User login
FDA will strengthen heart attack, stroke risk warnings for all NSAIDs
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
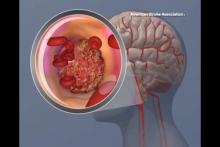
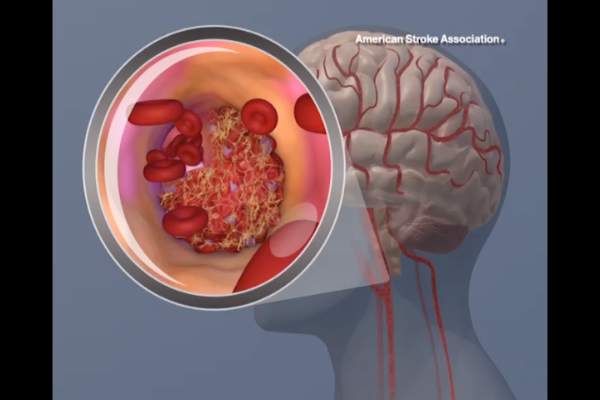
Updated acute stroke guideline boosts thrombectomy
Pivotal new high-quality evidence from randomized clinical trials and other sources published since 2013 has prompted the American Heart Association and the American Stroke Association to update their joint clinical practice guideline on endovascular treatment of acute ischemic stroke.
The revisions were published online June 29 in Stroke.
Unchanged is the key recommendation that intravenous recombinant tissue-type plasminogen activator (r-tPA) remain the mainstay of initial therapy, even if endovascular treatment is being considered. But a new recommendation adds that a period of observation to assess patients’ clinical response to r-tPA before proceeding with endovascular therapy is not necessary and is not advisable, said Dr. William J. Powers, chair of the guideline writing committee and professor and chairman of the department of neurology, University of North Carolina, Durham, and his associates.
Most of the updates pertain to the use of a stent retriever, which is now recommended for all patients with acute ischemic stroke who meet these seven criteria:
1. A prestroke modified Rankin scale (mRS) score of 0-1.
2. Receipt of r-tPA within 4.5 hours of symptom onset.
3. Causative occlusion of the internal carotid artery or proximal middle cerebral artery.
4. Age of 18 years or older.
5. A National Institutes of Health Stroke Scale (NIHSS) score of 6 or greater.
6. An Alberta Stroke Program Early CT Score (ASPECTS) of 6 or greater.
7. Initiation of the procedure within 6 hours of symptom onset.
Use of stent retrievers also is now considered “reasonable” in carefully selected patients with occlusion of the anterior circulation who have contraindications to r-tPA, such as current use of anticoagulants, prior stroke, serious head trauma, or hemorrhagic coagulopathy. It also may be reasonable in selected patients who have causative occlusion of the M2 or M3 portion of the middle cerebral arteries, anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries, although the benefits are “uncertain” in this patient population.
Similarly, endovascular therapy using stent retrievers may be reasonable for some patients younger than age 18 who otherwise meet the seven criteria, even though the benefits of treatment haven’t been established in this age group. And it likewise may be reasonable in patients with prestroke mRS scores greater than 1, an ASPECTS of less than 6, or NIHSS scores of less than 6 if there is causative occlusion of the internal carotid artery or the proximal middle cerebral artery.
The updated guideline also says that stent retrievers are preferable to the Merci device, but that other mechanical thrombectomy devices may be reasonable to use in some circumstances. And adjunctive use of a proximal balloon guide catheter or a large-bore distal access catheter rather than a cervical guide catheter along with stent retrievers also may be beneficial.
In addition, “the technical goal of the thrombectomy procedure should be a TICI [Thrombolysis in Cerebral Infarction] 2b/3 angiographic result to maximize the probability of a good functional outcome. Use of salvage technical adjuncts including intra-arterial fibrinolysis may be reasonable to achieve these angiographic results, if completed within 6 hours of symptom onset,” the guideline states (Stroke 2015 June 29 [doi:10.1161/STR.0000000000000074]).
Also with regard to intra-arterial rather than intravenous fibrinolysis, stent retrievers are now preferable to intra-arterial fibrinolysis as first-line therapy.
The updated guideline also has added the recommendation that conscious sedation may be preferable to general anesthesia during endovascular therapy, depending on patient risk factors, tolerance of the procedure, and other clinical characteristics. It also revised recommendations addressing imaging studies and systems of stroke care.
