a rate of 0.80% in 2004 to 1.09% in 2009.
Although planned home birth for women with a prior cesarean delivery is still rare, there is CDC evidence that VBAC at home is increasing in the United States as well (Obstet. Gynecol. 2012;119:737-44).
Although these increases may be considered small, the changes are part of a congruence of events in the United States and other developed countries that demand our attention and professional response. One such event is a 2010 ruling by the European Court of Human Rights that states that the decision to become a parent includes the right of "choosing the circumstances of becoming a parent." This right includes the right to professional assistance at home birth, according to the ruling.
The full ramifications of this court decision, which originated in Hungary when a pregnant woman alleged that she was not able to give birth at home because health professionals were dissuaded by law from assisting her, remain to be seen. However, recent statements from professional associations favor the woman’s right to choose planned home birth.
The Royal College of Obstetricians and Gynecologists (RCOG) and the Royal College of Midwives (RCM) issued a statement in 2007 in support of planned home birth for women with uncomplicated pregnancies, saying there is "no reason why home birth should not be offered to women at low risk of complications." Home birth in such cases may confer "considerable benefits" for the mother and her family, increasing the likelihood of a birth that is "both satisfying and safe," the statement says.
In addition, the American College of Obstetricians and Gynecologists said in a 2011 committee opinion (#476) that while it believes hospitals and birthing centers are the safest setting for birth, "it respects the right of a woman to make a medically informed decision about delivery." In doing so, ACOG qualified its previous statement, which recommended against home birth (Obstet. Gynecol. 2011;117:425-8).
In the meantime, articles in the consumer press have focused on the benefits of planned home birth, indicating that home birth has become fashionable and that the midwife is increasingly regarded as a status symbol.
Planned home birth has been debated for decades, but this recent recrudescence of support-motivated ethicist Laurence B. McCullough, myself, and a team of physicians – a U.S. neonatologist and a pediatric neurologist and perinatologist from Europe – to review the change in the context of professional responsibility (Am. J. Obstet. Gynecol. 2013:208;31-8).
Advocates for planned home birth emphasize patient satisfaction, patient safety, cost effectiveness, and respect for women’s rights. Yet, as we have described in detail, none of these reasons or causes of support for home birth can or should stand unchallenged. Most importantly, planned home birth does not meet current obstetric standards for patient safety. One of the largest and most current studies, for instance, shows a two- to threefold increased risk of neonatal death with planned home birth, compared with hospital birth.
Some advocates of planned home birth accept this finding as well as other studies showing adverse outcomes and maintain that the level of risk is ethically acceptable. However, we feel that such views are antithetical to our professional responsibility. As obstetricians, our professional responsibility is to both the pregnant woman and the fetal patient. An overwhelming emphasis on maternal rights over fetal rights – a form of rights-based reductionism – is ethically incomplete, clinically inadequate, and therefore unprofessional.
Safety issues
A systematic review published in 2010 identified a doubling of the overall rate of neonatal mortality, and a tripling of the neonatal mortality rate among nonanomalous neonates, in planned home birth vs. planned hospital birth. Dr. Joseph R. Wax and his associates called these findings "especially striking" because women planning home births were "of similar and often lower obstetric risk than those planning hospital births."
The meta-analysis, which included 12 studies from the United States, Canada, Europe, and Australia, showed that women who chose home birth are "in large part successful in achieving their goal of delivering with less morbidity and medical intervention than experienced during hospital-based childbirth," but at a significant cost, the authors said (Obstet. Gynecol. 2010:203;243.e1-8).
A population-based study from South Australia on all births and perinatal deaths between 1991 and 2006 – one of the studies included in the review – reported that the overall perinatal mortality rate of nonhospital deliveries was similar to that for planned hospital births. However, there was a 7-fold higher risk of intrapartum death and a 27-fold higher risk of death from intrapartum asphyxia (Med. J. Austr. 2010:192;76-80).
A key complicating factor in planned home birth is the frequent need for transport to the hospital. Maternal and fetal reasons for transport during labor include failure for labor to progress, unbearable labor pain, fetal malpresentation, abrupt deterioration of fetal heart rate, uterine rupture, acute bleeding, placental abruption, acute sepsis, and cord prolapse.
Neonatal reasons for transport include signs of respiratory distress, unexpected very low or very high birth weight, and acute sepsis. Indeed, in the 2010 meta-analysis, respiratory distress and failed resuscitation contributed disproportionately to neonatal deaths among planned home births.
The 2010 review concluded that more data are necessary before drawing any conclusions regarding maternal mortality in planned home vs. planned hospital delivery. Although rare, preventable maternal death may nevertheless sometimes occur. Just recently, an Australian midwife and home-birth advocate died from postpartum hemorrhage after attempting to deliver her second child at home.
These complications and high-risk conditions are often impossible to predict, even with the best possible prenatal screenings, risk assessments, and fetal surveillance during labor. Women need immediate access to in-hospital care and emergency cesarean delivery.
Even studies that generally support home birth have reported high rates of transport. For example, the recent Birthplace in England prospective cohort study reported transport rates from nonobstetric units to the hospital of 36%-45% for nulliparous women and 9%-13% for multiparous women (BMJ 2011:343;d7400).
Adverse outcomes were similarly much higher in this study in women having their first baby at home. For women "without any complicating factor at the start of care in labour," the adjusted odds ratio of a primary outcome event for births planned at home, compared with planned obstetric unit births, was 1.59. The primary outcome in this study was defined as a composite measure of perinatal mortality and intrapartum-related neonatal morbidities (which include early neonatal death, neonatal encephalopathy, meconium aspiration syndrome, and brachial plexus injury).
This adjusted odds ratio of an adverse event increased to 1.75 in a subgroup analysis of nulliparous women, and to 2.8 when the sample was restricted to nulliparous women with no complications at the start of labor. Although the authors did not elucidate on the issue of transport, the 59%-75% increase in a poor primary outcome may largely be attributed to the delay in access to hospital care from transport time.
In the Netherlands, where there is a long tradition of organized home birth with well-trained midwives, 49% of primiparous and 17% of multiparous women are transported during labor (BJOG 2008:115:570-8). Research done in the Netherlands also shows that women who are transferred to a hospital have a significantly higher rate of operative vaginal and secondary cesarean delivery.
In the United States, women tend to envision that any complications can be easily mitigated by a rapid and seamless transport and transition to the hospital, but in reality, even the best of transport systems experience unavoidable delays that can result in increased mortality and morbidity.
The standard of care in the United States is that "decision to incision" should take no more than 30 minutes, and ACOG has said in a recent practice bulletin that once a decision for operative delivery has been made in the context of a Category III EFM tracing, it should be accomplished as expeditiously as possible. The standards outside the United States are much the same, if not stricter. In Germany, for instance, 20 minutes is the standard used in the assessment of perinatal centers.
None of these standards of care can be consistently met when pregnant patients have started the labor process at home and then are transported to obstetric units, and the inherent problems with transport are largely irremediable even with significant investments of capital. Moreover, even if rates of emergency transport were low, there still should be considerable concern given the severity and frequency of the reasons for transfer.
Ethics, our response
The RCOG-RCM statement emphasizes the psychosocial importance of planned home birth and says that the focus should not be exclusively on the physical safety of planned home birth.
Other supporters of home birth, including some experts in the United States, focus on the absolute risk of planned home birth rather than the relative risk. According to these experts, in the broader context, the numbers of adverse outcomes are so small that it is ethically acceptable to support a patient’s desire for home birth. The ACOG, meanwhile, says that pregnant women should be informed of the risks of planned home birth, as summarized in the 2010 review.
It is antithetical to professional responsibility, however, to regard the risks of home birth, however small in the absolute sense, as ethically acceptable. Every life is important. The nature of a pregnant woman’s relationship to her soon-to-be-born child is primarily one of obligation to protect, not freedom. Hence, she does not have an unconditional, systematic right to control her body to the extent that her rights automatically override fetal rights. She does not have an unmitigated right to put her soon-to-be-born child at risk.
Supporting a woman’s autonomy-based rights at the expense of the rights of the fetal or neonatal patient is a form of "rights-based reductionism." Reductionism as an ethical model has an appealing simplicity, but it is ethically incomplete and unprofessional.
As professionals, obstetricians have the obligation, as a matter of professional integrity, to protect the pregnant, fetal, and neonatal patients. Under the ethical model that we call the "professional responsibility model of obstetric ethics," beneficence-based obligations must always be balanced against autonomy-based obligations to the pregnant patient. The obstetrician’s role is to identify and present medically reasonable alternatives for the management of pregnancy – that is, management for which there is evidence of a net clinical benefit. The patient has the right to select from among the medically reasonable alternatives.
Women’s questions about planned home birth should be respectfully addressed in an evidence-based manner. As obstetricians, we must inform women of the high transport rate and of the preventable risks of home birth to herself and the child. Women also should be made aware that emergency transport can be psychologically disruptive, even traumatizing. The risk of long-term harm was documented in a Dutch study in which 17% of all transported women reported having psychological difficulties up to 3 years after giving birth (Birth 2008:35;107-16).
Interestingly, the planned home-birth rate in the Netherlands has decreased from 38% to 23% in the last 20 years, largely because of an increased awareness of the media, patients, and physicians about the risks. This decline has occurred in spite of the fact that women have to pay additional fees for "nonindicated" hospital births.
Our professional response to women’s interest in planned home birth should be compassionate and understanding, taking into consideration some of the legitimate arguments supporting this method of delivery: The desire for empathetic caregivers and the comfort of home, greater control and undisrupted labor, and fewer interventions.
We must work to ensure that delivery in the hospital is safe, respectful and compassionate, as home-like as possible, and free of unnecessary operative deliveries, episiotomies, and other interventions. We need to scrutinize organizational policies and practices, and encourage and further develop collaborative models with nurse midwives, either within the hospital or at home-birth centers with access to full back-up. Simply put, we have to make hospital birth a more humane experience – without jeopardizing outcomes.
We have a clear professional obligation to provide excellent, nonjudgmental emergency care to women who are transported from planned home birth to the hospital. On the other hand, when a woman remains committed to planned home birth despite our communication, we must just say no to our participation, with the explanation that it is ethically unprofessional to participate in substandard care.
Dr. Chervenak is the Given Foundation Professor and chairman of the department of obstetrics and gynecology at Cornell University in New York. Dr. Chervenak reported that he has no disclosures relevant to this Master Class.
The vaginal approach to hysterectomy
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
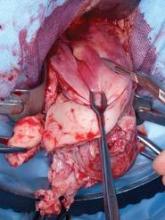
Removing the Large Uterus
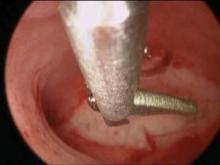
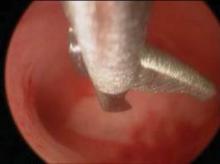
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
Removing the Large Uterus
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
Removing the Large Uterus
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.
Total Laparoscopic Hysterectomy
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.
Assessing Fetal Heart Rate
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
Cervical Length Screening to Reduce Preterm Birth
Preterm birth continues to be a major problem for our patients and for our society. Although the preterm birth rate in the United States fell for a fourth consecutive year in 2010, it remains alarmingly high. The incidence of preterm birth was almost 12% in 2009, according to the Centers for Disease Control and Prevention. It has been estimated, moreover, that two-thirds of preterm births in the United States are spontaneous.
Tocolysis can delay preterm birth by up to 48 hours and improve neonatal outcomes by allowing time for administration of corticosteroids. In general, however, tertiary prevention has been ineffective in decreasing the number of preterm births, and our attention has shifted to primary and secondary prevention. Several risk factors – from body mass index to various infections – have been studied, but their sensitivity or positive predictive value has been disappointing. However, two of these factors – a history of prior preterm birth and short cervical length – have proved to be important independent predictors of preterm delivery.
Term labor is preceded by cervical ripening and effacement by several days. It would make logical sense that preterm cervical effacement would similarly predict preterm delivery. Since the 1990s, research from various countries has shown that cervical effacement, detected as a short cervix by transvaginal ultrasound in the midtrimester, is indeed a powerful predictor of spontaneous preterm birth. Detecting women at high risk for preterm birth without proven effective therapies or interventions, however, would only create anxiety for the mother and frustration for the physician. Routine screening, therefore, traditionally was not offered.
Today, however, we have ample evidence from the past decade of the safety and benefit of vaginal progesterone – and possibly other interventions – in women with singleton gestation and cervical shortening. Multiple studies have demonstrated that vaginal progesterone can significantly cut the rate of spontaneous preterm birth among the approximately 2% of women who have asymptomatic short cervix (most commonly defined as less than or equal to 2 cm) in the midtrimester.
Transvaginal ultrasound, in the meantime, has been widely recognized as a relatively safe, easy-to-perform, sensitive, and reproducible method for detecting shortened cervical length. It can easily be performed at 18-22 weeks’ gestation in conjunction with the fetal anatomic survey.
We currently have the knowledge and tools, therefore, to further reduce the incidence of preterm birth. By screening for short cervix, we can offer hope to more women that preterm birth can be averted. We also can improve the rates of neonatal and infant morbidity and mortality as well as longer-term prematurity-related medical problems and societal issues. It is time to recommend routine cervical length screening of women with singleton gestation at midtrimester, and to offer prophylactic treatment to those with short cervical length.
Efficacy of Progesterone
The first meaningful advance with respect to progesterone and prevention of preterm birth came in 2003 with the da Fonseca study using daily vaginal progesterone (Am. J. Obstet. Gynecol. 2003;188:419-24) and the Meis study using weekly injections of 17-alpha-hydroxyprogesterone (17P). Both double-blind, placebo-controlled studies looked at prevention of recurrent preterm delivery in women with a singleton pregnancy who had previously delivered prematurely, and both showed efficacy of progesterone. Dr. Meis and colleagues, for instance, reported that progesterone treatment reduced the risk of delivery at less than 37 weeks’ gestation by approximately one-third (N. Engl. J. Med. 2003;348:2379-85).
The value of careful history taking and prophylactic intervention for women with a prior preterm birth was recognized and approved by medical organizations and widely implemented in practice.
Subsequently, several other studies demonstrated a benefit of vaginal progesterone, which is less painful than 17P, in women with a short cervix. The Fetal Medicine Foundation Second Trimester Screening Group from London, for instance, reported a 44% lower rate of spontaneous delivery before 34 weeks’ gestation in women who were randomized to receive daily vaginal progesterone than in those randomized to receive a placebo (19.2% vs. 34.4%). The 250 asymptomatic women who participated had a cervical length of 15 mm or less at 20-25 weeks’ gestation; they represented 1.7% of 24,620 women who were screened during routine prenatal care, but accounted for approximately 26% of all spontaneous preterm births (N. Engl. J. Med. 2007;357:462-9).
An even larger National Institutes of Health–collaborated, randomized, double-blind trial conducted at 44 centers in 10 countries documented a 45% reduction in the rate of preterm birth before 33 weeks’ gestation in women who received daily progesterone gel compared with placebo. The study enrolled 458 asymptomatic women with cervical length of 10-20 mm at 19-23 weeks’ gestation.
Vaginal progesterone was also associated in this trial with a 38% reduction in spontaneous preterm birth before 35 weeks’ gestation, and a 50% reduction before 28 weeks’ gestation. In addition, there was a significantly lower incidence of respiratory distress syndrome (RDS) and a lower rate of any neonatal morbidity or mortality in the vaginal progesterone group (Ultrasound Obstet. Gynecol. 2011;38:18-31).
The publication this year of an individual patient data meta-analysis of randomized controlled trials in asymptomatic women with a midtrimester cervical length of 25 mm or less provides added evidence of progesterone’s benefit (Am. J. Obstet. Gynecol. 2012;206:124.e1-19).
The meta-analysis by Dr. Roberto Romero and colleagues covered 775 women (723 singleton pregnancies) with a sonographic short cervix of 25 mm or less in the midtrimester. Overall, treatment with vaginal progesterone was associated with a 42% reduction in the rate of preterm birth at less than 33 weeks’ gestation. Women with singleton gestation and no prior preterm birth had a 40% reduction, and women with a singleton gestation and at least one prior preterm birth had a 46% reduction.
Treatment with vaginal progesterone also was associated in singletons with significant reductions in the rate of preterm birth at less than 35 weeks’ and less than 28 weeks’ gestation, the incidence of RDS, composite neonatal morbidity and mortality, birth weight, and NICU admission.
A beneficial role of pessary use in women with midtrimester short cervical length is also emerging, with benefits seen in the PECEP trial (Lancet 2012;379:1800-6).
Ready for Screening
In the past 3 years, at least several articles advocating universal cervical length screening and vaginal progesterone – and at least two cost-effectiveness analyses supporting such calls – have been published. One, by a team at Yale University, for instance, calculated significant cost-savings and numbers of neonatal deaths and long-term neurologic deficits that would be prevented with universal screening in low-risk pregnancies (Ultrasound Obstet. Gynecol. 2011;38:32-7).
Unfortunately, however, the use of transvaginal ultrasound cervical length screening in singleton gestations without a history of spontaneous preterm birth continues to be a controversial issue.
In a committee opinion on "Incidentally Detected Short Cervical Length" issued in April this year, the American College of Obstetricians and Gynecologists (ACOG) recommended that a cervical length measurement be performed at the time the ultrasound examination is undertaken for fetal anatomic survey at around 18-22 weeks’ gestation, and said "cervical length measured by transvaginal ultrasound examination is a useful screening test for predicting spontaneous preterm birth."
Interestingly, the ACOG opinion was withdrawn shortly after publication and the college issued a Practice Bulletin in October 2012 on "Prediction and Prevention of Preterm Birth." The bulletin reaffirms that "cervical length screening by transvaginal ultrasonography is safe, highly reproducible, and more predictive than transabdominal ultrasound screening" and digital examination.
Despite such conclusions, however, ACOG fell short of mandating routine transvaginal cervical length screening in women without prior preterm birth. The bulletin states instead that "this screening strategy may be considered" and that "practitioners who decide to implement universal cervical length screening should follow one of the protocols for transvaginal measurement of cervical length from the clinical trials" (Obstet. Gynecol. 2012;120:964-73).
There are no recommendations regarding specific management options or strategies for short cervix detected by transabdominal ultrasound or other methods, possibly because published studies have involved diagnoses confirmed by transvaginal ultrasound. It seems fair, therefore, to assume that if a short cervix is detected by any other method, transvaginal ultrasound will need to be performed for accuracy and to determine the prognosis and management plan.
The Society for Maternal-Fetal Medicine also recognized in a recently published guideline that universal cervical screening in singleton pregnancies without prior preterm birth is a "reasonable" option – one that can be considered by individual practitioners but "cannot be mandated" (Am. J. Obstet. Gynecol. 2012;206:376-86).
The SMFM guideline takes a more decisive stance with respect to singletons with prior preterm birth, recommending transvaginal ultrasound cervical length measurements every 2 weeks and providing management decisions for those who choose to offer universal screening in all singleton gestations. All told, the language suggests to me that SMFM is encouraging universal screening without mandating it.
Critics of universal screening have cited a concern that facilities for transvaginal ultrasound screening are not widely enough available. Others have spoken about an uncertainty that screening outcomes in actual practice will compare favorably with the outcomes of controlled trials. There also is concern about the potential misuse or overuse of technology, and concern that many women will undergo treatment unnecessarily.
I understand some of these concerns, but I do not believe they are justifiable reasons for not requiring the use of a cost-effective, beneficial screening test. Such concerns are inherent to the introduction of any new test or therapy. In the past, we have successfully implemented many advances and breakthrough findings that have involved similar concerns. Even if the degree of benefit is debated, the risk-benefit ratio balance still tips toward routine screening and the use of vaginal progesterone in women with shortened cervical length – especially because there are no significant side effects. Moreover, if the SMFM can so strongly recommend frequent transvaginal ultrasound measurements for women with prior preterm birth, then the concern regarding availability of technology may not be as significant or insurmountable as some have suggested. The infrastructure and equipment for transvaginal ultrasound already exists in most centers that perform fetal ultrasound.
Certainly, transvaginal ultrasound is preferable over transabdominal ultrasound. Although transabdominal screening would be better than no screening at all, it has been shown to have higher failure rates and lower sensitivity than the transvaginal approach.
Transvaginal ultrasound by far has been the main screening tool in studies on cervical length and the risk of preterm birth as well as studies looking at the impact of intervention. In the one major study that I could identify employing transabdominal ultrasound as the primary screening tool, the percentage of women identified with a cervical length of 15 mm or less was 0.6%, compared with 1.6%-1.7% in studies using transvaginal ultrasound as the front-line screening tool. The transvaginal approach also has been shown in numerous studies to be well accepted by patients.
Some physicians have advocated the use of CerviLenz, an instrument that measures the cervico-portio length without the use of ultrasound. In the one prospective trial of CerviLenz, the instrument diagnosed cervical length less than 3 cm with a sensitivity of 88% and a specificity of 92% (J. Reprod. Med. 2007;52:385-389). Transvaginal ultrasound was needed to confirm the findings. While CerviLenz could possibly be used in areas where transvaginal ultrasound is not available, the gold standard is transvaginal ultrasound. By screening with anything less than the gold standard, we would be providing suboptimal care.
In general, most agree that some form of cervical length screening in the midtrimester is required. The major point of debate, it seems, is whether it should be transabdominal or transvaginal ultrasound. The fact is that transabdominal scan identifies less than 50% of women with short cervix, and a majority of these women have severe cervical shortening (less than 10 mm) – a scenario where vaginal progesterone is minimally effective. Using this method will have little impact on the rate of preterm deliveries.
