User login
Should you order genetic testing to identify how patients metabolize antipsychotics?
Two months ago, Mr. P, age 20, experienced paranoid thoughts, anxiety, agitation, and auditory hallucinations. During a brief hospitalization 1 month later, he received IM haloperidol, 2 mg, which he said “made his neck stiff.” After he was discharged, Mr. P, who is White, stopped taking his antipsychotics. During a recent outpatient evaluation, the clinician gives Mr. P a working diagnosis of schizophrenia and prescribes risperidone, 2 mg/d, with plans to titrate to 4 mg/d in the next 2 weeks. However, a week later, Mr. P complains of extreme sedation and feeling “knocked out” and does not want to continue taking the medication. Physical exam reveals slight cogwheel rigidity. His delusional thought content is not improved. The treating physician considers ordering a genetic test to determine Mr. P’s cytochrome P450 (CYP) 2D6 metabolizer status.
Studies investigating relationships among genetic variants thought to impact pharmacokinetics and pharmacodynamics of psychotropic medications have had mixed results.1 Metabolism of most antipsychotics depends on the CYP450 enzyme system, which is expressed predominantly in the liver (Table 1). CYP2D6 is one of these enzymes and may be responsible for metabolizing approximately 20% to 50% of all medications, including a number of antipsychotics.2 Genetic variations of CYP2D6 are common and the frequencies of these variants differ among racial groups.3
The half-life and other pharmacokinetic parameters of an antipsychotic metabolized by CYP2D6 may differ based on whether someone is a poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM), or ultrarapid metabolizer (UM).4 Regarding CYP2D6 metabolism among Whites, 3% to 5% are UMs, 70% to 80% are EMs, 10% to 17% are IMs, and 5% to 10% are PMs.5 By contrast, the percentage of PMs and UMs in the Asian population is low—about 1% for each phenotype; the IM phenotype is more common (65% to 70% in the Chinese population).5,6 The percentage of PMs in African Americans is roughly 2% to 6%.2
- Genetic variants of CYP2D6 may result in decreased or increased metabolism of some drugs, including risperidone, iloperidone, perphenazine, haloperidol, and thioridazine.
- The effect of reduced CYP2D6 activity may increase a patient’s risk for dose-related adverse effects.
- It is currently unknown if clinical genotyping for CYP2D6 variants and using this information to guide drug selection or dosing improves patient outcomes.
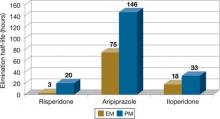
The clinical effect of altered metabolizer status depends on the extent the metabolism of a given agent is dependent on CYP2D6. PM status results in an approximately 2- to 6-fold increase in elimination half-life and overall exposure of aripiprazole,7 risperidone,8 and iloperidone9 (Figure). On the other end of the spectrum are UMs. Because of gene duplication, patients who fall into this category have enhanced metabolic activity. As a result, the therapeutic effect of several medications may be decreased because of faster clearance from the body, leading some physicians to label them as treatment-resistant.
Because side effects of many antipsychotics are dose-dependent, genotyping may be valuable for patients taking agents that are primarily metabolized by CYP2D6.10 Clinicians now have access to laboratory resources and FDA-approved methods for assessing CYP2D6 gene variants.11 It is debatable, however, whether this testing—which is expensive (≥$400) and may not be covered by health insurance—improves patient outcomes. In Mr. P’s case, if he had been genotyped as a CYP2D6 PM before treatment, his physicians might not have prescribed haloperidol and could have prevented a mild dystonic reaction. Also, they could have lowered the initial risperidone dose or chosen an antipsychotic such as ziprasidone, paliperidone, or quetiapine where the pharmacokinetic consequences of 2D6 poor metabolism are not as severe. Theoretically, one may argue that this could have reduced the risk for antipsychotic-associated side effects that now are a barrier to Mr. P’s desire to continue antipsychotics. On the other hand one may also reasonably argue that there may be other/additional reasons (genetic or non-genetic) that make some patients more sensitive to the side effects of antipsychotics and that simply assessing CYP2D6 status is not enough to guide drug selection and dosing.
Table 1
Cytochrome P450 (CYP) metabolism of commonly used antipsychotics*
| Drug | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4/5 |
|---|---|---|---|---|---|
| Aripiprazole | X | X | |||
| Asenapine | X | X | X | ||
| Chlorpromazine | X | X | X | ||
| Clozapine | X | X | X | X | X |
| Fluphenazine | X | ||||
| Haloperidol | X | X | X | ||
| Iloperidone | X | X | |||
| Olanzapine | X | X | |||
| Paliperidone | X† | X† | |||
| Perphenazine | X | X | X | X | X |
| Quetiapine | X | X | |||
| Risperidone | X | X | |||
| Thioridazine | X | X | |||
| Ziprasidone | X | X | |||
| *Information obtained from the most recent prescribing information available from each drug’s manufacturer †According to paliperidone’s prescribing information, in vitro studies identify that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism, but in vivo studies indicate that their role in eliminating paliperidone is minimal | |||||
Figure: Effects of CYP2D6 poor metabolizer status on the half-life of risperidone, aripiprazole, and iloperidone
EM: extensive metabolizer; PM: poor metabolizer
Source: References 7-9
Use in clinical practice
Proposed expert guidelines recommend halving the normal target dose of risperidone and avoiding haloperidol and phenothiazine antipsychotics in CYP2D6 PMs.12 These guidelines are based on expert review of the pharmacokinetic effects of CYP2D6 PM status as well as case reports and studies associating CYP2D6 with poor outcomes, usually side effects. Although these studies suggest that determining metabolizer status may be clinically important, many other studies—some very large—have not found evidence for associations between drug metabolizing enzyme variants and clinical outcomes from antipsychotics.13
There are 2 clinical scenarios in which one may consider obtaining CYP2D6 genotype information:
- before initiating treatment (Table 2)
- after trying ≥1 agent primarily dependent on CYP2D6 with evidence of dose-related side effect (Table 3).
Identifying PMs could influence drug selection and dosing if this information is available before antipsychotic exposure. Studies have found evidence that CYP2D6 PMs may be at greater risk of experiencing adverse reactions to risperidone compared with other metabolizer groups.14 Also, prescribing information for aripiprazole and iloperidone recommends halving the dose of these drugs in the presence of CYP2D6 inhibitors, a condition that pharmacokinetically mimics PM status.