Five prospective, randomized controlled trials have come out in the past few months, and triggered a revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with r-tPA.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, said Dr. Petr Widimsky, professor and chair of the cardiology department at Charles University in Prague, at the annual congress of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) held in Paris in May.
The American Academy of Neurology “affirms the value of this guideline as an educational tool for neurologists.” The revised guideline is endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the American Society of Neuroradiology, and the Society of Vascular and Interventional Neurology. A copy of the document is available at http://myamericanheart.org/statements.
Pivotal new high-quality evidence from randomized clinical trials and other sources published since 2013 has prompted the American Heart Association and the American Stroke Association to update their joint clinical practice guideline on endovascular treatment of acute ischemic stroke.
The revisions were published online June 29 in Stroke.
Unchanged is the key recommendation that intravenous recombinant tissue-type plasminogen activator (r-tPA) remain the mainstay of initial therapy, even if endovascular treatment is being considered. But a new recommendation adds that a period of observation to assess patients’ clinical response to r-tPA before proceeding with endovascular therapy is not necessary and is not advisable, said Dr. William J. Powers, chair of the guideline writing committee and professor and chairman of the department of neurology, University of North Carolina, Durham, and his associates.
Most of the updates pertain to the use of a stent retriever, which is now recommended for all patients with acute ischemic stroke who meet these seven criteria:
1. A prestroke modified Rankin scale (mRS) score of 0-1.
2. Receipt of r-tPA within 4.5 hours of symptom onset.
3. Causative occlusion of the internal carotid artery or proximal middle cerebral artery.
4. Age of 18 years or older.
5. A National Institutes of Health Stroke Scale (NIHSS) score of 6 or greater.
6. An Alberta Stroke Program Early CT Score (ASPECTS) of 6 or greater.
7. Initiation of the procedure within 6 hours of symptom onset.
Use of stent retrievers also is now considered “reasonable” in carefully selected patients with occlusion of the anterior circulation who have contraindications to r-tPA, such as current use of anticoagulants, prior stroke, serious head trauma, or hemorrhagic coagulopathy. It also may be reasonable in selected patients who have causative occlusion of the M2 or M3 portion of the middle cerebral arteries, anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries, although the benefits are “uncertain” in this patient population.
Similarly, endovascular therapy using stent retrievers may be reasonable for some patients younger than age 18 who otherwise meet the seven criteria, even though the benefits of treatment haven’t been established in this age group. And it likewise may be reasonable in patients with prestroke mRS scores greater than 1, an ASPECTS of less than 6, or NIHSS scores of less than 6 if there is causative occlusion of the internal carotid artery or the proximal middle cerebral artery.
The updated guideline also says that stent retrievers are preferable to the Merci device, but that other mechanical thrombectomy devices may be reasonable to use in some circumstances. And adjunctive use of a proximal balloon guide catheter or a large-bore distal access catheter rather than a cervical guide catheter along with stent retrievers also may be beneficial.
In addition, “the technical goal of the thrombectomy procedure should be a TICI [Thrombolysis in Cerebral Infarction] 2b/3 angiographic result to maximize the probability of a good functional outcome. Use of salvage technical adjuncts including intra-arterial fibrinolysis may be reasonable to achieve these angiographic results, if completed within 6 hours of symptom onset,” the guideline states (Stroke 2015 June 29 [doi:10.1161/STR.0000000000000074]).
Also with regard to intra-arterial rather than intravenous fibrinolysis, stent retrievers are now preferable to intra-arterial fibrinolysis as first-line therapy.
The updated guideline also has added the recommendation that conscious sedation may be preferable to general anesthesia during endovascular therapy, depending on patient risk factors, tolerance of the procedure, and other clinical characteristics. It also revised recommendations addressing imaging studies and systems of stroke care.
Five prospective, randomized controlled trials have come out in the past few months, and triggered a revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with r-tPA.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, said Dr. Petr Widimsky, professor and chair of the cardiology department at Charles University in Prague, at the annual congress of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) held in Paris in May.
The American Academy of Neurology “affirms the value of this guideline as an educational tool for neurologists.” The revised guideline is endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the American Society of Neuroradiology, and the Society of Vascular and Interventional Neurology. A copy of the document is available at http://myamericanheart.org/statements.
Pivotal new high-quality evidence from randomized clinical trials and other sources published since 2013 has prompted the American Heart Association and the American Stroke Association to update their joint clinical practice guideline on endovascular treatment of acute ischemic stroke.
The revisions were published online June 29 in Stroke.
