User login
Welcoming a new Section Editor for Sleep Strategies
Michelle Cao, DO, FCCP
Dr. Michelle Cao is a Clinical Associate Professor in the Division of Sleep Medicine and Division of Neuromuscular Medicine, at the Stanford University School of Medicine. Her clinical expertise is in complex sleep-related respiratory disorders and home mechanical ventilation for chronic respiratory failure syndromes. She oversees the Noninvasive Ventilation Program for the Stanford Neuromuscular Medicine Center. Dr. Cao also holds the position of Vice-Chair for the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork with CHEST and is a member of the Scientific Presentations and Awards Committee.
Michelle Cao, DO, FCCP
Dr. Michelle Cao is a Clinical Associate Professor in the Division of Sleep Medicine and Division of Neuromuscular Medicine, at the Stanford University School of Medicine. Her clinical expertise is in complex sleep-related respiratory disorders and home mechanical ventilation for chronic respiratory failure syndromes. She oversees the Noninvasive Ventilation Program for the Stanford Neuromuscular Medicine Center. Dr. Cao also holds the position of Vice-Chair for the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork with CHEST and is a member of the Scientific Presentations and Awards Committee.
Michelle Cao, DO, FCCP
Dr. Michelle Cao is a Clinical Associate Professor in the Division of Sleep Medicine and Division of Neuromuscular Medicine, at the Stanford University School of Medicine. Her clinical expertise is in complex sleep-related respiratory disorders and home mechanical ventilation for chronic respiratory failure syndromes. She oversees the Noninvasive Ventilation Program for the Stanford Neuromuscular Medicine Center. Dr. Cao also holds the position of Vice-Chair for the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork with CHEST and is a member of the Scientific Presentations and Awards Committee.
Sleep Strategies
Compared with obstructive sleep apnea (OSA), the prevalence of central sleep apnea (CSA) is low in the general population. However, in adults, CSA may be highly prevalent in certain conditions, most commonly among those with left ventricular systolic dysfunction, left ventricular diastolic dysfunction, atrial fibrillation, stroke, and opioid users (Javaheri S, et al. J Am Coll Cardiol. 2017; 69:841). CSA may also be found in patients with carotid artery stenosis, cervical neck injury, and renal dysfunction. CSA can occur when OSA is treated (treatment-emergent central sleep apnea, or TECA), notably, and most frequently, with continuous positive airway pressure (CPAP) devices. Though in many individuals, this frequently resolves with continued use of the device.
In addition, unlike OSA, adequate treatment of CSA has proven difficult. Specifically, the response to CPAP, oxygen, theophylline, acetazolamide, and adaptive-servo ventilation (ASV) is highly variable, with individuals who respond well, and individuals in whom therapy fails to fully suppress the disorder.
Our interest in phrenic nerve stimulation increased after it was shown that CPAP therapy failed to improve morbidity and mortality of CSA in patients with heart failure and reduced ejection fraction (HFrEF) (CANPAP trial, Bradley et al. N Engl J Med. 2005;353(19):2025). In fact, in this trial, treatment with CPAP was associated with significantly increased mortality during the first few months of therapy. We reason that a potential mechanism was positive airway pressure that had adverse cardiovascular effects (Javaheri S. J Clin Sleep Med. 2006;2:399). This is because positive airway pressure therapy decreases venous return to the right side of the heart and increases lung volume. This could increase pulmonary vascular resistance (right ventricular afterload), which is lung volume-dependent. Therefore, the subgroup of individuals with heart failure whose right ventricular function is preload-dependent and has pulmonary hypertension is at risk for premature mortality with any PAP device.
Interestingly, investigators of the SERVE-HF trial (Cowie MR, et al. N Engl J Med. 2015;373:1095) also hypothesized that one reason for excess mortality associated with ASV use might have been due to an ASV-associated excessive rise in intrathoracic pressure, similar to the hypothesis we proposed earlier for CPAP. We expanded on this hypothesis and reasoned that based on the algorithm of the device, in some patients, it could have generated excessive minute ventilation and pressure contributing to excess mortality, either at night or daytime (Javaheri S, et al. Chest. 2016;149:900). Other deficiencies of the algorithm of the ASV device could have contributed to excess mortality as well (Javaheri S, et al. Chest. 2014;146:514). These deficiencies of the ASV device used in the SERVE-HF trial have been significantly improved in the new generation of ASV devices.
Undoubtedly, therefore, mask therapy with positive airway pressures increases intrathoracic pressure and will adversely affect cardiovascular function in some patients with heart failure. Another issue for mask therapy is adherence to the device remains poor, as demonstrated both in the CANPAP and SERVE-HF trials, confirming the need for new approaches utilizing non-mask therapies both for CSA and OSA.
Given the limitations of mask-based therapies, over the last several years, we have performed studies exploring the use of oxygen, acetazolamide, theophylline, and, most recently, phrenic nerve stimulation (PNS). In general, these therapies are devoid of increasing intrathoracic pressure and are expected to be less reliant on patients’ adherence than PAP therapy. Long-term randomized clinical trials are needed, and, most recently, the NIH approved a phase 3 trial for a randomized placebo-controlled low flow oxygen therapy for treatment of CSA in HFrEF. This is a modified trial proposed by one of us more than 20 years ago!
Regarding PNS, CSA is characterized by intermittent phrenic nerve (and intercostal nerves) deactivation. It, therefore, makes sense to have an implanted stimulator for the phrenic nerve to prevent development of central apneas during sleep. This is not a new idea. In 1948, Sarnoff and colleagues demonstrated for the first time that artificial respiration could be effectively administered to the cat, dog, monkey, and rabbit in the absence of spontaneous respiration by electrical stimulation of one (or both) phrenic nerves (Sarnoff SJ, et al. Science. 1948;108:482). In later experiments, these investigators showed that unilateral phrenic nerve stimulation is also equally effective in man as that shown in animal models.
The phrenic nerves comes in contact with veins on both the right (brachiocephalic) and the left (pericardiophrenic vein) side of the mediastinum. Like a cardiac pacemaker, an electrophysiologist places the stimulator within the vein at the point of encounter with the phrenic nerve. Only unilateral stimulation is needed for the therapy. The device is typically placed on the right side of the chest as many patients may already have a cardiac implanted electronic device such as a pacemaker. Like the hypoglossal nerve stimulation, the FDA approved this device for the treatment of OSA. The system can be programmed using an external programmer in the office.
Phrenic nerve stimulation system is initially activated 1 month after the device is placed. It is programmed to be automatically activated at night when the patient is at rest. First, a time is set on the device for when the patient typically goes to bed and awakens. This allows the therapy to activate. The device contains a position sensor and accelerometer, which determine position and activity level. Once appropriate time, position, and activity are confirmed, the device activates automatically. Therapy comes on and can increase in level over several minutes. The device senses transthoracic impedance and can use this measurement to make changes in the therapy output and activity. If the patient gets up at night, the device automatically stops and restarts when the patient is back in a sleeping position. How quickly the therapy restarts and at what energy is programmable. The device may allow from 1 to 15 minutes for the patient to get back to sleep before beginning therapy. These programming changes allow for patient acceptance and comfort with the therapy even in very sensitive patients. Importantly, no patient activation is needed, so that therapy delivery is independent of patient’s adherence over time.
In the prospective, randomized pivotal trial (Costanzo et al. Lancet. 2016;388:974), 151 eligible patients with moderate-severe central sleep apnea were implanted and randomly assigned to the treatment (n=73) or control (n=78) groups. Participants in the active arm received PNS for 6 months. All polysomnograms were centrally and blindly scored. There were significant decreases in AHI (50 to 26/per hour of sleep), CAI (32 to 6), arousal index (46 to 25), and ODI (44 to 25). Two points should be emphasized: first, changes in AHI with PNS are similar to those in CANPAP trial, and there remained a significant number of hypopneas (some of these hypopneas are at least in part related to the speed of the titration when the subject sits up and the device automatically is deactivated, only to resume therapy in supine position); second, in contrast to the CANPAP trial, there was a significant reduction in arousals. Probably for this reason, subjective daytime sleepiness, as measured by the ESS, improved. In addition, PNS improved quality of life, in contrast to lack of effect of CPAP or ASV in this domain. Regarding side effects, 138 (91%) of 151 patients had no serious-related adverse events at 12 months. Seven (9%) cases of related-serious adverse events occurred in the control group and six (8%) cases were reported in the treatment group.—3.4% needed lead repositioning, a rate which is like that of cardiac implantable devices. Seven patients died (unrelated to implant, system, or therapy), four deaths (two in treatment group and two in control group) during the 6-month randomization period when neurostimulation was delivered to only the treatment and was off in the control group, and three deaths between 6 months and 12 months of follow-up when all patients received neurostimulation. Of 73 patients in the treatment group, 27 (37%) reported nonserious therapy-related discomfort that was resolved with simple system reprogramming in 26 (36%) patients but was unresolved in one (1%) patient.
Long-term studies have shown sustained effects of PNS on CSA with improvement in both sleep metrics and QOL, as measured by the Minnesota Living with Heart Failure Questionnaire (MLWHF) and patient global assessment (PGA). Furthermore, in the subgroup of patients with concomitant heart failure with LVEF ≤ 45%, PNS was associated with both improvements in LVEF and a trend toward lower hospitalization rates (Costanzo et al. Eur J Heart Fail. 2018; doi:10.1002/ejhf.1312).
Several issues must be emphasized. One advantage of PNS is complete adherence resulting in a major reduction in apnea burden across the whole night. Second, the mechanism of action prevents any potential adverse consequences related to increased intrathoracic pressure. However, the cost of this therapy is high, similar to that of hypoglossal nerve stimulation. Large scale, long-term studies related to mortality are not yet available, and continued research should help identify those patients most likely to benefit from this therapeutic approach.
Compared with obstructive sleep apnea (OSA), the prevalence of central sleep apnea (CSA) is low in the general population. However, in adults, CSA may be highly prevalent in certain conditions, most commonly among those with left ventricular systolic dysfunction, left ventricular diastolic dysfunction, atrial fibrillation, stroke, and opioid users (Javaheri S, et al. J Am Coll Cardiol. 2017; 69:841). CSA may also be found in patients with carotid artery stenosis, cervical neck injury, and renal dysfunction. CSA can occur when OSA is treated (treatment-emergent central sleep apnea, or TECA), notably, and most frequently, with continuous positive airway pressure (CPAP) devices. Though in many individuals, this frequently resolves with continued use of the device.
In addition, unlike OSA, adequate treatment of CSA has proven difficult. Specifically, the response to CPAP, oxygen, theophylline, acetazolamide, and adaptive-servo ventilation (ASV) is highly variable, with individuals who respond well, and individuals in whom therapy fails to fully suppress the disorder.
Our interest in phrenic nerve stimulation increased after it was shown that CPAP therapy failed to improve morbidity and mortality of CSA in patients with heart failure and reduced ejection fraction (HFrEF) (CANPAP trial, Bradley et al. N Engl J Med. 2005;353(19):2025). In fact, in this trial, treatment with CPAP was associated with significantly increased mortality during the first few months of therapy. We reason that a potential mechanism was positive airway pressure that had adverse cardiovascular effects (Javaheri S. J Clin Sleep Med. 2006;2:399). This is because positive airway pressure therapy decreases venous return to the right side of the heart and increases lung volume. This could increase pulmonary vascular resistance (right ventricular afterload), which is lung volume-dependent. Therefore, the subgroup of individuals with heart failure whose right ventricular function is preload-dependent and has pulmonary hypertension is at risk for premature mortality with any PAP device.
Interestingly, investigators of the SERVE-HF trial (Cowie MR, et al. N Engl J Med. 2015;373:1095) also hypothesized that one reason for excess mortality associated with ASV use might have been due to an ASV-associated excessive rise in intrathoracic pressure, similar to the hypothesis we proposed earlier for CPAP. We expanded on this hypothesis and reasoned that based on the algorithm of the device, in some patients, it could have generated excessive minute ventilation and pressure contributing to excess mortality, either at night or daytime (Javaheri S, et al. Chest. 2016;149:900). Other deficiencies of the algorithm of the ASV device could have contributed to excess mortality as well (Javaheri S, et al. Chest. 2014;146:514). These deficiencies of the ASV device used in the SERVE-HF trial have been significantly improved in the new generation of ASV devices.
Undoubtedly, therefore, mask therapy with positive airway pressures increases intrathoracic pressure and will adversely affect cardiovascular function in some patients with heart failure. Another issue for mask therapy is adherence to the device remains poor, as demonstrated both in the CANPAP and SERVE-HF trials, confirming the need for new approaches utilizing non-mask therapies both for CSA and OSA.
Given the limitations of mask-based therapies, over the last several years, we have performed studies exploring the use of oxygen, acetazolamide, theophylline, and, most recently, phrenic nerve stimulation (PNS). In general, these therapies are devoid of increasing intrathoracic pressure and are expected to be less reliant on patients’ adherence than PAP therapy. Long-term randomized clinical trials are needed, and, most recently, the NIH approved a phase 3 trial for a randomized placebo-controlled low flow oxygen therapy for treatment of CSA in HFrEF. This is a modified trial proposed by one of us more than 20 years ago!
Regarding PNS, CSA is characterized by intermittent phrenic nerve (and intercostal nerves) deactivation. It, therefore, makes sense to have an implanted stimulator for the phrenic nerve to prevent development of central apneas during sleep. This is not a new idea. In 1948, Sarnoff and colleagues demonstrated for the first time that artificial respiration could be effectively administered to the cat, dog, monkey, and rabbit in the absence of spontaneous respiration by electrical stimulation of one (or both) phrenic nerves (Sarnoff SJ, et al. Science. 1948;108:482). In later experiments, these investigators showed that unilateral phrenic nerve stimulation is also equally effective in man as that shown in animal models.
The phrenic nerves comes in contact with veins on both the right (brachiocephalic) and the left (pericardiophrenic vein) side of the mediastinum. Like a cardiac pacemaker, an electrophysiologist places the stimulator within the vein at the point of encounter with the phrenic nerve. Only unilateral stimulation is needed for the therapy. The device is typically placed on the right side of the chest as many patients may already have a cardiac implanted electronic device such as a pacemaker. Like the hypoglossal nerve stimulation, the FDA approved this device for the treatment of OSA. The system can be programmed using an external programmer in the office.
Phrenic nerve stimulation system is initially activated 1 month after the device is placed. It is programmed to be automatically activated at night when the patient is at rest. First, a time is set on the device for when the patient typically goes to bed and awakens. This allows the therapy to activate. The device contains a position sensor and accelerometer, which determine position and activity level. Once appropriate time, position, and activity are confirmed, the device activates automatically. Therapy comes on and can increase in level over several minutes. The device senses transthoracic impedance and can use this measurement to make changes in the therapy output and activity. If the patient gets up at night, the device automatically stops and restarts when the patient is back in a sleeping position. How quickly the therapy restarts and at what energy is programmable. The device may allow from 1 to 15 minutes for the patient to get back to sleep before beginning therapy. These programming changes allow for patient acceptance and comfort with the therapy even in very sensitive patients. Importantly, no patient activation is needed, so that therapy delivery is independent of patient’s adherence over time.
In the prospective, randomized pivotal trial (Costanzo et al. Lancet. 2016;388:974), 151 eligible patients with moderate-severe central sleep apnea were implanted and randomly assigned to the treatment (n=73) or control (n=78) groups. Participants in the active arm received PNS for 6 months. All polysomnograms were centrally and blindly scored. There were significant decreases in AHI (50 to 26/per hour of sleep), CAI (32 to 6), arousal index (46 to 25), and ODI (44 to 25). Two points should be emphasized: first, changes in AHI with PNS are similar to those in CANPAP trial, and there remained a significant number of hypopneas (some of these hypopneas are at least in part related to the speed of the titration when the subject sits up and the device automatically is deactivated, only to resume therapy in supine position); second, in contrast to the CANPAP trial, there was a significant reduction in arousals. Probably for this reason, subjective daytime sleepiness, as measured by the ESS, improved. In addition, PNS improved quality of life, in contrast to lack of effect of CPAP or ASV in this domain. Regarding side effects, 138 (91%) of 151 patients had no serious-related adverse events at 12 months. Seven (9%) cases of related-serious adverse events occurred in the control group and six (8%) cases were reported in the treatment group.—3.4% needed lead repositioning, a rate which is like that of cardiac implantable devices. Seven patients died (unrelated to implant, system, or therapy), four deaths (two in treatment group and two in control group) during the 6-month randomization period when neurostimulation was delivered to only the treatment and was off in the control group, and three deaths between 6 months and 12 months of follow-up when all patients received neurostimulation. Of 73 patients in the treatment group, 27 (37%) reported nonserious therapy-related discomfort that was resolved with simple system reprogramming in 26 (36%) patients but was unresolved in one (1%) patient.
Long-term studies have shown sustained effects of PNS on CSA with improvement in both sleep metrics and QOL, as measured by the Minnesota Living with Heart Failure Questionnaire (MLWHF) and patient global assessment (PGA). Furthermore, in the subgroup of patients with concomitant heart failure with LVEF ≤ 45%, PNS was associated with both improvements in LVEF and a trend toward lower hospitalization rates (Costanzo et al. Eur J Heart Fail. 2018; doi:10.1002/ejhf.1312).
Several issues must be emphasized. One advantage of PNS is complete adherence resulting in a major reduction in apnea burden across the whole night. Second, the mechanism of action prevents any potential adverse consequences related to increased intrathoracic pressure. However, the cost of this therapy is high, similar to that of hypoglossal nerve stimulation. Large scale, long-term studies related to mortality are not yet available, and continued research should help identify those patients most likely to benefit from this therapeutic approach.
Compared with obstructive sleep apnea (OSA), the prevalence of central sleep apnea (CSA) is low in the general population. However, in adults, CSA may be highly prevalent in certain conditions, most commonly among those with left ventricular systolic dysfunction, left ventricular diastolic dysfunction, atrial fibrillation, stroke, and opioid users (Javaheri S, et al. J Am Coll Cardiol. 2017; 69:841). CSA may also be found in patients with carotid artery stenosis, cervical neck injury, and renal dysfunction. CSA can occur when OSA is treated (treatment-emergent central sleep apnea, or TECA), notably, and most frequently, with continuous positive airway pressure (CPAP) devices. Though in many individuals, this frequently resolves with continued use of the device.
In addition, unlike OSA, adequate treatment of CSA has proven difficult. Specifically, the response to CPAP, oxygen, theophylline, acetazolamide, and adaptive-servo ventilation (ASV) is highly variable, with individuals who respond well, and individuals in whom therapy fails to fully suppress the disorder.
Our interest in phrenic nerve stimulation increased after it was shown that CPAP therapy failed to improve morbidity and mortality of CSA in patients with heart failure and reduced ejection fraction (HFrEF) (CANPAP trial, Bradley et al. N Engl J Med. 2005;353(19):2025). In fact, in this trial, treatment with CPAP was associated with significantly increased mortality during the first few months of therapy. We reason that a potential mechanism was positive airway pressure that had adverse cardiovascular effects (Javaheri S. J Clin Sleep Med. 2006;2:399). This is because positive airway pressure therapy decreases venous return to the right side of the heart and increases lung volume. This could increase pulmonary vascular resistance (right ventricular afterload), which is lung volume-dependent. Therefore, the subgroup of individuals with heart failure whose right ventricular function is preload-dependent and has pulmonary hypertension is at risk for premature mortality with any PAP device.
Interestingly, investigators of the SERVE-HF trial (Cowie MR, et al. N Engl J Med. 2015;373:1095) also hypothesized that one reason for excess mortality associated with ASV use might have been due to an ASV-associated excessive rise in intrathoracic pressure, similar to the hypothesis we proposed earlier for CPAP. We expanded on this hypothesis and reasoned that based on the algorithm of the device, in some patients, it could have generated excessive minute ventilation and pressure contributing to excess mortality, either at night or daytime (Javaheri S, et al. Chest. 2016;149:900). Other deficiencies of the algorithm of the ASV device could have contributed to excess mortality as well (Javaheri S, et al. Chest. 2014;146:514). These deficiencies of the ASV device used in the SERVE-HF trial have been significantly improved in the new generation of ASV devices.
Undoubtedly, therefore, mask therapy with positive airway pressures increases intrathoracic pressure and will adversely affect cardiovascular function in some patients with heart failure. Another issue for mask therapy is adherence to the device remains poor, as demonstrated both in the CANPAP and SERVE-HF trials, confirming the need for new approaches utilizing non-mask therapies both for CSA and OSA.
Given the limitations of mask-based therapies, over the last several years, we have performed studies exploring the use of oxygen, acetazolamide, theophylline, and, most recently, phrenic nerve stimulation (PNS). In general, these therapies are devoid of increasing intrathoracic pressure and are expected to be less reliant on patients’ adherence than PAP therapy. Long-term randomized clinical trials are needed, and, most recently, the NIH approved a phase 3 trial for a randomized placebo-controlled low flow oxygen therapy for treatment of CSA in HFrEF. This is a modified trial proposed by one of us more than 20 years ago!
Regarding PNS, CSA is characterized by intermittent phrenic nerve (and intercostal nerves) deactivation. It, therefore, makes sense to have an implanted stimulator for the phrenic nerve to prevent development of central apneas during sleep. This is not a new idea. In 1948, Sarnoff and colleagues demonstrated for the first time that artificial respiration could be effectively administered to the cat, dog, monkey, and rabbit in the absence of spontaneous respiration by electrical stimulation of one (or both) phrenic nerves (Sarnoff SJ, et al. Science. 1948;108:482). In later experiments, these investigators showed that unilateral phrenic nerve stimulation is also equally effective in man as that shown in animal models.
The phrenic nerves comes in contact with veins on both the right (brachiocephalic) and the left (pericardiophrenic vein) side of the mediastinum. Like a cardiac pacemaker, an electrophysiologist places the stimulator within the vein at the point of encounter with the phrenic nerve. Only unilateral stimulation is needed for the therapy. The device is typically placed on the right side of the chest as many patients may already have a cardiac implanted electronic device such as a pacemaker. Like the hypoglossal nerve stimulation, the FDA approved this device for the treatment of OSA. The system can be programmed using an external programmer in the office.
Phrenic nerve stimulation system is initially activated 1 month after the device is placed. It is programmed to be automatically activated at night when the patient is at rest. First, a time is set on the device for when the patient typically goes to bed and awakens. This allows the therapy to activate. The device contains a position sensor and accelerometer, which determine position and activity level. Once appropriate time, position, and activity are confirmed, the device activates automatically. Therapy comes on and can increase in level over several minutes. The device senses transthoracic impedance and can use this measurement to make changes in the therapy output and activity. If the patient gets up at night, the device automatically stops and restarts when the patient is back in a sleeping position. How quickly the therapy restarts and at what energy is programmable. The device may allow from 1 to 15 minutes for the patient to get back to sleep before beginning therapy. These programming changes allow for patient acceptance and comfort with the therapy even in very sensitive patients. Importantly, no patient activation is needed, so that therapy delivery is independent of patient’s adherence over time.
In the prospective, randomized pivotal trial (Costanzo et al. Lancet. 2016;388:974), 151 eligible patients with moderate-severe central sleep apnea were implanted and randomly assigned to the treatment (n=73) or control (n=78) groups. Participants in the active arm received PNS for 6 months. All polysomnograms were centrally and blindly scored. There were significant decreases in AHI (50 to 26/per hour of sleep), CAI (32 to 6), arousal index (46 to 25), and ODI (44 to 25). Two points should be emphasized: first, changes in AHI with PNS are similar to those in CANPAP trial, and there remained a significant number of hypopneas (some of these hypopneas are at least in part related to the speed of the titration when the subject sits up and the device automatically is deactivated, only to resume therapy in supine position); second, in contrast to the CANPAP trial, there was a significant reduction in arousals. Probably for this reason, subjective daytime sleepiness, as measured by the ESS, improved. In addition, PNS improved quality of life, in contrast to lack of effect of CPAP or ASV in this domain. Regarding side effects, 138 (91%) of 151 patients had no serious-related adverse events at 12 months. Seven (9%) cases of related-serious adverse events occurred in the control group and six (8%) cases were reported in the treatment group.—3.4% needed lead repositioning, a rate which is like that of cardiac implantable devices. Seven patients died (unrelated to implant, system, or therapy), four deaths (two in treatment group and two in control group) during the 6-month randomization period when neurostimulation was delivered to only the treatment and was off in the control group, and three deaths between 6 months and 12 months of follow-up when all patients received neurostimulation. Of 73 patients in the treatment group, 27 (37%) reported nonserious therapy-related discomfort that was resolved with simple system reprogramming in 26 (36%) patients but was unresolved in one (1%) patient.
Long-term studies have shown sustained effects of PNS on CSA with improvement in both sleep metrics and QOL, as measured by the Minnesota Living with Heart Failure Questionnaire (MLWHF) and patient global assessment (PGA). Furthermore, in the subgroup of patients with concomitant heart failure with LVEF ≤ 45%, PNS was associated with both improvements in LVEF and a trend toward lower hospitalization rates (Costanzo et al. Eur J Heart Fail. 2018; doi:10.1002/ejhf.1312).
Several issues must be emphasized. One advantage of PNS is complete adherence resulting in a major reduction in apnea burden across the whole night. Second, the mechanism of action prevents any potential adverse consequences related to increased intrathoracic pressure. However, the cost of this therapy is high, similar to that of hypoglossal nerve stimulation. Large scale, long-term studies related to mortality are not yet available, and continued research should help identify those patients most likely to benefit from this therapeutic approach.
The Emerging Role of Sleep in the Development of Alzheimer Disease
More than 5 million Americans are living with Alzheimer disease (AD), making this the leading cause of dementia in the United States. This number is projected to nearly triple to 14 million people by 2060 (Matthews KA, et al. Alzheimers Dement. 2018 Sep 17. doi: 10.1016/j.jalz.2018.06.3063. [Epub ahead of print]).
Experts predict estimated costs related to AD to be more than $500 billion annually starting in 2040 (Hurd MD, et al. N Engl J Med. 2013;368[14]:1326). AD is a neurodegenerative disorder characterized by gradual, progressive decline in memory along with other cognitive functions, eventually leading to impairment in activities of daily living. Most current treatments for AD are symptomatic and only minimally slow progression of disease. The increasing prevalence, overwhelming costs to society, and the absence of a cure for AD have created an impending national health crisis.
As the dementia progresses, sleep also tends to worsen. Currently, clinicians improve sleep in patients already diagnosed with AD through diagnosis and treatment of sleep disorders, such as insomnia and sleep apnea to improve overall functioning and quality of life. Treatment of obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) in patients diagnosed with AD has shown to improve cognition and other neurocognitive measures (Ancoli-Israel S, et al. J Am Geriatr Soc. 2008;56[11]:2076).
However, there is mounting interest in evaluating how poor sleep could lead to future development of AD or serve as a marker for AD disease in preclinical or asymptomatic populations. Sleep symptoms can be a precursor of other neurological diseases; for example, dream enactment (REM sleep behavior disorder) can precede onset of neurodegenerative disease (Parkinson disease) by decades. Increasing evidence suggests that sleep disruption seen in early or even preclinical AD contributes to its onset and progression. In response to this growing body of research, in June 2018, the American Academy of Sleep Medicine (AASM) issued a health advisory to patients and providers to consider early intervention to ensure sufficient sleep and to treat sleep disorders to assist prevention or delaying onset of AD.
Poor Sleep as a Risk Factor for Alzheimer Disease
Epidemiologic studies (both cross-sectional and prospective studies) have demonstrated that fragmented sleep in cognitively normal individuals is a risk factor for the future development of symptomatic AD (Bubu OM, et al. Sleep. 2017[Jan]:1;40). The pathogenesis of AD includes abnormal accumulation of the protein, amyloid-β (Aβ), in the brain as insoluble extracellular plaques followed by intracellular aggregation of tau, neuronal loss, and cognitive dysfunction. Aβ deposition in the brain begins approximately15 to 20 years before the onset of cognitive impairment and serves as an early biomarker of AD. Accumulation of Aβ results from imbalance between production and clearance of the protein from the central nervous system.
Numerous studies have demonstrated that people with disrupted sleep may show early evidence of AD disease, such as Aβ deposition compared with healthy sleepers. In one study, cognitively normal people with Aβ plaque disease had worse sleep efficiency and increased nap frequency measured by actigraphy as compared with cognitively normal individuals without Aβ plaques (Ju YE, et al. JAMA Neurol. 2013 [May];70[5]:587). Further, a recent study found that self-reported daytime sleepiness was associated with
Possible Mechanisms
Possible mechanisms have been suggested to explain how poor sleep may lead to AD. Over the past 10 years, sleep deprivation was found to increase Aβ concentrations in both a mouse model (Kang JE, et al. Science. 2009; 326:1005) and humans, most likely through increased production and/or release of Aβ (Lucey BP, et al. Ann Neurol. 2018; 83[1]:197). Sleep also appears to increase clearance of proteins and other molecules via bulk fluid flow (“glymphatic” clearance). Glymphatic clearance may enable the removal of interstitial toxic proteins, such as Aβ, through a dynamic interaction between the cerebrospinal fluid and the interstitial fluid, where astrocytes facilitate extracellular fluid transit though the brain during sleep (Xie L, et al. Science. 2013;342:373). Since Aβ deposition in the brain is concentration-dependent, higher Aβ levels from sleep disturbance could lead to greater deposition in the brain.
Circadian Rhythm and Alzheimer Disease
Another mechanism linking sleep to the pathogenesis of AD includes disruption of the circadian rhythm, which is commonly seen in patients with AD. Studies have linked populations who suffer from circadian rhythm disorders to higher rates of dementia (Tranah GJ, et al. Ann Neurol. 2011;70[5]:722). Circadian disruption may predispose the brain to neurodegenerative conditions by altering immune function, disrupting endocrine function, increasing inflammation and oxidative stress, or affecting neurogenesis (in specific areas such as the hippocampus). Thus, inadequate sleep could prime the brain for neurodegeneration by multiple pathways.
Obstructive Sleep Apnea and Alzheimer’s Disease
Sleep disruption and chronic intermittent hypoxia secondary to untreated OSA has also been associated with AD. Numerous studies have shown that sleep-disordered breathing is associated with AD risk and that AD patients have higher rates of OSA. For instance, a study in older women found that moderate and severe sleep-disordered breathing was associated with an increased risk of future cognitive impairment and dementia (Yaffe K, et al. JAMA. 2011[Aug]:10;306[6]:613). In addition to sleep disruption from sleep apnea affecting Aβ as detailed above, hypoxia from sleep apnea may also alter risk of future AD.
Future Directions
Studies support a clear bidirectional relationship between AD and sleep. As researchers continue to investigate sleep as a marker for AD, others are exploring the implications of using sleep interventions to prevent and/or delay the onset of AD. Patients with poor and disrupted sleep may be the ideal candidates for sleep interventions to lower the risk of AD, such as treating OSA with CPAP therapy or insomnia with hypnotic medication or cognitive behavioral therapy. These therapies are already well-studied and approved for human use, allowing for rapid translation to future intervention trials.
Dr. Malhotra is Associate Professor, Sleep Medicine Section; and Dr. Lucey is Assistant Professor, Director-Sleep Medicine Section; Department of Neurology, Washington University School of Medicine, St Louis, Missouri.
More than 5 million Americans are living with Alzheimer disease (AD), making this the leading cause of dementia in the United States. This number is projected to nearly triple to 14 million people by 2060 (Matthews KA, et al. Alzheimers Dement. 2018 Sep 17. doi: 10.1016/j.jalz.2018.06.3063. [Epub ahead of print]).
Experts predict estimated costs related to AD to be more than $500 billion annually starting in 2040 (Hurd MD, et al. N Engl J Med. 2013;368[14]:1326). AD is a neurodegenerative disorder characterized by gradual, progressive decline in memory along with other cognitive functions, eventually leading to impairment in activities of daily living. Most current treatments for AD are symptomatic and only minimally slow progression of disease. The increasing prevalence, overwhelming costs to society, and the absence of a cure for AD have created an impending national health crisis.
As the dementia progresses, sleep also tends to worsen. Currently, clinicians improve sleep in patients already diagnosed with AD through diagnosis and treatment of sleep disorders, such as insomnia and sleep apnea to improve overall functioning and quality of life. Treatment of obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) in patients diagnosed with AD has shown to improve cognition and other neurocognitive measures (Ancoli-Israel S, et al. J Am Geriatr Soc. 2008;56[11]:2076).
However, there is mounting interest in evaluating how poor sleep could lead to future development of AD or serve as a marker for AD disease in preclinical or asymptomatic populations. Sleep symptoms can be a precursor of other neurological diseases; for example, dream enactment (REM sleep behavior disorder) can precede onset of neurodegenerative disease (Parkinson disease) by decades. Increasing evidence suggests that sleep disruption seen in early or even preclinical AD contributes to its onset and progression. In response to this growing body of research, in June 2018, the American Academy of Sleep Medicine (AASM) issued a health advisory to patients and providers to consider early intervention to ensure sufficient sleep and to treat sleep disorders to assist prevention or delaying onset of AD.
Poor Sleep as a Risk Factor for Alzheimer Disease
Epidemiologic studies (both cross-sectional and prospective studies) have demonstrated that fragmented sleep in cognitively normal individuals is a risk factor for the future development of symptomatic AD (Bubu OM, et al. Sleep. 2017[Jan]:1;40). The pathogenesis of AD includes abnormal accumulation of the protein, amyloid-β (Aβ), in the brain as insoluble extracellular plaques followed by intracellular aggregation of tau, neuronal loss, and cognitive dysfunction. Aβ deposition in the brain begins approximately15 to 20 years before the onset of cognitive impairment and serves as an early biomarker of AD. Accumulation of Aβ results from imbalance between production and clearance of the protein from the central nervous system.
Numerous studies have demonstrated that people with disrupted sleep may show early evidence of AD disease, such as Aβ deposition compared with healthy sleepers. In one study, cognitively normal people with Aβ plaque disease had worse sleep efficiency and increased nap frequency measured by actigraphy as compared with cognitively normal individuals without Aβ plaques (Ju YE, et al. JAMA Neurol. 2013 [May];70[5]:587). Further, a recent study found that self-reported daytime sleepiness was associated with
Possible Mechanisms
Possible mechanisms have been suggested to explain how poor sleep may lead to AD. Over the past 10 years, sleep deprivation was found to increase Aβ concentrations in both a mouse model (Kang JE, et al. Science. 2009; 326:1005) and humans, most likely through increased production and/or release of Aβ (Lucey BP, et al. Ann Neurol. 2018; 83[1]:197). Sleep also appears to increase clearance of proteins and other molecules via bulk fluid flow (“glymphatic” clearance). Glymphatic clearance may enable the removal of interstitial toxic proteins, such as Aβ, through a dynamic interaction between the cerebrospinal fluid and the interstitial fluid, where astrocytes facilitate extracellular fluid transit though the brain during sleep (Xie L, et al. Science. 2013;342:373). Since Aβ deposition in the brain is concentration-dependent, higher Aβ levels from sleep disturbance could lead to greater deposition in the brain.