Knowing genotype information after ≥1 drugs have been tried may not be as useful. Clinicians often base drug switches or dose titrations on a patient’s experience with present or past doses of the antipsychotic. Examples include slowing titrations or reducing a target dose when a patient, such as Mr. P, experiences side effects, or selecting non-2D6 substrate agents after detecting a pattern of drug sensitivity.
Table 2
CYP2D6 testing before initiating antipsychotics: Benefits vs drawbacks
| Benefits | Drawbacks |
|---|---|
| Clinicians could avoid 2D6 substrate drugs with high likelihood for ADEs or increased risk of 2D6-based interactions in PMs | No empiric evidence shows that routine genotyping produces better clinical outcomes (eg, fewer side effects and better treatment adherence) |
| May lower initial dose, slow titration, and lower initial target dose to minimize risk of side effects in PMs | Many clinicians titrate slowly or adjust titration schedule and target doses based on initial tolerability as part of routine practice |
| The test would need to be done only once and the information may be useful for other therapy decisions | Patients who need immediate drug therapy may not be able to wait for test results |
| Testing may not be covered by a patient’s health insurance | |
| ADEs: adverse drug events; PMs: poor metabolizers | |
Table 3
Genotype testing after a patient experiences side effects
| Benefits |
| Identifying a biologic reason for side effect sensitivity may aid choice and dosing of subsequent antipsychotics and other medications |
| Drawbacks |
| In clinical practice, antipsychotic switching because of tolerability (and response) often is guided by outcomes experienced from previously used agents. In general, patients with a history of experiencing side effects at lower doses of antipsychotics are likely to be initiated at lower doses and titrated more cautiously during subsequent therapy choices regardless of whether side effects were caused by metabolizer status or other factor(s) |
Better patient outcomes?
It is not known if obtaining genotype information will provide better outcomes than a “trial and error” approach. Currently, obtaining genotype information before antipsychotic treatment is not standard clinical practice. Because this testing is expensive and requires prior approval from third party payers or out-of-pocket financial resources, testing is not recommended for all patients at this time.
However, a growing body of evidence suggests that knowing metabolizer status could be useful in drug selection or dosing for antipsychotics. This scientific knowledge continues to accumulate, and CYP2D6 genotyping may some day be integrated into routine clinical care. Currently, for patients and physicians with the resources to obtain and the ability to appropriately interpret the test results, this information may prove useful on an individual basis. However, additional studies are needed to support better outcomes from dosing and drug selection based on CYP2D6 genotype information.
Related Resources
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP). www.egappreviews.org.
- Pharmacogenomics Knowledge Base. www.pharmgkb.org.
- Indiana University School of Medicine. P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Bishop receives grant/research support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil-Janssen and has received honoraria from Eli Lilly and Company.
Ms. Chae reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Leckband SG, Bishop JR, Ellingrod VL. Pharmacogenomics in psychiatry. J Pharm Pract. 2007;20:252-264.
2. Neafsey P, Ginsberg G, Hattis D, et al. Genetic polymorphism in cytochrome P450 2D6 (CYP2D6): population distribution of CYP2D6 activity. J Toxicol Environ Health B Crit Rev. 2009;12(5-6):334-361.
3. Bradford LD, Gaedigk A, Leeder JS. High frequency of CYP2D6 poor and “intermediate” metabolizers in black populations: a review and preliminary data. Psychopharmacol Bull. 1998;34:797-804.
4. Gaedigk A, Simon SD, Pearce RE, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234-242.
5. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761-804.
6. Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17-22.
7. Risperdal [package insert]. Titusville, NJ: Janssen; 2010.
8. Abilify [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co.; 2009.
9. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals, Inc.; 2009.
10. Kirchheiner J, Rodriguez-Antona C. Cytochrome P450 2D6 genotyping: potential role in improving treatment outcomes in psychiatric disorders. CNS Drugs. 2009;23:181-191.
11. de Leon J, Susce MT, Murray-Carmichael E. The AmpliChip CYP450 genotyping test: integrating a new clinical tool. Mol Diagn Ther. 2006;10:135-151.
12. de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics. 2006;47:75-85.
13. Grossman I, Sullivan PF, Walley N, et al. Genetic determinants of variable metabolism have little impact on the clinical use of leading antipsychotics in the CATIE study. Genet Med. 2008;10:720-729.
14. Rodriguez-Antona C, Gurwitz D, de Leon J, et al. CYP2D6 genotyping for psychiatric patients treated with risperidone: considerations for cost-effectiveness studies. Pharmacogenomics. 2009;10:685-699.
Two months ago, Mr. P, age 20, experienced paranoid thoughts, anxiety, agitation, and auditory hallucinations. During a brief hospitalization 1 month later, he received IM haloperidol, 2 mg, which he said “made his neck stiff.” After he was discharged, Mr. P, who is White, stopped taking his antipsychotics. During a recent outpatient evaluation, the clinician gives Mr. P a working diagnosis of schizophrenia and prescribes risperidone, 2 mg/d, with plans to titrate to 4 mg/d in the next 2 weeks. However, a week later, Mr. P complains of extreme sedation and feeling “knocked out” and does not want to continue taking the medication. Physical exam reveals slight cogwheel rigidity. His delusional thought content is not improved. The treating physician considers ordering a genetic test to determine Mr. P’s cytochrome P450 (CYP) 2D6 metabolizer status.
Studies investigating relationships among genetic variants thought to impact pharmacokinetics and pharmacodynamics of psychotropic medications have had mixed results.1 Metabolism of most antipsychotics depends on the CYP450 enzyme system, which is expressed predominantly in the liver (Table 1). CYP2D6 is one of these enzymes and may be responsible for metabolizing approximately 20% to 50% of all medications, including a number of antipsychotics.2 Genetic variations of CYP2D6 are common and the frequencies of these variants differ among racial groups.3
The half-life and other pharmacokinetic parameters of an antipsychotic metabolized by CYP2D6 may differ based on whether someone is a poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM), or ultrarapid metabolizer (UM).4 Regarding CYP2D6 metabolism among Whites, 3% to 5% are UMs, 70% to 80% are EMs, 10% to 17% are IMs, and 5% to 10% are PMs.5 By contrast, the percentage of PMs and UMs in the Asian population is low—about 1% for each phenotype; the IM phenotype is more common (65% to 70% in the Chinese population).5,6 The percentage of PMs in African Americans is roughly 2% to 6%.2
- Genetic variants of CYP2D6 may result in decreased or increased metabolism of some drugs, including risperidone, iloperidone, perphenazine, haloperidol, and thioridazine.
- The effect of reduced CYP2D6 activity may increase a patient’s risk for dose-related adverse effects.
