User login
Are we failing to diagnose and treat the many faces of catatonia?
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
Catching the runner’s high: Anxiety and the endocannabinoid system
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
Administration of ketamine for depression should be limited to psychiatrists
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
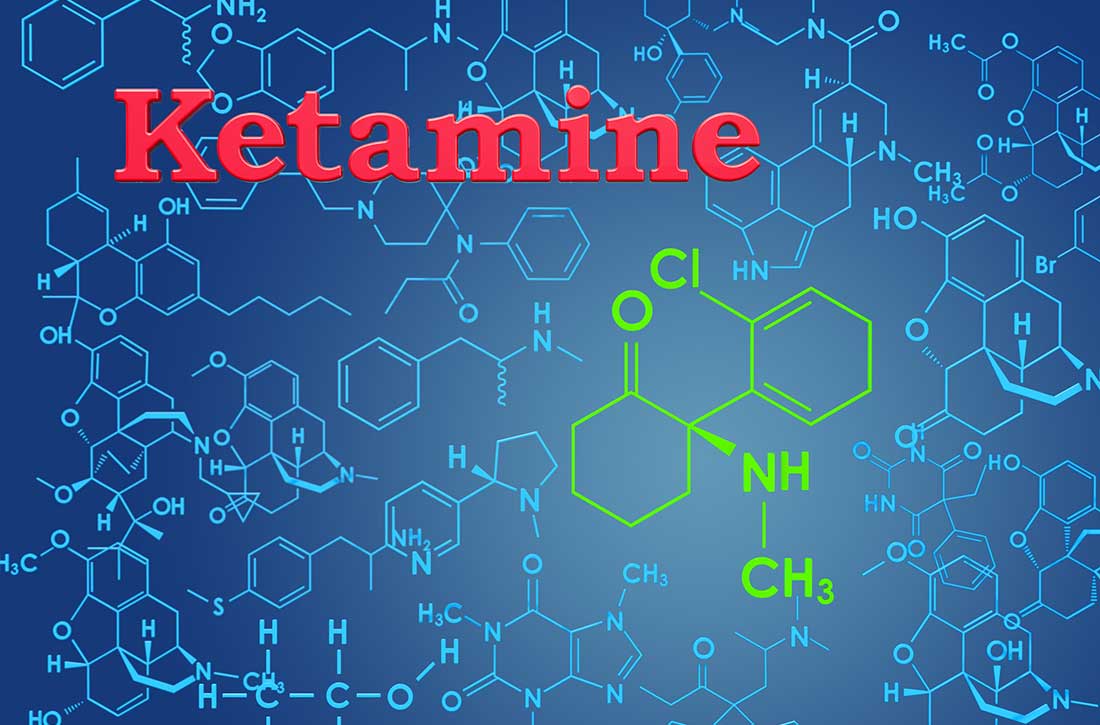
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
My palliative care rotation: Lessons of gratitude, mindfulness, and kindness
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
The challenge of ‘holding space’ while holding the pager
At morning shift change a few months ago on my consultation-liaison rotation, I thanked the night float resident who had been called to a case that was not at all psychiatrically acute. When I told my colleague I was sorry she had had such a “soft consult” during a busy shift, she graciously replied that the patient had been exceedingly pleasant. She said, “Sometimes we just offer our presence, and you know what? I’m glad I’m in that kind of field. The ‘being-present’ kind of field.”
As mental health professionals, we pride ourselves on being present for our patients and our colleagues alike. Winnicott1 originally coined the psychoanalytic term “holding” to denote one of the earliest stages of parental care, wherein an environment of both physical and emotional reliability allows a child to develop their sense of self. The complementary concept of “containing,” developed by Bion,2 indicates a parental figure’s receiving the child’s emotions, however difficult, and then processing them into a more tolerable form. I am frequently struck by how often our role as psychiatrists is not necessarily to offer a specific diagnosis or medication recommendation, but instead to “hold” by listening, “contain” whatever emotions emerge, and offer a sense of validation and perhaps a biopsychosocial formulation for the patient’s experience.3-5 In the consultation-liaison setting, we might assess the contribution of sleep cycle disturbance, postoperative opioids, and anticholinergic medications on a patient’s mental status. Just as important, we might help the patient and their primary team understand that the patient’s history of childhood trauma could, under stressful conditions such as a prolonged hospitalization, lead to affective dysregulation and result in projective identification through which the team felt just as frustrated and helpless as the patient.