For optimal care of pregnant women, we have instituted universal transvaginal cervical length screening of all women with singleton gestation at the time of their anatomy scan. If cervical length is 5-20 mm, vaginal progesterone is offered; if the woman had a prior preterm delivery, the choice of cervical cerclage is also offered. If the cervical length is 21-25 mm, transvaginal ultrasound is repeated in 1-2 weeks.
This column, Master Class, appears regularly in Ob.Gyn News. Dr. Khandelwal is professor of obstetrics and gynecology at Cooper Medical School of Rowan University, Camden, N.J. She was an investigator in the NIH-collaborated study on vaginal progesterone that is mentioned in this Master Class (Ultrasound Obstet. Gynecol. 2011:38:18-31), and served as a paid consultant to Watson Pharmaceuticals Inc. in its evaluation of and FDA submission in 2011 for the Prochieve vaginal progesterone gel.
Preterm birth continues to be a major problem for our patients and for our society. Although the preterm birth rate in the United States fell for a fourth consecutive year in 2010, it remains alarmingly high. The incidence of preterm birth was almost 12% in 2009, according to the Centers for Disease Control and Prevention. It has been estimated, moreover, that two-thirds of preterm births in the United States are spontaneous.
Tocolysis can delay preterm birth by up to 48 hours and improve neonatal outcomes by allowing time for administration of corticosteroids. In general, however, tertiary prevention has been ineffective in decreasing the number of preterm births, and our attention has shifted to primary and secondary prevention. Several risk factors – from body mass index to various infections – have been studied, but their sensitivity or positive predictive value has been disappointing. However, two of these factors – a history of prior preterm birth and short cervical length – have proved to be important independent predictors of preterm delivery.
Term labor is preceded by cervical ripening and effacement by several days. It would make logical sense that preterm cervical effacement would similarly predict preterm delivery. Since the 1990s, research from various countries has shown that cervical effacement, detected as a short cervix by transvaginal ultrasound in the midtrimester, is indeed a powerful predictor of spontaneous preterm birth. Detecting women at high risk for preterm birth without proven effective therapies or interventions, however, would only create anxiety for the mother and frustration for the physician. Routine screening, therefore, traditionally was not offered.
Today, however, we have ample evidence from the past decade of the safety and benefit of vaginal progesterone – and possibly other interventions – in women with singleton gestation and cervical shortening. Multiple studies have demonstrated that vaginal progesterone can significantly cut the rate of spontaneous preterm birth among the approximately 2% of women who have asymptomatic short cervix (most commonly defined as less than or equal to 2 cm) in the midtrimester.
Transvaginal ultrasound, in the meantime, has been widely recognized as a relatively safe, easy-to-perform, sensitive, and reproducible method for detecting shortened cervical length. It can easily be performed at 18-22 weeks’ gestation in conjunction with the fetal anatomic survey.
We currently have the knowledge and tools, therefore, to further reduce the incidence of preterm birth. By screening for short cervix, we can offer hope to more women that preterm birth can be averted. We also can improve the rates of neonatal and infant morbidity and mortality as well as longer-term prematurity-related medical problems and societal issues. It is time to recommend routine cervical length screening of women with singleton gestation at midtrimester, and to offer prophylactic treatment to those with short cervical length.
Efficacy of Progesterone
The first meaningful advance with respect to progesterone and prevention of preterm birth came in 2003 with the da Fonseca study using daily vaginal progesterone (Am. J. Obstet. Gynecol. 2003;188:419-24) and the Meis study using weekly injections of 17-alpha-hydroxyprogesterone (17P). Both double-blind, placebo-controlled studies looked at prevention of recurrent preterm delivery in women with a singleton pregnancy who had previously delivered prematurely, and both showed efficacy of progesterone. Dr. Meis and colleagues, for instance, reported that progesterone treatment reduced the risk of delivery at less than 37 weeks’ gestation by approximately one-third (N. Engl. J. Med. 2003;348:2379-85).
The value of careful history taking and prophylactic intervention for women with a prior preterm birth was recognized and approved by medical organizations and widely implemented in practice.
Subsequently, several other studies demonstrated a benefit of vaginal progesterone, which is less painful than 17P, in women with a short cervix. The Fetal Medicine Foundation Second Trimester Screening Group from London, for instance, reported a 44% lower rate of spontaneous delivery before 34 weeks’ gestation in women who were randomized to receive daily vaginal progesterone than in those randomized to receive a placebo (19.2% vs. 34.4%). The 250 asymptomatic women who participated had a cervical length of 15 mm or less at 20-25 weeks’ gestation; they represented 1.7% of 24,620 women who were screened during routine prenatal care, but accounted for approximately 26% of all spontaneous preterm births (N. Engl. J. Med. 2007;357:462-9).
An even larger National Institutes of Health–collaborated, randomized, double-blind trial conducted at 44 centers in 10 countries documented a 45% reduction in the rate of preterm birth before 33 weeks’ gestation in women who received daily progesterone gel compared with placebo. The study enrolled 458 asymptomatic women with cervical length of 10-20 mm at 19-23 weeks’ gestation.
Vaginal progesterone was also associated in this trial with a 38% reduction in spontaneous preterm birth before 35 weeks’ gestation, and a 50% reduction before 28 weeks’ gestation. In addition, there was a significantly lower incidence of respiratory distress syndrome (RDS) and a lower rate of any neonatal morbidity or mortality in the vaginal progesterone group (Ultrasound Obstet. Gynecol. 2011;38:18-31).
The publication this year of an individual patient data meta-analysis of randomized controlled trials in asymptomatic women with a midtrimester cervical length of 25 mm or less provides added evidence of progesterone’s benefit (Am. J. Obstet. Gynecol. 2012;206:124.e1-19).
The meta-analysis by Dr. Roberto Romero and colleagues covered 775 women (723 singleton pregnancies) with a sonographic short cervix of 25 mm or less in the midtrimester. Overall, treatment with vaginal progesterone was associated with a 42% reduction in the rate of preterm birth at less than 33 weeks’ gestation. Women with singleton gestation and no prior preterm birth had a 40% reduction, and women with a singleton gestation and at least one prior preterm birth had a 46% reduction.
Treatment with vaginal progesterone also was associated in singletons with significant reductions in the rate of preterm birth at less than 35 weeks’ and less than 28 weeks’ gestation, the incidence of RDS, composite neonatal morbidity and mortality, birth weight, and NICU admission.
A beneficial role of pessary use in women with midtrimester short cervical length is also emerging, with benefits seen in the PECEP trial (Lancet 2012;379:1800-6).
Ready for Screening
In the past 3 years, at least several articles advocating universal cervical length screening and vaginal progesterone – and at least two cost-effectiveness analyses supporting such calls – have been published. One, by a team at Yale University, for instance, calculated significant cost-savings and numbers of neonatal deaths and long-term neurologic deficits that would be prevented with universal screening in low-risk pregnancies (Ultrasound Obstet. Gynecol. 2011;38:32-7).
Unfortunately, however, the use of transvaginal ultrasound cervical length screening in singleton gestations without a history of spontaneous preterm birth continues to be a controversial issue.
In a committee opinion on "Incidentally Detected Short Cervical Length" issued in April this year, the American College of Obstetricians and Gynecologists (ACOG) recommended that a cervical length measurement be performed at the time the ultrasound examination is undertaken for fetal anatomic survey at around 18-22 weeks’ gestation, and said "cervical length measured by transvaginal ultrasound examination is a useful screening test for predicting spontaneous preterm birth."
Interestingly, the ACOG opinion was withdrawn shortly after publication and the college issued a Practice Bulletin in October 2012 on "Prediction and Prevention of Preterm Birth." The bulletin reaffirms that "cervical length screening by transvaginal ultrasonography is safe, highly reproducible, and more predictive than transabdominal ultrasound screening" and digital examination.
Despite such conclusions, however, ACOG fell short of mandating routine transvaginal cervical length screening in women without prior preterm birth. The bulletin states instead that "this screening strategy may be considered" and that "practitioners who decide to implement universal cervical length screening should follow one of the protocols for transvaginal measurement of cervical length from the clinical trials" (Obstet. Gynecol. 2012;120:964-73).
There are no recommendations regarding specific management options or strategies for short cervix detected by transabdominal ultrasound or other methods, possibly because published studies have involved diagnoses confirmed by transvaginal ultrasound. It seems fair, therefore, to assume that if a short cervix is detected by any other method, transvaginal ultrasound will need to be performed for accuracy and to determine the prognosis and management plan.
The Society for Maternal-Fetal Medicine also recognized in a recently published guideline that universal cervical screening in singleton pregnancies without prior preterm birth is a "reasonable" option – one that can be considered by individual practitioners but "cannot be mandated" (Am. J. Obstet. Gynecol. 2012;206:376-86).
The SMFM guideline takes a more decisive stance with respect to singletons with prior preterm birth, recommending transvaginal ultrasound cervical length measurements every 2 weeks and providing management decisions for those who choose to offer universal screening in all singleton gestations. All told, the language suggests to me that SMFM is encouraging universal screening without mandating it.
Critics of universal screening have cited a concern that facilities for transvaginal ultrasound screening are not widely enough available. Others have spoken about an uncertainty that screening outcomes in actual practice will compare favorably with the outcomes of controlled trials. There also is concern about the potential misuse or overuse of technology, and concern that many women will undergo treatment unnecessarily.
I understand some of these concerns, but I do not believe they are justifiable reasons for not requiring the use of a cost-effective, beneficial screening test. Such concerns are inherent to the introduction of any new test or therapy. In the past, we have successfully implemented many advances and breakthrough findings that have involved similar concerns. Even if the degree of benefit is debated, the risk-benefit ratio balance still tips toward routine screening and the use of vaginal progesterone in women with shortened cervical length – especially because there are no significant side effects. Moreover, if the SMFM can so strongly recommend frequent transvaginal ultrasound measurements for women with prior preterm birth, then the concern regarding availability of technology may not be as significant or insurmountable as some have suggested. The infrastructure and equipment for transvaginal ultrasound already exists in most centers that perform fetal ultrasound.
Certainly, transvaginal ultrasound is preferable over transabdominal ultrasound. Although transabdominal screening would be better than no screening at all, it has been shown to have higher failure rates and lower sensitivity than the transvaginal approach.
Transvaginal ultrasound by far has been the main screening tool in studies on cervical length and the risk of preterm birth as well as studies looking at the impact of intervention. In the one major study that I could identify employing transabdominal ultrasound as the primary screening tool, the percentage of women identified with a cervical length of 15 mm or less was 0.6%, compared with 1.6%-1.7% in studies using transvaginal ultrasound as the front-line screening tool. The transvaginal approach also has been shown in numerous studies to be well accepted by patients.
Some physicians have advocated the use of CerviLenz, an instrument that measures the cervico-portio length without the use of ultrasound. In the one prospective trial of CerviLenz, the instrument diagnosed cervical length less than 3 cm with a sensitivity of 88% and a specificity of 92% (J. Reprod. Med. 2007;52:385-389). Transvaginal ultrasound was needed to confirm the findings. While CerviLenz could possibly be used in areas where transvaginal ultrasound is not available, the gold standard is transvaginal ultrasound. By screening with anything less than the gold standard, we would be providing suboptimal care.
In general, most agree that some form of cervical length screening in the midtrimester is required. The major point of debate, it seems, is whether it should be transabdominal or transvaginal ultrasound. The fact is that transabdominal scan identifies less than 50% of women with short cervix, and a majority of these women have severe cervical shortening (less than 10 mm) – a scenario where vaginal progesterone is minimally effective. Using this method will have little impact on the rate of preterm deliveries.
For optimal care of pregnant women, we have instituted universal transvaginal cervical length screening of all women with singleton gestation at the time of their anatomy scan. If cervical length is 5-20 mm, vaginal progesterone is offered; if the woman had a prior preterm delivery, the choice of cervical cerclage is also offered. If the cervical length is 21-25 mm, transvaginal ultrasound is repeated in 1-2 weeks.
This column, Master Class, appears regularly in Ob.Gyn News. Dr. Khandelwal is professor of obstetrics and gynecology at Cooper Medical School of Rowan University, Camden, N.J. She was an investigator in the NIH-collaborated study on vaginal progesterone that is mentioned in this Master Class (Ultrasound Obstet. Gynecol. 2011:38:18-31), and served as a paid consultant to Watson Pharmaceuticals Inc. in its evaluation of and FDA submission in 2011 for the Prochieve vaginal progesterone gel.
Preterm birth continues to be a major problem for our patients and for our society. Although the preterm birth rate in the United States fell for a fourth consecutive year in 2010, it remains alarmingly high. The incidence of preterm birth was almost 12% in 2009, according to the Centers for Disease Control and Prevention. It has been estimated, moreover, that two-thirds of preterm births in the United States are spontaneous.
Tocolysis can delay preterm birth by up to 48 hours and improve neonatal outcomes by allowing time for administration of corticosteroids. In general, however, tertiary prevention has been ineffective in decreasing the number of preterm births, and our attention has shifted to primary and secondary prevention. Several risk factors – from body mass index to various infections – have been studied, but their sensitivity or positive predictive value has been disappointing. However, two of these factors – a history of prior preterm birth and short cervical length – have proved to be important independent predictors of preterm delivery.
Term labor is preceded by cervical ripening and effacement by several days. It would make logical sense that preterm cervical effacement would similarly predict preterm delivery. Since the 1990s, research from various countries has shown that cervical effacement, detected as a short cervix by transvaginal ultrasound in the midtrimester, is indeed a powerful predictor of spontaneous preterm birth. Detecting women at high risk for preterm birth without proven effective therapies or interventions, however, would only create anxiety for the mother and frustration for the physician. Routine screening, therefore, traditionally was not offered.
Today, however, we have ample evidence from the past decade of the safety and benefit of vaginal progesterone – and possibly other interventions – in women with singleton gestation and cervical shortening. Multiple studies have demonstrated that vaginal progesterone can significantly cut the rate of spontaneous preterm birth among the approximately 2% of women who have asymptomatic short cervix (most commonly defined as less than or equal to 2 cm) in the midtrimester.
Transvaginal ultrasound, in the meantime, has been widely recognized as a relatively safe, easy-to-perform, sensitive, and reproducible method for detecting shortened cervical length. It can easily be performed at 18-22 weeks’ gestation in conjunction with the fetal anatomic survey.
We currently have the knowledge and tools, therefore, to further reduce the incidence of preterm birth. By screening for short cervix, we can offer hope to more women that preterm birth can be averted. We also can improve the rates of neonatal and infant morbidity and mortality as well as longer-term prematurity-related medical problems and societal issues. It is time to recommend routine cervical length screening of women with singleton gestation at midtrimester, and to offer prophylactic treatment to those with short cervical length.
Efficacy of Progesterone
The first meaningful advance with respect to progesterone and prevention of preterm birth came in 2003 with the da Fonseca study using daily vaginal progesterone (Am. J. Obstet. Gynecol. 2003;188:419-24) and the Meis study using weekly injections of 17-alpha-hydroxyprogesterone (17P). Both double-blind, placebo-controlled studies looked at prevention of recurrent preterm delivery in women with a singleton pregnancy who had previously delivered prematurely, and both showed efficacy of progesterone. Dr. Meis and colleagues, for instance, reported that progesterone treatment reduced the risk of delivery at less than 37 weeks’ gestation by approximately one-third (N. Engl. J. Med. 2003;348:2379-85).
The value of careful history taking and prophylactic intervention for women with a prior preterm birth was recognized and approved by medical organizations and widely implemented in practice.
Subsequently, several other studies demonstrated a benefit of vaginal progesterone, which is less painful than 17P, in women with a short cervix. The Fetal Medicine Foundation Second Trimester Screening Group from London, for instance, reported a 44% lower rate of spontaneous delivery before 34 weeks’ gestation in women who were randomized to receive daily vaginal progesterone than in those randomized to receive a placebo (19.2% vs. 34.4%). The 250 asymptomatic women who participated had a cervical length of 15 mm or less at 20-25 weeks’ gestation; they represented 1.7% of 24,620 women who were screened during routine prenatal care, but accounted for approximately 26% of all spontaneous preterm births (N. Engl. J. Med. 2007;357:462-9).
An even larger National Institutes of Health–collaborated, randomized, double-blind trial conducted at 44 centers in 10 countries documented a 45% reduction in the rate of preterm birth before 33 weeks’ gestation in women who received daily progesterone gel compared with placebo. The study enrolled 458 asymptomatic women with cervical length of 10-20 mm at 19-23 weeks’ gestation.
Vaginal progesterone was also associated in this trial with a 38% reduction in spontaneous preterm birth before 35 weeks’ gestation, and a 50% reduction before 28 weeks’ gestation. In addition, there was a significantly lower incidence of respiratory distress syndrome (RDS) and a lower rate of any neonatal morbidity or mortality in the vaginal progesterone group (Ultrasound Obstet. Gynecol. 2011;38:18-31).
The publication this year of an individual patient data meta-analysis of randomized controlled trials in asymptomatic women with a midtrimester cervical length of 25 mm or less provides added evidence of progesterone’s benefit (Am. J. Obstet. Gynecol. 2012;206:124.e1-19).
The meta-analysis by Dr. Roberto Romero and colleagues covered 775 women (723 singleton pregnancies) with a sonographic short cervix of 25 mm or less in the midtrimester. Overall, treatment with vaginal progesterone was associated with a 42% reduction in the rate of preterm birth at less than 33 weeks’ gestation. Women with singleton gestation and no prior preterm birth had a 40% reduction, and women with a singleton gestation and at least one prior preterm birth had a 46% reduction.
Treatment with vaginal progesterone also was associated in singletons with significant reductions in the rate of preterm birth at less than 35 weeks’ and less than 28 weeks’ gestation, the incidence of RDS, composite neonatal morbidity and mortality, birth weight, and NICU admission.
A beneficial role of pessary use in women with midtrimester short cervical length is also emerging, with benefits seen in the PECEP trial (Lancet 2012;379:1800-6).
Ready for Screening
In the past 3 years, at least several articles advocating universal cervical length screening and vaginal progesterone – and at least two cost-effectiveness analyses supporting such calls – have been published. One, by a team at Yale University, for instance, calculated significant cost-savings and numbers of neonatal deaths and long-term neurologic deficits that would be prevented with universal screening in low-risk pregnancies (Ultrasound Obstet. Gynecol. 2011;38:32-7).
Unfortunately, however, the use of transvaginal ultrasound cervical length screening in singleton gestations without a history of spontaneous preterm birth continues to be a controversial issue.
In a committee opinion on "Incidentally Detected Short Cervical Length" issued in April this year, the American College of Obstetricians and Gynecologists (ACOG) recommended that a cervical length measurement be performed at the time the ultrasound examination is undertaken for fetal anatomic survey at around 18-22 weeks’ gestation, and said "cervical length measured by transvaginal ultrasound examination is a useful screening test for predicting spontaneous preterm birth."
Interestingly, the ACOG opinion was withdrawn shortly after publication and the college issued a Practice Bulletin in October 2012 on "Prediction and Prevention of Preterm Birth." The bulletin reaffirms that "cervical length screening by transvaginal ultrasonography is safe, highly reproducible, and more predictive than transabdominal ultrasound screening" and digital examination.
Despite such conclusions, however, ACOG fell short of mandating routine transvaginal cervical length screening in women without prior preterm birth. The bulletin states instead that "this screening strategy may be considered" and that "practitioners who decide to implement universal cervical length screening should follow one of the protocols for transvaginal measurement of cervical length from the clinical trials" (Obstet. Gynecol. 2012;120:964-73).
There are no recommendations regarding specific management options or strategies for short cervix detected by transabdominal ultrasound or other methods, possibly because published studies have involved diagnoses confirmed by transvaginal ultrasound. It seems fair, therefore, to assume that if a short cervix is detected by any other method, transvaginal ultrasound will need to be performed for accuracy and to determine the prognosis and management plan.
The Society for Maternal-Fetal Medicine also recognized in a recently published guideline that universal cervical screening in singleton pregnancies without prior preterm birth is a "reasonable" option – one that can be considered by individual practitioners but "cannot be mandated" (Am. J. Obstet. Gynecol. 2012;206:376-86).
The SMFM guideline takes a more decisive stance with respect to singletons with prior preterm birth, recommending transvaginal ultrasound cervical length measurements every 2 weeks and providing management decisions for those who choose to offer universal screening in all singleton gestations. All told, the language suggests to me that SMFM is encouraging universal screening without mandating it.
Critics of universal screening have cited a concern that facilities for transvaginal ultrasound screening are not widely enough available. Others have spoken about an uncertainty that screening outcomes in actual practice will compare favorably with the outcomes of controlled trials. There also is concern about the potential misuse or overuse of technology, and concern that many women will undergo treatment unnecessarily.
I understand some of these concerns, but I do not believe they are justifiable reasons for not requiring the use of a cost-effective, beneficial screening test. Such concerns are inherent to the introduction of any new test or therapy. In the past, we have successfully implemented many advances and breakthrough findings that have involved similar concerns. Even if the degree of benefit is debated, the risk-benefit ratio balance still tips toward routine screening and the use of vaginal progesterone in women with shortened cervical length – especially because there are no significant side effects. Moreover, if the SMFM can so strongly recommend frequent transvaginal ultrasound measurements for women with prior preterm birth, then the concern regarding availability of technology may not be as significant or insurmountable as some have suggested. The infrastructure and equipment for transvaginal ultrasound already exists in most centers that perform fetal ultrasound.
Certainly, transvaginal ultrasound is preferable over transabdominal ultrasound. Although transabdominal screening would be better than no screening at all, it has been shown to have higher failure rates and lower sensitivity than the transvaginal approach.
Transvaginal ultrasound by far has been the main screening tool in studies on cervical length and the risk of preterm birth as well as studies looking at the impact of intervention. In the one major study that I could identify employing transabdominal ultrasound as the primary screening tool, the percentage of women identified with a cervical length of 15 mm or less was 0.6%, compared with 1.6%-1.7% in studies using transvaginal ultrasound as the front-line screening tool. The transvaginal approach also has been shown in numerous studies to be well accepted by patients.
Some physicians have advocated the use of CerviLenz, an instrument that measures the cervico-portio length without the use of ultrasound. In the one prospective trial of CerviLenz, the instrument diagnosed cervical length less than 3 cm with a sensitivity of 88% and a specificity of 92% (J. Reprod. Med. 2007;52:385-389). Transvaginal ultrasound was needed to confirm the findings. While CerviLenz could possibly be used in areas where transvaginal ultrasound is not available, the gold standard is transvaginal ultrasound. By screening with anything less than the gold standard, we would be providing suboptimal care.
In general, most agree that some form of cervical length screening in the midtrimester is required. The major point of debate, it seems, is whether it should be transabdominal or transvaginal ultrasound. The fact is that transabdominal scan identifies less than 50% of women with short cervix, and a majority of these women have severe cervical shortening (less than 10 mm) – a scenario where vaginal progesterone is minimally effective. Using this method will have little impact on the rate of preterm deliveries.
For optimal care of pregnant women, we have instituted universal transvaginal cervical length screening of all women with singleton gestation at the time of their anatomy scan. If cervical length is 5-20 mm, vaginal progesterone is offered; if the woman had a prior preterm delivery, the choice of cervical cerclage is also offered. If the cervical length is 21-25 mm, transvaginal ultrasound is repeated in 1-2 weeks.
This column, Master Class, appears regularly in Ob.Gyn News. Dr. Khandelwal is professor of obstetrics and gynecology at Cooper Medical School of Rowan University, Camden, N.J. She was an investigator in the NIH-collaborated study on vaginal progesterone that is mentioned in this Master Class (Ultrasound Obstet. Gynecol. 2011:38:18-31), and served as a paid consultant to Watson Pharmaceuticals Inc. in its evaluation of and FDA submission in 2011 for the Prochieve vaginal progesterone gel.
Minilaparoscopy: The Best of Both Worlds
The clinical advantages of laparoscopy over laparotomy have been well established over the last several years. As the acceptance and use of laparoscopic approaches have increased among gynecologic surgeons, attention to cosmesis has also evolved.
Laparoscopy has been appealing for its cosmetic benefits as well as reductions in complications and recovery times. Women not only want to resume their normal activities sooner; they also want to take advantage of the smaller incisions that laparoscopy entails, as these incisions do not alter abdominal wall appearance as significantly as do laparotomy incisions.
Still, depending on an individual’s body mass index and wound healing, scars can be disturbing for patients. Some women who have had more than one laparoscopic surgery requiring different port placements have jokingly spoken about "connecting the dots," but such comments reflect concern about aesthetics and should be taken seriously. Although we have improved surgical outcomes with laparoscopy and reduced the size of the surgical scar, we can still create disfigured abdomens.
Last year, the AAGL Fellowship in Minimally Invasive Gynecologic Surgery at Newton-Wellesley (Mass.) Hospital published an article about a survey of women and their preferred abdominal incision choices, based solely on cosmesis (J. Minim. Invasive Gynecol. 2011;18:640-3). The survey participants were shown three photographs of incisions that were commonly performed at their institution and among several Boston-area gynecologic surgeons, and were asked to rank the incisions in order of preference.
The photograph depicting a single incision or single-site laparoscopy showed a vertical 25-mm incision. The robotic laparoscopy photo showed five incisions: umbilical and right midabdomen (paraumbilical) incisions of 12-mm length, and 8-mm incisions in the right lower quadrant, left lower quadrant, and left midabdomen. The incision configuration in the photo of a conventional four-port laparoscopy consisted of a 10-mm incision in the right lower quadrant and 5-mm incisions in the umbilicus, left lower quadrant, and suprapubic area.
The conventional laparoscopy incisions were most preferred by approximately 56% of the 241 women who completed the survey, whereas preference for a single incision was approximately 41% and for robotic surgery was 2.5%.
Granted, there are variable configurations and incision sizes for each of the laparoscopic techniques, and further strides are being made to make the approaches more cosmetically acceptable. Experienced gynecologic surgeons are developing techniques to minimize the number of ports used robotically, for instance, and smaller incision sizes for robotic laparoscopy are anticipated.
In any case, the overarching lesson from this survey is that aesthetics is of value to many women and should be an important consideration for us as treating physicians.
Miniaturization
The use of mini- or microlaparoscopic instrumentation enables us to address aesthetic concerns and take the issue of cosmesis to the next level, as well as to further minimizing trauma. Although conventional laparoscopy involves instruments of 5-mm diameter or larger, the term "minilaparoscopy" usually refers to the use of instruments greater than 2 mm up to less than 5 mm in diameter, and "microlaparoscopy" involves instrumentation of 2 mm or less in diameter.
In gynecology, minilaparoscopy has been utilized diagnostically since the mid-1990s to perform conscious pain mapping with local anesthesia. With a 2.5 mm–diameter scope and 3 mm–diameter trocars, surgeons have used a blunt probe to map trigger sites with the patient’s assistance and response, and to aid in identifying pelvic disease suspected of causing pain (Int. J. Gynaecol. Obstet. 2008;100:94-8).
The diagnostic accuracy of the mini- or microlaparoscope has been reported in various scenarios (including cases of endometriosis and adhesive disease) to be comparable to that of the 10-mm scope that is used in conventional laparoscopy.
In one small study, investigators compared the diagnostic accuracy of 2-mm and 10-mm laparoscopes, with two physicians independently reporting findings to a third person (Fertil. Steril. 1997;67:952-4). Although they had a small sample size, the researchers observed no significant differences between the two laparoscopes or the two physicians. (Scores for endometriosis and adnexal adhesions, for instance, did not differ in any significant way.) This suggested that the diagnostic accuracy achieved with the microlaparoscope was comparable to that of the standard 10-mm laparoscope.
Another study in which 87 consecutive women underwent microlaparoscopic evaluation for chronic pelvic pain similarly found that microlaparoscopy provides comparable efficacy for diagnosing endometriosis and evaluating for the presence of occlusive salpingitis isthmica nodosa. Evaluation through a 2-mm port affords "an excellent minimally invasive view of the pelvis," the investigator wrote (JSLS 2005;9:431-3).
Most of the literature for minilaparoscopy, however, has evolved from the nongynecologic specialties, particularly general surgery. It has been well documented that minilaparoscopy is safe for splenic and liver biopsies, as well as helpful in reducing port sizes and hernias when utilized in cholecystectomies and colorectal resections.
Most recently, the authors of a literature review spanning the last 30 years concluded that minilaparoscopy has been shown in almost all urologic indications to be feasible and safe, with better cosmetic results and reduced postoperative pain (Arch. Esp. Urol. 2012;65:366-83).
Collectively, these studies provide us with a solid body of evidence on the safety, feasibility, and benefits of minilaparoscopy. The findings noted in the surgical literature for minilaparoscopy include operative times, morbidity, and hospital lengths of stay that are comparable to those of conventional laparoscopy; improved cosmesis; a decrease in postoperative pain; and reductions in port-site complications such as bleeding, infection, and herniation.
Surpassing SIL
Single-incision laparoscopy (also known as "scarless" surgery, with the umbilicus viewed as a natural scar) was developed to improve cosmesis. An incision approximately 25 mm in size at the umbilicus allows a scope and two or three instruments to pass into the abdominal cavity.
A main challenge – and a limitation for many surgeons – is the lack of triangulation because of the parallel entry. Instruments are crossed, which is counterintuitive to conventional laparoscopic training, and hands and instruments can clash, which limits the use of instrumentation.
An array of curved and EndoWrist instruments (with flexible joints at the tips) was developed to help ease the technical difficulties of single-port surgery. Many advanced endoscopic surgeons have performed simple to complex single-incision procedures with low complication rates and with operative times and rates of blood loss that are comparable with those of conventional laparoscopy. One advantage to single-incision surgery is that the incision size allows the removal of larger tissue that has been resected from the abdomen.
A recent study in the Netherlands suggests that single-incision surgery may not be all that more difficult to learn (Surg. Endosc. 2012;26:1231-7). The simulation study looked at the performance/learning curves of 20 medical interns (none with laparoscopic experience) who were randomly assigned to perform single-incision or conventional laparoscopy. Each participant practiced each task 11 times. There were no significant differences between the two groups in terms of error or time in performing the tasks, and participants improved significantly in both laparoscopy settings.
There are still many concerns, however, that single-incision laparoscopy is indeed more technically challenging, and that a sizable learning curve for the single-incision approach –in addition to the learning curve of conventional laparoscopy – does exist. Without surgical volume and patience, this technique may be a struggle for many to adapt and teach.
Moreover, as shown in the study at Newton-Wellesley Hospital, the scars remain a concern for many women. The umbilicus is the easiest place to try to "hide" an incision and scar when the incision is made at its base, but a 2-1/2 cm incision can be difficult to hide in a shallow umbilicus; for some women, the larger single scar becomes a focal point of their abdomen and, thus, disfiguring.
Our Experience
With minilaparoscopy, we can maintain triangulation and employ the fundamental principles of conventional laparoscopy with greater technical ease. The approach also has the potential to reduce the risk of incision-site hernias and the incidence of wound complications.
The following drawbacks to minilaparoscopy have been cited:
• A smaller view of the operative field.
• Less tensile strength of the instruments.
• Electrosurgery instruments that are limited to small vessels/pathology.
• Difficulty with large-tissue extraction.
There are solutions to each of these challenges, many of which involve hybrid approaches that incorporate single-incision or conventional laparoscopic ports at the umbilicus to facilitate electrosurgery, tissue extraction, and visualization while maintaining cosmesis. The solutions include the following:
• Better optics. Significant strides have been made in the area of optics, and newer minilaparoscopes (Figure 1) have better resolution and optical clarity than does the previous generation of miniature scopes. We have found that we can zoom the image and reduce light to improve visualization and maximize the operative field.
• Improved instrument strength. As with miniaturized laparoscopes, a wide array of durable instrumentation (Figures 2 and 3) is now available. We employ instruments that are 2.3-3.3 mm in diameter and up to 36 cm in length, ports that are 3.5 mm in diameter and 10-15 cm in length, and scopes that are 2.9-3.3 mm in diameter and up to 25 cm in length.
For skin incisions, a 14-gauge needle is used instead of a scalpel to avoid an incision that is too large and would allow easy slippage of the port. On the 2.3-mm disposable instruments, the tip itself is a 13-gauge needle that is utilized to incise the skin and enter the abdominal cavity. (See Figure 4.) Wounds are not sutured closed, but are merely reapproximated with Steri-Strips at the end of the procedure.
• More versatile electrosurgery instruments. We utilize reusable and disposable bipolar coagulating forceps, as well as reusable instruments with monopolar electrosurgical capability, including dissecting and grasping forceps, scissors, hook, and spatula. For example, when we excise endometriosis, we will utilize a sharp grasping instrument to elevate the lesion away from vital structures, as well as monopolar scissors to excise the disease. Needle holders and knot pushers are sturdy for suturing, and a suction tip is also available in miniaturized form.
In procedures in which the vessel-sealing capacity of a 3-mm energy source is not sufficient, a 5-mm port can be placed at the umbilicus so that a standard 5-mm energy source can be utilized to improve or maintain hemostasis.
• Extraction of large tissues. For tissue extraction requiring an incision larger than 5 mm, a 10- to 12-mm trocar can be placed at the umbilicus for the possible use of an endoscopic specimen bag. We dilate the umbilicus with Hegar dilators to a size 16 to ease the extraction of tissue, if necessary, or for the placement of a laparoscopic morcellator.
Recently, however, we have been able to perform minilaparoscopic hysterectomies with the use of three 3-mm ports, a minilaparoscope, and other miniaturized instrumentation. Specifically, we utilize the 3-mm PKS MoLly forceps by Olympus Gyrus (Figure 5) to coagulate and monopolar scissors to transect the pedicles. A colpotomy is completed with the scissors, and the specimen is delivered through the colpotomy incision. The colpotomy incision is reapproximated with 0-PDS suture on CT-1–sized needles. The suture is backloaded through the miniport and is introduced into the abdomen without difficulty. The incision is sutured; the needle is parked in the upper abdominal wall, and is then cut free. The suture is then pulled through the port for extracorporeal knot tying. The needle is retrieved and pulled through the incision with the removal of the port, and is now ready for loading another suture.
A minilaparoscopic supracervical hysterectomy can be performed in a similar fashion. For tissue extraction, we perform transcervical morcellation. We have found that the morcellators with extra-long shafts (such as the Storz 12-mm extra-long Rotocut or Gynecare Morcellex) work best for transcervical morcellation. Once uterine amputation is completed, the cervical stump is dilated with cervical dilators to allow for the morcellator tip to advance through the cervix and into the pelvic cavity transvaginally. Under direct visualization and with the assistance of a laparoscopic grasper, the specimen is morcellated. Similarly, an endoscopic specimen bag can also be placed transcervically to retrieve specimens that are not to undergo morcellation.
Minilaparoscopy now has multiple applications in our practice. Diagnostically, we employ the use of minilaparoscopic instrumentation in conjunction with hysteroscopic metroplasty, adhesiolysis, or tubal cannulation. Operatively, we have utilized minilaparoscopic instrumentation for excision of endometriosis, ovarian cystectomy, adhesiolysis, enterolysis, tuboplasty, and supracervical and total hysterectomy with or without bilateral salpingo-oophorectomy.
Dr. Cholkeri-Singh is associate director of minimally invasive gynecologic surgery, director of gynecologic surgical education, and codirector of the AAGL/SRS (Society of Reproductive Surgeons) fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. In addition, she is a board member of the AAGL/SRS fellowship in minimally invasive gynecology and is chair of a postgraduate course at this year’s AAGL Global Congress on Minimally Invasive Gynecology. Dr. Cholkeri-Singh disclosed that she is a speaker for Conceptus Inc., Olympus Gyrus ACMI, and Ethicon Endo Surgery.
The clinical advantages of laparoscopy over laparotomy have been well established over the last several years. As the acceptance and use of laparoscopic approaches have increased among gynecologic surgeons, attention to cosmesis has also evolved.
Laparoscopy has been appealing for its cosmetic benefits as well as reductions in complications and recovery times. Women not only want to resume their normal activities sooner; they also want to take advantage of the smaller incisions that laparoscopy entails, as these incisions do not alter abdominal wall appearance as significantly as do laparotomy incisions.
Still, depending on an individual’s body mass index and wound healing, scars can be disturbing for patients. Some women who have had more than one laparoscopic surgery requiring different port placements have jokingly spoken about "connecting the dots," but such comments reflect concern about aesthetics and should be taken seriously. Although we have improved surgical outcomes with laparoscopy and reduced the size of the surgical scar, we can still create disfigured abdomens.
Last year, the AAGL Fellowship in Minimally Invasive Gynecologic Surgery at Newton-Wellesley (Mass.) Hospital published an article about a survey of women and their preferred abdominal incision choices, based solely on cosmesis (J. Minim. Invasive Gynecol. 2011;18:640-3). The survey participants were shown three photographs of incisions that were commonly performed at their institution and among several Boston-area gynecologic surgeons, and were asked to rank the incisions in order of preference.
The photograph depicting a single incision or single-site laparoscopy showed a vertical 25-mm incision. The robotic laparoscopy photo showed five incisions: umbilical and right midabdomen (paraumbilical) incisions of 12-mm length, and 8-mm incisions in the right lower quadrant, left lower quadrant, and left midabdomen. The incision configuration in the photo of a conventional four-port laparoscopy consisted of a 10-mm incision in the right lower quadrant and 5-mm incisions in the umbilicus, left lower quadrant, and suprapubic area.
The conventional laparoscopy incisions were most preferred by approximately 56% of the 241 women who completed the survey, whereas preference for a single incision was approximately 41% and for robotic surgery was 2.5%.
Granted, there are variable configurations and incision sizes for each of the laparoscopic techniques, and further strides are being made to make the approaches more cosmetically acceptable. Experienced gynecologic surgeons are developing techniques to minimize the number of ports used robotically, for instance, and smaller incision sizes for robotic laparoscopy are anticipated.
In any case, the overarching lesson from this survey is that aesthetics is of value to many women and should be an important consideration for us as treating physicians.
Miniaturization
The use of mini- or microlaparoscopic instrumentation enables us to address aesthetic concerns and take the issue of cosmesis to the next level, as well as to further minimizing trauma. Although conventional laparoscopy involves instruments of 5-mm diameter or larger, the term "minilaparoscopy" usually refers to the use of instruments greater than 2 mm up to less than 5 mm in diameter, and "microlaparoscopy" involves instrumentation of 2 mm or less in diameter.
In gynecology, minilaparoscopy has been utilized diagnostically since the mid-1990s to perform conscious pain mapping with local anesthesia. With a 2.5 mm–diameter scope and 3 mm–diameter trocars, surgeons have used a blunt probe to map trigger sites with the patient’s assistance and response, and to aid in identifying pelvic disease suspected of causing pain (Int. J. Gynaecol. Obstet. 2008;100:94-8).
The diagnostic accuracy of the mini- or microlaparoscope has been reported in various scenarios (including cases of endometriosis and adhesive disease) to be comparable to that of the 10-mm scope that is used in conventional laparoscopy.
In one small study, investigators compared the diagnostic accuracy of 2-mm and 10-mm laparoscopes, with two physicians independently reporting findings to a third person (Fertil. Steril. 1997;67:952-4). Although they had a small sample size, the researchers observed no significant differences between the two laparoscopes or the two physicians. (Scores for endometriosis and adnexal adhesions, for instance, did not differ in any significant way.) This suggested that the diagnostic accuracy achieved with the microlaparoscope was comparable to that of the standard 10-mm laparoscope.
Another study in which 87 consecutive women underwent microlaparoscopic evaluation for chronic pelvic pain similarly found that microlaparoscopy provides comparable efficacy for diagnosing endometriosis and evaluating for the presence of occlusive salpingitis isthmica nodosa. Evaluation through a 2-mm port affords "an excellent minimally invasive view of the pelvis," the investigator wrote (JSLS 2005;9:431-3).
Most of the literature for minilaparoscopy, however, has evolved from the nongynecologic specialties, particularly general surgery. It has been well documented that minilaparoscopy is safe for splenic and liver biopsies, as well as helpful in reducing port sizes and hernias when utilized in cholecystectomies and colorectal resections.
Most recently, the authors of a literature review spanning the last 30 years concluded that minilaparoscopy has been shown in almost all urologic indications to be feasible and safe, with better cosmetic results and reduced postoperative pain (Arch. Esp. Urol. 2012;65:366-83).
Collectively, these studies provide us with a solid body of evidence on the safety, feasibility, and benefits of minilaparoscopy. The findings noted in the surgical literature for minilaparoscopy include operative times, morbidity, and hospital lengths of stay that are comparable to those of conventional laparoscopy; improved cosmesis; a decrease in postoperative pain; and reductions in port-site complications such as bleeding, infection, and herniation.
Surpassing SIL
Single-incision laparoscopy (also known as "scarless" surgery, with the umbilicus viewed as a natural scar) was developed to improve cosmesis. An incision approximately 25 mm in size at the umbilicus allows a scope and two or three instruments to pass into the abdominal cavity.
A main challenge – and a limitation for many surgeons – is the lack of triangulation because of the parallel entry. Instruments are crossed, which is counterintuitive to conventional laparoscopic training, and hands and instruments can clash, which limits the use of instrumentation.
An array of curved and EndoWrist instruments (with flexible joints at the tips) was developed to help ease the technical difficulties of single-port surgery. Many advanced endoscopic surgeons have performed simple to complex single-incision procedures with low complication rates and with operative times and rates of blood loss that are comparable with those of conventional laparoscopy. One advantage to single-incision surgery is that the incision size allows the removal of larger tissue that has been resected from the abdomen.
A recent study in the Netherlands suggests that single-incision surgery may not be all that more difficult to learn (Surg. Endosc. 2012;26:1231-7). The simulation study looked at the performance/learning curves of 20 medical interns (none with laparoscopic experience) who were randomly assigned to perform single-incision or conventional laparoscopy. Each participant practiced each task 11 times. There were no significant differences between the two groups in terms of error or time in performing the tasks, and participants improved significantly in both laparoscopy settings.
There are still many concerns, however, that single-incision laparoscopy is indeed more technically challenging, and that a sizable learning curve for the single-incision approach –in addition to the learning curve of conventional laparoscopy – does exist. Without surgical volume and patience, this technique may be a struggle for many to adapt and teach.
Moreover, as shown in the study at Newton-Wellesley Hospital, the scars remain a concern for many women. The umbilicus is the easiest place to try to "hide" an incision and scar when the incision is made at its base, but a 2-1/2 cm incision can be difficult to hide in a shallow umbilicus; for some women, the larger single scar becomes a focal point of their abdomen and, thus, disfiguring.
Our Experience
With minilaparoscopy, we can maintain triangulation and employ the fundamental principles of conventional laparoscopy with greater technical ease. The approach also has the potential to reduce the risk of incision-site hernias and the incidence of wound complications.
The following drawbacks to minilaparoscopy have been cited:
• A smaller view of the operative field.
• Less tensile strength of the instruments.
• Electrosurgery instruments that are limited to small vessels/pathology.
• Difficulty with large-tissue extraction.
There are solutions to each of these challenges, many of which involve hybrid approaches that incorporate single-incision or conventional laparoscopic ports at the umbilicus to facilitate electrosurgery, tissue extraction, and visualization while maintaining cosmesis. The solutions include the following:
• Better optics. Significant strides have been made in the area of optics, and newer minilaparoscopes (Figure 1) have better resolution and optical clarity than does the previous generation of miniature scopes. We have found that we can zoom the image and reduce light to improve visualization and maximize the operative field.
• Improved instrument strength. As with miniaturized laparoscopes, a wide array of durable instrumentation (Figures 2 and 3) is now available. We employ instruments that are 2.3-3.3 mm in diameter and up to 36 cm in length, ports that are 3.5 mm in diameter and 10-15 cm in length, and scopes that are 2.9-3.3 mm in diameter and up to 25 cm in length.
For skin incisions, a 14-gauge needle is used instead of a scalpel to avoid an incision that is too large and would allow easy slippage of the port. On the 2.3-mm disposable instruments, the tip itself is a 13-gauge needle that is utilized to incise the skin and enter the abdominal cavity. (See Figure 4.) Wounds are not sutured closed, but are merely reapproximated with Steri-Strips at the end of the procedure.
• More versatile electrosurgery instruments. We utilize reusable and disposable bipolar coagulating forceps, as well as reusable instruments with monopolar electrosurgical capability, including dissecting and grasping forceps, scissors, hook, and spatula. For example, when we excise endometriosis, we will utilize a sharp grasping instrument to elevate the lesion away from vital structures, as well as monopolar scissors to excise the disease. Needle holders and knot pushers are sturdy for suturing, and a suction tip is also available in miniaturized form.
In procedures in which the vessel-sealing capacity of a 3-mm energy source is not sufficient, a 5-mm port can be placed at the umbilicus so that a standard 5-mm energy source can be utilized to improve or maintain hemostasis.
• Extraction of large tissues. For tissue extraction requiring an incision larger than 5 mm, a 10- to 12-mm trocar can be placed at the umbilicus for the possible use of an endoscopic specimen bag. We dilate the umbilicus with Hegar dilators to a size 16 to ease the extraction of tissue, if necessary, or for the placement of a laparoscopic morcellator.
Recently, however, we have been able to perform minilaparoscopic hysterectomies with the use of three 3-mm ports, a minilaparoscope, and other miniaturized instrumentation. Specifically, we utilize the 3-mm PKS MoLly forceps by Olympus Gyrus (Figure 5) to coagulate and monopolar scissors to transect the pedicles. A colpotomy is completed with the scissors, and the specimen is delivered through the colpotomy incision. The colpotomy incision is reapproximated with 0-PDS suture on CT-1–sized needles. The suture is backloaded through the miniport and is introduced into the abdomen without difficulty. The incision is sutured; the needle is parked in the upper abdominal wall, and is then cut free. The suture is then pulled through the port for extracorporeal knot tying. The needle is retrieved and pulled through the incision with the removal of the port, and is now ready for loading another suture.
A minilaparoscopic supracervical hysterectomy can be performed in a similar fashion. For tissue extraction, we perform transcervical morcellation. We have found that the morcellators with extra-long shafts (such as the Storz 12-mm extra-long Rotocut or Gynecare Morcellex) work best for transcervical morcellation. Once uterine amputation is completed, the cervical stump is dilated with cervical dilators to allow for the morcellator tip to advance through the cervix and into the pelvic cavity transvaginally. Under direct visualization and with the assistance of a laparoscopic grasper, the specimen is morcellated. Similarly, an endoscopic specimen bag can also be placed transcervically to retrieve specimens that are not to undergo morcellation.
Minilaparoscopy now has multiple applications in our practice. Diagnostically, we employ the use of minilaparoscopic instrumentation in conjunction with hysteroscopic metroplasty, adhesiolysis, or tubal cannulation. Operatively, we have utilized minilaparoscopic instrumentation for excision of endometriosis, ovarian cystectomy, adhesiolysis, enterolysis, tuboplasty, and supracervical and total hysterectomy with or without bilateral salpingo-oophorectomy.
Dr. Cholkeri-Singh is associate director of minimally invasive gynecologic surgery, director of gynecologic surgical education, and codirector of the AAGL/SRS (Society of Reproductive Surgeons) fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. In addition, she is a board member of the AAGL/SRS fellowship in minimally invasive gynecology and is chair of a postgraduate course at this year’s AAGL Global Congress on Minimally Invasive Gynecology. Dr. Cholkeri-Singh disclosed that she is a speaker for Conceptus Inc., Olympus Gyrus ACMI, and Ethicon Endo Surgery.
The clinical advantages of laparoscopy over laparotomy have been well established over the last several years. As the acceptance and use of laparoscopic approaches have increased among gynecologic surgeons, attention to cosmesis has also evolved.
Laparoscopy has been appealing for its cosmetic benefits as well as reductions in complications and recovery times. Women not only want to resume their normal activities sooner; they also want to take advantage of the smaller incisions that laparoscopy entails, as these incisions do not alter abdominal wall appearance as significantly as do laparotomy incisions.
Still, depending on an individual’s body mass index and wound healing, scars can be disturbing for patients. Some women who have had more than one laparoscopic surgery requiring different port placements have jokingly spoken about "connecting the dots," but such comments reflect concern about aesthetics and should be taken seriously. Although we have improved surgical outcomes with laparoscopy and reduced the size of the surgical scar, we can still create disfigured abdomens.
Last year, the AAGL Fellowship in Minimally Invasive Gynecologic Surgery at Newton-Wellesley (Mass.) Hospital published an article about a survey of women and their preferred abdominal incision choices, based solely on cosmesis (J. Minim. Invasive Gynecol. 2011;18:640-3). The survey participants were shown three photographs of incisions that were commonly performed at their institution and among several Boston-area gynecologic surgeons, and were asked to rank the incisions in order of preference.
The photograph depicting a single incision or single-site laparoscopy showed a vertical 25-mm incision. The robotic laparoscopy photo showed five incisions: umbilical and right midabdomen (paraumbilical) incisions of 12-mm length, and 8-mm incisions in the right lower quadrant, left lower quadrant, and left midabdomen. The incision configuration in the photo of a conventional four-port laparoscopy consisted of a 10-mm incision in the right lower quadrant and 5-mm incisions in the umbilicus, left lower quadrant, and suprapubic area.
The conventional laparoscopy incisions were most preferred by approximately 56% of the 241 women who completed the survey, whereas preference for a single incision was approximately 41% and for robotic surgery was 2.5%.
Granted, there are variable configurations and incision sizes for each of the laparoscopic techniques, and further strides are being made to make the approaches more cosmetically acceptable. Experienced gynecologic surgeons are developing techniques to minimize the number of ports used robotically, for instance, and smaller incision sizes for robotic laparoscopy are anticipated.
In any case, the overarching lesson from this survey is that aesthetics is of value to many women and should be an important consideration for us as treating physicians.
Miniaturization
The use of mini- or microlaparoscopic instrumentation enables us to address aesthetic concerns and take the issue of cosmesis to the next level, as well as to further minimizing trauma. Although conventional laparoscopy involves instruments of 5-mm diameter or larger, the term "minilaparoscopy" usually refers to the use of instruments greater than 2 mm up to less than 5 mm in diameter, and "microlaparoscopy" involves instrumentation of 2 mm or less in diameter.
In gynecology, minilaparoscopy has been utilized diagnostically since the mid-1990s to perform conscious pain mapping with local anesthesia. With a 2.5 mm–diameter scope and 3 mm–diameter trocars, surgeons have used a blunt probe to map trigger sites with the patient’s assistance and response, and to aid in identifying pelvic disease suspected of causing pain (Int. J. Gynaecol. Obstet. 2008;100:94-8).
The diagnostic accuracy of the mini- or microlaparoscope has been reported in various scenarios (including cases of endometriosis and adhesive disease) to be comparable to that of the 10-mm scope that is used in conventional laparoscopy.
In one small study, investigators compared the diagnostic accuracy of 2-mm and 10-mm laparoscopes, with two physicians independently reporting findings to a third person (Fertil. Steril. 1997;67:952-4). Although they had a small sample size, the researchers observed no significant differences between the two laparoscopes or the two physicians. (Scores for endometriosis and adnexal adhesions, for instance, did not differ in any significant way.) This suggested that the diagnostic accuracy achieved with the microlaparoscope was comparable to that of the standard 10-mm laparoscope.
Another study in which 87 consecutive women underwent microlaparoscopic evaluation for chronic pelvic pain similarly found that microlaparoscopy provides comparable efficacy for diagnosing endometriosis and evaluating for the presence of occlusive salpingitis isthmica nodosa. Evaluation through a 2-mm port affords "an excellent minimally invasive view of the pelvis," the investigator wrote (JSLS 2005;9:431-3).
Most of the literature for minilaparoscopy, however, has evolved from the nongynecologic specialties, particularly general surgery. It has been well documented that minilaparoscopy is safe for splenic and liver biopsies, as well as helpful in reducing port sizes and hernias when utilized in cholecystectomies and colorectal resections.
Most recently, the authors of a literature review spanning the last 30 years concluded that minilaparoscopy has been shown in almost all urologic indications to be feasible and safe, with better cosmetic results and reduced postoperative pain (Arch. Esp. Urol. 2012;65:366-83).
Collectively, these studies provide us with a solid body of evidence on the safety, feasibility, and benefits of minilaparoscopy. The findings noted in the surgical literature for minilaparoscopy include operative times, morbidity, and hospital lengths of stay that are comparable to those of conventional laparoscopy; improved cosmesis; a decrease in postoperative pain; and reductions in port-site complications such as bleeding, infection, and herniation.
Surpassing SIL
Single-incision laparoscopy (also known as "scarless" surgery, with the umbilicus viewed as a natural scar) was developed to improve cosmesis. An incision approximately 25 mm in size at the umbilicus allows a scope and two or three instruments to pass into the abdominal cavity.
A main challenge – and a limitation for many surgeons – is the lack of triangulation because of the parallel entry. Instruments are crossed, which is counterintuitive to conventional laparoscopic training, and hands and instruments can clash, which limits the use of instrumentation.
An array of curved and EndoWrist instruments (with flexible joints at the tips) was developed to help ease the technical difficulties of single-port surgery. Many advanced endoscopic surgeons have performed simple to complex single-incision procedures with low complication rates and with operative times and rates of blood loss that are comparable with those of conventional laparoscopy. One advantage to single-incision surgery is that the incision size allows the removal of larger tissue that has been resected from the abdomen.
A recent study in the Netherlands suggests that single-incision surgery may not be all that more difficult to learn (Surg. Endosc. 2012;26:1231-7). The simulation study looked at the performance/learning curves of 20 medical interns (none with laparoscopic experience) who were randomly assigned to perform single-incision or conventional laparoscopy. Each participant practiced each task 11 times. There were no significant differences between the two groups in terms of error or time in performing the tasks, and participants improved significantly in both laparoscopy settings.
There are still many concerns, however, that single-incision laparoscopy is indeed more technically challenging, and that a sizable learning curve for the single-incision approach –in addition to the learning curve of conventional laparoscopy – does exist. Without surgical volume and patience, this technique may be a struggle for many to adapt and teach.
Moreover, as shown in the study at Newton-Wellesley Hospital, the scars remain a concern for many women. The umbilicus is the easiest place to try to "hide" an incision and scar when the incision is made at its base, but a 2-1/2 cm incision can be difficult to hide in a shallow umbilicus; for some women, the larger single scar becomes a focal point of their abdomen and, thus, disfiguring.
Our Experience
With minilaparoscopy, we can maintain triangulation and employ the fundamental principles of conventional laparoscopy with greater technical ease. The approach also has the potential to reduce the risk of incision-site hernias and the incidence of wound complications.
The following drawbacks to minilaparoscopy have been cited:
• A smaller view of the operative field.
• Less tensile strength of the instruments.
• Electrosurgery instruments that are limited to small vessels/pathology.
• Difficulty with large-tissue extraction.
There are solutions to each of these challenges, many of which involve hybrid approaches that incorporate single-incision or conventional laparoscopic ports at the umbilicus to facilitate electrosurgery, tissue extraction, and visualization while maintaining cosmesis. The solutions include the following:
• Better optics. Significant strides have been made in the area of optics, and newer minilaparoscopes (Figure 1) have better resolution and optical clarity than does the previous generation of miniature scopes. We have found that we can zoom the image and reduce light to improve visualization and maximize the operative field.
• Improved instrument strength. As with miniaturized laparoscopes, a wide array of durable instrumentation (Figures 2 and 3) is now available. We employ instruments that are 2.3-3.3 mm in diameter and up to 36 cm in length, ports that are 3.5 mm in diameter and 10-15 cm in length, and scopes that are 2.9-3.3 mm in diameter and up to 25 cm in length.
For skin incisions, a 14-gauge needle is used instead of a scalpel to avoid an incision that is too large and would allow easy slippage of the port. On the 2.3-mm disposable instruments, the tip itself is a 13-gauge needle that is utilized to incise the skin and enter the abdominal cavity. (See Figure 4.) Wounds are not sutured closed, but are merely reapproximated with Steri-Strips at the end of the procedure.
• More versatile electrosurgery instruments. We utilize reusable and disposable bipolar coagulating forceps, as well as reusable instruments with monopolar electrosurgical capability, including dissecting and grasping forceps, scissors, hook, and spatula. For example, when we excise endometriosis, we will utilize a sharp grasping instrument to elevate the lesion away from vital structures, as well as monopolar scissors to excise the disease. Needle holders and knot pushers are sturdy for suturing, and a suction tip is also available in miniaturized form.
In procedures in which the vessel-sealing capacity of a 3-mm energy source is not sufficient, a 5-mm port can be placed at the umbilicus so that a standard 5-mm energy source can be utilized to improve or maintain hemostasis.
• Extraction of large tissues. For tissue extraction requiring an incision larger than 5 mm, a 10- to 12-mm trocar can be placed at the umbilicus for the possible use of an endoscopic specimen bag. We dilate the umbilicus with Hegar dilators to a size 16 to ease the extraction of tissue, if necessary, or for the placement of a laparoscopic morcellator.
Recently, however, we have been able to perform minilaparoscopic hysterectomies with the use of three 3-mm ports, a minilaparoscope, and other miniaturized instrumentation. Specifically, we utilize the 3-mm PKS MoLly forceps by Olympus Gyrus (Figure 5) to coagulate and monopolar scissors to transect the pedicles. A colpotomy is completed with the scissors, and the specimen is delivered through the colpotomy incision. The colpotomy incision is reapproximated with 0-PDS suture on CT-1–sized needles. The suture is backloaded through the miniport and is introduced into the abdomen without difficulty. The incision is sutured; the needle is parked in the upper abdominal wall, and is then cut free. The suture is then pulled through the port for extracorporeal knot tying. The needle is retrieved and pulled through the incision with the removal of the port, and is now ready for loading another suture.
A minilaparoscopic supracervical hysterectomy can be performed in a similar fashion. For tissue extraction, we perform transcervical morcellation. We have found that the morcellators with extra-long shafts (such as the Storz 12-mm extra-long Rotocut or Gynecare Morcellex) work best for transcervical morcellation. Once uterine amputation is completed, the cervical stump is dilated with cervical dilators to allow for the morcellator tip to advance through the cervix and into the pelvic cavity transvaginally. Under direct visualization and with the assistance of a laparoscopic grasper, the specimen is morcellated. Similarly, an endoscopic specimen bag can also be placed transcervically to retrieve specimens that are not to undergo morcellation.
Minilaparoscopy now has multiple applications in our practice. Diagnostically, we employ the use of minilaparoscopic instrumentation in conjunction with hysteroscopic metroplasty, adhesiolysis, or tubal cannulation. Operatively, we have utilized minilaparoscopic instrumentation for excision of endometriosis, ovarian cystectomy, adhesiolysis, enterolysis, tuboplasty, and supracervical and total hysterectomy with or without bilateral salpingo-oophorectomy.
Dr. Cholkeri-Singh is associate director of minimally invasive gynecologic surgery, director of gynecologic surgical education, and codirector of the AAGL/SRS (Society of Reproductive Surgeons) fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. In addition, she is a board member of the AAGL/SRS fellowship in minimally invasive gynecology and is chair of a postgraduate course at this year’s AAGL Global Congress on Minimally Invasive Gynecology. Dr. Cholkeri-Singh disclosed that she is a speaker for Conceptus Inc., Olympus Gyrus ACMI, and Ethicon Endo Surgery.
Twins
Pregnancies involving multiple births are an increasingly prevalent phenomenon in most obstetrical practices, with twin gestations now accounting for more than 3% of all births in the United States.
The incidence of twinning is attributable to two main social factors: older maternal age at childbirth, which is responsible for a third of the increase, and the increased use of assisted reproductive technology (ART), which is responsible for the other two-thirds.
Although refinements in ART and recommendations to limit the number of embryos transferred during in vitro procedures have led to reductions in the number of higher-order multiples, twin gestations are still common, occurring in up to one-third of IVF pregnancies. Ovulation induction, which is a much more commonly used ART procedure, is also associated with twinning rates of 5%-10%.
What must be appreciated is the reality that twin gestations carry significant risks and contribute disproportionately to our national rates of perinatal morbidity and mortality. Although twin gestations represent just over 3% of all live births, they account for 15% of all early preterm births and 25% of all very-low birth weight infants.
Approximately 15% of all neonates with respiratory distress syndrome are twins, and twins account for 12% of all cases of grade III or IV intraventricular hemorrhage. Of all neonatal deaths in the United States, approximately 15% are twins. Moreover, approximately 10% of all stillbirths in the United States are twins.
In short, twin gestations are one of the most common high-risk conditions in obstetrical practice. Twin pregnancies are usually exciting for parents and their families, but it is important to reflect on the significant risks they can present, and to consider how these pregnancies can be optimally managed in order to reduce these risks.
No Simple Diagnosis
The first step in the optimal management of twins is to make the early diagnosis of chorionicity. The American Journal of Obstetrics & Gynecology ran a striking editorial about the significance of establishing chorionicity by first-trimester ultrasound in multiple gestations. The editorial’s authors, Dr. Kenneth J. Moise Jr. and Dr. Anthony Johnson, reflected on a talk given by Dr. Krypos Nicolaides of King’s College London, in which he broke away from his discussion of fetal therapy, paused, and made the following statement:
"There is NO diagnosis of twins. There are only monochorionic twins or dichorionic twins. This diagnosis should be written in capital red letters across the top of the patient’s chart." (Am. J. Obstet. Gynecol. 2010;203:1-2).
It could not have been better stated. It is not appropriate in obstetrical medicine to make a simple diagnosis of "twins" any longer. Early identification of whether a twin gestation involves either a monochorionic, single-placenta placentation, or a dichorionic, dual-placenta placentation is essential for accurate risk assessment and optimal antepartum management. Monochorionic pregnancies, which represent just over 20% of all twin gestations, are at much greater risk for miscarriage, congenital anomalies, growth abnormalities (including twin-to-twin transfusion syndrome), preterm birth, stillbirth, and neurodevelopmental abnormalities.
Determining this single feature – the chorionicity of the twin gestations – will thus shape a host of decisions, from the frequency of ultrasound examinations to the type of late-pregnancy surveillance and the timing of delivery.
With proper training, chorionicity can be determined with almost 100% accuracy in the first trimester, and if a patient doesn’t present in the first trimester, it still can be determined in the second trimester with an accuracy approaching 100% using a variety of ultrasound markers and parameters.
Optimizing Nutrition
I firmly believe that maximizing the mother’s nutrition is one of the most important determinants of a healthy outcome in a twin pregnancy.
In its 2009 report titled "Weight Gain During Pregnancy: Reexamining the Guidelines," the Institute of Medicine for the first time offered body mass index–specific recommendations for women with multiple gestations. The IOM has long had recommendations for singleton pregnancies based on pregravid BMI status, but this latest report marked the first time that multiple pregnancies were addressed in such detail.
Clearly, our knowledge base has advanced. During the 1990s and 2000s, a number of investigators – most notably Barbara Luke, Sc.D., currently a professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing – have published articles on the role of nutrition and weight gain in women with multiple gestations, and the relationship of these factors to pregnancy outcomes. Studies have demonstrated the critical effect that appropriate maternal weight gain and optimal nutrition have on twin fetal growth, birth weight, length of gestation, and other outcomes.
Surprisingly, the IOM consensus panel did not issue recommendations for women who are underweight, citing a lack of evidence. I believe there is clear epidemiologic evidence, however, that women with twin pregnancies who are underweight before pregnancy and have poor gestational weight gain are at the highest risk for poor outcomes.
In a 2003 report, Dr. Luke and a team of investigators, myself included, developed BMI-specific weight gain guidelines for optimal birth weights in twin pregnancies. Our weight gain curves and recommendations, which were based on a multi-institution cohort study of 2,324 twin pregnancies, are similar to the IOM’s guidelines. We also addressed the category of underweight women, however, and advised a gain of 50-62 pounds for these women. (See box.)
Women with a pregravid underweight status who had a total weight gain within this range experienced optimal fetal growth and birth weight. (J. Reprod. Med. 2003;48:217-24).