- It is currently unknown if clinical genotyping for CYP2D6 variants and using this information to guide drug selection or dosing improves patient outcomes.
The clinical effect of altered metabolizer status depends on the extent the metabolism of a given agent is dependent on CYP2D6. PM status results in an approximately 2- to 6-fold increase in elimination half-life and overall exposure of aripiprazole,7 risperidone,8 and iloperidone9 (Figure). On the other end of the spectrum are UMs. Because of gene duplication, patients who fall into this category have enhanced metabolic activity. As a result, the therapeutic effect of several medications may be decreased because of faster clearance from the body, leading some physicians to label them as treatment-resistant.
Because side effects of many antipsychotics are dose-dependent, genotyping may be valuable for patients taking agents that are primarily metabolized by CYP2D6.10 Clinicians now have access to laboratory resources and FDA-approved methods for assessing CYP2D6 gene variants.11 It is debatable, however, whether this testing—which is expensive (≥$400) and may not be covered by health insurance—improves patient outcomes. In Mr. P’s case, if he had been genotyped as a CYP2D6 PM before treatment, his physicians might not have prescribed haloperidol and could have prevented a mild dystonic reaction. Also, they could have lowered the initial risperidone dose or chosen an antipsychotic such as ziprasidone, paliperidone, or quetiapine where the pharmacokinetic consequences of 2D6 poor metabolism are not as severe. Theoretically, one may argue that this could have reduced the risk for antipsychotic-associated side effects that now are a barrier to Mr. P’s desire to continue antipsychotics. On the other hand one may also reasonably argue that there may be other/additional reasons (genetic or non-genetic) that make some patients more sensitive to the side effects of antipsychotics and that simply assessing CYP2D6 status is not enough to guide drug selection and dosing.
Table 1
Cytochrome P450 (CYP) metabolism of commonly used antipsychotics*
| Drug | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4/5 |
|---|---|---|---|---|---|
| Aripiprazole | X | X | |||
| Asenapine | X | X | X | ||
| Chlorpromazine | X | X | X | ||
| Clozapine | X | X | X | X | X |
| Fluphenazine | X | ||||
| Haloperidol | X | X | X | ||
| Iloperidone | X | X | |||
| Olanzapine | X | X | |||
| Paliperidone | X† | X† | |||
| Perphenazine | X | X | X | X | X |
| Quetiapine | X | X | |||
| Risperidone | X | X | |||
| Thioridazine | X | X | |||
| Ziprasidone | X | X | |||
| *Information obtained from the most recent prescribing information available from each drug’s manufacturer †According to paliperidone’s prescribing information, in vitro studies identify that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism, but in vivo studies indicate that their role in eliminating paliperidone is minimal | |||||
Figure: Effects of CYP2D6 poor metabolizer status on the half-life of risperidone, aripiprazole, and iloperidone
EM: extensive metabolizer; PM: poor metabolizer
Source: References 7-9
Use in clinical practice
Proposed expert guidelines recommend halving the normal target dose of risperidone and avoiding haloperidol and phenothiazine antipsychotics in CYP2D6 PMs.12 These guidelines are based on expert review of the pharmacokinetic effects of CYP2D6 PM status as well as case reports and studies associating CYP2D6 with poor outcomes, usually side effects. Although these studies suggest that determining metabolizer status may be clinically important, many other studies—some very large—have not found evidence for associations between drug metabolizing enzyme variants and clinical outcomes from antipsychotics.13
There are 2 clinical scenarios in which one may consider obtaining CYP2D6 genotype information:
- before initiating treatment (Table 2)
- after trying ≥1 agent primarily dependent on CYP2D6 with evidence of dose-related side effect (Table 3).
Identifying PMs could influence drug selection and dosing if this information is available before antipsychotic exposure. Studies have found evidence that CYP2D6 PMs may be at greater risk of experiencing adverse reactions to risperidone compared with other metabolizer groups.14 Also, prescribing information for aripiprazole and iloperidone recommends halving the dose of these drugs in the presence of CYP2D6 inhibitors, a condition that pharmacokinetically mimics PM status.
Knowing genotype information after ≥1 drugs have been tried may not be as useful. Clinicians often base drug switches or dose titrations on a patient’s experience with present or past doses of the antipsychotic. Examples include slowing titrations or reducing a target dose when a patient, such as Mr. P, experiences side effects, or selecting non-2D6 substrate agents after detecting a pattern of drug sensitivity.
Table 2
CYP2D6 testing before initiating antipsychotics: Benefits vs drawbacks
| Benefits | Drawbacks |
|---|---|
| Clinicians could avoid 2D6 substrate drugs with high likelihood for ADEs or increased risk of 2D6-based interactions in PMs | No empiric evidence shows that routine genotyping produces better clinical outcomes (eg, fewer side effects and better treatment adherence) |
| May lower initial dose, slow titration, and lower initial target dose to minimize risk of side effects in PMs | Many clinicians titrate slowly or adjust titration schedule and target doses based on initial tolerability as part of routine practice |
| The test would need to be done only once and the information may be useful for other therapy decisions | Patients who need immediate drug therapy may not be able to wait for test results |
| Testing may not be covered by a patient’s health insurance | |
| ADEs: adverse drug events; PMs: poor metabolizers | |
Table 3
Genotype testing after a patient experiences side effects
| Benefits |
| Identifying a biologic reason for side effect sensitivity may aid choice and dosing of subsequent antipsychotics and other medications |
| Drawbacks |
| In clinical practice, antipsychotic switching because of tolerability (and response) often is guided by outcomes experienced from previously used agents. In general, patients with a history of experiencing side effects at lower doses of antipsychotics are likely to be initiated at lower doses and titrated more cautiously during subsequent therapy choices regardless of whether side effects were caused by metabolizer status or other factor(s) |
Better patient outcomes?
It is not known if obtaining genotype information will provide better outcomes than a “trial and error” approach. Currently, obtaining genotype information before antipsychotic treatment is not standard clinical practice. Because this testing is expensive and requires prior approval from third party payers or out-of-pocket financial resources, testing is not recommended for all patients at this time.
However, a growing body of evidence suggests that knowing metabolizer status could be useful in drug selection or dosing for antipsychotics. This scientific knowledge continues to accumulate, and CYP2D6 genotyping may some day be integrated into routine clinical care. Currently, for patients and physicians with the resources to obtain and the ability to appropriately interpret the test results, this information may prove useful on an individual basis. However, additional studies are needed to support better outcomes from dosing and drug selection based on CYP2D6 genotype information.
Related Resources
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP). www.egappreviews.org.
- Pharmacogenomics Knowledge Base. www.pharmgkb.org.
- Indiana University School of Medicine. P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Bishop receives grant/research support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil-Janssen and has received honoraria from Eli Lilly and Company.
Ms. Chae reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Two months ago, Mr. P, age 20, experienced paranoid thoughts, anxiety, agitation, and auditory hallucinations. During a brief hospitalization 1 month later, he received IM haloperidol, 2 mg, which he said “made his neck stiff.” After he was discharged, Mr. P, who is White, stopped taking his antipsychotics. During a recent outpatient evaluation, the clinician gives Mr. P a working diagnosis of schizophrenia and prescribes risperidone, 2 mg/d, with plans to titrate to 4 mg/d in the next 2 weeks. However, a week later, Mr. P complains of extreme sedation and feeling “knocked out” and does not want to continue taking the medication. Physical exam reveals slight cogwheel rigidity. His delusional thought content is not improved. The treating physician considers ordering a genetic test to determine Mr. P’s cytochrome P450 (CYP) 2D6 metabolizer status.
Studies investigating relationships among genetic variants thought to impact pharmacokinetics and pharmacodynamics of psychotropic medications have had mixed results.1 Metabolism of most antipsychotics depends on the CYP450 enzyme system, which is expressed predominantly in the liver (Table 1). CYP2D6 is one of these enzymes and may be responsible for metabolizing approximately 20% to 50% of all medications, including a number of antipsychotics.2 Genetic variations of CYP2D6 are common and the frequencies of these variants differ among racial groups.3
The half-life and other pharmacokinetic parameters of an antipsychotic metabolized by CYP2D6 may differ based on whether someone is a poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM), or ultrarapid metabolizer (UM).4 Regarding CYP2D6 metabolism among Whites, 3% to 5% are UMs, 70% to 80% are EMs, 10% to 17% are IMs, and 5% to 10% are PMs.5 By contrast, the percentage of PMs and UMs in the Asian population is low—about 1% for each phenotype; the IM phenotype is more common (65% to 70% in the Chinese population).5,6 The percentage of PMs in African Americans is roughly 2% to 6%.2
- Genetic variants of CYP2D6 may result in decreased or increased metabolism of some drugs, including risperidone, iloperidone, perphenazine, haloperidol, and thioridazine.
- The effect of reduced CYP2D6 activity may increase a patient’s risk for dose-related adverse effects.
- It is currently unknown if clinical genotyping for CYP2D6 variants and using this information to guide drug selection or dosing improves patient outcomes.
The clinical effect of altered metabolizer status depends on the extent the metabolism of a given agent is dependent on CYP2D6. PM status results in an approximately 2- to 6-fold increase in elimination half-life and overall exposure of aripiprazole,7 risperidone,8 and iloperidone9 (Figure). On the other end of the spectrum are UMs. Because of gene duplication, patients who fall into this category have enhanced metabolic activity. As a result, the therapeutic effect of several medications may be decreased because of faster clearance from the body, leading some physicians to label them as treatment-resistant.
Because side effects of many antipsychotics are dose-dependent, genotyping may be valuable for patients taking agents that are primarily metabolized by CYP2D6.10 Clinicians now have access to laboratory resources and FDA-approved methods for assessing CYP2D6 gene variants.11 It is debatable, however, whether this testing—which is expensive (≥$400) and may not be covered by health insurance—improves patient outcomes. In Mr. P’s case, if he had been genotyped as a CYP2D6 PM before treatment, his physicians might not have prescribed haloperidol and could have prevented a mild dystonic reaction. Also, they could have lowered the initial risperidone dose or chosen an antipsychotic such as ziprasidone, paliperidone, or quetiapine where the pharmacokinetic consequences of 2D6 poor metabolism are not as severe. Theoretically, one may argue that this could have reduced the risk for antipsychotic-associated side effects that now are a barrier to Mr. P’s desire to continue antipsychotics. On the other hand one may also reasonably argue that there may be other/additional reasons (genetic or non-genetic) that make some patients more sensitive to the side effects of antipsychotics and that simply assessing CYP2D6 status is not enough to guide drug selection and dosing.
Table 1
Cytochrome P450 (CYP) metabolism of commonly used antipsychotics*
| Drug | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4/5 |
|---|---|---|---|---|---|
| Aripiprazole | X | X | |||
| Asenapine | X | X | X | ||
| Chlorpromazine | X | X | X | ||
| Clozapine | X | X | X | X | X |
| Fluphenazine | X | ||||
| Haloperidol | X | X | X | ||
| Iloperidone | X | X | |||
| Olanzapine | X | X | |||
| Paliperidone | X† | X† | |||
| Perphenazine | X | X | X | X | X |
| Quetiapine | X | X | |||
| Risperidone | X | X | |||
| Thioridazine | X | X | |||
| Ziprasidone | X | X | |||
| *Information obtained from the most recent prescribing information available from each drug’s manufacturer †According to paliperidone’s prescribing information, in vitro studies identify that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism, but in vivo studies indicate that their role in eliminating paliperidone is minimal | |||||
Figure: Effects of CYP2D6 poor metabolizer status on the half-life of risperidone, aripiprazole, and iloperidone
EM: extensive metabolizer; PM: poor metabolizer
Source: References 7-9
Use in clinical practice
Proposed expert guidelines recommend halving the normal target dose of risperidone and avoiding haloperidol and phenothiazine antipsychotics in CYP2D6 PMs.12 These guidelines are based on expert review of the pharmacokinetic effects of CYP2D6 PM status as well as case reports and studies associating CYP2D6 with poor outcomes, usually side effects. Although these studies suggest that determining metabolizer status may be clinically important, many other studies—some very large—have not found evidence for associations between drug metabolizing enzyme variants and clinical outcomes from antipsychotics.13
There are 2 clinical scenarios in which one may consider obtaining CYP2D6 genotype information:
- before initiating treatment (Table 2)
- after trying ≥1 agent primarily dependent on CYP2D6 with evidence of dose-related side effect (Table 3).
Identifying PMs could influence drug selection and dosing if this information is available before antipsychotic exposure. Studies have found evidence that CYP2D6 PMs may be at greater risk of experiencing adverse reactions to risperidone compared with other metabolizer groups.14 Also, prescribing information for aripiprazole and iloperidone recommends halving the dose of these drugs in the presence of CYP2D6 inhibitors, a condition that pharmacokinetically mimics PM status.
Knowing genotype information after ≥1 drugs have been tried may not be as useful. Clinicians often base drug switches or dose titrations on a patient’s experience with present or past doses of the antipsychotic. Examples include slowing titrations or reducing a target dose when a patient, such as Mr. P, experiences side effects, or selecting non-2D6 substrate agents after detecting a pattern of drug sensitivity.
Table 2
CYP2D6 testing before initiating antipsychotics: Benefits vs drawbacks
| Benefits | Drawbacks |
|---|---|
| Clinicians could avoid 2D6 substrate drugs with high likelihood for ADEs or increased risk of 2D6-based interactions in PMs | No empiric evidence shows that routine genotyping produces better clinical outcomes (eg, fewer side effects and better treatment adherence) |
| May lower initial dose, slow titration, and lower initial target dose to minimize risk of side effects in PMs | Many clinicians titrate slowly or adjust titration schedule and target doses based on initial tolerability as part of routine practice |
| The test would need to be done only once and the information may be useful for other therapy decisions | Patients who need immediate drug therapy may not be able to wait for test results |
| Testing may not be covered by a patient’s health insurance | |
| ADEs: adverse drug events; PMs: poor metabolizers | |
Table 3
Genotype testing after a patient experiences side effects
| Benefits |
| Identifying a biologic reason for side effect sensitivity may aid choice and dosing of subsequent antipsychotics and other medications |
| Drawbacks |
| In clinical practice, antipsychotic switching because of tolerability (and response) often is guided by outcomes experienced from previously used agents. In general, patients with a history of experiencing side effects at lower doses of antipsychotics are likely to be initiated at lower doses and titrated more cautiously during subsequent therapy choices regardless of whether side effects were caused by metabolizer status or other factor(s) |
Better patient outcomes?
It is not known if obtaining genotype information will provide better outcomes than a “trial and error” approach. Currently, obtaining genotype information before antipsychotic treatment is not standard clinical practice. Because this testing is expensive and requires prior approval from third party payers or out-of-pocket financial resources, testing is not recommended for all patients at this time.
However, a growing body of evidence suggests that knowing metabolizer status could be useful in drug selection or dosing for antipsychotics. This scientific knowledge continues to accumulate, and CYP2D6 genotyping may some day be integrated into routine clinical care. Currently, for patients and physicians with the resources to obtain and the ability to appropriately interpret the test results, this information may prove useful on an individual basis. However, additional studies are needed to support better outcomes from dosing and drug selection based on CYP2D6 genotype information.
Related Resources
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP). www.egappreviews.org.
- Pharmacogenomics Knowledge Base. www.pharmgkb.org.
- Indiana University School of Medicine. P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Bishop receives grant/research support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil-Janssen and has received honoraria from Eli Lilly and Company.
Ms. Chae reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Leckband SG, Bishop JR, Ellingrod VL. Pharmacogenomics in psychiatry. J Pharm Pract. 2007;20:252-264.
2. Neafsey P, Ginsberg G, Hattis D, et al. Genetic polymorphism in cytochrome P450 2D6 (CYP2D6): population distribution of CYP2D6 activity. J Toxicol Environ Health B Crit Rev. 2009;12(5-6):334-361.
3. Bradford LD, Gaedigk A, Leeder JS. High frequency of CYP2D6 poor and “intermediate” metabolizers in black populations: a review and preliminary data. Psychopharmacol Bull. 1998;34:797-804.
4. Gaedigk A, Simon SD, Pearce RE, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234-242.
5. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761-804.
6. Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17-22.
7. Risperdal [package insert]. Titusville, NJ: Janssen; 2010.
8. Abilify [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co.; 2009.
9. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals, Inc.; 2009.
10. Kirchheiner J, Rodriguez-Antona C. Cytochrome P450 2D6 genotyping: potential role in improving treatment outcomes in psychiatric disorders. CNS Drugs. 2009;23:181-191.
11. de Leon J, Susce MT, Murray-Carmichael E. The AmpliChip CYP450 genotyping test: integrating a new clinical tool. Mol Diagn Ther. 2006;10:135-151.
12. de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics. 2006;47:75-85.
13. Grossman I, Sullivan PF, Walley N, et al. Genetic determinants of variable metabolism have little impact on the clinical use of leading antipsychotics in the CATIE study. Genet Med. 2008;10:720-729.
14. Rodriguez-Antona C, Gurwitz D, de Leon J, et al. CYP2D6 genotyping for psychiatric patients treated with risperidone: considerations for cost-effectiveness studies. Pharmacogenomics. 2009;10:685-699.
1. Leckband SG, Bishop JR, Ellingrod VL. Pharmacogenomics in psychiatry. J Pharm Pract. 2007;20:252-264.
2. Neafsey P, Ginsberg G, Hattis D, et al. Genetic polymorphism in cytochrome P450 2D6 (CYP2D6): population distribution of CYP2D6 activity. J Toxicol Environ Health B Crit Rev. 2009;12(5-6):334-361.
3. Bradford LD, Gaedigk A, Leeder JS. High frequency of CYP2D6 poor and “intermediate” metabolizers in black populations: a review and preliminary data. Psychopharmacol Bull. 1998;34:797-804.
4. Gaedigk A, Simon SD, Pearce RE, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234-242.
5. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761-804.
6. Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17-22.
7. Risperdal [package insert]. Titusville, NJ: Janssen; 2010.
8. Abilify [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co.; 2009.
9. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals, Inc.; 2009.
10. Kirchheiner J, Rodriguez-Antona C. Cytochrome P450 2D6 genotyping: potential role in improving treatment outcomes in psychiatric disorders. CNS Drugs. 2009;23:181-191.
11. de Leon J, Susce MT, Murray-Carmichael E. The AmpliChip CYP450 genotyping test: integrating a new clinical tool. Mol Diagn Ther. 2006;10:135-151.
12. de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics. 2006;47:75-85.
13. Grossman I, Sullivan PF, Walley N, et al. Genetic determinants of variable metabolism have little impact on the clinical use of leading antipsychotics in the CATIE study. Genet Med. 2008;10:720-729.
14. Rodriguez-Antona C, Gurwitz D, de Leon J, et al. CYP2D6 genotyping for psychiatric patients treated with risperidone: considerations for cost-effectiveness studies. Pharmacogenomics. 2009;10:685-699.