Circadian Rhythm and Alzheimer Disease
Another mechanism linking sleep to the pathogenesis of AD includes disruption of the circadian rhythm, which is commonly seen in patients with AD. Studies have linked populations who suffer from circadian rhythm disorders to higher rates of dementia (Tranah GJ, et al. Ann Neurol. 2011;70[5]:722). Circadian disruption may predispose the brain to neurodegenerative conditions by altering immune function, disrupting endocrine function, increasing inflammation and oxidative stress, or affecting neurogenesis (in specific areas such as the hippocampus). Thus, inadequate sleep could prime the brain for neurodegeneration by multiple pathways.
Obstructive Sleep Apnea and Alzheimer’s Disease
Sleep disruption and chronic intermittent hypoxia secondary to untreated OSA has also been associated with AD. Numerous studies have shown that sleep-disordered breathing is associated with AD risk and that AD patients have higher rates of OSA. For instance, a study in older women found that moderate and severe sleep-disordered breathing was associated with an increased risk of future cognitive impairment and dementia (Yaffe K, et al. JAMA. 2011[Aug]:10;306[6]:613). In addition to sleep disruption from sleep apnea affecting Aβ as detailed above, hypoxia from sleep apnea may also alter risk of future AD.
Future Directions
Studies support a clear bidirectional relationship between AD and sleep. As researchers continue to investigate sleep as a marker for AD, others are exploring the implications of using sleep interventions to prevent and/or delay the onset of AD. Patients with poor and disrupted sleep may be the ideal candidates for sleep interventions to lower the risk of AD, such as treating OSA with CPAP therapy or insomnia with hypnotic medication or cognitive behavioral therapy. These therapies are already well-studied and approved for human use, allowing for rapid translation to future intervention trials.
Dr. Malhotra is Associate Professor, Sleep Medicine Section; and Dr. Lucey is Assistant Professor, Director-Sleep Medicine Section; Department of Neurology, Washington University School of Medicine, St Louis, Missouri.
More than 5 million Americans are living with Alzheimer disease (AD), making this the leading cause of dementia in the United States. This number is projected to nearly triple to 14 million people by 2060 (Matthews KA, et al. Alzheimers Dement. 2018 Sep 17. doi: 10.1016/j.jalz.2018.06.3063. [Epub ahead of print]).
Experts predict estimated costs related to AD to be more than $500 billion annually starting in 2040 (Hurd MD, et al. N Engl J Med. 2013;368[14]:1326). AD is a neurodegenerative disorder characterized by gradual, progressive decline in memory along with other cognitive functions, eventually leading to impairment in activities of daily living. Most current treatments for AD are symptomatic and only minimally slow progression of disease. The increasing prevalence, overwhelming costs to society, and the absence of a cure for AD have created an impending national health crisis.
As the dementia progresses, sleep also tends to worsen. Currently, clinicians improve sleep in patients already diagnosed with AD through diagnosis and treatment of sleep disorders, such as insomnia and sleep apnea to improve overall functioning and quality of life. Treatment of obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) in patients diagnosed with AD has shown to improve cognition and other neurocognitive measures (Ancoli-Israel S, et al. J Am Geriatr Soc. 2008;56[11]:2076).
However, there is mounting interest in evaluating how poor sleep could lead to future development of AD or serve as a marker for AD disease in preclinical or asymptomatic populations. Sleep symptoms can be a precursor of other neurological diseases; for example, dream enactment (REM sleep behavior disorder) can precede onset of neurodegenerative disease (Parkinson disease) by decades. Increasing evidence suggests that sleep disruption seen in early or even preclinical AD contributes to its onset and progression. In response to this growing body of research, in June 2018, the American Academy of Sleep Medicine (AASM) issued a health advisory to patients and providers to consider early intervention to ensure sufficient sleep and to treat sleep disorders to assist prevention or delaying onset of AD.
Poor Sleep as a Risk Factor for Alzheimer Disease
Epidemiologic studies (both cross-sectional and prospective studies) have demonstrated that fragmented sleep in cognitively normal individuals is a risk factor for the future development of symptomatic AD (Bubu OM, et al. Sleep. 2017[Jan]:1;40). The pathogenesis of AD includes abnormal accumulation of the protein, amyloid-β (Aβ), in the brain as insoluble extracellular plaques followed by intracellular aggregation of tau, neuronal loss, and cognitive dysfunction. Aβ deposition in the brain begins approximately15 to 20 years before the onset of cognitive impairment and serves as an early biomarker of AD. Accumulation of Aβ results from imbalance between production and clearance of the protein from the central nervous system.
Numerous studies have demonstrated that people with disrupted sleep may show early evidence of AD disease, such as Aβ deposition compared with healthy sleepers. In one study, cognitively normal people with Aβ plaque disease had worse sleep efficiency and increased nap frequency measured by actigraphy as compared with cognitively normal individuals without Aβ plaques (Ju YE, et al. JAMA Neurol. 2013 [May];70[5]:587). Further, a recent study found that self-reported daytime sleepiness was associated with
Possible Mechanisms
Possible mechanisms have been suggested to explain how poor sleep may lead to AD. Over the past 10 years, sleep deprivation was found to increase Aβ concentrations in both a mouse model (Kang JE, et al. Science. 2009; 326:1005) and humans, most likely through increased production and/or release of Aβ (Lucey BP, et al. Ann Neurol. 2018; 83[1]:197). Sleep also appears to increase clearance of proteins and other molecules via bulk fluid flow (“glymphatic” clearance). Glymphatic clearance may enable the removal of interstitial toxic proteins, such as Aβ, through a dynamic interaction between the cerebrospinal fluid and the interstitial fluid, where astrocytes facilitate extracellular fluid transit though the brain during sleep (Xie L, et al. Science. 2013;342:373). Since Aβ deposition in the brain is concentration-dependent, higher Aβ levels from sleep disturbance could lead to greater deposition in the brain.
Circadian Rhythm and Alzheimer Disease
Another mechanism linking sleep to the pathogenesis of AD includes disruption of the circadian rhythm, which is commonly seen in patients with AD. Studies have linked populations who suffer from circadian rhythm disorders to higher rates of dementia (Tranah GJ, et al. Ann Neurol. 2011;70[5]:722). Circadian disruption may predispose the brain to neurodegenerative conditions by altering immune function, disrupting endocrine function, increasing inflammation and oxidative stress, or affecting neurogenesis (in specific areas such as the hippocampus). Thus, inadequate sleep could prime the brain for neurodegeneration by multiple pathways.
Obstructive Sleep Apnea and Alzheimer’s Disease
Sleep disruption and chronic intermittent hypoxia secondary to untreated OSA has also been associated with AD. Numerous studies have shown that sleep-disordered breathing is associated with AD risk and that AD patients have higher rates of OSA. For instance, a study in older women found that moderate and severe sleep-disordered breathing was associated with an increased risk of future cognitive impairment and dementia (Yaffe K, et al. JAMA. 2011[Aug]:10;306[6]:613). In addition to sleep disruption from sleep apnea affecting Aβ as detailed above, hypoxia from sleep apnea may also alter risk of future AD.
Future Directions
Studies support a clear bidirectional relationship between AD and sleep. As researchers continue to investigate sleep as a marker for AD, others are exploring the implications of using sleep interventions to prevent and/or delay the onset of AD. Patients with poor and disrupted sleep may be the ideal candidates for sleep interventions to lower the risk of AD, such as treating OSA with CPAP therapy or insomnia with hypnotic medication or cognitive behavioral therapy. These therapies are already well-studied and approved for human use, allowing for rapid translation to future intervention trials.
Dr. Malhotra is Associate Professor, Sleep Medicine Section; and Dr. Lucey is Assistant Professor, Director-Sleep Medicine Section; Department of Neurology, Washington University School of Medicine, St Louis, Missouri.
The link between suicide and sleep
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
Value-based sleep: understanding and maximizing value in sleep medicine care
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.
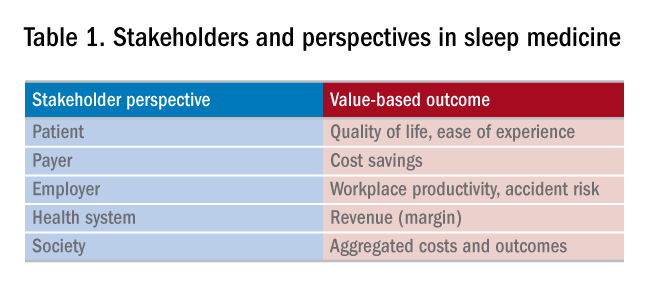
Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 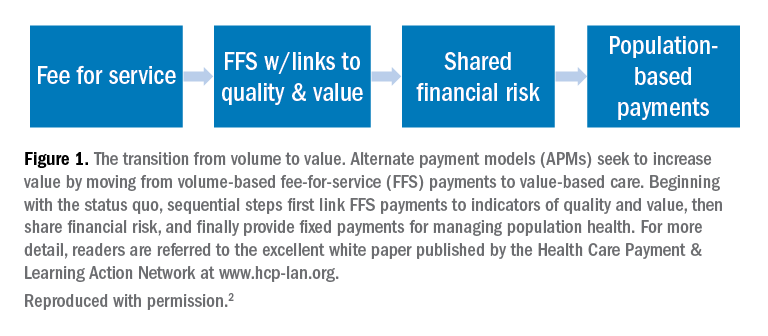
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.

Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
In addition to well-documented health consequences, obstructive sleep apnea (OSA) is associated with substantial economic costs borne by patients, payers, employers, and society at large. For example, in a recent white paper commissioned by the American Academy of Sleep Medicine, the total societal-level costs of OSA were estimated to exceed $150 billion per year in the United States alone. In addition to direct costs associated with OSA diagnosis and treatment, indirect costs were estimated at $86.9 billion for lost workplace productivity; $30 billion for increased health-care utilization (HCU); $26.2 billion for motor vehicle crashes (MVC); and $6.5 billion for workplace accidents and injuries.1
More important, evidence suggests that OSA treatments provide positive economic impact, for example reducing health-care utilization and reducing days missed from work. Our group at the University of Maryland is currently heavily involved in related research examining the health economic impact of sleep disorders and their treatments.
Value-based sleep is a concept that I created several years ago to guide a greater emphasis on health economic outcomes in order to advance our field. In addition to working with payers, industry partners, employers, and forward-thinking startups, we are investing much effort into provider education regarding the health economic aspects of sleep. This article examines what value-based sleep is, how to increase the value of sleep in your practice setting, and steps to prepare for payment models of the future.
Value is in the eye of the beholder
Unlike sleep medicine providers (and some patients), the majority of society views sleep as means to an end and not as an end-in-itself. That is, people only value sleep insofar as sleep will help them achieve their primary objectives, whatever they might be. In health economic terms, these distinct viewpoints are referred to as perspectives. For example, from the patient perspective, sleep is valued to the extent that it helps to increase quality of life. From the payer perspective, sleep is valued to the extent that reduces health-care utilization. From the employer perspective, sleep is valued to the extent that it increases workplace productivity and reduces health-care expenses. Table 1 summarizes common stakeholders and perspectives in sleep medicine.

Speaking the language of value
In order to define, demonstrate, and maximize the perceived value of sleep medicine services, sleep physicians must understand and clearly articulate the values of these multiple constituents. Most important, this means that sleep physicians must move beyond discussing the apnea-hypopnea index (AHI). To be clear, no one other than sleep medicine insiders care about the AHI! Of course, the AHI is an important (albeit imperfect) measure of OSA disease severity and treatment outcomes. However, when was the last time that a patient told you they woke up one morning dreaming about a lower AHI? It simply does not happen. Instead, stakeholders care about outcomes that matters to them, from their own unique perspectives. To speak directly to these interests and frame the value of sleep, sleep medicine providers must methodically develop value propositions with each unique target constituency in mind. Speak the language of your audience, and use terms that matter to them.
Adopting value-based payments
Much has been spoken about a transition from fee-for-service to value-based care in medicine. New health-care business models will soon impact patients, providers, payers, and health systems. To guide and ensure sustainable change, multi-stakeholder organizations, such as the Health Care Payment & Learning Action Network, are heavily engaged in the development and implementation of alternate payment models (APMs) to facilitate the transition from fee-for-service to population health. As depicted in Figure 1, sequential steps toward value-based care include increased fees corresponding to improved outcomes. A reimbursement model that is fully value-based centers on shared financial risks. Although private practitioners may be ill-equipped to provide population-level services or negotiate fully value-based models, sleep medicine providers should do well to increase familiarity with APMs and their impact on primary and specialty care services. 
Five steps to a value-based approach
In the modern health-care climate of increasing costs on the one hand and limited resources on the other, sleep medicine providers must embrace a value-based perspective to survive, thrive, and grow in a new world of value-based care. This will require sleep medicine providers to learn, adapt, and adjust. The good news is that regardless of your practice or organizational setting, these strategies and tactics will help guide you:
1. Know thyself. What are your personal and organization objectives? Where are you, career-wise? Where do you want to be in 2, 3, and 5 years?
2. Know your customer. Whom do you serve? More broadly, whom does sleep serve? Listen carefully and identify the outcomes that matter to your constituents. Make these your endpoints.
3. Develop customer-centric language. Develop scripts. Rehearse them.
4. Understand trends in payments and technology. Is your region adopting bundled payments or paying more for improved outcomes? How might telemedicine or preauthorization for PAP impact your practice?
5. Know your numbers. To negotiate with confidence, you need to know your numbers. What are your costs per patient, per test, per outcome, and lifetime value of the patient?
Summary and next steps
To survive and thrive in a value-based future, you need to define, demonstrate, and maximize your perceived value. This will require greater attention to the language that you use, the results that you emphasize, and the data that you use to make decisions, all while attending to the perspectives of diverse stakeholders. The need for sleep medicine services has never been greater. Adopt a value-based sleep approach to ensure your bright future.
References
1. American Academy of Sleep M. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Mountain View, CA: Frost & Sullivan; 2016.
2. Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881-884.
Dr. Wickwire is Associate Professor of Psychiatry and Medicine at the University of Maryland School of Medicine, where he directs the insomnia program. His current research interests include health and economic consequences of sleep disorders and their treatments and targeting sleep treatments for specific populations.
COPD-OSA overlap syndrome
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) each affect at least 10% of the general adult population and, thus, both disorders together, commonly referred to as the overlap syndrome, could be expected in at least 1% of adults by chance alone. However, there is evidence of important interactions between the disorders that influence the prevalence of the overlap, which have implications for the development of comorbidities,and also for management (McNicholas WT. Chest. 2017; 152[6]:1318). Furthermore, sleep quality is typically poor in COPD, which has been linked to worse pulmonary function and lung hyperinflation and may contribute to daytime fatigue.
Interactions between COPD and OSA that may influence the prevalence of overlap
Previous reports have presented conflicting results regarding the likely association between COPD and OSA, which may partly reflect different definitions of OSA, patient populations, and methodologies of investigation. However, COPD represents a spectrum of clinical phenotypes ranging from the hyperinflated patient with low BMI (predominant emphysema phenotype) to the patient with higher BMI and tendency to right-sided heart failure (predominant chronic bronchitis phenotype). The predominant emphysema phenotype may predispose to a lower likelihood of OSA, and there is recent evidence that lung hyperinflation is protective against the development of OSA by lowering the critical closing pressure of the upper airway during sleep. Furthermore, the degree of emphysema and gas trapping on CT scan of the thorax correlates inversely with apnea-hypopnia index in patients with severe COPD (Krachman SL et al. Ann Am Thorac Soc. 2016;13[7]:1129).
In contrast, the predominant chronic bronchitis phenotype predisposes to a higher likelihood of OSA because of higher BMI and likelihood of right-sided heart failure. Peripheral fluid retention in such patients predisposes to OSA because of the rostral fluid shift that occurs during sleep in the supine position, predisposing to upper airway obstruction by airway narrowing. The COPDGene study reports that the chronic bronchitis phenotype has a higher prevalence of OSA even in the absence of differences in BMI and lung function (Kim V et al. Chest. 2011;140[3]:626). Upper airway inflammation associated with cigarette smoking may also contribute to the development of OSA, and corticosteroid therapy may adversely affect upper airway muscle function. OSA also appears to exacerbate lower airway inflammation in COPD. In practice, most patients with COPD have a mixture of emphysema and chronic bronchitis, and the probability of OSA will represent the balance of these protective and promoting factors in individual patients (Fig 1).
While there is evidence of increased mortality in patients with COPD and OSA alone, a recent report based on the Sleep Heart Health Study somewhat surprisingly found that the incremental contribution of declining lung function to mortality diminished with increasing severity of SDB measured by AHI (Putcha N et al. Am J Respir Crit Care Med. 2016;194[8]:1007). Thus, the epidemiologic relationship of COPD and OSA and related clinical outcomes remains an important research topic comparing different clinical phenotypes.
Mechanisms of interaction in the overlap syndrome and implications for comorbidity
COPD and OSA are associated with several overlapping physiological and biological disturbances, including hypoxia and inflammation, which may contribute to cardiovascular and other comorbidities. Thus, the probability should be high that the overlap syndrome will be associated with a greater risk of comorbidity than with either disease alone. Patients with the overlap syndrome demonstrate greater degrees of oxygen desaturation predisposing to pulmonary hypertension, which is especially common in these patients.
COPD and OSA are each associated with systemic inflammation and oxidative stress, and C-reactive protein (CRP) has been identified as a measure of systemic inflammation that is commonly elevated in both disorders, although in OSA, concurrent obesity is an important confounding factor. Systemic inflammation contributes to the development of cardiovascular disease, which is a common complication of both COPD and OSA. Thus, one could expect that cardiovascular disease is particularly prevalent in patients with overlap syndrome, but there are limited data on this relationship, which represents an important research topic.