In a separate follow-up study of the value of using these BMI-specific weight gain goals, Dr. William Goodnight and I found that in our twins clinic, women who failed to achieve their BMI-specific weight-gain goals had a lower birth weight by nearly 200 g per twin and a length of gestation that was 1 week shorter than that of women who met weight gain goals (Am. J. Obstet. Gynecol. 2006;195[suppl]:S121).
As we described in a review published in 2009, achieving twin weight-gain goals should be part of a comprehensive approach to nutrition that also includes an appropriate caloric intake (3,000-4,000 kcal/day in underweight to normal weight women); supplementation with calcium, magnesium, folate, and zinc (beyond a usual prenatal vitamin); and a nutrient-dense diet that is high in iron-rich proteins and omega-3 fatty acids (Obstet. Gynecol. 2009;114:1121-34). In our practice, we emphasize the value of meat protein and of low-mercury fish.
Dr. Luke, who has coauthored a book with Tamara Eberlein that we often recommend to our patients, titled "When Youre Expecting Twins, Triplets or Quads" (New York: HarperCollins, 2011), has best demonstrated the extent to which intensive nutritional counseling and follow-up pays off in twin pregnancies.
In a cohort study published in 2003, she enrolled 190 women with twin pregnancies in a specialized prenatal program that involved twice-monthly visits for nutritional counseling and monitoring. Women were prescribed a diet of 3,000-4,000 kcal/day, with 20% of calories from protein, 40% from carbohydrates, and 40% from fat, as well as multimineral supplementation. The women were monitored for adequate weight gain and had serial ultrasound assessments.
Compared with 339 women with twin pregnancies who were followed by their physicians at the University of Michigan but not enrolled in the program, the program participants had significantly longer gestations (about a week), higher birth weights (220 g), a 23% reduction in preterm births, and significant reductions in preterm premature rupture of membranes, preterm labor, preeclampsia, ICU admission, and other poor outcomes.
Overall, the incidence of major neonatal morbidity was 17% for the nutritional program participants, compared with 32% for those women who did not receive the specialized care (Am. J. Obstet. Gynecol. 2003;189:934-8).
Measuring Cervical Length
One of the major clinical concerns with any twin pregnancy is the prevention of preterm birth and, by extension, the identification of those women with twins who, within this broader high-risk category, are at greatest risk for preterm birth.
Since the mid-1990s, research has shown that a short cervix detected in the midtrimester (defined as 16-24 weeks) by transvaginal ultrasound is a powerful predictor of preterm birth in women with either singleton or twin gestations. Studies of twin gestations have shown that as cervical length shortens to 25 mm or less, the risk of subsequent preterm birth (defined less than 34 weeks) rises dramatically.
In a report on twin pregnancies from the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, for instance, Dr. R.L. Goldenberg and associates demonstrated that cervical length less than or equal to 25 mm at 24 weeks was the best predictor of spontaneous preterm birth. In fact, of all 50 potential risk factors that were studied, a short cervix was the only factor that was consistently associated with preterm birth. The investigators also noted that this shorter cervical length was more common at both 24 and 28 weeks in twins, compared with singleton pregnancies (Am. J. Obstet. Gynecol. 1996;175:1047-53).
Through the 1990s and the next decade, investigators searched for a viable intervention. However, in numerous studies, the use of cerclage in mothers of twins with a short cervix was found to be of no benefit. Prophylactic tocolysis also failed to prevent preterm birth in published studies.
The use of bed rest has remained controversial. It is commonly prescribed to prevent preterm birth in women with a twin gestation and a short cervix, despite the fact that we have no published data demonstrating its effectiveness in prolonging pregnancy. Personally, I believe that bed rest can reduce the frequency of uterine contractions and help protect the cervix from the weight of the pregnancy.
Interest in the treatment of twin pregnancies with progesterone had waned after 2007, when a National Institutes of Health multicenter, randomized trial of injectable progesterone reported that 17-alpha-hydroxyprogesterone caproate did not reduce the rate of twin preterm birth (N. Engl. J. Med. 2007;357:454-61).
However, progesterone recently has reemerged as a treatment for women who are at higher risk of preterm birth, based on the publication this year of Dr. Roberto Romero’s review and meta-analysis of vaginal progesterone for women with a sonographic short cervix (Am. J. Obstet. Gynecol. 2012;206:124.e1-19). Indeed, this review provides the first evidence of a beneficial effect in women with a short cervix who are carrying a twin gestation. The meta-analysis covered five prospective, randomized trials of vaginal progesterone with a total of 775 women who had a sonographically confirmed short cervix (25 mm or less) in the midtrimester. The vast majority of women in these studies carried singleton pregnancies, but two of the studies enrolled twin as well as singleton gestations, and one of the five studies – albeit a small one – focused solely on twin pregnancies.
Overall, treatment with vaginal progesterone was associated with a highly significant 42% reduction in the rate of preterm birth at less than 33 weeks. Among twin gestations specifically, the reduction in preterm birth was 30% – a meaningful trend, but not statistically significant. When it came to neonatal morbidity and mortality, however, the effect of vaginal progesterone among twin gestations was far more striking: The group that received vaginal progesterone had a 48% reduction in the risk of composite neonatal morbidity and mortality.
Admittedly, a primary randomized, controlled trial in twin gestations is still needed. In the meantime, however, given the tremendous risk faced by women with a twin gestation and a short cervix, and the lack of any other proven treatment, I believe that vaginal progesterone (Prometrium, in either a 200-mg suppository or 90-mg gel) is an appropriate treatment for this condition.
In our practice, we routinely perform transvaginal cervical-length measurements with our twin pregnancies at the time of their anatomical survey at 18-20 weeks, and then every 2-4 weeks (depending on how short the cervix is) up to 26-28 weeks. If a short cervix is diagnosed, we restrict activity and start vaginal progesterone.
Surveillance, Delivery
Careful surveillance during the late gestational period is critical, as twins – particularly monochorionic twins – are at increased risk of growth restriction or growth discordancy, and have an increased risk of developing abnormalities in amniotic fluid volume. Compared with singletons, twins also are at increased risk of stillbirth in the third trimester; this risk, again, appears to be higher for monochorionic gestations.
We perform routine ultrasound evaluations every 3 weeks for our monochorionic twins and every 4 weeks for our dichorionic twins, in the absence of any abnormalities. If abnormalities in growth are suspected with standard ultrasound evaluation, we add umbilical artery Doppler studies to further assess well-being. We also routinely institute fetal nonstress testing at 32 weeks’ gestation for monochorionic twins and at 34 weeks for our dichorionic twins. Additional strategies are employed as necessary.
The overall risk of stillbirth for twin gestations is 0.2%-0.4% per week after 32 weeks’ gestation, and rises further beyond 38 weeks – a risk that makes surveillance critical.
Some investigators, however, have recently reported higher-than-expected stillbirth rates for "apparently uncomplicated" monochorionic twin gestations. This risk has ranged from 1% to 4% at 32 and 38 weeks’ gestation in various reports.
These studies were debated as part of a workshop held in 2011 by the National Institutes of Health and the Society for Maternal-Fetal Medicine on the "Timing of Indicated Late Preterm and Early Term Births." The findings of the higher stillbirth rates remain controversial, but on the basis of these concerns, it was recommended that monochorionic twins – even "uncomplicated" cases – should be offered elective delivery at 34-37 weeks’ gestation, with decisions made after careful discussion and informed consent.
Uncomplicated dichorionic twins, on the other hand, appear to have optimal outcomes when delivered at 38 weeks’ gestation (Obstet. Gynecol. 2011;118:323-3; Semin. Perinatol. 2011;35:277-85).
Recommended surveillance and delivery of monoamniotic twins – a rare but serious type of monochorionic twin gestation – has evolved recently in favor of intensive inpatient monitoring.
The benefits of hospitalization were demonstrated most strikingly in a multicenter cohort study that compared 43 women who were admitted at a median gestational age of 26.5 weeks for inpatient fetal testing two to three times daily vs. 44 women who were followed as outpatients with fetal testing one to three times weekly.
There were no stillbirths in the hospitalized group, but there was a 15% stillbirth rate in the outpatient group. The inpatient group also had significant improvements in birth weight, gestational age at delivery, and neonatal morbidity (Am. J. Obstet. Gynecol. 2005;192:96-101).
This and other evidence suggests that mothers of monoamniotic twins have the best possible outcome when their pregnancies are managed in a hospital setting with fetal monitoring two to three times a day, starting at 24-26 weeks’ gestation, with delivery timed between 32 and 34 weeks’ gestation. This was among the conclusions of the 2011 NIH-SMFM workshop.
Specialized Care
A final consideration regarding the antepartum care of multiples would be the benefit that might be achieved by establishing a specialized twins clinic.
The literature includes numerous reports, including one of our own (Semin. Perinatol 1995;19:387-403), describing improved perinatal outcomes for twins who are cared for by multidisciplinary teams using best practice protocols. Our team includes an obstetrician, a certified nurse-midwife, a nutritionist, an ultrasonographer, and a perinatal nurse.
In our experience, this approach significantly reduces perinatal mortality, primarily by reducing preterm premature rupture of membranes and very low birth weight delivery.
Dr. Newman is currently a professor and the Maas Chair for Reproductive Sciences in the department of obstetrics and gynecology, and vice chairman for academic affairs and women’s health research at the Medical University of South Carolina, Charleston. He established a multidisciplinary twins clinic in 1989 and since then has provided care for more than 1,000 women with twins. Dr. Rogers said he has no relevant financial disclosures.
Pregnancies involving multiple births are an increasingly prevalent phenomenon in most obstetrical practices, with twin gestations now accounting for more than 3% of all births in the United States.
The incidence of twinning is attributable to two main social factors: older maternal age at childbirth, which is responsible for a third of the increase, and the increased use of assisted reproductive technology (ART), which is responsible for the other two-thirds.
Although refinements in ART and recommendations to limit the number of embryos transferred during in vitro procedures have led to reductions in the number of higher-order multiples, twin gestations are still common, occurring in up to one-third of IVF pregnancies. Ovulation induction, which is a much more commonly used ART procedure, is also associated with twinning rates of 5%-10%.
What must be appreciated is the reality that twin gestations carry significant risks and contribute disproportionately to our national rates of perinatal morbidity and mortality. Although twin gestations represent just over 3% of all live births, they account for 15% of all early preterm births and 25% of all very-low birth weight infants.
Approximately 15% of all neonates with respiratory distress syndrome are twins, and twins account for 12% of all cases of grade III or IV intraventricular hemorrhage. Of all neonatal deaths in the United States, approximately 15% are twins. Moreover, approximately 10% of all stillbirths in the United States are twins.
In short, twin gestations are one of the most common high-risk conditions in obstetrical practice. Twin pregnancies are usually exciting for parents and their families, but it is important to reflect on the significant risks they can present, and to consider how these pregnancies can be optimally managed in order to reduce these risks.
No Simple Diagnosis
The first step in the optimal management of twins is to make the early diagnosis of chorionicity. The American Journal of Obstetrics & Gynecology ran a striking editorial about the significance of establishing chorionicity by first-trimester ultrasound in multiple gestations. The editorial’s authors, Dr. Kenneth J. Moise Jr. and Dr. Anthony Johnson, reflected on a talk given by Dr. Krypos Nicolaides of King’s College London, in which he broke away from his discussion of fetal therapy, paused, and made the following statement:
"There is NO diagnosis of twins. There are only monochorionic twins or dichorionic twins. This diagnosis should be written in capital red letters across the top of the patient’s chart." (Am. J. Obstet. Gynecol. 2010;203:1-2).
It could not have been better stated. It is not appropriate in obstetrical medicine to make a simple diagnosis of "twins" any longer. Early identification of whether a twin gestation involves either a monochorionic, single-placenta placentation, or a dichorionic, dual-placenta placentation is essential for accurate risk assessment and optimal antepartum management. Monochorionic pregnancies, which represent just over 20% of all twin gestations, are at much greater risk for miscarriage, congenital anomalies, growth abnormalities (including twin-to-twin transfusion syndrome), preterm birth, stillbirth, and neurodevelopmental abnormalities.
Determining this single feature – the chorionicity of the twin gestations – will thus shape a host of decisions, from the frequency of ultrasound examinations to the type of late-pregnancy surveillance and the timing of delivery.
With proper training, chorionicity can be determined with almost 100% accuracy in the first trimester, and if a patient doesn’t present in the first trimester, it still can be determined in the second trimester with an accuracy approaching 100% using a variety of ultrasound markers and parameters.
Optimizing Nutrition
I firmly believe that maximizing the mother’s nutrition is one of the most important determinants of a healthy outcome in a twin pregnancy.
In its 2009 report titled "Weight Gain During Pregnancy: Reexamining the Guidelines," the Institute of Medicine for the first time offered body mass index–specific recommendations for women with multiple gestations. The IOM has long had recommendations for singleton pregnancies based on pregravid BMI status, but this latest report marked the first time that multiple pregnancies were addressed in such detail.
Clearly, our knowledge base has advanced. During the 1990s and 2000s, a number of investigators – most notably Barbara Luke, Sc.D., currently a professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing – have published articles on the role of nutrition and weight gain in women with multiple gestations, and the relationship of these factors to pregnancy outcomes. Studies have demonstrated the critical effect that appropriate maternal weight gain and optimal nutrition have on twin fetal growth, birth weight, length of gestation, and other outcomes.
Surprisingly, the IOM consensus panel did not issue recommendations for women who are underweight, citing a lack of evidence. I believe there is clear epidemiologic evidence, however, that women with twin pregnancies who are underweight before pregnancy and have poor gestational weight gain are at the highest risk for poor outcomes.
In a 2003 report, Dr. Luke and a team of investigators, myself included, developed BMI-specific weight gain guidelines for optimal birth weights in twin pregnancies. Our weight gain curves and recommendations, which were based on a multi-institution cohort study of 2,324 twin pregnancies, are similar to the IOM’s guidelines. We also addressed the category of underweight women, however, and advised a gain of 50-62 pounds for these women. (See box.)
Women with a pregravid underweight status who had a total weight gain within this range experienced optimal fetal growth and birth weight. (J. Reprod. Med. 2003;48:217-24).
In a separate follow-up study of the value of using these BMI-specific weight gain goals, Dr. William Goodnight and I found that in our twins clinic, women who failed to achieve their BMI-specific weight-gain goals had a lower birth weight by nearly 200 g per twin and a length of gestation that was 1 week shorter than that of women who met weight gain goals (Am. J. Obstet. Gynecol. 2006;195[suppl]:S121).
As we described in a review published in 2009, achieving twin weight-gain goals should be part of a comprehensive approach to nutrition that also includes an appropriate caloric intake (3,000-4,000 kcal/day in underweight to normal weight women); supplementation with calcium, magnesium, folate, and zinc (beyond a usual prenatal vitamin); and a nutrient-dense diet that is high in iron-rich proteins and omega-3 fatty acids (Obstet. Gynecol. 2009;114:1121-34). In our practice, we emphasize the value of meat protein and of low-mercury fish.
Dr. Luke, who has coauthored a book with Tamara Eberlein that we often recommend to our patients, titled "When Youre Expecting Twins, Triplets or Quads" (New York: HarperCollins, 2011), has best demonstrated the extent to which intensive nutritional counseling and follow-up pays off in twin pregnancies.
In a cohort study published in 2003, she enrolled 190 women with twin pregnancies in a specialized prenatal program that involved twice-monthly visits for nutritional counseling and monitoring. Women were prescribed a diet of 3,000-4,000 kcal/day, with 20% of calories from protein, 40% from carbohydrates, and 40% from fat, as well as multimineral supplementation. The women were monitored for adequate weight gain and had serial ultrasound assessments.
Compared with 339 women with twin pregnancies who were followed by their physicians at the University of Michigan but not enrolled in the program, the program participants had significantly longer gestations (about a week), higher birth weights (220 g), a 23% reduction in preterm births, and significant reductions in preterm premature rupture of membranes, preterm labor, preeclampsia, ICU admission, and other poor outcomes.
Overall, the incidence of major neonatal morbidity was 17% for the nutritional program participants, compared with 32% for those women who did not receive the specialized care (Am. J. Obstet. Gynecol. 2003;189:934-8).
Measuring Cervical Length
One of the major clinical concerns with any twin pregnancy is the prevention of preterm birth and, by extension, the identification of those women with twins who, within this broader high-risk category, are at greatest risk for preterm birth.
Since the mid-1990s, research has shown that a short cervix detected in the midtrimester (defined as 16-24 weeks) by transvaginal ultrasound is a powerful predictor of preterm birth in women with either singleton or twin gestations. Studies of twin gestations have shown that as cervical length shortens to 25 mm or less, the risk of subsequent preterm birth (defined less than 34 weeks) rises dramatically.
In a report on twin pregnancies from the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, for instance, Dr. R.L. Goldenberg and associates demonstrated that cervical length less than or equal to 25 mm at 24 weeks was the best predictor of spontaneous preterm birth. In fact, of all 50 potential risk factors that were studied, a short cervix was the only factor that was consistently associated with preterm birth. The investigators also noted that this shorter cervical length was more common at both 24 and 28 weeks in twins, compared with singleton pregnancies (Am. J. Obstet. Gynecol. 1996;175:1047-53).
Through the 1990s and the next decade, investigators searched for a viable intervention. However, in numerous studies, the use of cerclage in mothers of twins with a short cervix was found to be of no benefit. Prophylactic tocolysis also failed to prevent preterm birth in published studies.
The use of bed rest has remained controversial. It is commonly prescribed to prevent preterm birth in women with a twin gestation and a short cervix, despite the fact that we have no published data demonstrating its effectiveness in prolonging pregnancy. Personally, I believe that bed rest can reduce the frequency of uterine contractions and help protect the cervix from the weight of the pregnancy.
Interest in the treatment of twin pregnancies with progesterone had waned after 2007, when a National Institutes of Health multicenter, randomized trial of injectable progesterone reported that 17-alpha-hydroxyprogesterone caproate did not reduce the rate of twin preterm birth (N. Engl. J. Med. 2007;357:454-61).
However, progesterone recently has reemerged as a treatment for women who are at higher risk of preterm birth, based on the publication this year of Dr. Roberto Romero’s review and meta-analysis of vaginal progesterone for women with a sonographic short cervix (Am. J. Obstet. Gynecol. 2012;206:124.e1-19). Indeed, this review provides the first evidence of a beneficial effect in women with a short cervix who are carrying a twin gestation. The meta-analysis covered five prospective, randomized trials of vaginal progesterone with a total of 775 women who had a sonographically confirmed short cervix (25 mm or less) in the midtrimester. The vast majority of women in these studies carried singleton pregnancies, but two of the studies enrolled twin as well as singleton gestations, and one of the five studies – albeit a small one – focused solely on twin pregnancies.
Overall, treatment with vaginal progesterone was associated with a highly significant 42% reduction in the rate of preterm birth at less than 33 weeks. Among twin gestations specifically, the reduction in preterm birth was 30% – a meaningful trend, but not statistically significant. When it came to neonatal morbidity and mortality, however, the effect of vaginal progesterone among twin gestations was far more striking: The group that received vaginal progesterone had a 48% reduction in the risk of composite neonatal morbidity and mortality.
Admittedly, a primary randomized, controlled trial in twin gestations is still needed. In the meantime, however, given the tremendous risk faced by women with a twin gestation and a short cervix, and the lack of any other proven treatment, I believe that vaginal progesterone (Prometrium, in either a 200-mg suppository or 90-mg gel) is an appropriate treatment for this condition.
In our practice, we routinely perform transvaginal cervical-length measurements with our twin pregnancies at the time of their anatomical survey at 18-20 weeks, and then every 2-4 weeks (depending on how short the cervix is) up to 26-28 weeks. If a short cervix is diagnosed, we restrict activity and start vaginal progesterone.
Surveillance, Delivery
Careful surveillance during the late gestational period is critical, as twins – particularly monochorionic twins – are at increased risk of growth restriction or growth discordancy, and have an increased risk of developing abnormalities in amniotic fluid volume. Compared with singletons, twins also are at increased risk of stillbirth in the third trimester; this risk, again, appears to be higher for monochorionic gestations.
We perform routine ultrasound evaluations every 3 weeks for our monochorionic twins and every 4 weeks for our dichorionic twins, in the absence of any abnormalities. If abnormalities in growth are suspected with standard ultrasound evaluation, we add umbilical artery Doppler studies to further assess well-being. We also routinely institute fetal nonstress testing at 32 weeks’ gestation for monochorionic twins and at 34 weeks for our dichorionic twins. Additional strategies are employed as necessary.
The overall risk of stillbirth for twin gestations is 0.2%-0.4% per week after 32 weeks’ gestation, and rises further beyond 38 weeks – a risk that makes surveillance critical.
Some investigators, however, have recently reported higher-than-expected stillbirth rates for "apparently uncomplicated" monochorionic twin gestations. This risk has ranged from 1% to 4% at 32 and 38 weeks’ gestation in various reports.
These studies were debated as part of a workshop held in 2011 by the National Institutes of Health and the Society for Maternal-Fetal Medicine on the "Timing of Indicated Late Preterm and Early Term Births." The findings of the higher stillbirth rates remain controversial, but on the basis of these concerns, it was recommended that monochorionic twins – even "uncomplicated" cases – should be offered elective delivery at 34-37 weeks’ gestation, with decisions made after careful discussion and informed consent.
Uncomplicated dichorionic twins, on the other hand, appear to have optimal outcomes when delivered at 38 weeks’ gestation (Obstet. Gynecol. 2011;118:323-3; Semin. Perinatol. 2011;35:277-85).
Recommended surveillance and delivery of monoamniotic twins – a rare but serious type of monochorionic twin gestation – has evolved recently in favor of intensive inpatient monitoring.
The benefits of hospitalization were demonstrated most strikingly in a multicenter cohort study that compared 43 women who were admitted at a median gestational age of 26.5 weeks for inpatient fetal testing two to three times daily vs. 44 women who were followed as outpatients with fetal testing one to three times weekly.
There were no stillbirths in the hospitalized group, but there was a 15% stillbirth rate in the outpatient group. The inpatient group also had significant improvements in birth weight, gestational age at delivery, and neonatal morbidity (Am. J. Obstet. Gynecol. 2005;192:96-101).
This and other evidence suggests that mothers of monoamniotic twins have the best possible outcome when their pregnancies are managed in a hospital setting with fetal monitoring two to three times a day, starting at 24-26 weeks’ gestation, with delivery timed between 32 and 34 weeks’ gestation. This was among the conclusions of the 2011 NIH-SMFM workshop.
Specialized Care
A final consideration regarding the antepartum care of multiples would be the benefit that might be achieved by establishing a specialized twins clinic.
The literature includes numerous reports, including one of our own (Semin. Perinatol 1995;19:387-403), describing improved perinatal outcomes for twins who are cared for by multidisciplinary teams using best practice protocols. Our team includes an obstetrician, a certified nurse-midwife, a nutritionist, an ultrasonographer, and a perinatal nurse.
In our experience, this approach significantly reduces perinatal mortality, primarily by reducing preterm premature rupture of membranes and very low birth weight delivery.
Dr. Newman is currently a professor and the Maas Chair for Reproductive Sciences in the department of obstetrics and gynecology, and vice chairman for academic affairs and women’s health research at the Medical University of South Carolina, Charleston. He established a multidisciplinary twins clinic in 1989 and since then has provided care for more than 1,000 women with twins. Dr. Rogers said he has no relevant financial disclosures.
Pregnancies involving multiple births are an increasingly prevalent phenomenon in most obstetrical practices, with twin gestations now accounting for more than 3% of all births in the United States.
The incidence of twinning is attributable to two main social factors: older maternal age at childbirth, which is responsible for a third of the increase, and the increased use of assisted reproductive technology (ART), which is responsible for the other two-thirds.
Although refinements in ART and recommendations to limit the number of embryos transferred during in vitro procedures have led to reductions in the number of higher-order multiples, twin gestations are still common, occurring in up to one-third of IVF pregnancies. Ovulation induction, which is a much more commonly used ART procedure, is also associated with twinning rates of 5%-10%.
What must be appreciated is the reality that twin gestations carry significant risks and contribute disproportionately to our national rates of perinatal morbidity and mortality. Although twin gestations represent just over 3% of all live births, they account for 15% of all early preterm births and 25% of all very-low birth weight infants.
Approximately 15% of all neonates with respiratory distress syndrome are twins, and twins account for 12% of all cases of grade III or IV intraventricular hemorrhage. Of all neonatal deaths in the United States, approximately 15% are twins. Moreover, approximately 10% of all stillbirths in the United States are twins.
In short, twin gestations are one of the most common high-risk conditions in obstetrical practice. Twin pregnancies are usually exciting for parents and their families, but it is important to reflect on the significant risks they can present, and to consider how these pregnancies can be optimally managed in order to reduce these risks.
No Simple Diagnosis
The first step in the optimal management of twins is to make the early diagnosis of chorionicity. The American Journal of Obstetrics & Gynecology ran a striking editorial about the significance of establishing chorionicity by first-trimester ultrasound in multiple gestations. The editorial’s authors, Dr. Kenneth J. Moise Jr. and Dr. Anthony Johnson, reflected on a talk given by Dr. Krypos Nicolaides of King’s College London, in which he broke away from his discussion of fetal therapy, paused, and made the following statement:
"There is NO diagnosis of twins. There are only monochorionic twins or dichorionic twins. This diagnosis should be written in capital red letters across the top of the patient’s chart." (Am. J. Obstet. Gynecol. 2010;203:1-2).
It could not have been better stated. It is not appropriate in obstetrical medicine to make a simple diagnosis of "twins" any longer. Early identification of whether a twin gestation involves either a monochorionic, single-placenta placentation, or a dichorionic, dual-placenta placentation is essential for accurate risk assessment and optimal antepartum management. Monochorionic pregnancies, which represent just over 20% of all twin gestations, are at much greater risk for miscarriage, congenital anomalies, growth abnormalities (including twin-to-twin transfusion syndrome), preterm birth, stillbirth, and neurodevelopmental abnormalities.
Determining this single feature – the chorionicity of the twin gestations – will thus shape a host of decisions, from the frequency of ultrasound examinations to the type of late-pregnancy surveillance and the timing of delivery.
With proper training, chorionicity can be determined with almost 100% accuracy in the first trimester, and if a patient doesn’t present in the first trimester, it still can be determined in the second trimester with an accuracy approaching 100% using a variety of ultrasound markers and parameters.
Optimizing Nutrition
I firmly believe that maximizing the mother’s nutrition is one of the most important determinants of a healthy outcome in a twin pregnancy.
In its 2009 report titled "Weight Gain During Pregnancy: Reexamining the Guidelines," the Institute of Medicine for the first time offered body mass index–specific recommendations for women with multiple gestations. The IOM has long had recommendations for singleton pregnancies based on pregravid BMI status, but this latest report marked the first time that multiple pregnancies were addressed in such detail.
Clearly, our knowledge base has advanced. During the 1990s and 2000s, a number of investigators – most notably Barbara Luke, Sc.D., currently a professor of obstetrics, gynecology, and reproductive biology at Michigan State University, East Lansing – have published articles on the role of nutrition and weight gain in women with multiple gestations, and the relationship of these factors to pregnancy outcomes. Studies have demonstrated the critical effect that appropriate maternal weight gain and optimal nutrition have on twin fetal growth, birth weight, length of gestation, and other outcomes.
Surprisingly, the IOM consensus panel did not issue recommendations for women who are underweight, citing a lack of evidence. I believe there is clear epidemiologic evidence, however, that women with twin pregnancies who are underweight before pregnancy and have poor gestational weight gain are at the highest risk for poor outcomes.
In a 2003 report, Dr. Luke and a team of investigators, myself included, developed BMI-specific weight gain guidelines for optimal birth weights in twin pregnancies. Our weight gain curves and recommendations, which were based on a multi-institution cohort study of 2,324 twin pregnancies, are similar to the IOM’s guidelines. We also addressed the category of underweight women, however, and advised a gain of 50-62 pounds for these women. (See box.)
Women with a pregravid underweight status who had a total weight gain within this range experienced optimal fetal growth and birth weight. (J. Reprod. Med. 2003;48:217-24).
In a separate follow-up study of the value of using these BMI-specific weight gain goals, Dr. William Goodnight and I found that in our twins clinic, women who failed to achieve their BMI-specific weight-gain goals had a lower birth weight by nearly 200 g per twin and a length of gestation that was 1 week shorter than that of women who met weight gain goals (Am. J. Obstet. Gynecol. 2006;195[suppl]:S121).
As we described in a review published in 2009, achieving twin weight-gain goals should be part of a comprehensive approach to nutrition that also includes an appropriate caloric intake (3,000-4,000 kcal/day in underweight to normal weight women); supplementation with calcium, magnesium, folate, and zinc (beyond a usual prenatal vitamin); and a nutrient-dense diet that is high in iron-rich proteins and omega-3 fatty acids (Obstet. Gynecol. 2009;114:1121-34). In our practice, we emphasize the value of meat protein and of low-mercury fish.
Dr. Luke, who has coauthored a book with Tamara Eberlein that we often recommend to our patients, titled "When Youre Expecting Twins, Triplets or Quads" (New York: HarperCollins, 2011), has best demonstrated the extent to which intensive nutritional counseling and follow-up pays off in twin pregnancies.
In a cohort study published in 2003, she enrolled 190 women with twin pregnancies in a specialized prenatal program that involved twice-monthly visits for nutritional counseling and monitoring. Women were prescribed a diet of 3,000-4,000 kcal/day, with 20% of calories from protein, 40% from carbohydrates, and 40% from fat, as well as multimineral supplementation. The women were monitored for adequate weight gain and had serial ultrasound assessments.
Compared with 339 women with twin pregnancies who were followed by their physicians at the University of Michigan but not enrolled in the program, the program participants had significantly longer gestations (about a week), higher birth weights (220 g), a 23% reduction in preterm births, and significant reductions in preterm premature rupture of membranes, preterm labor, preeclampsia, ICU admission, and other poor outcomes.
Overall, the incidence of major neonatal morbidity was 17% for the nutritional program participants, compared with 32% for those women who did not receive the specialized care (Am. J. Obstet. Gynecol. 2003;189:934-8).
Measuring Cervical Length
One of the major clinical concerns with any twin pregnancy is the prevention of preterm birth and, by extension, the identification of those women with twins who, within this broader high-risk category, are at greatest risk for preterm birth.
Since the mid-1990s, research has shown that a short cervix detected in the midtrimester (defined as 16-24 weeks) by transvaginal ultrasound is a powerful predictor of preterm birth in women with either singleton or twin gestations. Studies of twin gestations have shown that as cervical length shortens to 25 mm or less, the risk of subsequent preterm birth (defined less than 34 weeks) rises dramatically.
In a report on twin pregnancies from the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, for instance, Dr. R.L. Goldenberg and associates demonstrated that cervical length less than or equal to 25 mm at 24 weeks was the best predictor of spontaneous preterm birth. In fact, of all 50 potential risk factors that were studied, a short cervix was the only factor that was consistently associated with preterm birth. The investigators also noted that this shorter cervical length was more common at both 24 and 28 weeks in twins, compared with singleton pregnancies (Am. J. Obstet. Gynecol. 1996;175:1047-53).
Through the 1990s and the next decade, investigators searched for a viable intervention. However, in numerous studies, the use of cerclage in mothers of twins with a short cervix was found to be of no benefit. Prophylactic tocolysis also failed to prevent preterm birth in published studies.
The use of bed rest has remained controversial. It is commonly prescribed to prevent preterm birth in women with a twin gestation and a short cervix, despite the fact that we have no published data demonstrating its effectiveness in prolonging pregnancy. Personally, I believe that bed rest can reduce the frequency of uterine contractions and help protect the cervix from the weight of the pregnancy.
Interest in the treatment of twin pregnancies with progesterone had waned after 2007, when a National Institutes of Health multicenter, randomized trial of injectable progesterone reported that 17-alpha-hydroxyprogesterone caproate did not reduce the rate of twin preterm birth (N. Engl. J. Med. 2007;357:454-61).
However, progesterone recently has reemerged as a treatment for women who are at higher risk of preterm birth, based on the publication this year of Dr. Roberto Romero’s review and meta-analysis of vaginal progesterone for women with a sonographic short cervix (Am. J. Obstet. Gynecol. 2012;206:124.e1-19). Indeed, this review provides the first evidence of a beneficial effect in women with a short cervix who are carrying a twin gestation. The meta-analysis covered five prospective, randomized trials of vaginal progesterone with a total of 775 women who had a sonographically confirmed short cervix (25 mm or less) in the midtrimester. The vast majority of women in these studies carried singleton pregnancies, but two of the studies enrolled twin as well as singleton gestations, and one of the five studies – albeit a small one – focused solely on twin pregnancies.
Overall, treatment with vaginal progesterone was associated with a highly significant 42% reduction in the rate of preterm birth at less than 33 weeks. Among twin gestations specifically, the reduction in preterm birth was 30% – a meaningful trend, but not statistically significant. When it came to neonatal morbidity and mortality, however, the effect of vaginal progesterone among twin gestations was far more striking: The group that received vaginal progesterone had a 48% reduction in the risk of composite neonatal morbidity and mortality.
Admittedly, a primary randomized, controlled trial in twin gestations is still needed. In the meantime, however, given the tremendous risk faced by women with a twin gestation and a short cervix, and the lack of any other proven treatment, I believe that vaginal progesterone (Prometrium, in either a 200-mg suppository or 90-mg gel) is an appropriate treatment for this condition.
In our practice, we routinely perform transvaginal cervical-length measurements with our twin pregnancies at the time of their anatomical survey at 18-20 weeks, and then every 2-4 weeks (depending on how short the cervix is) up to 26-28 weeks. If a short cervix is diagnosed, we restrict activity and start vaginal progesterone.
Surveillance, Delivery
Careful surveillance during the late gestational period is critical, as twins – particularly monochorionic twins – are at increased risk of growth restriction or growth discordancy, and have an increased risk of developing abnormalities in amniotic fluid volume. Compared with singletons, twins also are at increased risk of stillbirth in the third trimester; this risk, again, appears to be higher for monochorionic gestations.
We perform routine ultrasound evaluations every 3 weeks for our monochorionic twins and every 4 weeks for our dichorionic twins, in the absence of any abnormalities. If abnormalities in growth are suspected with standard ultrasound evaluation, we add umbilical artery Doppler studies to further assess well-being. We also routinely institute fetal nonstress testing at 32 weeks’ gestation for monochorionic twins and at 34 weeks for our dichorionic twins. Additional strategies are employed as necessary.
The overall risk of stillbirth for twin gestations is 0.2%-0.4% per week after 32 weeks’ gestation, and rises further beyond 38 weeks – a risk that makes surveillance critical.
Some investigators, however, have recently reported higher-than-expected stillbirth rates for "apparently uncomplicated" monochorionic twin gestations. This risk has ranged from 1% to 4% at 32 and 38 weeks’ gestation in various reports.
These studies were debated as part of a workshop held in 2011 by the National Institutes of Health and the Society for Maternal-Fetal Medicine on the "Timing of Indicated Late Preterm and Early Term Births." The findings of the higher stillbirth rates remain controversial, but on the basis of these concerns, it was recommended that monochorionic twins – even "uncomplicated" cases – should be offered elective delivery at 34-37 weeks’ gestation, with decisions made after careful discussion and informed consent.
Uncomplicated dichorionic twins, on the other hand, appear to have optimal outcomes when delivered at 38 weeks’ gestation (Obstet. Gynecol. 2011;118:323-3; Semin. Perinatol. 2011;35:277-85).
Recommended surveillance and delivery of monoamniotic twins – a rare but serious type of monochorionic twin gestation – has evolved recently in favor of intensive inpatient monitoring.
The benefits of hospitalization were demonstrated most strikingly in a multicenter cohort study that compared 43 women who were admitted at a median gestational age of 26.5 weeks for inpatient fetal testing two to three times daily vs. 44 women who were followed as outpatients with fetal testing one to three times weekly.
There were no stillbirths in the hospitalized group, but there was a 15% stillbirth rate in the outpatient group. The inpatient group also had significant improvements in birth weight, gestational age at delivery, and neonatal morbidity (Am. J. Obstet. Gynecol. 2005;192:96-101).
This and other evidence suggests that mothers of monoamniotic twins have the best possible outcome when their pregnancies are managed in a hospital setting with fetal monitoring two to three times a day, starting at 24-26 weeks’ gestation, with delivery timed between 32 and 34 weeks’ gestation. This was among the conclusions of the 2011 NIH-SMFM workshop.
Specialized Care
A final consideration regarding the antepartum care of multiples would be the benefit that might be achieved by establishing a specialized twins clinic.
The literature includes numerous reports, including one of our own (Semin. Perinatol 1995;19:387-403), describing improved perinatal outcomes for twins who are cared for by multidisciplinary teams using best practice protocols. Our team includes an obstetrician, a certified nurse-midwife, a nutritionist, an ultrasonographer, and a perinatal nurse.
In our experience, this approach significantly reduces perinatal mortality, primarily by reducing preterm premature rupture of membranes and very low birth weight delivery.
Dr. Newman is currently a professor and the Maas Chair for Reproductive Sciences in the department of obstetrics and gynecology, and vice chairman for academic affairs and women’s health research at the Medical University of South Carolina, Charleston. He established a multidisciplinary twins clinic in 1989 and since then has provided care for more than 1,000 women with twins. Dr. Rogers said he has no relevant financial disclosures.
Establishing Standards in Minimally Invasive Gynecologic Surgery
Minimally invasive endoscopy, with and without robotics, has become a viable option for almost every operative gynecologic procedure.
Yet, despite a solid body of evidence demonstrating the benefits of minimally invasive approaches and despite tremendous progress in postgraduate education, many gynecologic surgeons are not incorporating endoscopic options into their practices – or even into their patient education. Others have adopted minimally invasive techniques, but sometimes without the essential or optimal knowledge and skills needed to perform minimally invasive gynecologic surgery with optimal outcomes and patient safety.
New gynecologic surgeons, in the meantime, are leaving residency programs with variable levels of experience and proficiency. Some have adequate opportunity to gain the skills and experience they need to be comfortable and confident in the endoscopic arena, but many do not.
As Dr. Charles E. Miller, past president of the AAGL (formerly known as the American Association of Gynecologic Laparoscopists), said in his presidential address in 2008, data have shown that one in three residents graduate with a paucity of experience in minimally invasive gynecologic procedures.
At the root of these realities are the lack of a defined knowledge base and the lack of a specific, unified endoscopy curriculum. There have been no minimal standards set for gynecologic endoscopy, no quantifiable measures defined for proficiency in minimally invasive gynecologic surgery, and no validated tools developed for gynecologic surgeons to learn and then document their competency.
Change is underway, however. For the past 2 years, the AAGL has worked with hundreds of physicians and experts to determine the essential knowledge needed for performing minimally invasive gynecologic surgery – and to develop an appropriate assessment test for gynecologic surgeons.
Called Essentials in Minimally Invasive Gynecology (EMIG), the test is based upon professional testing standards and guidelines established by professional testing societies. The process used to develop the test has incorporated the important principles of validity, reliability, and defensibility.
The EMIG cognitive assessment is currently in the beta-test stage and is scheduled to be administered for the first time in November 2012 at the AAGL annual clinical meeting. After that, physicians will be able to take the test through any one of multiple proctored testing centers operated worldwide by the consulting firm that helped develop and validate Fundamentals of Laparoscopic Surgery (FLS), the educational program that is now a standard part of all general surgery residencies.
The EMIG cognitive test covers the areas of applied anatomy and physiology, endoscopic principles, patient selection, instrumentation, energy sources, operating room/equipment setup, laparoscopy, hysteroscopy, and complications. A skills testing portion of the EMIG is under development.
Most immediately, for practicing gynecologic surgeons, EMIG will give them the opportunity to demonstrate and validate their laparoscopic skills. Then, as training becomes more standardized, gynecologists will be able to acquire skills, or enhance their skills, through postgraduate courses that incorporate EMIG principles in a unified, standardized manner.
Continuous Progress
It was gynecology that pioneered use of the laparoscope and brought it to our general surgery colleagues. And over the years, gynecology has been cognizant of the need to develop a standardized assessment, curriculum, and training to meet the demands of the dynamic developments in endoscopy.
In the early 1990s, the American College of Obstetricians and Gynecologists issued basic guidelines for credentialing gynecologists to perform laparoscopy, and the Accreditation Council for Gynecologic Endoscopy (now the Council for Gynecologic Endoscopy and part of the AAGL) started a registry identifying gynecologists skilled at laparoscopy and hysteroscopy.
In 1998, Dr. Andrew Brill and Dr. Robert Rogers outlined a comprehensive program for resident training in the Journal of the American Association of Gynecologic Laparoscopists (J. Am. Assoc. Gynecol. Laparosc. 1998;5:223-8). Recently, the Fellowship in Minimally Invasive Gynecologic Surgery program issued guidelines as well for fellow and resident education, hands-on training, written examinations and skills testing, and supervised surgery.
In the world of general surgery, in the meantime, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) began developing the FLS in the late 1990s; the FLS is a comprehensive web-based education module designed to teach and assess basic cognitive, technical, and psychomotor skills required to perform laparoscopic surgery.
FLS has two parts: didactic testing, which is online multiple choice testing, and skills testing, which is hands-on. Endorsed by the American College of Surgeons (ACS), FLS was designed to give surgical residents, fellows, and practicing physicians an opportunity to learn the fundamentals of laparoscopic surgery in a consistent, scientific format.
The goal was not only to increase familiarity with and access to minimally invasive techniques, but to ensure good outcomes and patient safety.
Since the FLS test became available in 2004, the number of physicians seeking FLS certification rose annually. A study of the first 5 years of FLS certification testing (2004-2009) shows an overall pass rate of 88% on the examination – close to the target pass rate of 90% established during the test-setting process. Almost 20% of the test participants over this time period were attending surgeons; the rest were senior residents or fellows (the largest participant group) and junior residents. A small number – 4% – were gynecologists (Surg. Endosc. 2011;25:1192-8).
In 2008, the ACS went one step further by requiring all general surgery residents, starting in 2009, to complete and pass the FLS course before achieving board certification. This mandate set minimum standards for basic cognitive and technical skills, and encouraged a uniform framework for laparoscopy training in general surgery residency programs.
Urology has similarly deliberated and addressed the idea that standard training and credentialing initiatives are critical to have and to update as surgical technology and techniques evolve. The American Urologic Association has developed and is validating a Basic Laparoscopic Urologic Surgery (BLUS) skills curriculum similar to FLS for laparoscopic cognitive and technical skills. The association also has created a Simulation Advisory Board that will vet current and future skills courses and provide guidance for matching learning objectives with effective simulation-based technologies. In addition, the AUA is expanding a preexisting urology-specific robotic skills curriculum and, along with AAGL, SAGES, and the Minimally Invasive Robotic Association, is developing a Fundamentals of Robotic Skills curriculum.
Essential Gynecologic Knowledge
As minimally invasive surgery becomes mainstream, it is important for gynecologic surgeons to be familiar with the fundamentals of laparoscopic gynecologic surgery and hysteroscopy, whether or not they are utilizing or planning to utilize robotic technology.
Development of the cognitive portion of EMIG is the first step of an AAGL-led effort that will ensure that gynecology meets the need for a standardized, demonstrable set of skills. As it takes shape and gains acceptance, the EMIG assessment should provide a standardized tool for physicians, patients, hospitals and payers to identify gynecologic surgeons who have demonstrated the knowledge and skills necessary to understand the intricacies of minimally invasive gynecology.
Unlike the FLS, the EMIG test is specifically focused on gynecology and includes hysteroscopy. Hundreds of physicians were involved in completing a survey, writing psychometrically correct items, and reviewing questions. A test development psychometrician directed the AAGL through test development and technical review and performed a psychometric edit.
As of May 2012, beta testers were scheduled to answer approximately 380 questions, over two sessions totaling 6-8 hours, in a proctored environment. From the results, two forms of the test, with approximately 125 questions each, will be created. A passing score will be determined, and the test will be made available in online test delivery software so it can be taken anywhere in the world – after its first run at the AAGL meeting this fall.
Concurrently, the AAGL has been developing an approach to testing psychomotor skills, which is needed, along with cognitive assessment, to ascertain the qualifications of a surgeon. The AAGL identified essential skills by asking more than 1,000 physicians to rank the importance and frequency of the skills. This information will be used to evaluate skills trainers and testing instruments.
Eventually, it is hoped, a standardized core curriculum – based on the EMIG knowledge base – will be developed and incorporated into residency programs, just as it has been for general surgery. Such a curriculum could also be implemented in the Fellowship in Minimally Invasive Gynecologic Surgery, cosponsored by the AAGL and the Society of Reproductive Surgeons (an affiliate society of the American Society for Reproductive Medicine), in which case it could be longer in duration.
A standardized core curriculum could be implemented as well in the growing number of postgraduate courses that are available to practicing gynecologic surgeons; in this case, the curriculum would be shorter in duration.
A proposal for a formal gynecologic endoscopy curriculum, published a few years ago in The Journal of Minimally Invasive Gynecology (J. Minim. Invasive Gynecol. 2009;16:416-21), envisions a quarterly system with an online examination given after quarters 1 and 2, a hands-on examination given after quarter 3, and a demonstration of leadership and teaching skills in the operating room after quarter 4.
As envisioned in the proposed curriculum, the first quarter would focus on knowledge of pelvic and abdominal anatomy that is specific to laparoscopy and gynecology, including the major branches of anterior and posterior division of the internal iliac artery and the major nerve supplies to the pelvis. Knowledge of the physiology and principles of creating and maintaining pneumoperitoneum and the principles and application of electrosurgery, ultrasonic energy, and various laser energy sources, for instance, also would be acquired.
The second quarter would encompass patient selection, preoperative planning and preparation, and patient counseling. Technical skills for performing safe diagnostic laparoscopy and hysteroscopy would be a focus, along with an understanding of potential complications and appropriate management. The third quarter would be dedicated to acquiring specific surgical skills – knowing the boundaries of pelvic spaces and using techniques such as hydrodissection and traction/countertraction, for instance, as well as performing laparoscopic suturing and using agents for adhesion prevention. By the end of the fourth quarter, the trainee would be able to independently implement appropriate surgical and medical plans as well as teach and assist other surgeons.
It is important to appreciate that robotic surgery is, in fact, a form of laparoscopy, and the best preparation for robotic surgery lies in basic laparoscopic training. There are physicians who are performing both laparoscopic and robotic-assisted laparoscopic surgery without having a basic knowledge of laparoscopy.
Looking back to the general surgery literature of the late 1980s and early 1990s, there were reports of complications relating to laparoscopy that were new to general surgeons but were well known to gynecologists, such as problems with abdominal entry, port-related injuries, and laser and electrosurgery injuries. Currently, a look at the robotic urology literature shows the same known laparoscopy-related complications being reported as robotic surgery complications.
Dr. Nezhat is the current secretary/treasurer of the AAGL; adjunct clinical associate professor of gynecology and obstetrics at Emory University, Atlanta; adjunct clinical associate professor of obstetrics and gynecology, Stanford (Calif.) University; and fellowship director, Center for Special Minimally Invasive Surgery & Reproductive Medicine, Atlanta. He is a pioneer in the field of laparoscopic surgery, specializing in infertility and endometriosis, as well as urologic and pelvic reconstruction. He has authored more than 100 publications including refereed journal articles, book chapters, and two books. Dr. Nezhat is a frequent lecturer and educator and regularly serves as a medical volunteer in underdeveloped countries. He had no disclosures to report.
Minimally invasive endoscopy, with and without robotics, has become a viable option for almost every operative gynecologic procedure.
Yet, despite a solid body of evidence demonstrating the benefits of minimally invasive approaches and despite tremendous progress in postgraduate education, many gynecologic surgeons are not incorporating endoscopic options into their practices – or even into their patient education. Others have adopted minimally invasive techniques, but sometimes without the essential or optimal knowledge and skills needed to perform minimally invasive gynecologic surgery with optimal outcomes and patient safety.
New gynecologic surgeons, in the meantime, are leaving residency programs with variable levels of experience and proficiency. Some have adequate opportunity to gain the skills and experience they need to be comfortable and confident in the endoscopic arena, but many do not.
As Dr. Charles E. Miller, past president of the AAGL (formerly known as the American Association of Gynecologic Laparoscopists), said in his presidential address in 2008, data have shown that one in three residents graduate with a paucity of experience in minimally invasive gynecologic procedures.
At the root of these realities are the lack of a defined knowledge base and the lack of a specific, unified endoscopy curriculum. There have been no minimal standards set for gynecologic endoscopy, no quantifiable measures defined for proficiency in minimally invasive gynecologic surgery, and no validated tools developed for gynecologic surgeons to learn and then document their competency.
Change is underway, however. For the past 2 years, the AAGL has worked with hundreds of physicians and experts to determine the essential knowledge needed for performing minimally invasive gynecologic surgery – and to develop an appropriate assessment test for gynecologic surgeons.
Called Essentials in Minimally Invasive Gynecology (EMIG), the test is based upon professional testing standards and guidelines established by professional testing societies. The process used to develop the test has incorporated the important principles of validity, reliability, and defensibility.
The EMIG cognitive assessment is currently in the beta-test stage and is scheduled to be administered for the first time in November 2012 at the AAGL annual clinical meeting. After that, physicians will be able to take the test through any one of multiple proctored testing centers operated worldwide by the consulting firm that helped develop and validate Fundamentals of Laparoscopic Surgery (FLS), the educational program that is now a standard part of all general surgery residencies.
The EMIG cognitive test covers the areas of applied anatomy and physiology, endoscopic principles, patient selection, instrumentation, energy sources, operating room/equipment setup, laparoscopy, hysteroscopy, and complications. A skills testing portion of the EMIG is under development.
Most immediately, for practicing gynecologic surgeons, EMIG will give them the opportunity to demonstrate and validate their laparoscopic skills. Then, as training becomes more standardized, gynecologists will be able to acquire skills, or enhance their skills, through postgraduate courses that incorporate EMIG principles in a unified, standardized manner.
Continuous Progress
It was gynecology that pioneered use of the laparoscope and brought it to our general surgery colleagues. And over the years, gynecology has been cognizant of the need to develop a standardized assessment, curriculum, and training to meet the demands of the dynamic developments in endoscopy.
In the early 1990s, the American College of Obstetricians and Gynecologists issued basic guidelines for credentialing gynecologists to perform laparoscopy, and the Accreditation Council for Gynecologic Endoscopy (now the Council for Gynecologic Endoscopy and part of the AAGL) started a registry identifying gynecologists skilled at laparoscopy and hysteroscopy.
In 1998, Dr. Andrew Brill and Dr. Robert Rogers outlined a comprehensive program for resident training in the Journal of the American Association of Gynecologic Laparoscopists (J. Am. Assoc. Gynecol. Laparosc. 1998;5:223-8). Recently, the Fellowship in Minimally Invasive Gynecologic Surgery program issued guidelines as well for fellow and resident education, hands-on training, written examinations and skills testing, and supervised surgery.
In the world of general surgery, in the meantime, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) began developing the FLS in the late 1990s; the FLS is a comprehensive web-based education module designed to teach and assess basic cognitive, technical, and psychomotor skills required to perform laparoscopic surgery.
FLS has two parts: didactic testing, which is online multiple choice testing, and skills testing, which is hands-on. Endorsed by the American College of Surgeons (ACS), FLS was designed to give surgical residents, fellows, and practicing physicians an opportunity to learn the fundamentals of laparoscopic surgery in a consistent, scientific format.
The goal was not only to increase familiarity with and access to minimally invasive techniques, but to ensure good outcomes and patient safety.
Since the FLS test became available in 2004, the number of physicians seeking FLS certification rose annually. A study of the first 5 years of FLS certification testing (2004-2009) shows an overall pass rate of 88% on the examination – close to the target pass rate of 90% established during the test-setting process. Almost 20% of the test participants over this time period were attending surgeons; the rest were senior residents or fellows (the largest participant group) and junior residents. A small number – 4% – were gynecologists (Surg. Endosc. 2011;25:1192-8).
In 2008, the ACS went one step further by requiring all general surgery residents, starting in 2009, to complete and pass the FLS course before achieving board certification. This mandate set minimum standards for basic cognitive and technical skills, and encouraged a uniform framework for laparoscopy training in general surgery residency programs.
Urology has similarly deliberated and addressed the idea that standard training and credentialing initiatives are critical to have and to update as surgical technology and techniques evolve. The American Urologic Association has developed and is validating a Basic Laparoscopic Urologic Surgery (BLUS) skills curriculum similar to FLS for laparoscopic cognitive and technical skills. The association also has created a Simulation Advisory Board that will vet current and future skills courses and provide guidance for matching learning objectives with effective simulation-based technologies. In addition, the AUA is expanding a preexisting urology-specific robotic skills curriculum and, along with AAGL, SAGES, and the Minimally Invasive Robotic Association, is developing a Fundamentals of Robotic Skills curriculum.
Essential Gynecologic Knowledge
As minimally invasive surgery becomes mainstream, it is important for gynecologic surgeons to be familiar with the fundamentals of laparoscopic gynecologic surgery and hysteroscopy, whether or not they are utilizing or planning to utilize robotic technology.
Development of the cognitive portion of EMIG is the first step of an AAGL-led effort that will ensure that gynecology meets the need for a standardized, demonstrable set of skills. As it takes shape and gains acceptance, the EMIG assessment should provide a standardized tool for physicians, patients, hospitals and payers to identify gynecologic surgeons who have demonstrated the knowledge and skills necessary to understand the intricacies of minimally invasive gynecology.
Unlike the FLS, the EMIG test is specifically focused on gynecology and includes hysteroscopy. Hundreds of physicians were involved in completing a survey, writing psychometrically correct items, and reviewing questions. A test development psychometrician directed the AAGL through test development and technical review and performed a psychometric edit.
As of May 2012, beta testers were scheduled to answer approximately 380 questions, over two sessions totaling 6-8 hours, in a proctored environment. From the results, two forms of the test, with approximately 125 questions each, will be created. A passing score will be determined, and the test will be made available in online test delivery software so it can be taken anywhere in the world – after its first run at the AAGL meeting this fall.
Concurrently, the AAGL has been developing an approach to testing psychomotor skills, which is needed, along with cognitive assessment, to ascertain the qualifications of a surgeon. The AAGL identified essential skills by asking more than 1,000 physicians to rank the importance and frequency of the skills. This information will be used to evaluate skills trainers and testing instruments.
Eventually, it is hoped, a standardized core curriculum – based on the EMIG knowledge base – will be developed and incorporated into residency programs, just as it has been for general surgery. Such a curriculum could also be implemented in the Fellowship in Minimally Invasive Gynecologic Surgery, cosponsored by the AAGL and the Society of Reproductive Surgeons (an affiliate society of the American Society for Reproductive Medicine), in which case it could be longer in duration.
A standardized core curriculum could be implemented as well in the growing number of postgraduate courses that are available to practicing gynecologic surgeons; in this case, the curriculum would be shorter in duration.
A proposal for a formal gynecologic endoscopy curriculum, published a few years ago in The Journal of Minimally Invasive Gynecology (J. Minim. Invasive Gynecol. 2009;16:416-21), envisions a quarterly system with an online examination given after quarters 1 and 2, a hands-on examination given after quarter 3, and a demonstration of leadership and teaching skills in the operating room after quarter 4.
As envisioned in the proposed curriculum, the first quarter would focus on knowledge of pelvic and abdominal anatomy that is specific to laparoscopy and gynecology, including the major branches of anterior and posterior division of the internal iliac artery and the major nerve supplies to the pelvis. Knowledge of the physiology and principles of creating and maintaining pneumoperitoneum and the principles and application of electrosurgery, ultrasonic energy, and various laser energy sources, for instance, also would be acquired.
The second quarter would encompass patient selection, preoperative planning and preparation, and patient counseling. Technical skills for performing safe diagnostic laparoscopy and hysteroscopy would be a focus, along with an understanding of potential complications and appropriate management. The third quarter would be dedicated to acquiring specific surgical skills – knowing the boundaries of pelvic spaces and using techniques such as hydrodissection and traction/countertraction, for instance, as well as performing laparoscopic suturing and using agents for adhesion prevention. By the end of the fourth quarter, the trainee would be able to independently implement appropriate surgical and medical plans as well as teach and assist other surgeons.
It is important to appreciate that robotic surgery is, in fact, a form of laparoscopy, and the best preparation for robotic surgery lies in basic laparoscopic training. There are physicians who are performing both laparoscopic and robotic-assisted laparoscopic surgery without having a basic knowledge of laparoscopy.
Looking back to the general surgery literature of the late 1980s and early 1990s, there were reports of complications relating to laparoscopy that were new to general surgeons but were well known to gynecologists, such as problems with abdominal entry, port-related injuries, and laser and electrosurgery injuries. Currently, a look at the robotic urology literature shows the same known laparoscopy-related complications being reported as robotic surgery complications.
Dr. Nezhat is the current secretary/treasurer of the AAGL; adjunct clinical associate professor of gynecology and obstetrics at Emory University, Atlanta; adjunct clinical associate professor of obstetrics and gynecology, Stanford (Calif.) University; and fellowship director, Center for Special Minimally Invasive Surgery & Reproductive Medicine, Atlanta. He is a pioneer in the field of laparoscopic surgery, specializing in infertility and endometriosis, as well as urologic and pelvic reconstruction. He has authored more than 100 publications including refereed journal articles, book chapters, and two books. Dr. Nezhat is a frequent lecturer and educator and regularly serves as a medical volunteer in underdeveloped countries. He had no disclosures to report.
Minimally invasive endoscopy, with and without robotics, has become a viable option for almost every operative gynecologic procedure.
Yet, despite a solid body of evidence demonstrating the benefits of minimally invasive approaches and despite tremendous progress in postgraduate education, many gynecologic surgeons are not incorporating endoscopic options into their practices – or even into their patient education. Others have adopted minimally invasive techniques, but sometimes without the essential or optimal knowledge and skills needed to perform minimally invasive gynecologic surgery with optimal outcomes and patient safety.
New gynecologic surgeons, in the meantime, are leaving residency programs with variable levels of experience and proficiency. Some have adequate opportunity to gain the skills and experience they need to be comfortable and confident in the endoscopic arena, but many do not.
As Dr. Charles E. Miller, past president of the AAGL (formerly known as the American Association of Gynecologic Laparoscopists), said in his presidential address in 2008, data have shown that one in three residents graduate with a paucity of experience in minimally invasive gynecologic procedures.
At the root of these realities are the lack of a defined knowledge base and the lack of a specific, unified endoscopy curriculum. There have been no minimal standards set for gynecologic endoscopy, no quantifiable measures defined for proficiency in minimally invasive gynecologic surgery, and no validated tools developed for gynecologic surgeons to learn and then document their competency.
Change is underway, however. For the past 2 years, the AAGL has worked with hundreds of physicians and experts to determine the essential knowledge needed for performing minimally invasive gynecologic surgery – and to develop an appropriate assessment test for gynecologic surgeons.
Called Essentials in Minimally Invasive Gynecology (EMIG), the test is based upon professional testing standards and guidelines established by professional testing societies. The process used to develop the test has incorporated the important principles of validity, reliability, and defensibility.
The EMIG cognitive assessment is currently in the beta-test stage and is scheduled to be administered for the first time in November 2012 at the AAGL annual clinical meeting. After that, physicians will be able to take the test through any one of multiple proctored testing centers operated worldwide by the consulting firm that helped develop and validate Fundamentals of Laparoscopic Surgery (FLS), the educational program that is now a standard part of all general surgery residencies.
The EMIG cognitive test covers the areas of applied anatomy and physiology, endoscopic principles, patient selection, instrumentation, energy sources, operating room/equipment setup, laparoscopy, hysteroscopy, and complications. A skills testing portion of the EMIG is under development.
Most immediately, for practicing gynecologic surgeons, EMIG will give them the opportunity to demonstrate and validate their laparoscopic skills. Then, as training becomes more standardized, gynecologists will be able to acquire skills, or enhance their skills, through postgraduate courses that incorporate EMIG principles in a unified, standardized manner.
Continuous Progress
It was gynecology that pioneered use of the laparoscope and brought it to our general surgery colleagues. And over the years, gynecology has been cognizant of the need to develop a standardized assessment, curriculum, and training to meet the demands of the dynamic developments in endoscopy.
In the early 1990s, the American College of Obstetricians and Gynecologists issued basic guidelines for credentialing gynecologists to perform laparoscopy, and the Accreditation Council for Gynecologic Endoscopy (now the Council for Gynecologic Endoscopy and part of the AAGL) started a registry identifying gynecologists skilled at laparoscopy and hysteroscopy.
In 1998, Dr. Andrew Brill and Dr. Robert Rogers outlined a comprehensive program for resident training in the Journal of the American Association of Gynecologic Laparoscopists (J. Am. Assoc. Gynecol. Laparosc. 1998;5:223-8). Recently, the Fellowship in Minimally Invasive Gynecologic Surgery program issued guidelines as well for fellow and resident education, hands-on training, written examinations and skills testing, and supervised surgery.
In the world of general surgery, in the meantime, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) began developing the FLS in the late 1990s; the FLS is a comprehensive web-based education module designed to teach and assess basic cognitive, technical, and psychomotor skills required to perform laparoscopic surgery.
FLS has two parts: didactic testing, which is online multiple choice testing, and skills testing, which is hands-on. Endorsed by the American College of Surgeons (ACS), FLS was designed to give surgical residents, fellows, and practicing physicians an opportunity to learn the fundamentals of laparoscopic surgery in a consistent, scientific format.
The goal was not only to increase familiarity with and access to minimally invasive techniques, but to ensure good outcomes and patient safety.
Since the FLS test became available in 2004, the number of physicians seeking FLS certification rose annually. A study of the first 5 years of FLS certification testing (2004-2009) shows an overall pass rate of 88% on the examination – close to the target pass rate of 90% established during the test-setting process. Almost 20% of the test participants over this time period were attending surgeons; the rest were senior residents or fellows (the largest participant group) and junior residents. A small number – 4% – were gynecologists (Surg. Endosc. 2011;25:1192-8).
In 2008, the ACS went one step further by requiring all general surgery residents, starting in 2009, to complete and pass the FLS course before achieving board certification. This mandate set minimum standards for basic cognitive and technical skills, and encouraged a uniform framework for laparoscopy training in general surgery residency programs.
Urology has similarly deliberated and addressed the idea that standard training and credentialing initiatives are critical to have and to update as surgical technology and techniques evolve. The American Urologic Association has developed and is validating a Basic Laparoscopic Urologic Surgery (BLUS) skills curriculum similar to FLS for laparoscopic cognitive and technical skills. The association also has created a Simulation Advisory Board that will vet current and future skills courses and provide guidance for matching learning objectives with effective simulation-based technologies. In addition, the AUA is expanding a preexisting urology-specific robotic skills curriculum and, along with AAGL, SAGES, and the Minimally Invasive Robotic Association, is developing a Fundamentals of Robotic Skills curriculum.
Essential Gynecologic Knowledge
As minimally invasive surgery becomes mainstream, it is important for gynecologic surgeons to be familiar with the fundamentals of laparoscopic gynecologic surgery and hysteroscopy, whether or not they are utilizing or planning to utilize robotic technology.
Development of the cognitive portion of EMIG is the first step of an AAGL-led effort that will ensure that gynecology meets the need for a standardized, demonstrable set of skills. As it takes shape and gains acceptance, the EMIG assessment should provide a standardized tool for physicians, patients, hospitals and payers to identify gynecologic surgeons who have demonstrated the knowledge and skills necessary to understand the intricacies of minimally invasive gynecology.
Unlike the FLS, the EMIG test is specifically focused on gynecology and includes hysteroscopy. Hundreds of physicians were involved in completing a survey, writing psychometrically correct items, and reviewing questions. A test development psychometrician directed the AAGL through test development and technical review and performed a psychometric edit.
As of May 2012, beta testers were scheduled to answer approximately 380 questions, over two sessions totaling 6-8 hours, in a proctored environment. From the results, two forms of the test, with approximately 125 questions each, will be created. A passing score will be determined, and the test will be made available in online test delivery software so it can be taken anywhere in the world – after its first run at the AAGL meeting this fall.
Concurrently, the AAGL has been developing an approach to testing psychomotor skills, which is needed, along with cognitive assessment, to ascertain the qualifications of a surgeon. The AAGL identified essential skills by asking more than 1,000 physicians to rank the importance and frequency of the skills. This information will be used to evaluate skills trainers and testing instruments.
Eventually, it is hoped, a standardized core curriculum – based on the EMIG knowledge base – will be developed and incorporated into residency programs, just as it has been for general surgery. Such a curriculum could also be implemented in the Fellowship in Minimally Invasive Gynecologic Surgery, cosponsored by the AAGL and the Society of Reproductive Surgeons (an affiliate society of the American Society for Reproductive Medicine), in which case it could be longer in duration.
A standardized core curriculum could be implemented as well in the growing number of postgraduate courses that are available to practicing gynecologic surgeons; in this case, the curriculum would be shorter in duration.
A proposal for a formal gynecologic endoscopy curriculum, published a few years ago in The Journal of Minimally Invasive Gynecology (J. Minim. Invasive Gynecol. 2009;16:416-21), envisions a quarterly system with an online examination given after quarters 1 and 2, a hands-on examination given after quarter 3, and a demonstration of leadership and teaching skills in the operating room after quarter 4.
As envisioned in the proposed curriculum, the first quarter would focus on knowledge of pelvic and abdominal anatomy that is specific to laparoscopy and gynecology, including the major branches of anterior and posterior division of the internal iliac artery and the major nerve supplies to the pelvis. Knowledge of the physiology and principles of creating and maintaining pneumoperitoneum and the principles and application of electrosurgery, ultrasonic energy, and various laser energy sources, for instance, also would be acquired.
The second quarter would encompass patient selection, preoperative planning and preparation, and patient counseling. Technical skills for performing safe diagnostic laparoscopy and hysteroscopy would be a focus, along with an understanding of potential complications and appropriate management. The third quarter would be dedicated to acquiring specific surgical skills – knowing the boundaries of pelvic spaces and using techniques such as hydrodissection and traction/countertraction, for instance, as well as performing laparoscopic suturing and using agents for adhesion prevention. By the end of the fourth quarter, the trainee would be able to independently implement appropriate surgical and medical plans as well as teach and assist other surgeons.
It is important to appreciate that robotic surgery is, in fact, a form of laparoscopy, and the best preparation for robotic surgery lies in basic laparoscopic training. There are physicians who are performing both laparoscopic and robotic-assisted laparoscopic surgery without having a basic knowledge of laparoscopy.
Looking back to the general surgery literature of the late 1980s and early 1990s, there were reports of complications relating to laparoscopy that were new to general surgeons but were well known to gynecologists, such as problems with abdominal entry, port-related injuries, and laser and electrosurgery injuries. Currently, a look at the robotic urology literature shows the same known laparoscopy-related complications being reported as robotic surgery complications.
Dr. Nezhat is the current secretary/treasurer of the AAGL; adjunct clinical associate professor of gynecology and obstetrics at Emory University, Atlanta; adjunct clinical associate professor of obstetrics and gynecology, Stanford (Calif.) University; and fellowship director, Center for Special Minimally Invasive Surgery & Reproductive Medicine, Atlanta. He is a pioneer in the field of laparoscopic surgery, specializing in infertility and endometriosis, as well as urologic and pelvic reconstruction. He has authored more than 100 publications including refereed journal articles, book chapters, and two books. Dr. Nezhat is a frequent lecturer and educator and regularly serves as a medical volunteer in underdeveloped countries. He had no disclosures to report.
A Sound Model of Obstetric Ethics
Ethics is an essential dimension of day-to-day obstetric practice, and not merely something to be left to administrators, lawyers, or clergy. A sound, acceptable model of ethics must be an integral part of our thinking, our clinical judgment, and our navigation of the sometimes clashing demands of fetal and maternal "rights."
Obstetric ethics has too often been characterized by polarized assertions, claiming either that the rights of the fetus or the rights of the pregnant woman must take precedence. These ethical models – one favoring fetal rights and one favoring the pregnant woman’s rights – are overly simplistic and highly flawed. Rather, obstetricians must embrace a "professional responsibility model" of ethics in which they care for and protect both the pregnant and the fetal patient.
The obstetrician-gynecologist’s professional obligations originate in the same ethical concept of the profession of medicine, and physicians as professionals. This concept emanated in large part from the work of two remarkable physician-philosophers, John Gregory and Thomas Percival, who practiced in Great Britain in the 18th century.
At that time, there was rampant entrepreneurialism in medicine. It was treated primarily as a business and was characterized by an overriding drive to obtain money and compete effectively. Gregory and Percival were both troubled by this model and argued that the patient should always come first. The concept they put forth was that physicians should be scientifically, ethically, and clinically competent and should protect and promote the patient as their primary concern and motivation.
Each spoke of medicine as a "public trust," and maintained that self-interest is fine – as long as such interest is a secondary consideration. Their work was incorporated into the first code of ethics of the American Medical Association in 1847, and the views of both these physician-philosophers were translated throughout the world. Today, as we face ever-increasing fiscal pressures and attempt to reshape the construct of obstetric ethics and move away from rights-based extremes, their work is very much alive and relevant.
The Clashing of Rights
The ethical issues we face as obstetricians are especially challenging, as we frequently have two patients. We often are faced with two extreme positions in obstetrics – and two models of ethics based on these positions – that emphasize overwhelmingly fetal or exclusively maternal rights.
However, these rights-based models are overly simplistic, embracing what is known as "reductionism." George Engel made a landmark contribution to medicine when he argued against biological reductionism. He developed what’s referred to as the biopsychosocial model of health and disease, which considers clinically relevant psychosocial aspects of medicine as well as anatomic and pathophysiologic factors.
Engel argued against clinical tunnel vision where only biologic factors are considered; he warned that a purely biomedical model is scientifically and clinically incomplete and, therefore, misleading. His proposed biopsychosocial model is an integral part of medicine today. It reminds the ob.gyn. that many factors can contribute toward reproductive failure, for instance, and that comprehensive clinical judgment during pregnancy requires attention to psychological and social dimensions as well as biological fundamentals.
Moreover, Engel’s case against biologic reductionism in medicine is analogous to the challenge we face today in obstetric ethics, with frequent use of two rights-based reductionism models for ethical thinking. As my associates and I have detailed in a previously published article on the professional responsibility model of obstetric ethics, reductionism has an appealing simplicity in either extreme, but is ethically incomplete, clinically inadequate, and, therefore, unprofessional (Am. J. Obstet. Gynecol. 2011;205:315.e1-5).
Under the fetal rights reductionism model of obstetric ethics, fetal rights systematically override the pregnant woman’s rights. This model emphasizes the fetal right to life, for instance, from the moment of conception; the option of abortion is prohibited regardless of gestational age or whether the pregnancy is voluntary or not.
At the other extreme, under the pregnant woman’s rights model, pregnant women have an unconditional, systematic right to control their own bodies; their rights automatically override fetal rights. This model would support a woman’s right for her obstetrician to attend her planned home birth, for instance, or her right to refuse cesarean delivery or to have a clinically nonindicated cesarean delivery.
The Professional Responsibility Model of Obstetric Ethics
A third model – what my associates and I call the professional responsibility model of obstetric ethics – emphasizes the relationship of the physician to the patient and the physician’s responsibility to and for his or her patients. As obstetricians, our professional obligations are owed to both the pregnant woman and the fetal patient. The two patients, and our obligations to them, are inexorably linked.
To the pregnant woman, we have both autonomy-based and beneficence-based obligations. We also have beneficence-based obligations to the fetus when there are linkages between the fetus and the child the fetus will become. One such linkage is viability. Another such linkage occurs when the pregnant women confers on her pre-viable fetus the moral status of being a patient, based on her beliefs and values.
Our obligations to the fetal patient are not absolute obligations; they must in all cases be balanced with autonomy- and beneficence-based obligations to the pregnant woman. Conversely, obligations to the pregnant woman must be balanced in all cases with obligations to the fetal patient.
The view that the fetus has rights, such as an unconditional "right" to life, does not consider the fact that there are irreconcilable differences among and within the major religions of the world – and among cultures, philosophers, and other authoritative sources – on the status of a pre-viable fetus and on fetal rights.
The woman’s "right" to be attended by her obstetrician for her planned home birth as viewed through the pregnant woman’s rights model, on the other hand, does not consider that there are obligations of the medical profession and also of the pregnant woman to the soon-to-be-born child (Obstet. Gynecol. 2011:117:1183-7). Neither does a woman’s "right" to smoke during pregnancy, nor her "right" to have cesarean delivery that is not medically indicated. In the latter case, for example, the professional responsibility model of obstetric ethics tells us we have a strong ethical obligation to try to persuade the pregnant woman not to have a procedure that is medically disadvantageous – an obligation that rights-based models ignore.
A woman who does not avail herself of the option of abortion before fetal viability subsequently has obligations to the soon-to-be-born child. The pregnant woman, in other words, should not be viewed as a person who has absolute rights, but as one who also has obligations to her soon-to-be-born child (Am. J. Obstet. Gynecol. 2009;201:560.e1-6).
Rights-based approaches obscure professional obligations. A professional responsibility model, on the other hand, is professional; it emphasizes obligations we have to both the pregnant woman and the fetal patient, and enables us to avoid extremes that promote unprofessional behavior. The model creates a solid foundation for our care of both the pregnant and fetal patient.
Dr. Chervenak said he has no relevant financial disclosures.
Ethics is an essential dimension of day-to-day obstetric practice, and not merely something to be left to administrators, lawyers, or clergy. A sound, acceptable model of ethics must be an integral part of our thinking, our clinical judgment, and our navigation of the sometimes clashing demands of fetal and maternal "rights."
Obstetric ethics has too often been characterized by polarized assertions, claiming either that the rights of the fetus or the rights of the pregnant woman must take precedence. These ethical models – one favoring fetal rights and one favoring the pregnant woman’s rights – are overly simplistic and highly flawed. Rather, obstetricians must embrace a "professional responsibility model" of ethics in which they care for and protect both the pregnant and the fetal patient.
The obstetrician-gynecologist’s professional obligations originate in the same ethical concept of the profession of medicine, and physicians as professionals. This concept emanated in large part from the work of two remarkable physician-philosophers, John Gregory and Thomas Percival, who practiced in Great Britain in the 18th century.
At that time, there was rampant entrepreneurialism in medicine. It was treated primarily as a business and was characterized by an overriding drive to obtain money and compete effectively. Gregory and Percival were both troubled by this model and argued that the patient should always come first. The concept they put forth was that physicians should be scientifically, ethically, and clinically competent and should protect and promote the patient as their primary concern and motivation.
Each spoke of medicine as a "public trust," and maintained that self-interest is fine – as long as such interest is a secondary consideration. Their work was incorporated into the first code of ethics of the American Medical Association in 1847, and the views of both these physician-philosophers were translated throughout the world. Today, as we face ever-increasing fiscal pressures and attempt to reshape the construct of obstetric ethics and move away from rights-based extremes, their work is very much alive and relevant.
The Clashing of Rights
The ethical issues we face as obstetricians are especially challenging, as we frequently have two patients. We often are faced with two extreme positions in obstetrics – and two models of ethics based on these positions – that emphasize overwhelmingly fetal or exclusively maternal rights.
However, these rights-based models are overly simplistic, embracing what is known as "reductionism." George Engel made a landmark contribution to medicine when he argued against biological reductionism. He developed what’s referred to as the biopsychosocial model of health and disease, which considers clinically relevant psychosocial aspects of medicine as well as anatomic and pathophysiologic factors.
Engel argued against clinical tunnel vision where only biologic factors are considered; he warned that a purely biomedical model is scientifically and clinically incomplete and, therefore, misleading. His proposed biopsychosocial model is an integral part of medicine today. It reminds the ob.gyn. that many factors can contribute toward reproductive failure, for instance, and that comprehensive clinical judgment during pregnancy requires attention to psychological and social dimensions as well as biological fundamentals.
Moreover, Engel’s case against biologic reductionism in medicine is analogous to the challenge we face today in obstetric ethics, with frequent use of two rights-based reductionism models for ethical thinking. As my associates and I have detailed in a previously published article on the professional responsibility model of obstetric ethics, reductionism has an appealing simplicity in either extreme, but is ethically incomplete, clinically inadequate, and, therefore, unprofessional (Am. J. Obstet. Gynecol. 2011;205:315.e1-5).
Under the fetal rights reductionism model of obstetric ethics, fetal rights systematically override the pregnant woman’s rights. This model emphasizes the fetal right to life, for instance, from the moment of conception; the option of abortion is prohibited regardless of gestational age or whether the pregnancy is voluntary or not.
At the other extreme, under the pregnant woman’s rights model, pregnant women have an unconditional, systematic right to control their own bodies; their rights automatically override fetal rights. This model would support a woman’s right for her obstetrician to attend her planned home birth, for instance, or her right to refuse cesarean delivery or to have a clinically nonindicated cesarean delivery.
The Professional Responsibility Model of Obstetric Ethics
A third model – what my associates and I call the professional responsibility model of obstetric ethics – emphasizes the relationship of the physician to the patient and the physician’s responsibility to and for his or her patients. As obstetricians, our professional obligations are owed to both the pregnant woman and the fetal patient. The two patients, and our obligations to them, are inexorably linked.
To the pregnant woman, we have both autonomy-based and beneficence-based obligations. We also have beneficence-based obligations to the fetus when there are linkages between the fetus and the child the fetus will become. One such linkage is viability. Another such linkage occurs when the pregnant women confers on her pre-viable fetus the moral status of being a patient, based on her beliefs and values.
Our obligations to the fetal patient are not absolute obligations; they must in all cases be balanced with autonomy- and beneficence-based obligations to the pregnant woman. Conversely, obligations to the pregnant woman must be balanced in all cases with obligations to the fetal patient.
The view that the fetus has rights, such as an unconditional "right" to life, does not consider the fact that there are irreconcilable differences among and within the major religions of the world – and among cultures, philosophers, and other authoritative sources – on the status of a pre-viable fetus and on fetal rights.
The woman’s "right" to be attended by her obstetrician for her planned home birth as viewed through the pregnant woman’s rights model, on the other hand, does not consider that there are obligations of the medical profession and also of the pregnant woman to the soon-to-be-born child (Obstet. Gynecol. 2011:117:1183-7). Neither does a woman’s "right" to smoke during pregnancy, nor her "right" to have cesarean delivery that is not medically indicated. In the latter case, for example, the professional responsibility model of obstetric ethics tells us we have a strong ethical obligation to try to persuade the pregnant woman not to have a procedure that is medically disadvantageous – an obligation that rights-based models ignore.
A woman who does not avail herself of the option of abortion before fetal viability subsequently has obligations to the soon-to-be-born child. The pregnant woman, in other words, should not be viewed as a person who has absolute rights, but as one who also has obligations to her soon-to-be-born child (Am. J. Obstet. Gynecol. 2009;201:560.e1-6).
Rights-based approaches obscure professional obligations. A professional responsibility model, on the other hand, is professional; it emphasizes obligations we have to both the pregnant woman and the fetal patient, and enables us to avoid extremes that promote unprofessional behavior. The model creates a solid foundation for our care of both the pregnant and fetal patient.
Dr. Chervenak said he has no relevant financial disclosures.
Ethics is an essential dimension of day-to-day obstetric practice, and not merely something to be left to administrators, lawyers, or clergy. A sound, acceptable model of ethics must be an integral part of our thinking, our clinical judgment, and our navigation of the sometimes clashing demands of fetal and maternal "rights."
Obstetric ethics has too often been characterized by polarized assertions, claiming either that the rights of the fetus or the rights of the pregnant woman must take precedence. These ethical models – one favoring fetal rights and one favoring the pregnant woman’s rights – are overly simplistic and highly flawed. Rather, obstetricians must embrace a "professional responsibility model" of ethics in which they care for and protect both the pregnant and the fetal patient.
The obstetrician-gynecologist’s professional obligations originate in the same ethical concept of the profession of medicine, and physicians as professionals. This concept emanated in large part from the work of two remarkable physician-philosophers, John Gregory and Thomas Percival, who practiced in Great Britain in the 18th century.
At that time, there was rampant entrepreneurialism in medicine. It was treated primarily as a business and was characterized by an overriding drive to obtain money and compete effectively. Gregory and Percival were both troubled by this model and argued that the patient should always come first. The concept they put forth was that physicians should be scientifically, ethically, and clinically competent and should protect and promote the patient as their primary concern and motivation.
Each spoke of medicine as a "public trust," and maintained that self-interest is fine – as long as such interest is a secondary consideration. Their work was incorporated into the first code of ethics of the American Medical Association in 1847, and the views of both these physician-philosophers were translated throughout the world. Today, as we face ever-increasing fiscal pressures and attempt to reshape the construct of obstetric ethics and move away from rights-based extremes, their work is very much alive and relevant.
The Clashing of Rights
The ethical issues we face as obstetricians are especially challenging, as we frequently have two patients. We often are faced with two extreme positions in obstetrics – and two models of ethics based on these positions – that emphasize overwhelmingly fetal or exclusively maternal rights.
However, these rights-based models are overly simplistic, embracing what is known as "reductionism." George Engel made a landmark contribution to medicine when he argued against biological reductionism. He developed what’s referred to as the biopsychosocial model of health and disease, which considers clinically relevant psychosocial aspects of medicine as well as anatomic and pathophysiologic factors.
Engel argued against clinical tunnel vision where only biologic factors are considered; he warned that a purely biomedical model is scientifically and clinically incomplete and, therefore, misleading. His proposed biopsychosocial model is an integral part of medicine today. It reminds the ob.gyn. that many factors can contribute toward reproductive failure, for instance, and that comprehensive clinical judgment during pregnancy requires attention to psychological and social dimensions as well as biological fundamentals.
Moreover, Engel’s case against biologic reductionism in medicine is analogous to the challenge we face today in obstetric ethics, with frequent use of two rights-based reductionism models for ethical thinking. As my associates and I have detailed in a previously published article on the professional responsibility model of obstetric ethics, reductionism has an appealing simplicity in either extreme, but is ethically incomplete, clinically inadequate, and, therefore, unprofessional (Am. J. Obstet. Gynecol. 2011;205:315.e1-5).
Under the fetal rights reductionism model of obstetric ethics, fetal rights systematically override the pregnant woman’s rights. This model emphasizes the fetal right to life, for instance, from the moment of conception; the option of abortion is prohibited regardless of gestational age or whether the pregnancy is voluntary or not.
At the other extreme, under the pregnant woman’s rights model, pregnant women have an unconditional, systematic right to control their own bodies; their rights automatically override fetal rights. This model would support a woman’s right for her obstetrician to attend her planned home birth, for instance, or her right to refuse cesarean delivery or to have a clinically nonindicated cesarean delivery.
The Professional Responsibility Model of Obstetric Ethics
A third model – what my associates and I call the professional responsibility model of obstetric ethics – emphasizes the relationship of the physician to the patient and the physician’s responsibility to and for his or her patients. As obstetricians, our professional obligations are owed to both the pregnant woman and the fetal patient. The two patients, and our obligations to them, are inexorably linked.
To the pregnant woman, we have both autonomy-based and beneficence-based obligations. We also have beneficence-based obligations to the fetus when there are linkages between the fetus and the child the fetus will become. One such linkage is viability. Another such linkage occurs when the pregnant women confers on her pre-viable fetus the moral status of being a patient, based on her beliefs and values.
Our obligations to the fetal patient are not absolute obligations; they must in all cases be balanced with autonomy- and beneficence-based obligations to the pregnant woman. Conversely, obligations to the pregnant woman must be balanced in all cases with obligations to the fetal patient.
The view that the fetus has rights, such as an unconditional "right" to life, does not consider the fact that there are irreconcilable differences among and within the major religions of the world – and among cultures, philosophers, and other authoritative sources – on the status of a pre-viable fetus and on fetal rights.
The woman’s "right" to be attended by her obstetrician for her planned home birth as viewed through the pregnant woman’s rights model, on the other hand, does not consider that there are obligations of the medical profession and also of the pregnant woman to the soon-to-be-born child (Obstet. Gynecol. 2011:117:1183-7). Neither does a woman’s "right" to smoke during pregnancy, nor her "right" to have cesarean delivery that is not medically indicated. In the latter case, for example, the professional responsibility model of obstetric ethics tells us we have a strong ethical obligation to try to persuade the pregnant woman not to have a procedure that is medically disadvantageous – an obligation that rights-based models ignore.
A woman who does not avail herself of the option of abortion before fetal viability subsequently has obligations to the soon-to-be-born child. The pregnant woman, in other words, should not be viewed as a person who has absolute rights, but as one who also has obligations to her soon-to-be-born child (Am. J. Obstet. Gynecol. 2009;201:560.e1-6).
Rights-based approaches obscure professional obligations. A professional responsibility model, on the other hand, is professional; it emphasizes obligations we have to both the pregnant woman and the fetal patient, and enables us to avoid extremes that promote unprofessional behavior. The model creates a solid foundation for our care of both the pregnant and fetal patient.
Dr. Chervenak said he has no relevant financial disclosures.
Managing the Septate Uterus
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.