How to manage your patient’s dementia by discontinuing medications
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
How differences among generics might affect your patient’s response
- Keep current with new developments in psychopharmacology
- Learn more about pharmacodynamics, pharmacokinetics, drug-drug interactions, and prescribing for special populations
- Collaborate with psychiatric pharmacists to solve or prevent problems patients may have with their medications
Mr. X, age 47, suffers from major depressive disorder, which he developed 1 year ago after experiencing a myocardial infarction. At that time, Mr. X received brand-name fluoxetine (Prozac), 20 mg/d. After 4 weeks, his mood improved, but he experienced delayed ejaculation, which resolved spontaneously after 12 weeks of treatment.
Because Mr. X recently lost his job and health insurance, he inquires about lowering his health care costs. Discontinuing fluoxetine is not advised, so you recommend changing to a generic formulation. Mr. X tolerates this conversion without difficulty; however, 9 months later he reports he is experiencing delayed ejaculation again. He has had no changes in medical history and has not started any new medications. Mr. X claims he has been compliant with his medication, although he mentions that the fluoxetine tablets looked different when he refilled his prescription 2 weeks ago. You call the pharmacy and discover that they started dispensing generic fluoxetine from a different manufacturer around the time Mr. X refilled his prescription. You prescribe Mr. X his old version of generic fluoxetine, and his sexual dysfunction resolves within 2 weeks.
- For most patients, using generic medications poses no problems and offers an appropriate therapeutic option at a lower cost.
- If problems arise during treatment, consider differences among generic brands. Although each generic must be tested against the brand-name product for bioequivalence, they do not need to be tested against each other.
- Different generic formations may have different inert ingredients, which may cause problems if patients are allergic to a specific inactive ingredient.
- Consult the ‘Orange Book’ for information on approved drugs and their generic interchangeability or the patient’s local pharmacist or a board-certified psychiatric pharmacist if you have questions about generic formulations.
In the United States, 2.6 billion prescriptions—approximately 70% of all prescriptions—are filled using generic versions of brand-name products.1 For most patients, generic substitution is acceptable and reduces costs. Although the practice has become routine, certain circumstances may make switching to a generic medication or between generic medication problematic. To understand why, it is important to discuss the FDA’s generic drug approval process.2
Bioequivalence
Pharmaceutical manufacturers developing a generic drug must create a product that will deliver the same amount of medication at the same rate and in the same form (ie, tablet, capsule, suspension, etc.) as the brand-name product. The FDA requires bioequivalence (BE) studies.2 These studies usually include fewer than 40 healthy individuals and must show that the generic product has the same pharmacokinetic profile as the brand-name drug (the active ingredient already has been shown to be safe and effective). The generic product can deviate from the brand product’s profile by a set amount—currently a 90% confidence interval limit of 80% to 125%.2 This means that pharmacokinetic parameters such as max concentration (Cmax), time to max concentration (Tmax), mean absorption time, and area under the curve (AUC)—which is a measure of overall drug exposure—are no less than 80% and no more than 125% of the parameters seen with the brand-name product. This may seem like a large deviation, but the FDA reports that generally “small differences in blood levels—<4%—may exist in some cases between a brand and its generic equivalent.”1
A new generic formulation does not have to be tested against other generic formulations, only against the brand-name drug. Therefore, 2 generic formulations may differ pharmacokinetically by more than a 4% difference if one product is on the low side of the BE limit and the other is on the high side. If a patient starts 1 generic and then switches to another, efficacy may be lost or side effects may emerge because of BE differences. In Mr. X’s case, it is possible that the new generic version of fluoxetine resulted in higher plasma drug levels that lead to recurrence of sexual dysfunction.
Exceptions
Generic medications are not recommended for certain medical conditions, such as epilepsy and some hormone replacement therapy, because of lack of satisfactory BE to the brand-name drug.3 For these conditions, generic medications may be used, but should not be substituted for the brand-name product without careful monitoring. Additionally, switching between different generic manufacturers should be avoided.
If you have a question about generic substitution, consult the FDA’s Approved drug products with therapeutic equivalence evaluations4—also known as the “Orange Book,” which is available online (see Related Resources). This resource provides guidance about which drugs are interchangeable. The Orange Book is the “gold standard” on approved drug products and their interchangeability.
The Table lists certain psychiatric medications that may have issues with generic substitutions. Most pharmacies stock and dispense only generic drugs that the FDA considers bioequivalent to the brand-name product.
Allergic reactions may occur because of different inert ingredients within each generic or brand-name drug. Generic drug manufacturers are not required to use the same inactive or “filler” ingredients. Some patients may be allergic to 1 version and may require a specific brand or generic version to overcome this potential allergy.3
Although most generic substitutions occur without incident, consider BE differences among products and your patient’s medical condition before initiating a switch. When switching between generics, carefully monitor your patient as you would when switching from the brand-name product to a generic. If new treatment-related issues arise or lack of efficacy occurs, ask your patient if the pharmacy switched to a new generic formulation.
Table
Bioequivalence among generic psychotropics: What to know before you switch
| Medication | Comments |
|---|---|
| Amitriptyline/perphenazine | Generic formulations may not be interchangeable* |
| Anticonvulsants | Because these medications have a narrow therapeutic index when used for seizure disorders, patients are recommended to not switch formulations. When used for psychiatric disorders, the margin of safety is unknown and switching may be appropriate |
| Chlorpromazine | Generic formulations are not bioequivalent |
| Clozapine | Generic formulations may not be bioequivalent at all dosages* Dosage adjustments may be needed in patients who need to switch formulations during treatment |
| Venlafaxine | Some formulations may not be interchangeable* |
| *Consult the FDA’s Approved drug products with therapeutic equivalence evaluations to determine if generics are interchangeable | |
| Source: Reference 4 | |
- Food and Drug Administration. www.fda.gov/Drugs/default.htm.
- Generic Pharmaceutical Association. www.gphaonline.org.
- Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/default.cfm.
Drug brand names
- Amitriptyline/Perphenazine • Triavil
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Venlafaxine • Effexor
Disclosure
Dr. Ellingrod is a member of an advisory board for Eli Lilly and Company.
1. Generic Pharmaceutical Association. Bioequivalence. Available at: http://www.gphaonline.org/issues/bioequivalence. Accessed January 4, 2010.
2. Food and Drug Administration. Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. Rockville, MD: U.S. Department of Health and Human Services; 2003.
3. Silverman HM. Bioequivalence and interchangeability of generic drugs. In: Merck manual home edition online. 2007. Available at: http://www.merck.com/mmhe/sec02/ch017/ch017b.html. Accessed January 4, 2010.
4. Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed January 4, 2010.
- Keep current with new developments in psychopharmacology
- Learn more about pharmacodynamics, pharmacokinetics, drug-drug interactions, and prescribing for special populations
- Collaborate with psychiatric pharmacists to solve or prevent problems patients may have with their medications
Mr. X, age 47, suffers from major depressive disorder, which he developed 1 year ago after experiencing a myocardial infarction. At that time, Mr. X received brand-name fluoxetine (Prozac), 20 mg/d. After 4 weeks, his mood improved, but he experienced delayed ejaculation, which resolved spontaneously after 12 weeks of treatment.
Because Mr. X recently lost his job and health insurance, he inquires about lowering his health care costs. Discontinuing fluoxetine is not advised, so you recommend changing to a generic formulation. Mr. X tolerates this conversion without difficulty; however, 9 months later he reports he is experiencing delayed ejaculation again. He has had no changes in medical history and has not started any new medications. Mr. X claims he has been compliant with his medication, although he mentions that the fluoxetine tablets looked different when he refilled his prescription 2 weeks ago. You call the pharmacy and discover that they started dispensing generic fluoxetine from a different manufacturer around the time Mr. X refilled his prescription. You prescribe Mr. X his old version of generic fluoxetine, and his sexual dysfunction resolves within 2 weeks.
- For most patients, using generic medications poses no problems and offers an appropriate therapeutic option at a lower cost.
- If problems arise during treatment, consider differences among generic brands. Although each generic must be tested against the brand-name product for bioequivalence, they do not need to be tested against each other.
- Different generic formations may have different inert ingredients, which may cause problems if patients are allergic to a specific inactive ingredient.
- Consult the ‘Orange Book’ for information on approved drugs and their generic interchangeability or the patient’s local pharmacist or a board-certified psychiatric pharmacist if you have questions about generic formulations.
In the United States, 2.6 billion prescriptions—approximately 70% of all prescriptions—are filled using generic versions of brand-name products.1 For most patients, generic substitution is acceptable and reduces costs. Although the practice has become routine, certain circumstances may make switching to a generic medication or between generic medication problematic. To understand why, it is important to discuss the FDA’s generic drug approval process.2
Bioequivalence
Pharmaceutical manufacturers developing a generic drug must create a product that will deliver the same amount of medication at the same rate and in the same form (ie, tablet, capsule, suspension, etc.) as the brand-name product. The FDA requires bioequivalence (BE) studies.2 These studies usually include fewer than 40 healthy individuals and must show that the generic product has the same pharmacokinetic profile as the brand-name drug (the active ingredient already has been shown to be safe and effective). The generic product can deviate from the brand product’s profile by a set amount—currently a 90% confidence interval limit of 80% to 125%.2 This means that pharmacokinetic parameters such as max concentration (Cmax), time to max concentration (Tmax), mean absorption time, and area under the curve (AUC)—which is a measure of overall drug exposure—are no less than 80% and no more than 125% of the parameters seen with the brand-name product. This may seem like a large deviation, but the FDA reports that generally “small differences in blood levels—<4%—may exist in some cases between a brand and its generic equivalent.”1
A new generic formulation does not have to be tested against other generic formulations, only against the brand-name drug. Therefore, 2 generic formulations may differ pharmacokinetically by more than a 4% difference if one product is on the low side of the BE limit and the other is on the high side. If a patient starts 1 generic and then switches to another, efficacy may be lost or side effects may emerge because of BE differences. In Mr. X’s case, it is possible that the new generic version of fluoxetine resulted in higher plasma drug levels that lead to recurrence of sexual dysfunction.
Exceptions
Generic medications are not recommended for certain medical conditions, such as epilepsy and some hormone replacement therapy, because of lack of satisfactory BE to the brand-name drug.3 For these conditions, generic medications may be used, but should not be substituted for the brand-name product without careful monitoring. Additionally, switching between different generic manufacturers should be avoided.
If you have a question about generic substitution, consult the FDA’s Approved drug products with therapeutic equivalence evaluations4—also known as the “Orange Book,” which is available online (see Related Resources). This resource provides guidance about which drugs are interchangeable. The Orange Book is the “gold standard” on approved drug products and their interchangeability.
The Table lists certain psychiatric medications that may have issues with generic substitutions. Most pharmacies stock and dispense only generic drugs that the FDA considers bioequivalent to the brand-name product.
Allergic reactions may occur because of different inert ingredients within each generic or brand-name drug. Generic drug manufacturers are not required to use the same inactive or “filler” ingredients. Some patients may be allergic to 1 version and may require a specific brand or generic version to overcome this potential allergy.3
Although most generic substitutions occur without incident, consider BE differences among products and your patient’s medical condition before initiating a switch. When switching between generics, carefully monitor your patient as you would when switching from the brand-name product to a generic. If new treatment-related issues arise or lack of efficacy occurs, ask your patient if the pharmacy switched to a new generic formulation.
Table
Bioequivalence among generic psychotropics: What to know before you switch
| Medication | Comments |
|---|---|
| Amitriptyline/perphenazine | Generic formulations may not be interchangeable* |
| Anticonvulsants | Because these medications have a narrow therapeutic index when used for seizure disorders, patients are recommended to not switch formulations. When used for psychiatric disorders, the margin of safety is unknown and switching may be appropriate |
| Chlorpromazine | Generic formulations are not bioequivalent |
| Clozapine | Generic formulations may not be bioequivalent at all dosages* Dosage adjustments may be needed in patients who need to switch formulations during treatment |
| Venlafaxine | Some formulations may not be interchangeable* |
| *Consult the FDA’s Approved drug products with therapeutic equivalence evaluations to determine if generics are interchangeable | |
| Source: Reference 4 | |
- Food and Drug Administration. www.fda.gov/Drugs/default.htm.
- Generic Pharmaceutical Association. www.gphaonline.org.
- Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/default.cfm.
Drug brand names
- Amitriptyline/Perphenazine • Triavil
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Venlafaxine • Effexor
Disclosure
Dr. Ellingrod is a member of an advisory board for Eli Lilly and Company.
- Keep current with new developments in psychopharmacology
- Learn more about pharmacodynamics, pharmacokinetics, drug-drug interactions, and prescribing for special populations
- Collaborate with psychiatric pharmacists to solve or prevent problems patients may have with their medications
Mr. X, age 47, suffers from major depressive disorder, which he developed 1 year ago after experiencing a myocardial infarction. At that time, Mr. X received brand-name fluoxetine (Prozac), 20 mg/d. After 4 weeks, his mood improved, but he experienced delayed ejaculation, which resolved spontaneously after 12 weeks of treatment.
Because Mr. X recently lost his job and health insurance, he inquires about lowering his health care costs. Discontinuing fluoxetine is not advised, so you recommend changing to a generic formulation. Mr. X tolerates this conversion without difficulty; however, 9 months later he reports he is experiencing delayed ejaculation again. He has had no changes in medical history and has not started any new medications. Mr. X claims he has been compliant with his medication, although he mentions that the fluoxetine tablets looked different when he refilled his prescription 2 weeks ago. You call the pharmacy and discover that they started dispensing generic fluoxetine from a different manufacturer around the time Mr. X refilled his prescription. You prescribe Mr. X his old version of generic fluoxetine, and his sexual dysfunction resolves within 2 weeks.
- For most patients, using generic medications poses no problems and offers an appropriate therapeutic option at a lower cost.
- If problems arise during treatment, consider differences among generic brands. Although each generic must be tested against the brand-name product for bioequivalence, they do not need to be tested against each other.
- Different generic formations may have different inert ingredients, which may cause problems if patients are allergic to a specific inactive ingredient.
- Consult the ‘Orange Book’ for information on approved drugs and their generic interchangeability or the patient’s local pharmacist or a board-certified psychiatric pharmacist if you have questions about generic formulations.
In the United States, 2.6 billion prescriptions—approximately 70% of all prescriptions—are filled using generic versions of brand-name products.1 For most patients, generic substitution is acceptable and reduces costs. Although the practice has become routine, certain circumstances may make switching to a generic medication or between generic medication problematic. To understand why, it is important to discuss the FDA’s generic drug approval process.2
Bioequivalence
Pharmaceutical manufacturers developing a generic drug must create a product that will deliver the same amount of medication at the same rate and in the same form (ie, tablet, capsule, suspension, etc.) as the brand-name product. The FDA requires bioequivalence (BE) studies.2 These studies usually include fewer than 40 healthy individuals and must show that the generic product has the same pharmacokinetic profile as the brand-name drug (the active ingredient already has been shown to be safe and effective). The generic product can deviate from the brand product’s profile by a set amount—currently a 90% confidence interval limit of 80% to 125%.2 This means that pharmacokinetic parameters such as max concentration (Cmax), time to max concentration (Tmax), mean absorption time, and area under the curve (AUC)—which is a measure of overall drug exposure—are no less than 80% and no more than 125% of the parameters seen with the brand-name product. This may seem like a large deviation, but the FDA reports that generally “small differences in blood levels—<4%—may exist in some cases between a brand and its generic equivalent.”1
A new generic formulation does not have to be tested against other generic formulations, only against the brand-name drug. Therefore, 2 generic formulations may differ pharmacokinetically by more than a 4% difference if one product is on the low side of the BE limit and the other is on the high side. If a patient starts 1 generic and then switches to another, efficacy may be lost or side effects may emerge because of BE differences. In Mr. X’s case, it is possible that the new generic version of fluoxetine resulted in higher plasma drug levels that lead to recurrence of sexual dysfunction.
Exceptions
Generic medications are not recommended for certain medical conditions, such as epilepsy and some hormone replacement therapy, because of lack of satisfactory BE to the brand-name drug.3 For these conditions, generic medications may be used, but should not be substituted for the brand-name product without careful monitoring. Additionally, switching between different generic manufacturers should be avoided.
If you have a question about generic substitution, consult the FDA’s Approved drug products with therapeutic equivalence evaluations4—also known as the “Orange Book,” which is available online (see Related Resources). This resource provides guidance about which drugs are interchangeable. The Orange Book is the “gold standard” on approved drug products and their interchangeability.
The Table lists certain psychiatric medications that may have issues with generic substitutions. Most pharmacies stock and dispense only generic drugs that the FDA considers bioequivalent to the brand-name product.
Allergic reactions may occur because of different inert ingredients within each generic or brand-name drug. Generic drug manufacturers are not required to use the same inactive or “filler” ingredients. Some patients may be allergic to 1 version and may require a specific brand or generic version to overcome this potential allergy.3
Although most generic substitutions occur without incident, consider BE differences among products and your patient’s medical condition before initiating a switch. When switching between generics, carefully monitor your patient as you would when switching from the brand-name product to a generic. If new treatment-related issues arise or lack of efficacy occurs, ask your patient if the pharmacy switched to a new generic formulation.
Table
Bioequivalence among generic psychotropics: What to know before you switch
| Medication | Comments |
|---|---|
| Amitriptyline/perphenazine | Generic formulations may not be interchangeable* |
| Anticonvulsants | Because these medications have a narrow therapeutic index when used for seizure disorders, patients are recommended to not switch formulations. When used for psychiatric disorders, the margin of safety is unknown and switching may be appropriate |
| Chlorpromazine | Generic formulations are not bioequivalent |
| Clozapine | Generic formulations may not be bioequivalent at all dosages* Dosage adjustments may be needed in patients who need to switch formulations during treatment |
| Venlafaxine | Some formulations may not be interchangeable* |
| *Consult the FDA’s Approved drug products with therapeutic equivalence evaluations to determine if generics are interchangeable | |
| Source: Reference 4 | |
- Food and Drug Administration. www.fda.gov/Drugs/default.htm.
- Generic Pharmaceutical Association. www.gphaonline.org.
- Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/default.cfm.
Drug brand names
- Amitriptyline/Perphenazine • Triavil
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluoxetine • Prozac
- Venlafaxine • Effexor
Disclosure
Dr. Ellingrod is a member of an advisory board for Eli Lilly and Company.
1. Generic Pharmaceutical Association. Bioequivalence. Available at: http://www.gphaonline.org/issues/bioequivalence. Accessed January 4, 2010.
2. Food and Drug Administration. Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. Rockville, MD: U.S. Department of Health and Human Services; 2003.
3. Silverman HM. Bioequivalence and interchangeability of generic drugs. In: Merck manual home edition online. 2007. Available at: http://www.merck.com/mmhe/sec02/ch017/ch017b.html. Accessed January 4, 2010.
4. Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed January 4, 2010.
1. Generic Pharmaceutical Association. Bioequivalence. Available at: http://www.gphaonline.org/issues/bioequivalence. Accessed January 4, 2010.
2. Food and Drug Administration. Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. Rockville, MD: U.S. Department of Health and Human Services; 2003.
3. Silverman HM. Bioequivalence and interchangeability of generic drugs. In: Merck manual home edition online. 2007. Available at: http://www.merck.com/mmhe/sec02/ch017/ch017b.html. Accessed January 4, 2010.
4. Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed January 4, 2010.