The relentless pursuit of efficiency vs time spent with patients
In inpatient work, I may serve as short-term psychotherapist for the patient, their family members, or a consulting team, and I treasure the time spent in those roles. But I concurrently hold various other responsibilities during my shift, including the roles of triage clinician, medical ethicist, and psychopharmacology expert (or, in the case of a newly-third-year resident such as myself, a nonexpert trying to build her knowledge base). I am also literally holding a pager, which intrudes—with aggressive cacophony, vibration, or both—upon the sanctity of any space. The pager is a reminder of a myriad of tasks: calling collateral, answering questions from team members, pre-charting, note-writing, ordering labs, checking labs, updating the handoff, reconciling medication lists, filling out legal paperwork, triaging the next consult. These are unavoidable and generally necessary parts of clinical work, but sometimes they veer into sheer drudgery.
As a medical student, learning to complete tasks is a substantial part of each clinical rotation, and task completion provides plenty of dopaminergic reinforcements that could masquerade as job satisfaction. Through my first year and a half of residency, I pushed hard to build “efficiency” in my workflow, but eventually, task completion stopped providing sufficient inherent satisfaction. It has been a relief to find that amid the stream of checkboxes, the true work of psychiatric care (the interactions with patients, their clinical presentations, and considering their differential diagnoses and treatment options) feels deeply meaningful and ever more fascinating.
At times, I am angered by the reality of limited clinician bandwidth. This frustration motivates me to seek system-level improvements that can enable us to deliver quality psychiatric care while mitigating the risk of clinician burnout. What ends up shortchanged in the relentless pursuit of efficiency is the time spent with patients. This is never more apparent than during a busy inpatient shift, when I often need to compress patient interactions and focus only on the most acute clinical questions. When I have to apologize for stepping out of the interview room to answer yet another page, I marvel at seeing attending psychiatrists who—with apparent ease—make patients feel as if they have all the time in the world, and I wonder when I will be able to do the same.
And yet, there are other times when my pager stays blessedly quiet, time can slow down in the room, and I can make a patient feel heard, held, and contained. In those moments, I also hold my own need for connection with the patient, and can recall what my colleague reminded me: what a privilege it is to be in the “being-present” kind of field.
1. Winnicott DW. The theory of the parent-infant relationship. Int J Psychoanal. 1961;41:585-595.
2. Bion WR. Learning from experience. William Heinemann Medical Books; 1962.
3. Green SA. Psychotherapeutic principles and techniques: principles of medical psychotherapy. In: Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 3rd ed. Oxford University Press; 2015:191-204.
4. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116. doi:10.1176/appi.psy.46.2.109
5. Nash SS, Kent LK, Muskin PR. Psychodynamics in medically ill patients. Harv Rev Psychiatry. 2009;17(6):389-397. doi:10.3109/10673220903465726
At morning shift change a few months ago on my consultation-liaison rotation, I thanked the night float resident who had been called to a case that was not at all psychiatrically acute. When I told my colleague I was sorry she had had such a “soft consult” during a busy shift, she graciously replied that the patient had been exceedingly pleasant. She said, “Sometimes we just offer our presence, and you know what? I’m glad I’m in that kind of field. The ‘being-present’ kind of field.”