Unchanged is the key recommendation that intravenous recombinant tissue-type plasminogen activator (r-tPA) remain the mainstay of initial therapy, even if endovascular treatment is being considered. But a new recommendation adds that a period of observation to assess patients’ clinical response to r-tPA before proceeding with endovascular therapy is not necessary and is not advisable, said Dr. William J. Powers, chair of the guideline writing committee and professor and chairman of the department of neurology, University of North Carolina, Durham, and his associates.
Most of the updates pertain to the use of a stent retriever, which is now recommended for all patients with acute ischemic stroke who meet these seven criteria:
1. A prestroke modified Rankin scale (mRS) score of 0-1.
2. Receipt of r-tPA within 4.5 hours of symptom onset.
3. Causative occlusion of the internal carotid artery or proximal middle cerebral artery.
4. Age of 18 years or older.
5. A National Institutes of Health Stroke Scale (NIHSS) score of 6 or greater.
6. An Alberta Stroke Program Early CT Score (ASPECTS) of 6 or greater.
7. Initiation of the procedure within 6 hours of symptom onset.
Use of stent retrievers also is now considered “reasonable” in carefully selected patients with occlusion of the anterior circulation who have contraindications to r-tPA, such as current use of anticoagulants, prior stroke, serious head trauma, or hemorrhagic coagulopathy. It also may be reasonable in selected patients who have causative occlusion of the M2 or M3 portion of the middle cerebral arteries, anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries, although the benefits are “uncertain” in this patient population.
Similarly, endovascular therapy using stent retrievers may be reasonable for some patients younger than age 18 who otherwise meet the seven criteria, even though the benefits of treatment haven’t been established in this age group. And it likewise may be reasonable in patients with prestroke mRS scores greater than 1, an ASPECTS of less than 6, or NIHSS scores of less than 6 if there is causative occlusion of the internal carotid artery or the proximal middle cerebral artery.
The updated guideline also says that stent retrievers are preferable to the Merci device, but that other mechanical thrombectomy devices may be reasonable to use in some circumstances. And adjunctive use of a proximal balloon guide catheter or a large-bore distal access catheter rather than a cervical guide catheter along with stent retrievers also may be beneficial.
In addition, “the technical goal of the thrombectomy procedure should be a TICI [Thrombolysis in Cerebral Infarction] 2b/3 angiographic result to maximize the probability of a good functional outcome. Use of salvage technical adjuncts including intra-arterial fibrinolysis may be reasonable to achieve these angiographic results, if completed within 6 hours of symptom onset,” the guideline states (Stroke 2015 June 29 [doi:10.1161/STR.0000000000000074]).
Also with regard to intra-arterial rather than intravenous fibrinolysis, stent retrievers are now preferable to intra-arterial fibrinolysis as first-line therapy.
The updated guideline also has added the recommendation that conscious sedation may be preferable to general anesthesia during endovascular therapy, depending on patient risk factors, tolerance of the procedure, and other clinical characteristics. It also revised recommendations addressing imaging studies and systems of stroke care.
Five prospective, randomized controlled trials have come out in the past few months, and triggered a revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with r-tPA.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, said Dr. Petr Widimsky, professor and chair of the cardiology department at Charles University in Prague, at the annual congress of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR) held in Paris in May.
The American Academy of Neurology “affirms the value of this guideline as an educational tool for neurologists.” The revised guideline is endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the American Society of Neuroradiology, and the Society of Vascular and Interventional Neurology. A copy of the document is available at http://myamericanheart.org/statements.
FROM STROKE
Key clinical point: Pivotal new evidence prompted several changes in the 2013 AHA/ASA clinical practice guideline for early endovascular treatment of acute ischemic stroke.
Major finding: Most of the updates pertain to use of stent retrievers, which is now recommended for all patients with acute ischemic stroke who meet seven criteria.
Data source: A detailed review of eight randomized clinical trials and other relevant data published since 2013.
Disclosures: This work was supported by the American Heart Association and the American Stroke Association; Medtronic/Covidien, maker of the stent retriever newly recommended in this guideline, is a corporate sponsor of both the AHA and the ASA. Dr. Powers reported having no relevant financial disclosures; his associates on the writing committee reported ties to Microvention, Penumbra, Silk Road, Pulse Therapeutics, Covidien, Genentech, Stryker, Roche, Sequent, Lazarus, Codman, and Aldagn/Cytomedix.
Stent-retriever thrombectomy reduces poststroke disability
For patients with proximal large-vessel anterior stroke, neurovascular thrombectomy with a stent retriever plus medical therapy in the REVASCAT trial reduced the severity of poststroke disability and raised the rate of functional independence, compared with medical therapy alone, according to a report in the New England Journal of Medicine.
