User login
American Academy of Sleep Medicine (AASM) advocates for year-round standard time
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Sleep-disordered breathing in neuromuscular disease
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
Sleep-disordered breathing (SDB) is a common sleep disturbance in neuromuscular disease (NMD) affecting 36% to 53% of diagnosed adults (Arens R, et al. Paediatr Respir Rev. 2010;11[1]:24). Disturbances in sleep may serve as the earliest sign of muscle weakness in these patients, at times being detected before their underlying neuromuscular disease is diagnosed. This is of paramount importance to sleep medicine and pulmonary physicians who may be among the first specialists to evaluate these patients and can play a vital role in the recognition and diagnosis of neuromuscular disease. Herein, we will provide a guide to aid the reader in recognizing the early signs and symptoms of NMD as it pertains to sleep, as earlier diagnosis may lead to improved quality of life or possibly even survival, in some cases.
Pathophysiology
To begin, it is important to understand the pathophysiology of NMD and how it is altered during the sleep state. Sleep-related physiologic changes in healthy humans include reduction in upper airway muscle tone, blunting of chemoreceptors associated with pharyngeal dilator augmentation, and sleep stage-specific changes in skeletal muscle tone. In patients with NMD, these changes may not be adequately compensated for, leading to sleep-disordered breathing that can present as sleep apnea, hypoventilation, or hypoxia (Govindarajan R, et al. Sleep Issues in Neuromuscular Disorders: A Clinical Guide. Springer International Publishing AG, Springer Nature 2018).
Central respiratory control
The respiratory centers in the pons and medulla are generally spared from the primary effects of most NMD; however, over time, they may be affected secondarily. Similar to obesity hypoventilation syndrome (OHS), untreated chronic sleep-related hypoventilation from NMD can impair the sensitivity of respiratory chemoreceptors leading to worsening hypoventilation.
Upper airway resistance
Pharyngeal muscle tone is key to maintaining a patent airway during sleep. In some NMD, bulbar muscle weakness with pharyngeal dilator muscle hypotonia leads to increased upper airway resistance, especially during REM sleep, which can result in obstructive sleep apnea (OSA). In addition to weakness affecting the upper airway musculature, anatomical changes may also contribute to sleep-disordered breathing. In Pompe disease, for example, macroglossia and fibro-fatty replacement of tongue muscles may occur, leading to the development of OSA.
Diaphragm weakness
In NMD that affects the diaphragm, there is an increased reliance on the skeletal muscles of respiration to maintain adequate ventilation as the underlying disease progresses. Generally, weakness of the diaphragm will cause disturbances in REM sleep first as, during REM, ventilation predominately depends on the diaphragm and patients lose the assistance of their skeletal muscles. However, over time, the progressive weakening of the diaphragm will progress to involve NREM sleep as well, clinically manifesting with frank sleep apnea, hypoventilation, and, ultimately, chronic hypercapnic respiratory failure.
Inspiratory muscle weakness
As noted above, there are many other muscles used in inspiration in addition to the diaphragm. Other primary muscles include the intercostal and scalene muscles, and accessory muscles include the sternocleidomastoid, pectoralis, latissimus dorsi, erector spinae, and trapezius muscles. While sleep and breathing problems may begin early in the course of a neuromuscular disease, the complex restrictive lung disease pattern that we see in these patients may not develop until the respiratory muscles of the chest wall are involved. This restriction, which corresponds to lower lung volumes, leads to a fall in the caudal traction force of the airways which can lead to reduction in the pharyngeal airway cross section. Because these issues are worsened in the supine position, their pathophysiologic effects on respiration are most notable during sleep, putting patients at higher risk of OSA.
Cardiac abnormalities
Lastly, it should be noted that diseases such as the muscular dystrophies, myotonic dystrophy, mitochondriopathies, and nemaline myopathy can be associated with a cardiomyopathy ,which can lead to central sleep apnea in the form of Cheyne-Stokes breathing.
Sleep-disordered breathing in specific NMDs
In amyotrophic lateral sclerosis (ALS), up to 75% of patients may have SDB, the majority of which is central sleep apnea (CSA) and hypoventilation although they still have a higher prevalence of obstructive sleep apnea (OSA) than the general population. Whether the diaphragm or the pharyngeal muscles are predominantly affected may have something to do with the type of apnea a patient experiences; however, studies have shown that even in bulbar ALS, CSA is most common. It should be noted, that this is not Cheyne-Stokes CSA, but rather lack of chest wall and abdominal movement due to weakness. (David WS, et al. J Neurol Sci. 1997;152[suppl 1]:S29-35).
In myasthenia gravis (MG), about 40% to 60% of patients have SDB, and about 30% develop overt respiratory weakness, generally late in the course of their disease. Many of these patients report excessive daytime sleepiness, often attributed to myasthenic fatigue requiring treatment with corticosteroids. It is important to evaluate for sleep apnea, given that if diagnosed and treated, their generalized fatigue may improve and the need for steroids may be reduced or eliminated altogether. It is also important to note that the respiratory and sleep issues MG patients face may not correlate with the severity of their overall disease, such that patients well-controlled on medications from a generalized weakness standpoint may still require home noninvasive ventilation (NIV) for chronic respiratory failure due to weakness of the respiratory system muscles.
Duchenne muscular dystrophy (DMD), an X-linked disease associated with dysfunction of dystrophin synthesis, is often diagnosed in early childhood and gradually progresses over years. Their initial sleep and respiratory symptoms can be subtle and may start with increased nighttime awakenings and daytime somnolence. Generally, these patients will develop OSA in the first decade of life and progress to hypoventilation in their second decade and beyond. These patients are especially important to recognize, as studies have shown appropriate NIV therapy may significantly prolong their life (Finder JD, et al; American Thoracic Society. Am J Respir Crit Care Med. 2004(Aug 15);170[4]:456-465).
In addition to the well-known motor neuron and neuromuscular diseases mentioned above, neuropathic diseases can lead to sleep disturbances, as well. In Charcot-Marie-Tooth (CMT), pharyngeal and laryngeal neuropathy, as well as hypoglossal nerve dysfunction, lead to OSA. Similar to ALS and MG, there is a significant amount of CSA and hypoventilation, likely related to phrenic neuropathy. In contrast to MG, in CMT, the severity of neuropathic disease does correlate to the severity of sleep apnea.
Testing
Testing can range from overnight oximetry to polysomnogram (PSG) with CO2 monitoring. Generally, all patients with a rapidly progressive neuromuscular disease should get pulmonary function testing (PFT) (upright and supine) to evaluate forced vital capacity (FVC) every 3 to 6 months to monitor for respiratory failure. Laboratory studies that can be helpful in assessing for SDB are the PaCO2 (> 45 mm Hg) measured on an arterial blood gas and serum bicarbonate levels (>27 mmol/L or a base excess >4 mmol/L). Patients can qualify for NIV with an overnight SaO2 less than or equal to 88% for greater than or equal to 5 minutes in a 2-hour recording period, PaCO2 greater than or equal to 45 mm Hg, forced vital capacity (FVC) < 50% of predicted, or maximal inspiratory pressure (MIP) <60 cm H2O. For ALS specifically, sniff nasal pressure < 40 cm H2O and orthopnea are additional criteria that can be used. It is worth noting that a PSG is not required for NIV qualification in neuromuscular respiratory insufficiency. However, PSG is beneficial in patients with preserved PFTs but suspected of having early nocturnal respiratory impairment.
Therapy
NIV is the mainstay of therapy for SDB in patients with NMD and has been associated with a slower decline in FVC and improved survival in some cases, as demonstrated in studies of patients with DMD or ALS. Generally, a bi-level PAP mode is preferred; the expiratory positive airway pressure prevents micro-atelectasis and improves V/Q matching and the inspiratory positive airway pressure reduces inspiratory muscle load and optimizes ventilation. As weakness progresses, patients may have difficulty creating enough negative force to initiate a spontaneous breath, thus a mode with a set respiratory rate is preferred that can be implemented in bi-level PAP or more advanced modes such as volume-assured pressure support (VAPS) modality. For patients who are unable to tolerate NIV, particularly those with severe bulbar disease and difficult to manage respiratory secretions, tracheostomy with mechanical ventilation may ultimately be needed. This decision should be made as part of a multidisciplinary shared decision-making conversation with the patient, their family, and their team of providers.
Summary
Sleep is a particularly vulnerable state for patients with NMD, and in many patients, disturbances in sleep may be the first clue to their ultimate diagnosis. It is important that sleep medicine and pulmonary specialists understand the pathophysiology and management of NMD as they can play a vital role in the interdisciplinary care of these patients.
Dr. Greer is a Sleep Medicine Fellow, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine; Dr. Collop is Professor of Medicine and Neurology, Director, Emory Sleep Center; Emory University, Atlanta, Georgia.
An update on the pharmacologic treatment of hypersomnia
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
COVID-19 and the future of telehealth for sleep medicine
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 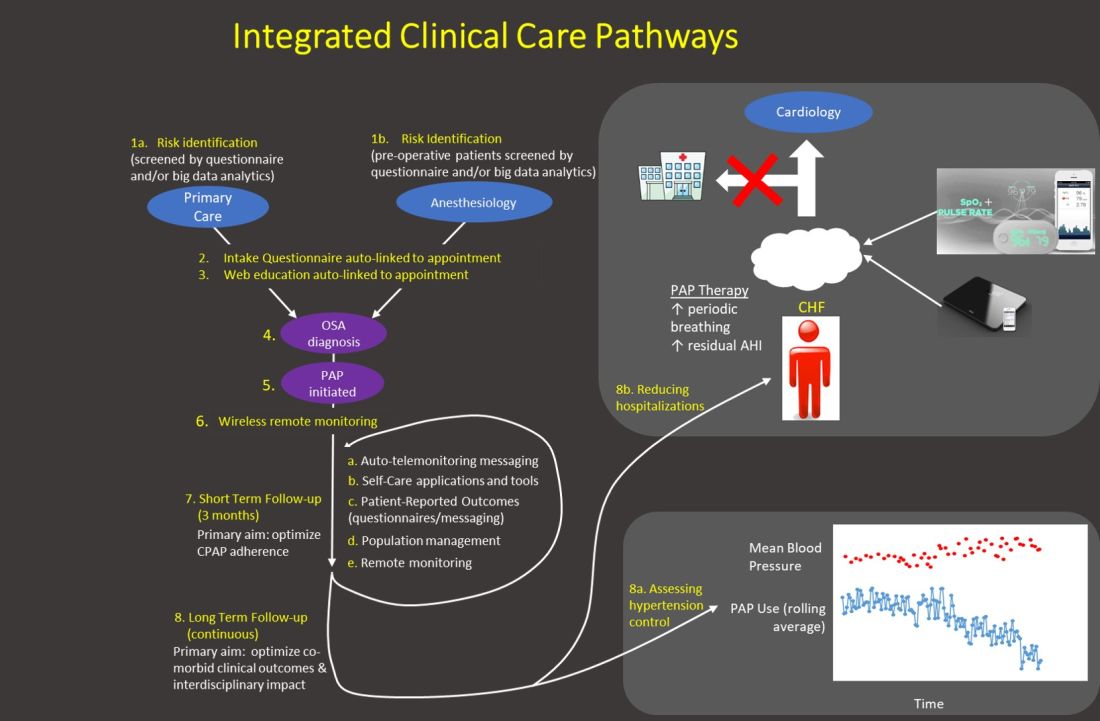
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
COVID-19 and impact on sleep medicine practices
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Stimulation to titration: An update on hypoglossal nerve stimulation for OSA
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
CPAP vs noninvasive ventilation for obesity hypoventilation syndrome
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.
The conventional approach to treat hypoventilation has been to use noninvasive ventilation (NIV), while continuous positive airway pressure (CPAP) that does not augment alveolar ventilation improves gas exchange by maintaining upper airway patency and increasing functional residual capacity. To understand this rationale, it is important to first review the pathophysiology of OHS.
The hallmark of OHS is resting daytime awake arterial PaCO2of 45 mm Hg or greater in an obese patient (BMI > 30 kg/m2) in absence of any other identifiable cause. To recognize why only some but not all obese subjects develop OHS, it is important to understand the different components of pathophysiology that contribute to hypoventilation: (1) obesity-related reduction in functional residual capacity and lung compliance with resultant increase in work of breathing; (2) central hypoventilation related to leptin resistance and reduction in respiratory drive with REM hypoventilation; and (3) upper airway obstruction caused by upper airway fat deposition along with low FRC contributing to pharyngeal airway narrowing and increased airway collapsibility (Masa JF, et al. Eur Respir Rev. 2019; 28:180097).
CPAP vs NIV for OHS
Let us examine some of the studies that have compared the short-term efficacy of CPAP vs NIV in patients with OHS. In a small randomized controlled trial (RCT), the effectiveness of CPAP and NIV was compared in 36 patients with OHS (Piper AJ, et al. Thorax. 2008;63:395). Reduction in PaCO2 at 3 months was similar between the two groups. However, patients with persistent nocturnal desaturation despite optimal CPAP were excluded from the study. In another RCT of 60 patients with OHS who were either in stable condition or after an episode of acute on chronic hypercapnic respiratory failure, the use of CPAP or NIV showed similar improvements at 3 months in daytime PaCO2, quality of life, and sleep parameters (Howard ME, et al. Thorax. 2017;72:437).
In one of the largest randomized control trials, the Spanish Pickwick study randomized 221 patients with OHS and AHI >30/h to NIV, CPAP, and lifestyle modification (Masa JF, et al. Am J Respir Crit Care Med. 2015:192:86). PAP therapy included NIV that consisted of in-lab titration with bilevel PAP therapy targeted to tidal volume 5-6 mL/kg of actual body weight or CPAP. Life style modification served as the control group. Primary outcome was the change in PaCO2 at 2 months. Secondary outcomes were symptoms, HRQOL, polysomnographic parameters, spirometry, and 6-min walk distance (6 MWD). Mean AHI was 69/h, and mean PAP settings for NIV and CPAP were 20/7.7 cm and 11 cm H2O, respectively. NIV provided the greatest improvement in PaCO2 and serum HCO3 as compared with control group but not relative to CPAP group. CPAP improved PaCO2 as compared with control group only after adjustment of PAP use. Spirometry and 6 MWD and some HRQOL measures improved slightly more with NIV as compared to CPAP. Improvement in symptoms and polysomnographic parameters was similar between the two groups.
In another related study by the same group (Masa JF, et al. Thorax. 2016;71:899), 86 patients with OHS and mild OSA (AHI <30/h), were randomized to NIV and lifestyle modification. Mean AHI was 14/h and mean baseline PaCO2 was 49 +/-4 mm Hg. The NIV group with mean PAP adherence at 6 hours showed greater improvement in PaCO2 as compared with lifestyle modification (6 mm vs 2.8 mm Hg). They concluded that NIV was better than lifestyle modification in patients with OHS and mild OSA.
To determine the long-term clinical effectiveness of CPAP vs NIV, patients in the Pickwick study, who were initially assigned to either CPAP or NIV treatment group, were continued on their respective treatments, while subjects in the control group were again randomized at 2 months to either CPAP or NIV (Masa JF, et al. Lancet. 2019;393:1721). All subjects (CPAP n=107; NIV n=97) were followed for a minimum of 3 years. CPAP and NIV settings (pressure-targeted to desired tidal volume) were determined by in-lab titration without transcutaneous CO2 monitor, and daytime adjustment of PAP to improve oxygen saturation. Primary outcome was the number of hospitalization days per year. Mean CPAP was 10.7 cm H2O pressure and NIV 19.7/8.18 cm H2O pressure with an average respiratory rate of 14/min. Median PAP use and adherence > 4 h, respectively, were similar between the two groups (CPAP 6.0 h, adherence > 4 h 67% vs NIV 6.0/h, adherence >4 h 61%). Median duration of follow-up was 5.44 years (IOR 4.45-6.37 years) for both groups. Mean hospitalization days per patient-year were similar between the two groups (CPAP 1.63 vs NIV 1.44 days; adj RR 0.78, 95% CI 0.34-1.77; p=0.561). Overall mortality, adverse cardiovascular events, and arterial blood gas parameters were similar between the two groups, suggesting equal efficacy of CPAP and NIV in this group of stable patients with OHS with an AHI >30/h. Given the low complexity and cost of CPAP vs NIV, the authors concluded that CPAP may be the preferred PAP treatment modality until more studies are available.
An accompanying editorial (Murphy PB, et al. Lancet. 2019; 393:1674), discussed that since this study was powered for superiority as opposed to noninferiority of NIV (20% reduction in hospitalization with NIV when compared with CPAP), superiority could not be shown, due to the low event rate for hospitalization (NIV 1.44 days vs CPAP 1.63 days). It is also possible optimum NIV titration may not have been determined since TCO2 was not used. Furthermore, since this study was done only in patients with OHS and AHI >30/h, these results may not be applicable to patients with OHS and low AHI < 30/h that are more likely to have central hypoventilation and comorbidities, and this group may benefit from NIV as compared with CPAP.
Novel modes of bi-level PAP therapy
There are limited data on the use of the new bi-level PAP modalities, such as volume-targeted pressure support ventilation (PS) with fixed or auto-EPAP. The use of intelligent volume-assured pressure support ventilation (iVAPS) vs standard fixed pressure support ventilation in select OHS patients (n=18) showed equivalent control of chronic respiratory failure with no worsening of sleep quality and better PAP adherence (Kelly JL, et al. Respirology. 2014;19:596). In another small randomized, double-blind, crossover study, done on two consecutive nights in 11 patients with OHS, the use of auto-adjusting EPAP was noninferior to fixed EPAP (10.8 cm vs 11.8 cm H2O pressure), with no differences in sleep quality and patient preference (McArdle N. Sleep. 2017;40:1). Although the data are limited, these small studies suggest the use of new PAP modalities, such as variable PS to deliver target volumes and auto EPAP could offer the potential to initiate bi-level PAP therapy in outpatients without the in-lab titration. More studies are needed before bi-level PAP therapy can be safely initiated in outpatients with OHS.
Summary
In summary, how can we utilize the most effective PAP therapy for patients with OHS? Can we use a phenotype-dependent approach to PAP treatment options? The answer is probably yes. Recently published ATS Clinical Practice Guideline (Am J Respir Crit Care Med. 2019;200:e6-e24) suggests the use of PAP therapy for stable ambulatory patients with OHS as compared with no PAP therapy, and patients with OHS with AHI >30/h (approximately 70% of the OHS patients) can be initially started on CPAP instead of NIV. Patients who have persistent nocturnal desaturation despite optimum CPAP can be switched to NIV. On the other hand, data are limited on the use of CPAP in patients with OHS with AHI <30/h, and these patients can be started on NIV. PAP adherence >5-6 h, and weight loss using a multidisciplinary approach should be encouraged for all patients with OHS.
Dr. Dewan is Professor and Program Director, Sleep Medicine; Division of Pulmonary, Critical Care and Sleep Medicine; Chief, Pulmonary Section VA Medical Center; Creighton University, Omaha, Nebraska.
Noninvasive ventilation: Redefining insurance guidelines
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Restless legs syndrome: Update on evaluation and treatment
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
The burgeoning role of sleep-related chronic hypoxia in long-term outcomes
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.
Clinicians are well aware of the acute effects of hypoxemia when encountered in conditions such as pulmonary embolism, pulmonary edema, COPD exacerbation, and others, whereas effects of chronic hypoxemia, such as pulmonary hypertension and polycythemia, are more difficult to recognize. Chronic hypoxemia is frequent in chronic lung diseases, such as COPD, but how it leads to increased mortality in severe COPD is unknown (NHLBI Working Group for LTOT in COPD. Am J Respir Crit Care Med. 2006;174:373). Chronic hypoxemia following high altitude exposure tends to have more unpredictable effects. Chronic hypoxemia, greater than that expected for the altitude of residence, is encountered frequently in high altitude dwellers. Here it has been implicated in the pathophysiology of chronic mountain sickness (Villafeurte and Corante. High Alt Med Biol. 2016;17[2]:61) and low birth weights (Maatta J, et al. Sci Rep. 2018;8[1]:13583), even though high altitude residence has been linked to better cardiovascular outcomes and reduced cancer-related deaths (Burstcher M. Aging Dis. 2013;5[4]:274). Chronic hypoxia effects at high altitude may, therefore, be variegated depending on a number of factors that include organ-system-specific effects, severity of chronic hypoxia, and a propensity to disease determined by genetic background and generations of residence.
Such diverse effects of chronic sleep-related hypoxemia are also being reported with obstructive sleep apnea (OSA). While sleep can result in sustained drops in ventilation and consequent hypoxemia similar to what is seen in COPD, OSA is typified by a form of sleep-related hypoxemia in a pattern termed as chronic intermittent hypoxia (CIH). CIH is characterized by rapid fluctuations in oxygen saturations (Figure 1) that is virtually pathognomonic of sleep apnea either from recurrent upper airway obstructions (as in OSA) or pauses in respiratory generator firing (as in central sleep apnea). OSA-driven CIH has received most attention, given its purported role in in the causation of the wide range of pathologic conditions associated with OSA. Outcomes from cross-sectional and longitudinal studies have correlated time spent below 90% or recurrent oxygen desaturations to a number of OSA-related outcomes such as cardiovascular disease, diabetes, and cognitive dysfunction (Dewan et al. Chest. 2015;147[1]:266). While these effects of OSA-related intermittent hypoxemia occur over long periods of time, as with other forms of chronic hypoxia, some effects, such as hypertension, are demonstrable in animal models after much shorter durations of sleep-related intermittent hypoxia exposure. As seen with other forms of chronic hypoxemia, an opposing beneficial effect has also been demonstrated on the size of myocardial infarct during acute coronary events and from mild OSA-related mortality in elderly subjects (Javaheri et al. J Am Coll Cardiol. 2017;69[7]:841).
Given how common sleep-related hypoxemia and OSA are, it is important to understand the implications of different patterns of sleep-related hypoxemia that a vast segment of the population experiences on a nightly basis. A number of factors may determine chronic outcomes with sleep-related hypoxemia that include the pattern of sleep-related hypoxemia (chronic sustained hypoxemia associated with sleep-related hypoventilation vs chronic intermittent hypoxemia of OSA), degree of hypoxemia, presence of underlying disease, and hitherto undescribed individual factors. While a correlation between hypoxemic burden secondary to sleep-disordered breathing and cardiovascular outcomes has been shown (Azabarzin A, et al. Eur Heart J. 2018 Oct 30), CPAP interventional studies that address OSA-related CIH have shown mixed results for prevention of cardiovascular disease (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919). It has also been difficult to draw upon results of oxygen supplementation in other forms of hypoxemia, such as COPD, when specifically targeted to addressing the hypoxemia seen only at night or with exercise (LOTT Research Group. N Engl J Med. 2016;375:1617 ). To complicate this further, high altitude residence (that may result in similar levels of sleep-related hypoxemia) is not associated with any differences in life-expectancy but may provide a reduction in cardiovascular outcomes (Ezzati, et al. J Epidemiol Community Health. 2012;66[7]:e17).
How do we reconcile such disparate effects of chronic hypoxemia? Part of difference may be in the pattern of chronic intermittent hypoxemia noted with OSA characterized not only by rapid drops in oxygen but also rapid reoxygenation events secondary to arousals terminating an apnea – these reoxygenation events have been attributed to the increased oxidant stress demonstrable in multiple tissues. While chronic hypoxia itself may cause increased oxidant stress, such effects seen with sustained forms of hypoxia, such as sleep-related hypoventilation or high altitude residence, may be more gradual resulting in lesser degrees of tissue effects and regulation of antioxidant defenses with sustained exposure. Herein lies the importance of understanding physiologic and biological effects stemming from chronic hypoxia to explain its variegated effects on different organ systems. In this regard, the role of carotid body, a structure with unique vascular supply and with the ability to respond to minor changes in oxygen saturation as is seen in patients with OSA is key to the causation of hypertension associated with OSA (Shell et al. Curr Hyperten Rep. 2016;18[3]:19). Carotid body activation by intermittent hypoxia and long-term sensory facilitation drives the elevated sympathetic activity and consequent increases in blood pressure that can be improved by supplemental oxygen (Turnbull CD, et al. Am J Respir Crit Care Med. 2019;199[2]:211).
While carotid body responses are key to the pathophysiology of OSA, every organ in the body (in fact, every cell within the body) has the ability to sense and respond to hypoxia. This ability to sense oxygen tensions is ingrained in every cell by virtue of oxygen’s critical role in the genesis of life and evolution. These cellular responses to hypoxia are mediated by hypoxia-inducible factors (HIFs), isoforms of which include the more ubiquitous HIF-1 found in all parenchymal cells and HIF-2 found in specialized erythropoietin-producing cells of the kidney and the pulmonary circulation (the polycythemia and pulmonary vasoconstrictive responses from hypoxia are mediated through HIF-2 ). HIFs mediate the transcription of hundreds of genes, and they have been implicated in the pathobiology of a wide range of phenomena, from cancer to atherosclerotic vascular disease, metabolic syndrome, neurodegenerative disorders, pulmonary hypertension, and nonalcoholic fatty liver disease (Prabhakar and Semenza. Physiol Rev. 2012;92[3]:967). While HIF activation is an attractive target for examining the effects of chronic hypoxia of high altitude and sleep-disordered breathing, HIF activation varies from tissue to tissue and interacts with a number of other cellular systems in leading to differential effects. The short half-life of HIF proteins make them difficult to detect in tissues, so a number of secondary HIF-effects has been measured with mixed results depending on animal model utilized, pattern and degree of hypoxia studied, and the target effect measured. Comparative effects of intermittent vs sustained hypoxemia need to be systematically studied in different organ systems in different species, given the differing oxygen thresholds of individual cells due to unique blood flows and variations in the system of co-factors and prolyl hydroxylases that regulate the activation of HIFs. While the thrust of the work has been centered on HIF-related effects and the role of NF-kB-driven inflammation seen in OSA, there is substantial evidence to the role of oxidant stress that may be directly related to reoxygenation events occurring with CIH (Lavie L. Sleep Med Rev. 2015;20:27).
For life that has been intricately involved with oxygen from its genesis, it is not unreasonable to expect adaptations of cells, organs, and the whole individual to a wide range of oxygen tensions. Attempts to understand the import of sleep-disordered breathing has led to a need to unravel the implications of OSA-related chronic intermittent hypoxia and sleep-hypoventilation. This has led to a resurgence of interest in hypoxia-related research. Whether such chronic sleep-related sustained and intermittent hypoxemia is a harbinger of chronic disease is still not fully clear. A number of challenges exist with the understanding of these chronic hypoxia effects that include the long time needed for disease occurrence, its differential effects on organ systems, the role of hypoxia vs reoxygenation injury, importance of local blood flow, etc. Understanding these pathways will be crucial in prognosticating the role of sleep-related hypoxemia, the recognition of which has become part and parcel of routine management in sleep medicine.
Dr. Sundar is Medical Director, Sleep-Wake Center, Clinical Professor, Pulmonary, Critical Care & Sleep Medicine, Department of Medicine, University of Utah, Salt Lake City, Utah.