As mental health professionals, we pride ourselves on being present for our patients and our colleagues alike. Winnicott1 originally coined the psychoanalytic term “holding” to denote one of the earliest stages of parental care, wherein an environment of both physical and emotional reliability allows a child to develop their sense of self. The complementary concept of “containing,” developed by Bion,2 indicates a parental figure’s receiving the child’s emotions, however difficult, and then processing them into a more tolerable form. I am frequently struck by how often our role as psychiatrists is not necessarily to offer a specific diagnosis or medication recommendation, but instead to “hold” by listening, “contain” whatever emotions emerge, and offer a sense of validation and perhaps a biopsychosocial formulation for the patient’s experience.3-5 In the consultation-liaison setting, we might assess the contribution of sleep cycle disturbance, postoperative opioids, and anticholinergic medications on a patient’s mental status. Just as important, we might help the patient and their primary team understand that the patient’s history of childhood trauma could, under stressful conditions such as a prolonged hospitalization, lead to affective dysregulation and result in projective identification through which the team felt just as frustrated and helpless as the patient.
The relentless pursuit of efficiency vs time spent with patients
In inpatient work, I may serve as short-term psychotherapist for the patient, their family members, or a consulting team, and I treasure the time spent in those roles. But I concurrently hold various other responsibilities during my shift, including the roles of triage clinician, medical ethicist, and psychopharmacology expert (or, in the case of a newly-third-year resident such as myself, a nonexpert trying to build her knowledge base). I am also literally holding a pager, which intrudes—with aggressive cacophony, vibration, or both—upon the sanctity of any space. The pager is a reminder of a myriad of tasks: calling collateral, answering questions from team members, pre-charting, note-writing, ordering labs, checking labs, updating the handoff, reconciling medication lists, filling out legal paperwork, triaging the next consult. These are unavoidable and generally necessary parts of clinical work, but sometimes they veer into sheer drudgery.
As a medical student, learning to complete tasks is a substantial part of each clinical rotation, and task completion provides plenty of dopaminergic reinforcements that could masquerade as job satisfaction. Through my first year and a half of residency, I pushed hard to build “efficiency” in my workflow, but eventually, task completion stopped providing sufficient inherent satisfaction. It has been a relief to find that amid the stream of checkboxes, the true work of psychiatric care (the interactions with patients, their clinical presentations, and considering their differential diagnoses and treatment options) feels deeply meaningful and ever more fascinating.
At times, I am angered by the reality of limited clinician bandwidth. This frustration motivates me to seek system-level improvements that can enable us to deliver quality psychiatric care while mitigating the risk of clinician burnout. What ends up shortchanged in the relentless pursuit of efficiency is the time spent with patients. This is never more apparent than during a busy inpatient shift, when I often need to compress patient interactions and focus only on the most acute clinical questions. When I have to apologize for stepping out of the interview room to answer yet another page, I marvel at seeing attending psychiatrists who—with apparent ease—make patients feel as if they have all the time in the world, and I wonder when I will be able to do the same.
And yet, there are other times when my pager stays blessedly quiet, time can slow down in the room, and I can make a patient feel heard, held, and contained. In those moments, I also hold my own need for connection with the patient, and can recall what my colleague reminded me: what a privilege it is to be in the “being-present” kind of field.
At morning shift change a few months ago on my consultation-liaison rotation, I thanked the night float resident who had been called to a case that was not at all psychiatrically acute. When I told my colleague I was sorry she had had such a “soft consult” during a busy shift, she graciously replied that the patient had been exceedingly pleasant. She said, “Sometimes we just offer our presence, and you know what? I’m glad I’m in that kind of field. The ‘being-present’ kind of field.”
As mental health professionals, we pride ourselves on being present for our patients and our colleagues alike. Winnicott1 originally coined the psychoanalytic term “holding” to denote one of the earliest stages of parental care, wherein an environment of both physical and emotional reliability allows a child to develop their sense of self. The complementary concept of “containing,” developed by Bion,2 indicates a parental figure’s receiving the child’s emotions, however difficult, and then processing them into a more tolerable form. I am frequently struck by how often our role as psychiatrists is not necessarily to offer a specific diagnosis or medication recommendation, but instead to “hold” by listening, “contain” whatever emotions emerge, and offer a sense of validation and perhaps a biopsychosocial formulation for the patient’s experience.3-5 In the consultation-liaison setting, we might assess the contribution of sleep cycle disturbance, postoperative opioids, and anticholinergic medications on a patient’s mental status. Just as important, we might help the patient and their primary team understand that the patient’s history of childhood trauma could, under stressful conditions such as a prolonged hospitalization, lead to affective dysregulation and result in projective identification through which the team felt just as frustrated and helpless as the patient.