To assess the efficacy and safety of thrombectomy with a stent retriever, investigators performed a prospective, open-label, phase III clinical trial involving 206 adults up to 85 years of age treated at four designated comprehensive stroke centers in Catalonia, Spain.
All the participants in the Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset (REVASCAT) had either not responded to intravenous alteplase administered within 4.5 hours of symptom onset or had contraindications to alteplase therapy. They were randomly assigned in equal numbers to undergo endovascular treatment with a stent retriever or medical therapy alone, said Dr. Tudor G. Jovin, director of the Stroke Institute, University of Pittsburgh Medical Center, and his associates.
The trial was halted early when the first interim analysis showed “lack of equipoise” between the two study groups, and because emerging results from three other studies demonstrated the superior efficacy of thrombectomy. The primary efficacy outcome measure – severity of disability at 90 days, as measured by expert assessors blinded to treatment assignment – significantly favored thrombectomy over medical therapy. The proportion of patients who achieved functional independence by day 90 on the modified Rankin scale also demonstrated the clear superiority of thrombectomy (43.7%) over medical therapy (28.2%).
Only 6.5 patients would need to be treated with thrombectomy to prevent 1 case of functional dependency or death. In addition, “thrombectomy was associated with a shift toward better outcomes across the entire spectrum of disability,” Dr. Jovin and his associates said (N. Engl. J. Med. 2015 [doi:10.1056/NEJMoa1503780]).
Regarding safety, the rate of death at 90 days did not differ significantly between patients who underwent thrombectomy (18.4%) and control subjects (15.5%). Rates of intracranial hemorrhage were the same, 1.9%, in both groups, and rates of other serious adverse events also were similar.
These findings are consistent with those of several other recently reported clinical trials and show that “in patients with acute stroke caused by a proximal large-vessel occlusion and an absence of a large infarct on baseline imaging, mechanical thrombectomy with [a] stent retriever was safe and led to improved clinical outcomes, as compared with medical therapy alone,” the investigators said.
REVASCAT was funded by an unrestricted grant from Covidien, maker of the stent retriever, and by grants from several Spanish research institutes. Dr. Jovin reported ties to Covidien, Silk Road Medical, Air Liquide, Medtronic, and Stryker Neurovascular.
For patients with proximal large-vessel anterior stroke, neurovascular thrombectomy with a stent retriever plus medical therapy in the REVASCAT trial reduced the severity of poststroke disability and raised the rate of functional independence, compared with medical therapy alone, according to a report in the New England Journal of Medicine.
To assess the efficacy and safety of thrombectomy with a stent retriever, investigators performed a prospective, open-label, phase III clinical trial involving 206 adults up to 85 years of age treated at four designated comprehensive stroke centers in Catalonia, Spain.
All the participants in the Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset (REVASCAT) had either not responded to intravenous alteplase administered within 4.5 hours of symptom onset or had contraindications to alteplase therapy. They were randomly assigned in equal numbers to undergo endovascular treatment with a stent retriever or medical therapy alone, said Dr. Tudor G. Jovin, director of the Stroke Institute, University of Pittsburgh Medical Center, and his associates.
The trial was halted early when the first interim analysis showed “lack of equipoise” between the two study groups, and because emerging results from three other studies demonstrated the superior efficacy of thrombectomy. The primary efficacy outcome measure – severity of disability at 90 days, as measured by expert assessors blinded to treatment assignment – significantly favored thrombectomy over medical therapy. The proportion of patients who achieved functional independence by day 90 on the modified Rankin scale also demonstrated the clear superiority of thrombectomy (43.7%) over medical therapy (28.2%).
Only 6.5 patients would need to be treated with thrombectomy to prevent 1 case of functional dependency or death. In addition, “thrombectomy was associated with a shift toward better outcomes across the entire spectrum of disability,” Dr. Jovin and his associates said (N. Engl. J. Med. 2015 [doi:10.1056/NEJMoa1503780]).
Regarding safety, the rate of death at 90 days did not differ significantly between patients who underwent thrombectomy (18.4%) and control subjects (15.5%). Rates of intracranial hemorrhage were the same, 1.9%, in both groups, and rates of other serious adverse events also were similar.
These findings are consistent with those of several other recently reported clinical trials and show that “in patients with acute stroke caused by a proximal large-vessel occlusion and an absence of a large infarct on baseline imaging, mechanical thrombectomy with [a] stent retriever was safe and led to improved clinical outcomes, as compared with medical therapy alone,” the investigators said.