Clinical assessment
Patients with the overlap syndrome present with typical clinical features of each disorder and additional features that reflect the higher prevalence of hypoxemia, hypercapnia, and pulmonary hypertension. Thus, morning headaches reflecting hypercapnia and peripheral edema reflecting right-sided heart failure may be especially common. Screening questionnaires may be helpful in the initial evaluation of likely OSA in patients with COPD, and objective clinical data, including anthropometrics such as age, sex, and BMI, and medical history such as cardiovascular comorbidity, are especially useful in clinical prediction (McNicholas WT. Lancet Respir Med. 2016;4[9]:683). Thus, screening for OSA in patients with COPD should not be complicated, and the widespread failure to do so may reflect a lack of awareness of the possible association by the clinician involved.
The specific diagnosis of OSA in COPD requires some form of overnight sleep study, and there is a growing move toward ambulatory studies that focus on cardiorespiratory variables. Overnight monitoring of oxygen saturation is especially useful, particularly if linked to special analysis software, and may be sufficient in many cases. Full polysomnography can be reserved for select cases where the diagnosis remains in doubt.
Management and outcomes
Nocturnal hypoxemia in patients with COPD benefits from inhaled, long-acting beta-agonist and anticholinergic therapy, and mean nocturnal oxygen saturation is 2% to 3% higher on each medication compared with placebo. Supplemental oxygen may be indicated when nocturnal oxygen desaturation persists despite optimum pharmacotherapy and does not appear to be associated with significant additional risk of hypercapnia.
However, in patients with COPD-OSA overlap, nonnvasive pressure support is the most appropriate management option. In patients with predominant OSA, continuous positive airway pressure therapy (CPAP) is the preferred option, but where COPD is the dominant component, noninvasive ventilation (NIV) in the form of bi-level positive airway pressure (BIPAP) may be more appropriate. Recent reports in severe COPD indicate that NIV targeted to markedly reduce hypercapnia is associated with improved quality of life and prolonged survival (Köhnlein T et al. Lancet Respir Med. 2014;2[9]:698), and patients with COPD with persistent hypercapnia following hospitalization with an acute exacerbation show improved clinical outcomes and survival with continuing home NIV (Murphy PB et al. JAMA. 2017;317[21]:2177).
The recognition of co-existing OSA in patients with COPD has important clinical relevance as the management of patients with overlap syndrome is different from COPD alone, and the long-term survival of patients with overlap syndrome not treated with nocturnal positive airway pressure is significantly inferior to those patients with overlap syndrome appropriately treated (Marin JM et al. Am J Respir Crit Care Med. 2010;182[3]:325).
Dr. McNicholas is with the Department of Respiratory and Sleep Medicine, St. Vincent’s University Hospital, Dublin School of Medicine, University College Dublin, Ireland.
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) each affect at least 10% of the general adult population and, thus, both disorders together, commonly referred to as the overlap syndrome, could be expected in at least 1% of adults by chance alone. However, there is evidence of important interactions between the disorders that influence the prevalence of the overlap, which have implications for the development of comorbidities,and also for management (McNicholas WT. Chest. 2017; 152[6]:1318). Furthermore, sleep quality is typically poor in COPD, which has been linked to worse pulmonary function and lung hyperinflation and may contribute to daytime fatigue.
Interactions between COPD and OSA that may influence the prevalence of overlap
Previous reports have presented conflicting results regarding the likely association between COPD and OSA, which may partly reflect different definitions of OSA, patient populations, and methodologies of investigation. However, COPD represents a spectrum of clinical phenotypes ranging from the hyperinflated patient with low BMI (predominant emphysema phenotype) to the patient with higher BMI and tendency to right-sided heart failure (predominant chronic bronchitis phenotype). The predominant emphysema phenotype may predispose to a lower likelihood of OSA, and there is recent evidence that lung hyperinflation is protective against the development of OSA by lowering the critical closing pressure of the upper airway during sleep. Furthermore, the degree of emphysema and gas trapping on CT scan of the thorax correlates inversely with apnea-hypopnia index in patients with severe COPD (Krachman SL et al. Ann Am Thorac Soc. 2016;13[7]:1129).
In contrast, the predominant chronic bronchitis phenotype predisposes to a higher likelihood of OSA because of higher BMI and likelihood of right-sided heart failure. Peripheral fluid retention in such patients predisposes to OSA because of the rostral fluid shift that occurs during sleep in the supine position, predisposing to upper airway obstruction by airway narrowing. The COPDGene study reports that the chronic bronchitis phenotype has a higher prevalence of OSA even in the absence of differences in BMI and lung function (Kim V et al. Chest. 2011;140[3]:626). Upper airway inflammation associated with cigarette smoking may also contribute to the development of OSA, and corticosteroid therapy may adversely affect upper airway muscle function. OSA also appears to exacerbate lower airway inflammation in COPD. In practice, most patients with COPD have a mixture of emphysema and chronic bronchitis, and the probability of OSA will represent the balance of these protective and promoting factors in individual patients (Fig 1).
While there is evidence of increased mortality in patients with COPD and OSA alone, a recent report based on the Sleep Heart Health Study somewhat surprisingly found that the incremental contribution of declining lung function to mortality diminished with increasing severity of SDB measured by AHI (Putcha N et al. Am J Respir Crit Care Med. 2016;194[8]:1007). Thus, the epidemiologic relationship of COPD and OSA and related clinical outcomes remains an important research topic comparing different clinical phenotypes.
Mechanisms of interaction in the overlap syndrome and implications for comorbidity
COPD and OSA are associated with several overlapping physiological and biological disturbances, including hypoxia and inflammation, which may contribute to cardiovascular and other comorbidities. Thus, the probability should be high that the overlap syndrome will be associated with a greater risk of comorbidity than with either disease alone. Patients with the overlap syndrome demonstrate greater degrees of oxygen desaturation predisposing to pulmonary hypertension, which is especially common in these patients.
COPD and OSA are each associated with systemic inflammation and oxidative stress, and C-reactive protein (CRP) has been identified as a measure of systemic inflammation that is commonly elevated in both disorders, although in OSA, concurrent obesity is an important confounding factor. Systemic inflammation contributes to the development of cardiovascular disease, which is a common complication of both COPD and OSA. Thus, one could expect that cardiovascular disease is particularly prevalent in patients with overlap syndrome, but there are limited data on this relationship, which represents an important research topic.
Clinical assessment
Patients with the overlap syndrome present with typical clinical features of each disorder and additional features that reflect the higher prevalence of hypoxemia, hypercapnia, and pulmonary hypertension. Thus, morning headaches reflecting hypercapnia and peripheral edema reflecting right-sided heart failure may be especially common. Screening questionnaires may be helpful in the initial evaluation of likely OSA in patients with COPD, and objective clinical data, including anthropometrics such as age, sex, and BMI, and medical history such as cardiovascular comorbidity, are especially useful in clinical prediction (McNicholas WT. Lancet Respir Med. 2016;4[9]:683). Thus, screening for OSA in patients with COPD should not be complicated, and the widespread failure to do so may reflect a lack of awareness of the possible association by the clinician involved.
The specific diagnosis of OSA in COPD requires some form of overnight sleep study, and there is a growing move toward ambulatory studies that focus on cardiorespiratory variables. Overnight monitoring of oxygen saturation is especially useful, particularly if linked to special analysis software, and may be sufficient in many cases. Full polysomnography can be reserved for select cases where the diagnosis remains in doubt.
Management and outcomes
Nocturnal hypoxemia in patients with COPD benefits from inhaled, long-acting beta-agonist and anticholinergic therapy, and mean nocturnal oxygen saturation is 2% to 3% higher on each medication compared with placebo. Supplemental oxygen may be indicated when nocturnal oxygen desaturation persists despite optimum pharmacotherapy and does not appear to be associated with significant additional risk of hypercapnia.
However, in patients with COPD-OSA overlap, nonnvasive pressure support is the most appropriate management option. In patients with predominant OSA, continuous positive airway pressure therapy (CPAP) is the preferred option, but where COPD is the dominant component, noninvasive ventilation (NIV) in the form of bi-level positive airway pressure (BIPAP) may be more appropriate. Recent reports in severe COPD indicate that NIV targeted to markedly reduce hypercapnia is associated with improved quality of life and prolonged survival (Köhnlein T et al. Lancet Respir Med. 2014;2[9]:698), and patients with COPD with persistent hypercapnia following hospitalization with an acute exacerbation show improved clinical outcomes and survival with continuing home NIV (Murphy PB et al. JAMA. 2017;317[21]:2177).
The recognition of co-existing OSA in patients with COPD has important clinical relevance as the management of patients with overlap syndrome is different from COPD alone, and the long-term survival of patients with overlap syndrome not treated with nocturnal positive airway pressure is significantly inferior to those patients with overlap syndrome appropriately treated (Marin JM et al. Am J Respir Crit Care Med. 2010;182[3]:325).
Dr. McNicholas is with the Department of Respiratory and Sleep Medicine, St. Vincent’s University Hospital, Dublin School of Medicine, University College Dublin, Ireland.
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) each affect at least 10% of the general adult population and, thus, both disorders together, commonly referred to as the overlap syndrome, could be expected in at least 1% of adults by chance alone. However, there is evidence of important interactions between the disorders that influence the prevalence of the overlap, which have implications for the development of comorbidities,and also for management (McNicholas WT. Chest. 2017; 152[6]:1318). Furthermore, sleep quality is typically poor in COPD, which has been linked to worse pulmonary function and lung hyperinflation and may contribute to daytime fatigue.
Interactions between COPD and OSA that may influence the prevalence of overlap
Previous reports have presented conflicting results regarding the likely association between COPD and OSA, which may partly reflect different definitions of OSA, patient populations, and methodologies of investigation. However, COPD represents a spectrum of clinical phenotypes ranging from the hyperinflated patient with low BMI (predominant emphysema phenotype) to the patient with higher BMI and tendency to right-sided heart failure (predominant chronic bronchitis phenotype). The predominant emphysema phenotype may predispose to a lower likelihood of OSA, and there is recent evidence that lung hyperinflation is protective against the development of OSA by lowering the critical closing pressure of the upper airway during sleep. Furthermore, the degree of emphysema and gas trapping on CT scan of the thorax correlates inversely with apnea-hypopnia index in patients with severe COPD (Krachman SL et al. Ann Am Thorac Soc. 2016;13[7]:1129).
In contrast, the predominant chronic bronchitis phenotype predisposes to a higher likelihood of OSA because of higher BMI and likelihood of right-sided heart failure. Peripheral fluid retention in such patients predisposes to OSA because of the rostral fluid shift that occurs during sleep in the supine position, predisposing to upper airway obstruction by airway narrowing. The COPDGene study reports that the chronic bronchitis phenotype has a higher prevalence of OSA even in the absence of differences in BMI and lung function (Kim V et al. Chest. 2011;140[3]:626). Upper airway inflammation associated with cigarette smoking may also contribute to the development of OSA, and corticosteroid therapy may adversely affect upper airway muscle function. OSA also appears to exacerbate lower airway inflammation in COPD. In practice, most patients with COPD have a mixture of emphysema and chronic bronchitis, and the probability of OSA will represent the balance of these protective and promoting factors in individual patients (Fig 1).
While there is evidence of increased mortality in patients with COPD and OSA alone, a recent report based on the Sleep Heart Health Study somewhat surprisingly found that the incremental contribution of declining lung function to mortality diminished with increasing severity of SDB measured by AHI (Putcha N et al. Am J Respir Crit Care Med. 2016;194[8]:1007). Thus, the epidemiologic relationship of COPD and OSA and related clinical outcomes remains an important research topic comparing different clinical phenotypes.
Mechanisms of interaction in the overlap syndrome and implications for comorbidity
COPD and OSA are associated with several overlapping physiological and biological disturbances, including hypoxia and inflammation, which may contribute to cardiovascular and other comorbidities. Thus, the probability should be high that the overlap syndrome will be associated with a greater risk of comorbidity than with either disease alone. Patients with the overlap syndrome demonstrate greater degrees of oxygen desaturation predisposing to pulmonary hypertension, which is especially common in these patients.
COPD and OSA are each associated with systemic inflammation and oxidative stress, and C-reactive protein (CRP) has been identified as a measure of systemic inflammation that is commonly elevated in both disorders, although in OSA, concurrent obesity is an important confounding factor. Systemic inflammation contributes to the development of cardiovascular disease, which is a common complication of both COPD and OSA. Thus, one could expect that cardiovascular disease is particularly prevalent in patients with overlap syndrome, but there are limited data on this relationship, which represents an important research topic.
Clinical assessment
Patients with the overlap syndrome present with typical clinical features of each disorder and additional features that reflect the higher prevalence of hypoxemia, hypercapnia, and pulmonary hypertension. Thus, morning headaches reflecting hypercapnia and peripheral edema reflecting right-sided heart failure may be especially common. Screening questionnaires may be helpful in the initial evaluation of likely OSA in patients with COPD, and objective clinical data, including anthropometrics such as age, sex, and BMI, and medical history such as cardiovascular comorbidity, are especially useful in clinical prediction (McNicholas WT. Lancet Respir Med. 2016;4[9]:683). Thus, screening for OSA in patients with COPD should not be complicated, and the widespread failure to do so may reflect a lack of awareness of the possible association by the clinician involved.
The specific diagnosis of OSA in COPD requires some form of overnight sleep study, and there is a growing move toward ambulatory studies that focus on cardiorespiratory variables. Overnight monitoring of oxygen saturation is especially useful, particularly if linked to special analysis software, and may be sufficient in many cases. Full polysomnography can be reserved for select cases where the diagnosis remains in doubt.
Management and outcomes
Nocturnal hypoxemia in patients with COPD benefits from inhaled, long-acting beta-agonist and anticholinergic therapy, and mean nocturnal oxygen saturation is 2% to 3% higher on each medication compared with placebo. Supplemental oxygen may be indicated when nocturnal oxygen desaturation persists despite optimum pharmacotherapy and does not appear to be associated with significant additional risk of hypercapnia.
However, in patients with COPD-OSA overlap, nonnvasive pressure support is the most appropriate management option. In patients with predominant OSA, continuous positive airway pressure therapy (CPAP) is the preferred option, but where COPD is the dominant component, noninvasive ventilation (NIV) in the form of bi-level positive airway pressure (BIPAP) may be more appropriate. Recent reports in severe COPD indicate that NIV targeted to markedly reduce hypercapnia is associated with improved quality of life and prolonged survival (Köhnlein T et al. Lancet Respir Med. 2014;2[9]:698), and patients with COPD with persistent hypercapnia following hospitalization with an acute exacerbation show improved clinical outcomes and survival with continuing home NIV (Murphy PB et al. JAMA. 2017;317[21]:2177).
The recognition of co-existing OSA in patients with COPD has important clinical relevance as the management of patients with overlap syndrome is different from COPD alone, and the long-term survival of patients with overlap syndrome not treated with nocturnal positive airway pressure is significantly inferior to those patients with overlap syndrome appropriately treated (Marin JM et al. Am J Respir Crit Care Med. 2010;182[3]:325).
Dr. McNicholas is with the Department of Respiratory and Sleep Medicine, St. Vincent’s University Hospital, Dublin School of Medicine, University College Dublin, Ireland.
OSA Endotypes and Phenotypes: Toward Personalized OSA Care
Obstructive sleep apnea (OSA) contributes a major health burden to society due to its high prevalence and substantial neurocognitive and cardiovascular consequences. Estimates suggest that at least 10% of adults in North America are afflicted with OSA, making it probably the most common respiratory disease in the developed world (Peppard et al. Am J Epidemiol. 2013;177[9]:1006). Nasal CPAP is a highly efficacious therapy that has been shown to improve neurocognitive and cardiovascular outcomes. However, CPAP is not always well tolerated. Alternative therapies, such as oral appliances and upper airway surgery, have highly variable efficacy, and evidence of important clinical benefits are uncertain. Therefore, efforts are ongoing to determine optimal alternative strategies for therapy.
In order to treat any condition optimally, one needs to be able to predict who is at highest risk of developing the condition, then to assess the consequences if left untreated, and finally to be able to predict response to various treatment options. Currently, the OSA field is still in its early stages of our understanding. Clinically, we are often faced with patients who have varying presentations and manifestations, but, for reasons that are unclear. For instance, two individuals with the same body mass index may have very different clinical manifestations, one with severe OSA and one without any OSA. Similarly, two individuals with an apnea hypopnea index of 40 events per hour (ie, severe OSA) may have very different symptoms attributable to OSA, eg, one could be asymptomatic and the other could be debilitated from sleepiness. We and others have been making efforts to determine why these phenomenon occur. At present, the techniques to define mechanisms underlying OSA are labor-intensive, requiring one or two overnight experiments to gather meaningful data. Although we are gathering new insights based on these techniques, efforts are ongoing to simplify these approaches and to make assessment of pathophysiologic characteristics more accessible to the clinician (Orr et al. Am J Respir Crit Care Med. 2017 Nov 30. doi: 10.1164/rccm.201707-1357LE. [Epub ahead of print]).
We ultimately believe that a thorough analysis of a sleep recording combined with demographic data and other readily available clinical data (perhaps plasma biomarkers) may yield sufficient information for us to know why OSA is occurring and what interventions might be helpful for an individual patient. Currently, our use of the polysomnogram to derive only an apnea hypopnea index does not take full advantage of the available data. An apnea hypopnea index can be readily obtained from home sleep testing and does not truly provide much insight into why a given individual has OSA, what symptoms are attributable to OSA, and what interventions might be considered for the afflicted individual. By analogy, if the only useful data derived from an ECG were a heart rate, the test would rapidly become obsolete. Along these lines, if the only role for the sleep clinician was to prescribe CPAP to everyone with an AHI greater than 5/h, there would be little need or interest in specialized training. In contrast, we suggest that rich insights regarding pathophysiology and mechanisms should be gathered and may influence clinical management of patients afflicted with OSA. Thus, we encourage more thorough analyses of available data to maximize information gleaned and, ultimately, to optimize clinical outcomes.
Recent studies suggest that sleep apnea occurs for varying reasons, a concept that is now thought to be clinically important (Jordan et al. Lancet. 2014;383[9918]:736). We draw a crucial distinction between endotypes (mechanisms underlying disease) and phenotypes (clinical expression of disease). Important endotypes include compromised upper airway anatomy, dysfunction in pharyngeal dilator muscles, unstable ventilatory control (high loop gain), and low arousal threshold (wake up easily), among others. Important phenotypes of sleep apnea are emerging and still evolving to include minimally symptomatic OSA, OSA with daytime sleepiness, and OSA with major cardiometabolic risk, among others. Several important concepts have emerged regarding different OSA endotypes and phenotypes:
1 The mechanism underlying OSA may predict potential response to therapeutic interventions. For instance, the endotype of OSA with unstable ventilatory control (high loop gain) may respond to agents such as oxygen and acetazolamide, which serve to stabilize control of breathing. In patients with anatomical compromise at the level of the velopharynx, uvulopalatopharyngoplasty may be an effective intervention. For patients with multiple pathophysiologic abnormalities, combination therapy may be required to alleviate OSA (Edwards et al. Sleep. 2016;9[11]:1973).
2 Given that OSA has many underlying etiologies, efforts are underway to determine whether individuals with different risk factors for OSA develop their disease based on varying mechanisms. As an example, people with posttraumatic stress disorder (PTSD) may be at increased risk of OSA perhaps on the basis of a low threshold for arousal (Orr et al. JCSM. 2017, 13[1]: 57-63). Another example would be patients with neuromuscular disease who may be at risk of OSA primarily based on impaired pharyngeal dilator muscle function.
3 A new concept is emerging whereby endotypes of OSA may actually predict differing OSA phenotypes. In theory, loop gain-driven OSA may have different consequences from OSA driven by compromise of pharyngeal anatomy. To this point, data suggest that OSA in the elderly may not have as many consequences as OSA in younger people matched on severity of illness. OSA in the elderly has lower loop gain than OSA in younger people and is associated with less negative intrathoracic pressure at the time of arousal as compared with younger individuals with OSA (Kobayashi et al. Chest. 2010; 137[6]:1310). As such, the endotype of OSA in the elderly may explain why the clinical consequences are fewer than in the younger OSA counterparts.
4 The mechanism underlying OSA may be important in determining response to clinical interventions, such as nasal CPAP. Patients with a low arousal threshold may be prone to insomnia when placed on CPAP and could theoretically be poorly tolerant of therapy based on disrupted sleep architecture. Such patients may benefit from non-myorelaxant hypnotic therapy to consolidate sleep and improve CPAP adherence. In addition, patients with high loop gain (unstable ventilatory control) may be prone to develop central apneas when placed on CPAP therapy (Stanchina et al. Ann Am Thorac Soc. 2015;12[9]:1351). These patients may benefit from newer technologies, eg, auto or adaptive servo ventilation - ASV. High loop gain has also been shown to predict failure of upper airway surgery as a treatment for OSA by several groups (Li et al. JCSM. 2017;13[9]:1029). Such patients should, perhaps, undergo nonsurgical therapies for OSA.
We emphasize that some of the points being made are somewhat speculative and, thus, encourage further basic and clinical research to test our assumptions. Robust, multicenter clinical trials assessing hard outcomes will ultimately be required to change the current standard of care. Nonetheless, we believe that a more thorough understanding of OSA pathogenesis can help guide clinical care today and will be critical to the optimal treatment of afflicted individuals tomorrow.
Dr. Owens is Assistant Clinical Professor of Medicine; Dr. Deacon is a Post-Doctoral Research Scholar; and Dr. Malhotra is Kenneth M. Moser Professor of Medicine and Chief, Division of Pulmonary, Critical Care and Sleep Medicine, University of California San Diego.
Obstructive sleep apnea (OSA) contributes a major health burden to society due to its high prevalence and substantial neurocognitive and cardiovascular consequences. Estimates suggest that at least 10% of adults in North America are afflicted with OSA, making it probably the most common respiratory disease in the developed world (Peppard et al. Am J Epidemiol. 2013;177[9]:1006). Nasal CPAP is a highly efficacious therapy that has been shown to improve neurocognitive and cardiovascular outcomes. However, CPAP is not always well tolerated. Alternative therapies, such as oral appliances and upper airway surgery, have highly variable efficacy, and evidence of important clinical benefits are uncertain. Therefore, efforts are ongoing to determine optimal alternative strategies for therapy.
In order to treat any condition optimally, one needs to be able to predict who is at highest risk of developing the condition, then to assess the consequences if left untreated, and finally to be able to predict response to various treatment options. Currently, the OSA field is still in its early stages of our understanding. Clinically, we are often faced with patients who have varying presentations and manifestations, but, for reasons that are unclear. For instance, two individuals with the same body mass index may have very different clinical manifestations, one with severe OSA and one without any OSA. Similarly, two individuals with an apnea hypopnea index of 40 events per hour (ie, severe OSA) may have very different symptoms attributable to OSA, eg, one could be asymptomatic and the other could be debilitated from sleepiness. We and others have been making efforts to determine why these phenomenon occur. At present, the techniques to define mechanisms underlying OSA are labor-intensive, requiring one or two overnight experiments to gather meaningful data. Although we are gathering new insights based on these techniques, efforts are ongoing to simplify these approaches and to make assessment of pathophysiologic characteristics more accessible to the clinician (Orr et al. Am J Respir Crit Care Med. 2017 Nov 30. doi: 10.1164/rccm.201707-1357LE. [Epub ahead of print]).
We ultimately believe that a thorough analysis of a sleep recording combined with demographic data and other readily available clinical data (perhaps plasma biomarkers) may yield sufficient information for us to know why OSA is occurring and what interventions might be helpful for an individual patient. Currently, our use of the polysomnogram to derive only an apnea hypopnea index does not take full advantage of the available data. An apnea hypopnea index can be readily obtained from home sleep testing and does not truly provide much insight into why a given individual has OSA, what symptoms are attributable to OSA, and what interventions might be considered for the afflicted individual. By analogy, if the only useful data derived from an ECG were a heart rate, the test would rapidly become obsolete. Along these lines, if the only role for the sleep clinician was to prescribe CPAP to everyone with an AHI greater than 5/h, there would be little need or interest in specialized training. In contrast, we suggest that rich insights regarding pathophysiology and mechanisms should be gathered and may influence clinical management of patients afflicted with OSA. Thus, we encourage more thorough analyses of available data to maximize information gleaned and, ultimately, to optimize clinical outcomes.
Recent studies suggest that sleep apnea occurs for varying reasons, a concept that is now thought to be clinically important (Jordan et al. Lancet. 2014;383[9918]:736). We draw a crucial distinction between endotypes (mechanisms underlying disease) and phenotypes (clinical expression of disease). Important endotypes include compromised upper airway anatomy, dysfunction in pharyngeal dilator muscles, unstable ventilatory control (high loop gain), and low arousal threshold (wake up easily), among others. Important phenotypes of sleep apnea are emerging and still evolving to include minimally symptomatic OSA, OSA with daytime sleepiness, and OSA with major cardiometabolic risk, among others. Several important concepts have emerged regarding different OSA endotypes and phenotypes:
1 The mechanism underlying OSA may predict potential response to therapeutic interventions. For instance, the endotype of OSA with unstable ventilatory control (high loop gain) may respond to agents such as oxygen and acetazolamide, which serve to stabilize control of breathing. In patients with anatomical compromise at the level of the velopharynx, uvulopalatopharyngoplasty may be an effective intervention. For patients with multiple pathophysiologic abnormalities, combination therapy may be required to alleviate OSA (Edwards et al. Sleep. 2016;9[11]:1973).
2 Given that OSA has many underlying etiologies, efforts are underway to determine whether individuals with different risk factors for OSA develop their disease based on varying mechanisms. As an example, people with posttraumatic stress disorder (PTSD) may be at increased risk of OSA perhaps on the basis of a low threshold for arousal (Orr et al. JCSM. 2017, 13[1]: 57-63). Another example would be patients with neuromuscular disease who may be at risk of OSA primarily based on impaired pharyngeal dilator muscle function.
3 A new concept is emerging whereby endotypes of OSA may actually predict differing OSA phenotypes. In theory, loop gain-driven OSA may have different consequences from OSA driven by compromise of pharyngeal anatomy. To this point, data suggest that OSA in the elderly may not have as many consequences as OSA in younger people matched on severity of illness. OSA in the elderly has lower loop gain than OSA in younger people and is associated with less negative intrathoracic pressure at the time of arousal as compared with younger individuals with OSA (Kobayashi et al. Chest. 2010; 137[6]:1310). As such, the endotype of OSA in the elderly may explain why the clinical consequences are fewer than in the younger OSA counterparts.
4 The mechanism underlying OSA may be important in determining response to clinical interventions, such as nasal CPAP. Patients with a low arousal threshold may be prone to insomnia when placed on CPAP and could theoretically be poorly tolerant of therapy based on disrupted sleep architecture. Such patients may benefit from non-myorelaxant hypnotic therapy to consolidate sleep and improve CPAP adherence. In addition, patients with high loop gain (unstable ventilatory control) may be prone to develop central apneas when placed on CPAP therapy (Stanchina et al. Ann Am Thorac Soc. 2015;12[9]:1351). These patients may benefit from newer technologies, eg, auto or adaptive servo ventilation - ASV. High loop gain has also been shown to predict failure of upper airway surgery as a treatment for OSA by several groups (Li et al. JCSM. 2017;13[9]:1029). Such patients should, perhaps, undergo nonsurgical therapies for OSA.
We emphasize that some of the points being made are somewhat speculative and, thus, encourage further basic and clinical research to test our assumptions. Robust, multicenter clinical trials assessing hard outcomes will ultimately be required to change the current standard of care. Nonetheless, we believe that a more thorough understanding of OSA pathogenesis can help guide clinical care today and will be critical to the optimal treatment of afflicted individuals tomorrow.
Dr. Owens is Assistant Clinical Professor of Medicine; Dr. Deacon is a Post-Doctoral Research Scholar; and Dr. Malhotra is Kenneth M. Moser Professor of Medicine and Chief, Division of Pulmonary, Critical Care and Sleep Medicine, University of California San Diego.
Obstructive sleep apnea (OSA) contributes a major health burden to society due to its high prevalence and substantial neurocognitive and cardiovascular consequences. Estimates suggest that at least 10% of adults in North America are afflicted with OSA, making it probably the most common respiratory disease in the developed world (Peppard et al. Am J Epidemiol. 2013;177[9]:1006). Nasal CPAP is a highly efficacious therapy that has been shown to improve neurocognitive and cardiovascular outcomes. However, CPAP is not always well tolerated. Alternative therapies, such as oral appliances and upper airway surgery, have highly variable efficacy, and evidence of important clinical benefits are uncertain. Therefore, efforts are ongoing to determine optimal alternative strategies for therapy.
In order to treat any condition optimally, one needs to be able to predict who is at highest risk of developing the condition, then to assess the consequences if left untreated, and finally to be able to predict response to various treatment options. Currently, the OSA field is still in its early stages of our understanding. Clinically, we are often faced with patients who have varying presentations and manifestations, but, for reasons that are unclear. For instance, two individuals with the same body mass index may have very different clinical manifestations, one with severe OSA and one without any OSA. Similarly, two individuals with an apnea hypopnea index of 40 events per hour (ie, severe OSA) may have very different symptoms attributable to OSA, eg, one could be asymptomatic and the other could be debilitated from sleepiness. We and others have been making efforts to determine why these phenomenon occur. At present, the techniques to define mechanisms underlying OSA are labor-intensive, requiring one or two overnight experiments to gather meaningful data. Although we are gathering new insights based on these techniques, efforts are ongoing to simplify these approaches and to make assessment of pathophysiologic characteristics more accessible to the clinician (Orr et al. Am J Respir Crit Care Med. 2017 Nov 30. doi: 10.1164/rccm.201707-1357LE. [Epub ahead of print]).
We ultimately believe that a thorough analysis of a sleep recording combined with demographic data and other readily available clinical data (perhaps plasma biomarkers) may yield sufficient information for us to know why OSA is occurring and what interventions might be helpful for an individual patient. Currently, our use of the polysomnogram to derive only an apnea hypopnea index does not take full advantage of the available data. An apnea hypopnea index can be readily obtained from home sleep testing and does not truly provide much insight into why a given individual has OSA, what symptoms are attributable to OSA, and what interventions might be considered for the afflicted individual. By analogy, if the only useful data derived from an ECG were a heart rate, the test would rapidly become obsolete. Along these lines, if the only role for the sleep clinician was to prescribe CPAP to everyone with an AHI greater than 5/h, there would be little need or interest in specialized training. In contrast, we suggest that rich insights regarding pathophysiology and mechanisms should be gathered and may influence clinical management of patients afflicted with OSA. Thus, we encourage more thorough analyses of available data to maximize information gleaned and, ultimately, to optimize clinical outcomes.
Recent studies suggest that sleep apnea occurs for varying reasons, a concept that is now thought to be clinically important (Jordan et al. Lancet. 2014;383[9918]:736). We draw a crucial distinction between endotypes (mechanisms underlying disease) and phenotypes (clinical expression of disease). Important endotypes include compromised upper airway anatomy, dysfunction in pharyngeal dilator muscles, unstable ventilatory control (high loop gain), and low arousal threshold (wake up easily), among others. Important phenotypes of sleep apnea are emerging and still evolving to include minimally symptomatic OSA, OSA with daytime sleepiness, and OSA with major cardiometabolic risk, among others. Several important concepts have emerged regarding different OSA endotypes and phenotypes:
1 The mechanism underlying OSA may predict potential response to therapeutic interventions. For instance, the endotype of OSA with unstable ventilatory control (high loop gain) may respond to agents such as oxygen and acetazolamide, which serve to stabilize control of breathing. In patients with anatomical compromise at the level of the velopharynx, uvulopalatopharyngoplasty may be an effective intervention. For patients with multiple pathophysiologic abnormalities, combination therapy may be required to alleviate OSA (Edwards et al. Sleep. 2016;9[11]:1973).
2 Given that OSA has many underlying etiologies, efforts are underway to determine whether individuals with different risk factors for OSA develop their disease based on varying mechanisms. As an example, people with posttraumatic stress disorder (PTSD) may be at increased risk of OSA perhaps on the basis of a low threshold for arousal (Orr et al. JCSM. 2017, 13[1]: 57-63). Another example would be patients with neuromuscular disease who may be at risk of OSA primarily based on impaired pharyngeal dilator muscle function.
3 A new concept is emerging whereby endotypes of OSA may actually predict differing OSA phenotypes. In theory, loop gain-driven OSA may have different consequences from OSA driven by compromise of pharyngeal anatomy. To this point, data suggest that OSA in the elderly may not have as many consequences as OSA in younger people matched on severity of illness. OSA in the elderly has lower loop gain than OSA in younger people and is associated with less negative intrathoracic pressure at the time of arousal as compared with younger individuals with OSA (Kobayashi et al. Chest. 2010; 137[6]:1310). As such, the endotype of OSA in the elderly may explain why the clinical consequences are fewer than in the younger OSA counterparts.
4 The mechanism underlying OSA may be important in determining response to clinical interventions, such as nasal CPAP. Patients with a low arousal threshold may be prone to insomnia when placed on CPAP and could theoretically be poorly tolerant of therapy based on disrupted sleep architecture. Such patients may benefit from non-myorelaxant hypnotic therapy to consolidate sleep and improve CPAP adherence. In addition, patients with high loop gain (unstable ventilatory control) may be prone to develop central apneas when placed on CPAP therapy (Stanchina et al. Ann Am Thorac Soc. 2015;12[9]:1351). These patients may benefit from newer technologies, eg, auto or adaptive servo ventilation - ASV. High loop gain has also been shown to predict failure of upper airway surgery as a treatment for OSA by several groups (Li et al. JCSM. 2017;13[9]:1029). Such patients should, perhaps, undergo nonsurgical therapies for OSA.
We emphasize that some of the points being made are somewhat speculative and, thus, encourage further basic and clinical research to test our assumptions. Robust, multicenter clinical trials assessing hard outcomes will ultimately be required to change the current standard of care. Nonetheless, we believe that a more thorough understanding of OSA pathogenesis can help guide clinical care today and will be critical to the optimal treatment of afflicted individuals tomorrow.
Dr. Owens is Assistant Clinical Professor of Medicine; Dr. Deacon is a Post-Doctoral Research Scholar; and Dr. Malhotra is Kenneth M. Moser Professor of Medicine and Chief, Division of Pulmonary, Critical Care and Sleep Medicine, University of California San Diego.
Role of Obstructive Sleep Apnea in HTN
Heart disease and stroke are leading causes of death and disability. High blood pressure (BP) is a major risk factor for both.
The 2017 guidelines regarding “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure” (JNC 7) were recently published, which is an update incorporating new information from studies regarding BP-related risk of cardiovascular disease (CVD) and strategies to improve hypertension (HTN) treatment and control.
Screening for secondary causes of HTN is necessary for new-onset or uncontrolled HTN in adults, including drug-resistant HTN. Screening includes testing for obstructive sleep apnea, which is highly prevalent in this population.
Obstructive sleep apnea is a common chronic condition characterized by recurrent collapse of upper airways during sleep, inducing intermittent episodes of apnea/hypopnea, hypoxemia, and sleep disruption (Pedrosa RP, et al. Chest. 2013;144[5]:1487).
It is estimated to affect 17% of US adults but is overwhelmingly underrecognized and untreated (JAMA. 2012;307[20]:2169). The prevalence is higher in men than women. The major risk factors for OSA are obesity, male sex, and advancing age. Since these conditions oftentimes predispose to and are concomitant with HTN, it can be challenging to determine the independent effects of OSA on the development of HTN.
The relationship between obstructive sleep apnea (OSA) and HTN has been a point of interest for decades, with untreated OSA being associated with an increased risk for developing new-onset HTN (JAMA. 2012;307[20]:2169).
There have been several landmark trials that have sought to determine the extent of a causal relationship between OSAS and HTN. Sleep Heart Health Study (Sleep. 2006;29;1009) was one such study, which was limited by the inability to prove that OSA preceded the onset of HTN.
Wisconsin Sleep Cohort (N Engl J Med. 2000;342:1378) was another landmark prospective longitudinal study that implicates OSA as a possible causal factor in HTN. The notable limitation of the study was the presence of HTN after initial assessment was found to be dependent upon the severity of OSA at baseline.
While these two cohort studies found an association between OSA and HTN, the Vitoria Sleep Cohort out of Spain (Am J Respir Crit Care Med. 2011;184[11]:1299), the third and most recent longitudinal cohort study, looked at younger and thinner patients than the SHHS and the Wisconsin Sleep Cohort, failed to show a significant association between OSA and incident HTN. Methodologic differences may help to explain the disparity in results.
NREM sleep has normal circadian variation of BP, causing “dipping” of both systolic and diastolic BP at night due to decreased sympathetic and increased parasympathetic activity. REM sleep has predominant sympathetic activity and transient nocturnal BP surges.
OSA results in hypoxemia, which causes nocturnal catecholamine surges, resulting in nocturnal increase in heart rate and BP that is most prominent during post-apneic hyperventilation.
Reduced nocturnal BP (nondipping) or even higher nocturnal BP than daytime BP is an undoubted risk factor for hypertensive patients due to the end-organ damage and subsequent cardiovascular events. With sleep apnea, sleep quality is decreased due to frequent arousal from sleep (Hypertension. 2006;47[5]:833).
Sleep duration of less than or equal to 5 hours per night was shown to significantly increase risk for HTN in patients less than or equal to 60 years of age, even after controlling for obesity and diabetes.
Sleep Heart Health Study suggests that sleep duration above or below a median of 7 to 8 hours per night is associated with a higher prevalence of HTN (Sleep. 2006;29:1009). Thus, improving duration and quality of sleep in sleep apnea patients may help decrease the risk of developing HTN.
Key question: Will treatment of OSA appreciably alter BP?
Continuous positive airway pressure (CPAP) is an efficacious treatment of choice for OSA. Interventional trials, though limited by issues related to compliance, have shown CPAP to acutely reduce sympathetic drive and BP during sleep. However, this improvement in BP control is not entirely consistent in all patients with the data being less clear-cut regarding nighttime CPAP therapy and impact on daytime BP.
A randomized controlled trial from Barbe et al suggests that normotensive subjects with severe OSA but without demonstrable daytime sleepiness are immune to the BP-reducing effects of CPAP (Ann Intern Med. 2001;134:1015); those who were objectively sleepy had a more robust response to the BP lowering effects of CPAP with better cardiovascular outcomes among patients who were adherent to CPAP therapy (≥4 hours per night).
Sleep Apnea Cardiovascular Endpoints (SAVE) study looked at CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea (N Engl J Med. 2016;375:919). CPAP significantly reduced snoring and daytime sleepiness and improved health-related quality of life and mood, but the risk of serious cardiovascular events was not lower among patients who received treatment with CPAP in addition to usual care compared with usual care alone. This study was not powered to provide definitive answers regarding the effects of CPAP on secondary cardiovascular end points, and the use of PAP was less than 4 hours.
A recent systematic review and meta-analysis looked at “Association of Positive Airway Pressure with Cardiovascular Events and Death in Adults with Sleep Apnea” (JAMA. 2017;318(2):156). No significant associations between PAP treatment and a range of cardiovascular events were noted in this meta-analysis.
It is possible that the limited adherence to therapy in many trials was insufficient to drive protection, along with short follow-up duration of most trials that may have given insufficient time for PAP to have affected vascular outcomes.
In a cross-over study of valsartan and CPAP, combining drug treatment with CPAP appeared to have a more synergistic effect in reducing BP than either agent alone (Am J Respir Crit Care Med. 2010;182:954).
The beneficial effect of CPAP remains an open question. Considering the multifactorial pathophysiology of OSA-associated HTN, proven therapies, such as BP lowering, lipid lowering, and antiplatelet therapy, along with PAP therapy, should be utilized. This combination strategy is likely to be more effective in improving both nocturnal and daytime BP control in OSA.
Dr. Singh is Director, Sleep Disorder and Research Center, Michael E. DeBakey VA Medical Center; and Dr. Singh is Assistant Professor and Dr. Velamuri is Associate Professor, Pulmonary, Critical Care and Sleep Medicine, Baylor College of Medicine. Houston, Texas.
Heart disease and stroke are leading causes of death and disability. High blood pressure (BP) is a major risk factor for both.
The 2017 guidelines regarding “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure” (JNC 7) were recently published, which is an update incorporating new information from studies regarding BP-related risk of cardiovascular disease (CVD) and strategies to improve hypertension (HTN) treatment and control.
Screening for secondary causes of HTN is necessary for new-onset or uncontrolled HTN in adults, including drug-resistant HTN. Screening includes testing for obstructive sleep apnea, which is highly prevalent in this population.
Obstructive sleep apnea is a common chronic condition characterized by recurrent collapse of upper airways during sleep, inducing intermittent episodes of apnea/hypopnea, hypoxemia, and sleep disruption (Pedrosa RP, et al. Chest. 2013;144[5]:1487).
It is estimated to affect 17% of US adults but is overwhelmingly underrecognized and untreated (JAMA. 2012;307[20]:2169). The prevalence is higher in men than women. The major risk factors for OSA are obesity, male sex, and advancing age. Since these conditions oftentimes predispose to and are concomitant with HTN, it can be challenging to determine the independent effects of OSA on the development of HTN.
The relationship between obstructive sleep apnea (OSA) and HTN has been a point of interest for decades, with untreated OSA being associated with an increased risk for developing new-onset HTN (JAMA. 2012;307[20]:2169).
There have been several landmark trials that have sought to determine the extent of a causal relationship between OSAS and HTN. Sleep Heart Health Study (Sleep. 2006;29;1009) was one such study, which was limited by the inability to prove that OSA preceded the onset of HTN.
Wisconsin Sleep Cohort (N Engl J Med. 2000;342:1378) was another landmark prospective longitudinal study that implicates OSA as a possible causal factor in HTN. The notable limitation of the study was the presence of HTN after initial assessment was found to be dependent upon the severity of OSA at baseline.
While these two cohort studies found an association between OSA and HTN, the Vitoria Sleep Cohort out of Spain (Am J Respir Crit Care Med. 2011;184[11]:1299), the third and most recent longitudinal cohort study, looked at younger and thinner patients than the SHHS and the Wisconsin Sleep Cohort, failed to show a significant association between OSA and incident HTN. Methodologic differences may help to explain the disparity in results.
NREM sleep has normal circadian variation of BP, causing “dipping” of both systolic and diastolic BP at night due to decreased sympathetic and increased parasympathetic activity. REM sleep has predominant sympathetic activity and transient nocturnal BP surges.
OSA results in hypoxemia, which causes nocturnal catecholamine surges, resulting in nocturnal increase in heart rate and BP that is most prominent during post-apneic hyperventilation.
Reduced nocturnal BP (nondipping) or even higher nocturnal BP than daytime BP is an undoubted risk factor for hypertensive patients due to the end-organ damage and subsequent cardiovascular events. With sleep apnea, sleep quality is decreased due to frequent arousal from sleep (Hypertension. 2006;47[5]:833).
Sleep duration of less than or equal to 5 hours per night was shown to significantly increase risk for HTN in patients less than or equal to 60 years of age, even after controlling for obesity and diabetes.
Sleep Heart Health Study suggests that sleep duration above or below a median of 7 to 8 hours per night is associated with a higher prevalence of HTN (Sleep. 2006;29:1009). Thus, improving duration and quality of sleep in sleep apnea patients may help decrease the risk of developing HTN.
Key question: Will treatment of OSA appreciably alter BP?
Continuous positive airway pressure (CPAP) is an efficacious treatment of choice for OSA. Interventional trials, though limited by issues related to compliance, have shown CPAP to acutely reduce sympathetic drive and BP during sleep. However, this improvement in BP control is not entirely consistent in all patients with the data being less clear-cut regarding nighttime CPAP therapy and impact on daytime BP.
A randomized controlled trial from Barbe et al suggests that normotensive subjects with severe OSA but without demonstrable daytime sleepiness are immune to the BP-reducing effects of CPAP (Ann Intern Med. 2001;134:1015); those who were objectively sleepy had a more robust response to the BP lowering effects of CPAP with better cardiovascular outcomes among patients who were adherent to CPAP therapy (≥4 hours per night).
Sleep Apnea Cardiovascular Endpoints (SAVE) study looked at CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea (N Engl J Med. 2016;375:919). CPAP significantly reduced snoring and daytime sleepiness and improved health-related quality of life and mood, but the risk of serious cardiovascular events was not lower among patients who received treatment with CPAP in addition to usual care compared with usual care alone. This study was not powered to provide definitive answers regarding the effects of CPAP on secondary cardiovascular end points, and the use of PAP was less than 4 hours.
A recent systematic review and meta-analysis looked at “Association of Positive Airway Pressure with Cardiovascular Events and Death in Adults with Sleep Apnea” (JAMA. 2017;318(2):156). No significant associations between PAP treatment and a range of cardiovascular events were noted in this meta-analysis.
It is possible that the limited adherence to therapy in many trials was insufficient to drive protection, along with short follow-up duration of most trials that may have given insufficient time for PAP to have affected vascular outcomes.
In a cross-over study of valsartan and CPAP, combining drug treatment with CPAP appeared to have a more synergistic effect in reducing BP than either agent alone (Am J Respir Crit Care Med. 2010;182:954).
The beneficial effect of CPAP remains an open question. Considering the multifactorial pathophysiology of OSA-associated HTN, proven therapies, such as BP lowering, lipid lowering, and antiplatelet therapy, along with PAP therapy, should be utilized. This combination strategy is likely to be more effective in improving both nocturnal and daytime BP control in OSA.
Dr. Singh is Director, Sleep Disorder and Research Center, Michael E. DeBakey VA Medical Center; and Dr. Singh is Assistant Professor and Dr. Velamuri is Associate Professor, Pulmonary, Critical Care and Sleep Medicine, Baylor College of Medicine. Houston, Texas.
Heart disease and stroke are leading causes of death and disability. High blood pressure (BP) is a major risk factor for both.
The 2017 guidelines regarding “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure” (JNC 7) were recently published, which is an update incorporating new information from studies regarding BP-related risk of cardiovascular disease (CVD) and strategies to improve hypertension (HTN) treatment and control.
Screening for secondary causes of HTN is necessary for new-onset or uncontrolled HTN in adults, including drug-resistant HTN. Screening includes testing for obstructive sleep apnea, which is highly prevalent in this population.
Obstructive sleep apnea is a common chronic condition characterized by recurrent collapse of upper airways during sleep, inducing intermittent episodes of apnea/hypopnea, hypoxemia, and sleep disruption (Pedrosa RP, et al. Chest. 2013;144[5]:1487).
It is estimated to affect 17% of US adults but is overwhelmingly underrecognized and untreated (JAMA. 2012;307[20]:2169). The prevalence is higher in men than women. The major risk factors for OSA are obesity, male sex, and advancing age. Since these conditions oftentimes predispose to and are concomitant with HTN, it can be challenging to determine the independent effects of OSA on the development of HTN.
The relationship between obstructive sleep apnea (OSA) and HTN has been a point of interest for decades, with untreated OSA being associated with an increased risk for developing new-onset HTN (JAMA. 2012;307[20]:2169).
There have been several landmark trials that have sought to determine the extent of a causal relationship between OSAS and HTN. Sleep Heart Health Study (Sleep. 2006;29;1009) was one such study, which was limited by the inability to prove that OSA preceded the onset of HTN.
Wisconsin Sleep Cohort (N Engl J Med. 2000;342:1378) was another landmark prospective longitudinal study that implicates OSA as a possible causal factor in HTN. The notable limitation of the study was the presence of HTN after initial assessment was found to be dependent upon the severity of OSA at baseline.
While these two cohort studies found an association between OSA and HTN, the Vitoria Sleep Cohort out of Spain (Am J Respir Crit Care Med. 2011;184[11]:1299), the third and most recent longitudinal cohort study, looked at younger and thinner patients than the SHHS and the Wisconsin Sleep Cohort, failed to show a significant association between OSA and incident HTN. Methodologic differences may help to explain the disparity in results.
NREM sleep has normal circadian variation of BP, causing “dipping” of both systolic and diastolic BP at night due to decreased sympathetic and increased parasympathetic activity. REM sleep has predominant sympathetic activity and transient nocturnal BP surges.
OSA results in hypoxemia, which causes nocturnal catecholamine surges, resulting in nocturnal increase in heart rate and BP that is most prominent during post-apneic hyperventilation.
Reduced nocturnal BP (nondipping) or even higher nocturnal BP than daytime BP is an undoubted risk factor for hypertensive patients due to the end-organ damage and subsequent cardiovascular events. With sleep apnea, sleep quality is decreased due to frequent arousal from sleep (Hypertension. 2006;47[5]:833).
Sleep duration of less than or equal to 5 hours per night was shown to significantly increase risk for HTN in patients less than or equal to 60 years of age, even after controlling for obesity and diabetes.
Sleep Heart Health Study suggests that sleep duration above or below a median of 7 to 8 hours per night is associated with a higher prevalence of HTN (Sleep. 2006;29:1009). Thus, improving duration and quality of sleep in sleep apnea patients may help decrease the risk of developing HTN.
Key question: Will treatment of OSA appreciably alter BP?
Continuous positive airway pressure (CPAP) is an efficacious treatment of choice for OSA. Interventional trials, though limited by issues related to compliance, have shown CPAP to acutely reduce sympathetic drive and BP during sleep. However, this improvement in BP control is not entirely consistent in all patients with the data being less clear-cut regarding nighttime CPAP therapy and impact on daytime BP.
A randomized controlled trial from Barbe et al suggests that normotensive subjects with severe OSA but without demonstrable daytime sleepiness are immune to the BP-reducing effects of CPAP (Ann Intern Med. 2001;134:1015); those who were objectively sleepy had a more robust response to the BP lowering effects of CPAP with better cardiovascular outcomes among patients who were adherent to CPAP therapy (≥4 hours per night).
Sleep Apnea Cardiovascular Endpoints (SAVE) study looked at CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea (N Engl J Med. 2016;375:919). CPAP significantly reduced snoring and daytime sleepiness and improved health-related quality of life and mood, but the risk of serious cardiovascular events was not lower among patients who received treatment with CPAP in addition to usual care compared with usual care alone. This study was not powered to provide definitive answers regarding the effects of CPAP on secondary cardiovascular end points, and the use of PAP was less than 4 hours.
A recent systematic review and meta-analysis looked at “Association of Positive Airway Pressure with Cardiovascular Events and Death in Adults with Sleep Apnea” (JAMA. 2017;318(2):156). No significant associations between PAP treatment and a range of cardiovascular events were noted in this meta-analysis.
It is possible that the limited adherence to therapy in many trials was insufficient to drive protection, along with short follow-up duration of most trials that may have given insufficient time for PAP to have affected vascular outcomes.
In a cross-over study of valsartan and CPAP, combining drug treatment with CPAP appeared to have a more synergistic effect in reducing BP than either agent alone (Am J Respir Crit Care Med. 2010;182:954).
The beneficial effect of CPAP remains an open question. Considering the multifactorial pathophysiology of OSA-associated HTN, proven therapies, such as BP lowering, lipid lowering, and antiplatelet therapy, along with PAP therapy, should be utilized. This combination strategy is likely to be more effective in improving both nocturnal and daytime BP control in OSA.
Dr. Singh is Director, Sleep Disorder and Research Center, Michael E. DeBakey VA Medical Center; and Dr. Singh is Assistant Professor and Dr. Velamuri is Associate Professor, Pulmonary, Critical Care and Sleep Medicine, Baylor College of Medicine. Houston, Texas.
Lessons Learned From SERVE-HF
Great attention has been paid to the SERVE-HF trial (“Treatment of Sleep-disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure”), which showed increased all-cause mortality and cardiovascular mortality in the Adaptive Servo-ventilation (ASV) group compared with the control group of conventional heart failure management alone. The results of this trial led to the recommendation by multiple ASV manufacturers and medical societies to withdraw clinical use of ASV from patients with heart failure and a reduced ejection fraction (HFrEF) less than 45%.
Sleep-disordered breathing is common in patients with HFrEF with prevalence rates of 50% to 75%. Central sleep apnea (CSA) is associated with increased mortality in heart failure (HF) and is found in 25% to 40% of this subpopulation. It is estimated that the severity of CSA increases in parallel with the severity of the HF. For several years, treatment of CSA with positive pressure ventilation was believed to favor outcomes in HFrEF with a protective effect.
In the Canadian Positive Airway Pressure for Patients with CSA and HF (CANPAP) trial, subjects were randomized to treatment with CPAP or no CPAP. This trial was terminated early; it did not show an advantage of CPAP in morbidity or mortality. A post-hoc analysis suggested that mortality might be reduced if the frequency of respiratory events per hour or apnea hypopnea index (AHI) is reduced to 15/hour or less while using CPAP.
Hoping to improve the outcomes of HF, SERVE-HF was the first randomized, large scale, multinational trial directed to treat CSA in patients with HFrEF < 45% and concomitant clinically significant sleep apnea with AHI > 15/hour of central predominance (CSA index >10/hour). Treatment arms compared the addition the ASV, one of the most effective noninvasive positive pressure ventilation technologies for central apneas that offers automated inspiratory pressure support in addition to expiratory positive pressure vs conventional medical treatment alone in the control group.