The relentless pursuit of efficiency vs time spent with patients
In inpatient work, I may serve as short-term psychotherapist for the patient, their family members, or a consulting team, and I treasure the time spent in those roles. But I concurrently hold various other responsibilities during my shift, including the roles of triage clinician, medical ethicist, and psychopharmacology expert (or, in the case of a newly-third-year resident such as myself, a nonexpert trying to build her knowledge base). I am also literally holding a pager, which intrudes—with aggressive cacophony, vibration, or both—upon the sanctity of any space. The pager is a reminder of a myriad of tasks: calling collateral, answering questions from team members, pre-charting, note-writing, ordering labs, checking labs, updating the handoff, reconciling medication lists, filling out legal paperwork, triaging the next consult. These are unavoidable and generally necessary parts of clinical work, but sometimes they veer into sheer drudgery.
As a medical student, learning to complete tasks is a substantial part of each clinical rotation, and task completion provides plenty of dopaminergic reinforcements that could masquerade as job satisfaction. Through my first year and a half of residency, I pushed hard to build “efficiency” in my workflow, but eventually, task completion stopped providing sufficient inherent satisfaction. It has been a relief to find that amid the stream of checkboxes, the true work of psychiatric care (the interactions with patients, their clinical presentations, and considering their differential diagnoses and treatment options) feels deeply meaningful and ever more fascinating.
At times, I am angered by the reality of limited clinician bandwidth. This frustration motivates me to seek system-level improvements that can enable us to deliver quality psychiatric care while mitigating the risk of clinician burnout. What ends up shortchanged in the relentless pursuit of efficiency is the time spent with patients. This is never more apparent than during a busy inpatient shift, when I often need to compress patient interactions and focus only on the most acute clinical questions. When I have to apologize for stepping out of the interview room to answer yet another page, I marvel at seeing attending psychiatrists who—with apparent ease—make patients feel as if they have all the time in the world, and I wonder when I will be able to do the same.
And yet, there are other times when my pager stays blessedly quiet, time can slow down in the room, and I can make a patient feel heard, held, and contained. In those moments, I also hold my own need for connection with the patient, and can recall what my colleague reminded me: what a privilege it is to be in the “being-present” kind of field.
1. Winnicott DW. The theory of the parent-infant relationship. Int J Psychoanal. 1961;41:585-595.
2. Bion WR. Learning from experience. William Heinemann Medical Books; 1962.
3. Green SA. Psychotherapeutic principles and techniques: principles of medical psychotherapy. In: Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 3rd ed. Oxford University Press; 2015:191-204.
4. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116. doi:10.1176/appi.psy.46.2.109
5. Nash SS, Kent LK, Muskin PR. Psychodynamics in medically ill patients. Harv Rev Psychiatry. 2009;17(6):389-397. doi:10.3109/10673220903465726
1. Winnicott DW. The theory of the parent-infant relationship. Int J Psychoanal. 1961;41:585-595.
2. Bion WR. Learning from experience. William Heinemann Medical Books; 1962.
3. Green SA. Psychotherapeutic principles and techniques: principles of medical psychotherapy. In: Fogel BS, Greenberg DB, eds. Psychiatric care of the medical patient. 3rd ed. Oxford University Press; 2015:191-204.
4. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116. doi:10.1176/appi.psy.46.2.109
5. Nash SS, Kent LK, Muskin PR. Psychodynamics in medically ill patients. Harv Rev Psychiatry. 2009;17(6):389-397. doi:10.3109/10673220903465726
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook