User login
Things We Do for No Reason: Prescribing Docusate for Constipation in Hospitalized Adults
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
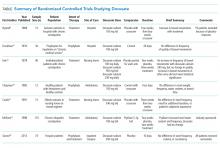
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Sliding-Scale Insulin as Monotherapy for Glycemic Control in Hospitalized Patients
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
© 2018 Society of Hospital Medicine
Things We Do for No Reason: Intermittent Pneumatic Compression for Medical Ward Patients?
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
© 2019 Society of Hospital Medicine