The study published in the New England Journal of Medicine in September 2015 was designed in an intention-to-treat basis with the primary end point of time to first event, a composite of death from any cause, lifesaving cardiovascular intervention (heart transplant, implantation of LVAD, resuscitation after sudden cardiac arrest, or defibrillation for ventricular arrhythmia), or unplanned hospitalization for heart failure. The study did not show significant differences in the primary end point between the ASV and control group (54.1% and 50.8%, respectively; hazard ratio, 1.13; 95% confidence interval, 0.97 to 1.31; P=.10).1
The most interesting and unexpected outcome was an increase in the all cause mortality and cardiovascular mortality in the ASV group (hazard ratio for death from any cause, 1.28; 95% CI, 1.06 to 1.55; P=.01; and hazard ratio from cardiovascular death, 1.34; 95% CI, 1.09 to 1.65; P=.006).1 These findings led to the above recommendations from manufacturers, as well as a position statement from the American Academy of Sleep Medicine. These findings cannot be extrapolated to the obstructive sleep apnea population with concomitant HFrEF or to patients with HF with preserved ejection fraction, where positive pressure ventilation has offered an advantage1 likely by a different physiologic mechanism not fully uncovered at this time, believed to be an overall effect of afterload reduction.
Selection and self-selection bias in this study was addressed in a new analysis by the same SERVE-HF investigator group published August 2017, where a time-dependent model of on-treatment analysis (done to tease out if the original results were related to the treatment assignment or to poor adherence) was conducted to understand potential causes of the initial findings in the original study. It showed patients randomized to ASV who crossed over to the control group were at higher risk of cardiovascular death than control subjects; also the control patients with crossover to ASV had a signal of lower risk of cardiovascular death risk compared with patients assigned to ASV.2 Reduced adherence to ASV treatment during SERVE-HF was a concern, since it resulted in a reduced exposure to the treatment. The on-treatment analysis showed again an increase of cardiovascular death in HFrEF patients with predominant CSA treated with ASV in addition to conventional heart failure treatment compared with the control group.2 There was no increase in cardiovascular death risk associated with ASV use intervals (dose effect). This effect is not related to the amount of hours used per night.
The effect of the recommended withdrawal of treatment in HFrEF patients with EF<45% and moderate to severe central predominant sleep apnea is being addressed in smaller studies. A single center retrospective analysis observed the effects after ASV discontinuation in this population. Thirteen out of 126 patients treated with ASV who met SERVE-HF criteria were followed for at least a year; 93% of the subjects who met criteria had ASV removed; immediate recurrence of the central apnea was observed in most (except two patients), while adverse events were not identified (defined as need for emergency hospitalization). Day and nighttime symptoms were reported by 61% of the group, and they were started on alternative treatments.3 At 1 year after ASV removal, 88% of patients were still alive, overall cardiac function did not change in 1 year (P=0.17), and seven patients required adjustment of medications for heart failure. Symptomatic patients were treated with oxygen supplementation for nocturnal symptoms or CPAP if they had daytime sleepiness. None was treated with bi-level PAP, acetazolamide, or phrenic nerve stimulation. Four patients insisted on continuation of ASV despite understanding physician concerns. 3 This study helps to demonstrate that ASV discontinuation is feasible but requires close follow-up. However, larger, long-term prospective reviews are required to draw statistically meaningful conclusions about the consequences and safety of ASV removal; these studies will be difficult to conduct under the current indications for ASV in the interest group.
At this time, investigators have shifted to further understand the causes of the increase in cardiovascular mortality, overall mortality, and the understanding of the pathophysiologic processes associated with ASV use in HFrEF. It is not known whether the effect in mortality is related to the specific ASV device/algorithm used to suppress CSA or is related to the ASV principle itself. Upcoming studies will assist in clarifying these details. Currently, there is an ongoing trial looking at the effect of ASV on survival and hospital admissions in heart failure (ADVENT–HF) using a different ASV device; this study will hopefully elucidate the impact of class effect vs device effect. It may also provide better insight of the etiology of mortality and the impact of improved ASV compliance, first addressed by the on-treatment analysis of the SERVE-HF.4
Although the reasons for increased mortality related to ASV are unclear, proposed hypotheses include: central apnea is an adaptive mechanism to HFrEF and the reversal of central apneas might adversely affect the underlying disease process,1 low adherence to ASV may impact outcomes, and specific devices may induce hyper-/hypoventilation generated by the algorithm designs of the specific ASV device and this may result in electrolyte abnormalities that generate arrhythmias.
The ADVENT-HF trial, although similar in design, has significant differences from SERVE-HF: different ASV devices may have a different impact on cardiac output and ventilation, recruited patients included those with less daytime sleepiness, and the potential to assess the effect of ASV in patients with OSA and low daytime sleepiness in patients with reduced EF.5,6 This ongoing study may help us to further understand why there is increased mortality and what effect ASV has on the treatment of sleep apnea in patients with HFrEF.
References
1. Cowie MR, et al. N Engl J Med. 2015;373(12):1095-1105.
2. Woehrle H, et al. Eur Respir J. 2017; 50:1601.
3. Brill AK, et al. Sleep Med. 2017;37:201-207.
4. Bradley TD, et al. Can Respir. 2015;22(6):313.
5. Lyons OD, et al. Eur J Heart Fail. 2017;19(4):579-587.
6. Haruki N, et al. Can J Cardiol. 2016;32(12):1402-1410.
Dr. Barrantes is an assistant professor, Department of Pulmonary, Critical Care, and Sleep Medicine, Baylor College of Medicine, Houston, Texas.
Great attention has been paid to the SERVE-HF trial (“Treatment of Sleep-disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure”), which showed increased all-cause mortality and cardiovascular mortality in the Adaptive Servo-ventilation (ASV) group compared with the control group of conventional heart failure management alone. The results of this trial led to the recommendation by multiple ASV manufacturers and medical societies to withdraw clinical use of ASV from patients with heart failure and a reduced ejection fraction (HFrEF) less than 45%.
Sleep-disordered breathing is common in patients with HFrEF with prevalence rates of 50% to 75%. Central sleep apnea (CSA) is associated with increased mortality in heart failure (HF) and is found in 25% to 40% of this subpopulation. It is estimated that the severity of CSA increases in parallel with the severity of the HF. For several years, treatment of CSA with positive pressure ventilation was believed to favor outcomes in HFrEF with a protective effect.
In the Canadian Positive Airway Pressure for Patients with CSA and HF (CANPAP) trial, subjects were randomized to treatment with CPAP or no CPAP. This trial was terminated early; it did not show an advantage of CPAP in morbidity or mortality. A post-hoc analysis suggested that mortality might be reduced if the frequency of respiratory events per hour or apnea hypopnea index (AHI) is reduced to 15/hour or less while using CPAP.
Hoping to improve the outcomes of HF, SERVE-HF was the first randomized, large scale, multinational trial directed to treat CSA in patients with HFrEF < 45% and concomitant clinically significant sleep apnea with AHI > 15/hour of central predominance (CSA index >10/hour). Treatment arms compared the addition the ASV, one of the most effective noninvasive positive pressure ventilation technologies for central apneas that offers automated inspiratory pressure support in addition to expiratory positive pressure vs conventional medical treatment alone in the control group.
The study published in the New England Journal of Medicine in September 2015 was designed in an intention-to-treat basis with the primary end point of time to first event, a composite of death from any cause, lifesaving cardiovascular intervention (heart transplant, implantation of LVAD, resuscitation after sudden cardiac arrest, or defibrillation for ventricular arrhythmia), or unplanned hospitalization for heart failure. The study did not show significant differences in the primary end point between the ASV and control group (54.1% and 50.8%, respectively; hazard ratio, 1.13; 95% confidence interval, 0.97 to 1.31; P=.10).1
The most interesting and unexpected outcome was an increase in the all cause mortality and cardiovascular mortality in the ASV group (hazard ratio for death from any cause, 1.28; 95% CI, 1.06 to 1.55; P=.01; and hazard ratio from cardiovascular death, 1.34; 95% CI, 1.09 to 1.65; P=.006).1 These findings led to the above recommendations from manufacturers, as well as a position statement from the American Academy of Sleep Medicine. These findings cannot be extrapolated to the obstructive sleep apnea population with concomitant HFrEF or to patients with HF with preserved ejection fraction, where positive pressure ventilation has offered an advantage1 likely by a different physiologic mechanism not fully uncovered at this time, believed to be an overall effect of afterload reduction.
Selection and self-selection bias in this study was addressed in a new analysis by the same SERVE-HF investigator group published August 2017, where a time-dependent model of on-treatment analysis (done to tease out if the original results were related to the treatment assignment or to poor adherence) was conducted to understand potential causes of the initial findings in the original study. It showed patients randomized to ASV who crossed over to the control group were at higher risk of cardiovascular death than control subjects; also the control patients with crossover to ASV had a signal of lower risk of cardiovascular death risk compared with patients assigned to ASV.2 Reduced adherence to ASV treatment during SERVE-HF was a concern, since it resulted in a reduced exposure to the treatment. The on-treatment analysis showed again an increase of cardiovascular death in HFrEF patients with predominant CSA treated with ASV in addition to conventional heart failure treatment compared with the control group.2 There was no increase in cardiovascular death risk associated with ASV use intervals (dose effect). This effect is not related to the amount of hours used per night.
The effect of the recommended withdrawal of treatment in HFrEF patients with EF<45% and moderate to severe central predominant sleep apnea is being addressed in smaller studies. A single center retrospective analysis observed the effects after ASV discontinuation in this population. Thirteen out of 126 patients treated with ASV who met SERVE-HF criteria were followed for at least a year; 93% of the subjects who met criteria had ASV removed; immediate recurrence of the central apnea was observed in most (except two patients), while adverse events were not identified (defined as need for emergency hospitalization). Day and nighttime symptoms were reported by 61% of the group, and they were started on alternative treatments.3 At 1 year after ASV removal, 88% of patients were still alive, overall cardiac function did not change in 1 year (P=0.17), and seven patients required adjustment of medications for heart failure. Symptomatic patients were treated with oxygen supplementation for nocturnal symptoms or CPAP if they had daytime sleepiness. None was treated with bi-level PAP, acetazolamide, or phrenic nerve stimulation. Four patients insisted on continuation of ASV despite understanding physician concerns. 3 This study helps to demonstrate that ASV discontinuation is feasible but requires close follow-up. However, larger, long-term prospective reviews are required to draw statistically meaningful conclusions about the consequences and safety of ASV removal; these studies will be difficult to conduct under the current indications for ASV in the interest group.
At this time, investigators have shifted to further understand the causes of the increase in cardiovascular mortality, overall mortality, and the understanding of the pathophysiologic processes associated with ASV use in HFrEF. It is not known whether the effect in mortality is related to the specific ASV device/algorithm used to suppress CSA or is related to the ASV principle itself. Upcoming studies will assist in clarifying these details. Currently, there is an ongoing trial looking at the effect of ASV on survival and hospital admissions in heart failure (ADVENT–HF) using a different ASV device; this study will hopefully elucidate the impact of class effect vs device effect. It may also provide better insight of the etiology of mortality and the impact of improved ASV compliance, first addressed by the on-treatment analysis of the SERVE-HF.4
Although the reasons for increased mortality related to ASV are unclear, proposed hypotheses include: central apnea is an adaptive mechanism to HFrEF and the reversal of central apneas might adversely affect the underlying disease process,1 low adherence to ASV may impact outcomes, and specific devices may induce hyper-/hypoventilation generated by the algorithm designs of the specific ASV device and this may result in electrolyte abnormalities that generate arrhythmias.
The ADVENT-HF trial, although similar in design, has significant differences from SERVE-HF: different ASV devices may have a different impact on cardiac output and ventilation, recruited patients included those with less daytime sleepiness, and the potential to assess the effect of ASV in patients with OSA and low daytime sleepiness in patients with reduced EF.5,6 This ongoing study may help us to further understand why there is increased mortality and what effect ASV has on the treatment of sleep apnea in patients with HFrEF.
References
1. Cowie MR, et al. N Engl J Med. 2015;373(12):1095-1105.
2. Woehrle H, et al. Eur Respir J. 2017; 50:1601.
3. Brill AK, et al. Sleep Med. 2017;37:201-207.
4. Bradley TD, et al. Can Respir. 2015;22(6):313.
5. Lyons OD, et al. Eur J Heart Fail. 2017;19(4):579-587.
6. Haruki N, et al. Can J Cardiol. 2016;32(12):1402-1410.
Dr. Barrantes is an assistant professor, Department of Pulmonary, Critical Care, and Sleep Medicine, Baylor College of Medicine, Houston, Texas.
Great attention has been paid to the SERVE-HF trial (“Treatment of Sleep-disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure”), which showed increased all-cause mortality and cardiovascular mortality in the Adaptive Servo-ventilation (ASV) group compared with the control group of conventional heart failure management alone. The results of this trial led to the recommendation by multiple ASV manufacturers and medical societies to withdraw clinical use of ASV from patients with heart failure and a reduced ejection fraction (HFrEF) less than 45%.
Sleep-disordered breathing is common in patients with HFrEF with prevalence rates of 50% to 75%. Central sleep apnea (CSA) is associated with increased mortality in heart failure (HF) and is found in 25% to 40% of this subpopulation. It is estimated that the severity of CSA increases in parallel with the severity of the HF. For several years, treatment of CSA with positive pressure ventilation was believed to favor outcomes in HFrEF with a protective effect.
In the Canadian Positive Airway Pressure for Patients with CSA and HF (CANPAP) trial, subjects were randomized to treatment with CPAP or no CPAP. This trial was terminated early; it did not show an advantage of CPAP in morbidity or mortality. A post-hoc analysis suggested that mortality might be reduced if the frequency of respiratory events per hour or apnea hypopnea index (AHI) is reduced to 15/hour or less while using CPAP.
Hoping to improve the outcomes of HF, SERVE-HF was the first randomized, large scale, multinational trial directed to treat CSA in patients with HFrEF < 45% and concomitant clinically significant sleep apnea with AHI > 15/hour of central predominance (CSA index >10/hour). Treatment arms compared the addition the ASV, one of the most effective noninvasive positive pressure ventilation technologies for central apneas that offers automated inspiratory pressure support in addition to expiratory positive pressure vs conventional medical treatment alone in the control group.
The study published in the New England Journal of Medicine in September 2015 was designed in an intention-to-treat basis with the primary end point of time to first event, a composite of death from any cause, lifesaving cardiovascular intervention (heart transplant, implantation of LVAD, resuscitation after sudden cardiac arrest, or defibrillation for ventricular arrhythmia), or unplanned hospitalization for heart failure. The study did not show significant differences in the primary end point between the ASV and control group (54.1% and 50.8%, respectively; hazard ratio, 1.13; 95% confidence interval, 0.97 to 1.31; P=.10).1
The most interesting and unexpected outcome was an increase in the all cause mortality and cardiovascular mortality in the ASV group (hazard ratio for death from any cause, 1.28; 95% CI, 1.06 to 1.55; P=.01; and hazard ratio from cardiovascular death, 1.34; 95% CI, 1.09 to 1.65; P=.006).1 These findings led to the above recommendations from manufacturers, as well as a position statement from the American Academy of Sleep Medicine. These findings cannot be extrapolated to the obstructive sleep apnea population with concomitant HFrEF or to patients with HF with preserved ejection fraction, where positive pressure ventilation has offered an advantage1 likely by a different physiologic mechanism not fully uncovered at this time, believed to be an overall effect of afterload reduction.
Selection and self-selection bias in this study was addressed in a new analysis by the same SERVE-HF investigator group published August 2017, where a time-dependent model of on-treatment analysis (done to tease out if the original results were related to the treatment assignment or to poor adherence) was conducted to understand potential causes of the initial findings in the original study. It showed patients randomized to ASV who crossed over to the control group were at higher risk of cardiovascular death than control subjects; also the control patients with crossover to ASV had a signal of lower risk of cardiovascular death risk compared with patients assigned to ASV.2 Reduced adherence to ASV treatment during SERVE-HF was a concern, since it resulted in a reduced exposure to the treatment. The on-treatment analysis showed again an increase of cardiovascular death in HFrEF patients with predominant CSA treated with ASV in addition to conventional heart failure treatment compared with the control group.2 There was no increase in cardiovascular death risk associated with ASV use intervals (dose effect). This effect is not related to the amount of hours used per night.
The effect of the recommended withdrawal of treatment in HFrEF patients with EF<45% and moderate to severe central predominant sleep apnea is being addressed in smaller studies. A single center retrospective analysis observed the effects after ASV discontinuation in this population. Thirteen out of 126 patients treated with ASV who met SERVE-HF criteria were followed for at least a year; 93% of the subjects who met criteria had ASV removed; immediate recurrence of the central apnea was observed in most (except two patients), while adverse events were not identified (defined as need for emergency hospitalization). Day and nighttime symptoms were reported by 61% of the group, and they were started on alternative treatments.3 At 1 year after ASV removal, 88% of patients were still alive, overall cardiac function did not change in 1 year (P=0.17), and seven patients required adjustment of medications for heart failure. Symptomatic patients were treated with oxygen supplementation for nocturnal symptoms or CPAP if they had daytime sleepiness. None was treated with bi-level PAP, acetazolamide, or phrenic nerve stimulation. Four patients insisted on continuation of ASV despite understanding physician concerns. 3 This study helps to demonstrate that ASV discontinuation is feasible but requires close follow-up. However, larger, long-term prospective reviews are required to draw statistically meaningful conclusions about the consequences and safety of ASV removal; these studies will be difficult to conduct under the current indications for ASV in the interest group.
At this time, investigators have shifted to further understand the causes of the increase in cardiovascular mortality, overall mortality, and the understanding of the pathophysiologic processes associated with ASV use in HFrEF. It is not known whether the effect in mortality is related to the specific ASV device/algorithm used to suppress CSA or is related to the ASV principle itself. Upcoming studies will assist in clarifying these details. Currently, there is an ongoing trial looking at the effect of ASV on survival and hospital admissions in heart failure (ADVENT–HF) using a different ASV device; this study will hopefully elucidate the impact of class effect vs device effect. It may also provide better insight of the etiology of mortality and the impact of improved ASV compliance, first addressed by the on-treatment analysis of the SERVE-HF.4
Although the reasons for increased mortality related to ASV are unclear, proposed hypotheses include: central apnea is an adaptive mechanism to HFrEF and the reversal of central apneas might adversely affect the underlying disease process,1 low adherence to ASV may impact outcomes, and specific devices may induce hyper-/hypoventilation generated by the algorithm designs of the specific ASV device and this may result in electrolyte abnormalities that generate arrhythmias.
The ADVENT-HF trial, although similar in design, has significant differences from SERVE-HF: different ASV devices may have a different impact on cardiac output and ventilation, recruited patients included those with less daytime sleepiness, and the potential to assess the effect of ASV in patients with OSA and low daytime sleepiness in patients with reduced EF.5,6 This ongoing study may help us to further understand why there is increased mortality and what effect ASV has on the treatment of sleep apnea in patients with HFrEF.
References
1. Cowie MR, et al. N Engl J Med. 2015;373(12):1095-1105.
2. Woehrle H, et al. Eur Respir J. 2017; 50:1601.
3. Brill AK, et al. Sleep Med. 2017;37:201-207.
4. Bradley TD, et al. Can Respir. 2015;22(6):313.
5. Lyons OD, et al. Eur J Heart Fail. 2017;19(4):579-587.
6. Haruki N, et al. Can J Cardiol. 2016;32(12):1402-1410.
Dr. Barrantes is an assistant professor, Department of Pulmonary, Critical Care, and Sleep Medicine, Baylor College of Medicine, Houston, Texas.
Sleep Strategies
The definition of mild obstructive sleep apnea (OSA) has varied over the years depending upon several factors, but based upon all definitions, it is highly prevalent. Depending upon presence of symptoms and gender, the prevalence may be as high 28% in men and 26% in women. (Young et al. N Engl J Med. 1993;328:1230).
Typically, a combination of symptoms and frequency of respiratory events is required to make the diagnosis. Based upon the International Classification of Sleep Disorders-3rd edition (ICSD-3), the threshold apnea hypopnea index (AHI) for diagnosis depends upon the presence or absence of symptoms. If an individual has no symptoms, an AHI of 15 events per hour or more is required to make a diagnosis of OSA. However, there are several concerns about whether or not an individual may be “symptomatic.” This is most relevant when driving privileges may be at risk, such as with a commercial drivers’ licensing.
The presence of other comorbid disease can be used as criteria, including hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, and type 2 diabetes mellitus. If no signs, symptoms, or comorbid diseases are present, then an AHI greater than 15 events per hour or more is required to make the diagnosis of OSA (Chowdrui et al. Am J Respir Crit Care Med. 2016;193:e37).
There is still debate regarding the association of mild OSA and cardiovascular disease and whether treatment may prevent or reduce cardiovascular outcomes. The four main clinical outcomes typically reported are hypertension, cardiovascular events, cardiovascular and all-cause mortality, and arrhythmias.
A large clinical cohort of patients referred for sleep studies showed no association of mild OSA with different composite outcomes. Kendzerska and colleagues evaluated a composite outcome (myocardial infarction, stroke, CHF, revascularization procedures, or death from any cause) during a median follow-up of 68 months. No association of mild OSA with the composite cardiovascular endpoint was identified compared with those without OSA (Kendzerska et al. PLoS Med. 2014;11[2]:e1001599). Only one population-based study (MrOS Sleep Study) looked at the association between mild OSA and nocturnal arrhythmias in elderly men. The study did not find an increased risk for atrial fibrillation or complex ventricular ectopy in patients with mild OSA vs no OSA (Mehra et al. Arch Intern Med. 2009; 169:1147).
Several cohort studies have reported mild OSA is not associated with increased cardiovascular mortality. In the 18-year follow-up of the Wisconsin Cohort Study, it was found that mild OSA was not associated with cardiovascular mortality (HR, 1.8; 95% CI, 0.7–4.9). All-cause mortality was also not significantly increased in the mild OSA group compared with the no-OSA group in the Wisconsin cohort after 8 years of follow-up (adjusted HR, 1.6; 95% CI, 0.8–2.8). In summary, compared with subjects without OSA, available evidence from population-based longitudinal studies indicates that mild OSA is not associated with increased cardiovascular or all-cause mortality.
Does treatment of mild OSA vs no treatment change cardiovascular or mortality outcomes? This is still debated with no definitive answer. There have been several studies that have examined different therapies for OSA to reduce cardiovascular events. Typical events include coronary artery disease, hypertension, heart failure, stroke, arrhythmias, and cardiovascular disease-related mortality. However, most studies have examined cohorts with moderate to severe OSA with limited evaluation in the mild OSA category.
An observational study evaluated the effects of CPAP specifically in patients with mild OSA. There was no significant difference in the risk of developing hypertension among those patients ineligible for CPAP therapy, active on therapy, or those who declined therapy (Marin et al. JAMA. 2012; 307:2169). In contrast, a retrospective longitudinal cohort with normal blood pressure at baseline (mild OSA without preexisting cardiovascular disease, diabetes, or hyperlipidemia) did show decrease in mean arterial blood pressure of 2 mm Hg in the treatment group (Jaimchariyatam et al. Sleep Med. 2010;11:837). The MOSAIC trial was a multicenter randomized trial that evaluated the effects of CPAP on cardiac function in minimally symptomatic patients with OSA. The use of CPAP reduced the oxygen desaturation index (ODI) and Epworth Sleepiness Scale values. However, 6 months of therapy did not change functional or structural parameters measured by echocardiogram or cardiac magnetic resonance scanning in patients with mild to moderate OSA (Craig et al. J Clin Sleep Med. 2015;11[9]:967). A single retrospective study reported the effects of CPAP in patients with mild OSA and all-cause mortality. The study compared treatment with patients using CPAP more than 4 hours vs a combined group of nonadherent and those who refused therapy (Hudgel et al. J Clin Sleep Med. 2012;8:9). There was no significant difference in all-cause mortality in the two groups. However, this study did not analyze the impact of therapy on cardiovascular-specific mortality.
To date, there have been no studies that have evaluated the impact of treatment of mild OSA on cardiovascular events, arrhythmias, or stroke. In addition, there have been no randomized studies assessing treatment of mild OSA on fatal and nonfatal cardiovascular events. There is inadequate evidence regarding the effect of mild OSA on elevated blood pressure, neurologic cognition, quality of life, and cardiovascular consequences. Future research is needed to investigate the impact of mild OSA on these outcomes.
In summary, mild OSA is a very prevalent disease but the association with hypertension remains unclear and the literature to date suggests no association with other cardiovascular outcomes. In addition, no clear prevention of cardiovascular outcomes with treatment has been proven in the setting of mild OSA.
Dr. Duthuluru is Assistant Professor, Dr. Nazir is Assistant Professor, and Dr. Stevens is Associate Professor at the University of Kansas Medical Center.
The definition of mild obstructive sleep apnea (OSA) has varied over the years depending upon several factors, but based upon all definitions, it is highly prevalent. Depending upon presence of symptoms and gender, the prevalence may be as high 28% in men and 26% in women. (Young et al. N Engl J Med. 1993;328:1230).
Typically, a combination of symptoms and frequency of respiratory events is required to make the diagnosis. Based upon the International Classification of Sleep Disorders-3rd edition (ICSD-3), the threshold apnea hypopnea index (AHI) for diagnosis depends upon the presence or absence of symptoms. If an individual has no symptoms, an AHI of 15 events per hour or more is required to make a diagnosis of OSA. However, there are several concerns about whether or not an individual may be “symptomatic.” This is most relevant when driving privileges may be at risk, such as with a commercial drivers’ licensing.
The presence of other comorbid disease can be used as criteria, including hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, and type 2 diabetes mellitus. If no signs, symptoms, or comorbid diseases are present, then an AHI greater than 15 events per hour or more is required to make the diagnosis of OSA (Chowdrui et al. Am J Respir Crit Care Med. 2016;193:e37).
There is still debate regarding the association of mild OSA and cardiovascular disease and whether treatment may prevent or reduce cardiovascular outcomes. The four main clinical outcomes typically reported are hypertension, cardiovascular events, cardiovascular and all-cause mortality, and arrhythmias.
A large clinical cohort of patients referred for sleep studies showed no association of mild OSA with different composite outcomes. Kendzerska and colleagues evaluated a composite outcome (myocardial infarction, stroke, CHF, revascularization procedures, or death from any cause) during a median follow-up of 68 months. No association of mild OSA with the composite cardiovascular endpoint was identified compared with those without OSA (Kendzerska et al. PLoS Med. 2014;11[2]:e1001599). Only one population-based study (MrOS Sleep Study) looked at the association between mild OSA and nocturnal arrhythmias in elderly men. The study did not find an increased risk for atrial fibrillation or complex ventricular ectopy in patients with mild OSA vs no OSA (Mehra et al. Arch Intern Med. 2009; 169:1147).
Several cohort studies have reported mild OSA is not associated with increased cardiovascular mortality. In the 18-year follow-up of the Wisconsin Cohort Study, it was found that mild OSA was not associated with cardiovascular mortality (HR, 1.8; 95% CI, 0.7–4.9). All-cause mortality was also not significantly increased in the mild OSA group compared with the no-OSA group in the Wisconsin cohort after 8 years of follow-up (adjusted HR, 1.6; 95% CI, 0.8–2.8). In summary, compared with subjects without OSA, available evidence from population-based longitudinal studies indicates that mild OSA is not associated with increased cardiovascular or all-cause mortality.
Does treatment of mild OSA vs no treatment change cardiovascular or mortality outcomes? This is still debated with no definitive answer. There have been several studies that have examined different therapies for OSA to reduce cardiovascular events. Typical events include coronary artery disease, hypertension, heart failure, stroke, arrhythmias, and cardiovascular disease-related mortality. However, most studies have examined cohorts with moderate to severe OSA with limited evaluation in the mild OSA category.
An observational study evaluated the effects of CPAP specifically in patients with mild OSA. There was no significant difference in the risk of developing hypertension among those patients ineligible for CPAP therapy, active on therapy, or those who declined therapy (Marin et al. JAMA. 2012; 307:2169). In contrast, a retrospective longitudinal cohort with normal blood pressure at baseline (mild OSA without preexisting cardiovascular disease, diabetes, or hyperlipidemia) did show decrease in mean arterial blood pressure of 2 mm Hg in the treatment group (Jaimchariyatam et al. Sleep Med. 2010;11:837). The MOSAIC trial was a multicenter randomized trial that evaluated the effects of CPAP on cardiac function in minimally symptomatic patients with OSA. The use of CPAP reduced the oxygen desaturation index (ODI) and Epworth Sleepiness Scale values. However, 6 months of therapy did not change functional or structural parameters measured by echocardiogram or cardiac magnetic resonance scanning in patients with mild to moderate OSA (Craig et al. J Clin Sleep Med. 2015;11[9]:967). A single retrospective study reported the effects of CPAP in patients with mild OSA and all-cause mortality. The study compared treatment with patients using CPAP more than 4 hours vs a combined group of nonadherent and those who refused therapy (Hudgel et al. J Clin Sleep Med. 2012;8:9). There was no significant difference in all-cause mortality in the two groups. However, this study did not analyze the impact of therapy on cardiovascular-specific mortality.
To date, there have been no studies that have evaluated the impact of treatment of mild OSA on cardiovascular events, arrhythmias, or stroke. In addition, there have been no randomized studies assessing treatment of mild OSA on fatal and nonfatal cardiovascular events. There is inadequate evidence regarding the effect of mild OSA on elevated blood pressure, neurologic cognition, quality of life, and cardiovascular consequences. Future research is needed to investigate the impact of mild OSA on these outcomes.
In summary, mild OSA is a very prevalent disease but the association with hypertension remains unclear and the literature to date suggests no association with other cardiovascular outcomes. In addition, no clear prevention of cardiovascular outcomes with treatment has been proven in the setting of mild OSA.
Dr. Duthuluru is Assistant Professor, Dr. Nazir is Assistant Professor, and Dr. Stevens is Associate Professor at the University of Kansas Medical Center.
The definition of mild obstructive sleep apnea (OSA) has varied over the years depending upon several factors, but based upon all definitions, it is highly prevalent. Depending upon presence of symptoms and gender, the prevalence may be as high 28% in men and 26% in women. (Young et al. N Engl J Med. 1993;328:1230).
Typically, a combination of symptoms and frequency of respiratory events is required to make the diagnosis. Based upon the International Classification of Sleep Disorders-3rd edition (ICSD-3), the threshold apnea hypopnea index (AHI) for diagnosis depends upon the presence or absence of symptoms. If an individual has no symptoms, an AHI of 15 events per hour or more is required to make a diagnosis of OSA. However, there are several concerns about whether or not an individual may be “symptomatic.” This is most relevant when driving privileges may be at risk, such as with a commercial drivers’ licensing.
The presence of other comorbid disease can be used as criteria, including hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, and type 2 diabetes mellitus. If no signs, symptoms, or comorbid diseases are present, then an AHI greater than 15 events per hour or more is required to make the diagnosis of OSA (Chowdrui et al. Am J Respir Crit Care Med. 2016;193:e37).
There is still debate regarding the association of mild OSA and cardiovascular disease and whether treatment may prevent or reduce cardiovascular outcomes. The four main clinical outcomes typically reported are hypertension, cardiovascular events, cardiovascular and all-cause mortality, and arrhythmias.
A large clinical cohort of patients referred for sleep studies showed no association of mild OSA with different composite outcomes. Kendzerska and colleagues evaluated a composite outcome (myocardial infarction, stroke, CHF, revascularization procedures, or death from any cause) during a median follow-up of 68 months. No association of mild OSA with the composite cardiovascular endpoint was identified compared with those without OSA (Kendzerska et al. PLoS Med. 2014;11[2]:e1001599). Only one population-based study (MrOS Sleep Study) looked at the association between mild OSA and nocturnal arrhythmias in elderly men. The study did not find an increased risk for atrial fibrillation or complex ventricular ectopy in patients with mild OSA vs no OSA (Mehra et al. Arch Intern Med. 2009; 169:1147).
Several cohort studies have reported mild OSA is not associated with increased cardiovascular mortality. In the 18-year follow-up of the Wisconsin Cohort Study, it was found that mild OSA was not associated with cardiovascular mortality (HR, 1.8; 95% CI, 0.7–4.9). All-cause mortality was also not significantly increased in the mild OSA group compared with the no-OSA group in the Wisconsin cohort after 8 years of follow-up (adjusted HR, 1.6; 95% CI, 0.8–2.8). In summary, compared with subjects without OSA, available evidence from population-based longitudinal studies indicates that mild OSA is not associated with increased cardiovascular or all-cause mortality.
Does treatment of mild OSA vs no treatment change cardiovascular or mortality outcomes? This is still debated with no definitive answer. There have been several studies that have examined different therapies for OSA to reduce cardiovascular events. Typical events include coronary artery disease, hypertension, heart failure, stroke, arrhythmias, and cardiovascular disease-related mortality. However, most studies have examined cohorts with moderate to severe OSA with limited evaluation in the mild OSA category.
An observational study evaluated the effects of CPAP specifically in patients with mild OSA. There was no significant difference in the risk of developing hypertension among those patients ineligible for CPAP therapy, active on therapy, or those who declined therapy (Marin et al. JAMA. 2012; 307:2169). In contrast, a retrospective longitudinal cohort with normal blood pressure at baseline (mild OSA without preexisting cardiovascular disease, diabetes, or hyperlipidemia) did show decrease in mean arterial blood pressure of 2 mm Hg in the treatment group (Jaimchariyatam et al. Sleep Med. 2010;11:837). The MOSAIC trial was a multicenter randomized trial that evaluated the effects of CPAP on cardiac function in minimally symptomatic patients with OSA. The use of CPAP reduced the oxygen desaturation index (ODI) and Epworth Sleepiness Scale values. However, 6 months of therapy did not change functional or structural parameters measured by echocardiogram or cardiac magnetic resonance scanning in patients with mild to moderate OSA (Craig et al. J Clin Sleep Med. 2015;11[9]:967). A single retrospective study reported the effects of CPAP in patients with mild OSA and all-cause mortality. The study compared treatment with patients using CPAP more than 4 hours vs a combined group of nonadherent and those who refused therapy (Hudgel et al. J Clin Sleep Med. 2012;8:9). There was no significant difference in all-cause mortality in the two groups. However, this study did not analyze the impact of therapy on cardiovascular-specific mortality.
To date, there have been no studies that have evaluated the impact of treatment of mild OSA on cardiovascular events, arrhythmias, or stroke. In addition, there have been no randomized studies assessing treatment of mild OSA on fatal and nonfatal cardiovascular events. There is inadequate evidence regarding the effect of mild OSA on elevated blood pressure, neurologic cognition, quality of life, and cardiovascular consequences. Future research is needed to investigate the impact of mild OSA on these outcomes.
In summary, mild OSA is a very prevalent disease but the association with hypertension remains unclear and the literature to date suggests no association with other cardiovascular outcomes. In addition, no clear prevention of cardiovascular outcomes with treatment has been proven in the setting of mild OSA.
Dr. Duthuluru is Assistant Professor, Dr. Nazir is Assistant Professor, and Dr. Stevens is Associate Professor at the University of Kansas Medical Center.





