REVASCAT was funded by an unrestricted grant from Covidien, maker of the stent retriever, and by grants from several Spanish research institutes. Dr. Jovin reported ties to Covidien, Silk Road Medical, Air Liquide, Medtronic, and Stryker Neurovascular.
For patients with proximal large-vessel anterior stroke, neurovascular thrombectomy with a stent retriever plus medical therapy in the REVASCAT trial reduced the severity of poststroke disability and raised the rate of functional independence, compared with medical therapy alone, according to a report in the New England Journal of Medicine.
To assess the efficacy and safety of thrombectomy with a stent retriever, investigators performed a prospective, open-label, phase III clinical trial involving 206 adults up to 85 years of age treated at four designated comprehensive stroke centers in Catalonia, Spain.
All the participants in the Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset (REVASCAT) had either not responded to intravenous alteplase administered within 4.5 hours of symptom onset or had contraindications to alteplase therapy. They were randomly assigned in equal numbers to undergo endovascular treatment with a stent retriever or medical therapy alone, said Dr. Tudor G. Jovin, director of the Stroke Institute, University of Pittsburgh Medical Center, and his associates.
The trial was halted early when the first interim analysis showed “lack of equipoise” between the two study groups, and because emerging results from three other studies demonstrated the superior efficacy of thrombectomy. The primary efficacy outcome measure – severity of disability at 90 days, as measured by expert assessors blinded to treatment assignment – significantly favored thrombectomy over medical therapy. The proportion of patients who achieved functional independence by day 90 on the modified Rankin scale also demonstrated the clear superiority of thrombectomy (43.7%) over medical therapy (28.2%).
Only 6.5 patients would need to be treated with thrombectomy to prevent 1 case of functional dependency or death. In addition, “thrombectomy was associated with a shift toward better outcomes across the entire spectrum of disability,” Dr. Jovin and his associates said (N. Engl. J. Med. 2015 [doi:10.1056/NEJMoa1503780]).
Regarding safety, the rate of death at 90 days did not differ significantly between patients who underwent thrombectomy (18.4%) and control subjects (15.5%). Rates of intracranial hemorrhage were the same, 1.9%, in both groups, and rates of other serious adverse events also were similar.
These findings are consistent with those of several other recently reported clinical trials and show that “in patients with acute stroke caused by a proximal large-vessel occlusion and an absence of a large infarct on baseline imaging, mechanical thrombectomy with [a] stent retriever was safe and led to improved clinical outcomes, as compared with medical therapy alone,” the investigators said.
REVASCAT was funded by an unrestricted grant from Covidien, maker of the stent retriever, and by grants from several Spanish research institutes. Dr. Jovin reported ties to Covidien, Silk Road Medical, Air Liquide, Medtronic, and Stryker Neurovascular.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Thrombectomy with a stent retriever reduced poststroke disability in patients with occlusion of the proximal anterior circulation.
Major finding: The proportion of patients who achieved functional independence by day 90 showed the clear superiority of thrombectomy (43.7%) over medical therapy (28.2%); only 6.5 patients would need to be treated with thrombectomy to prevent 1 case of functional dependency or death.
Data source: REVASCAT, a prospective, open-label, randomized phase III trial involving 206 adults treated during a 4-year period at four stroke centers in Spain.
Disclosures: REVASCAT was funded by an unrestricted grant from Covidien, maker of the stent retriever, and by grants from several Spanish research institutes. Dr. Jovin reported ties to Covidien, Silk Road Medical, Air Liquide, Medtronic, and Stryker Neurovascular.
Hyperglycemia may predict prognosis after ischemic stroke
VIENNA – Patients who have had an ischemic stroke and have high blood sugar levels without being diabetic may be more likely to experience functional impairments than those already diagnosed with diabetes, according to results from a large secondary stroke prevention trial performed in China.
In the trial, conducted at 47 hospitals, high levels of fasting blood glucose (FBG) were associated with worse disability at 6 months in the study, but the association only held in patients who did not have a diagnosis of diabetes at the time of their stroke. The odds ratios for poor functional outcome assessed using the modified Rankin scale (mRS) as a score of greater than 2 versus up to 2 were 1.09 (P = .031) in nondiabetics and 0.98 (P = 0.65) in those previously known to have diabetes.
“We think that a high FBG level after stroke might be better for predicting prognosis in patients without prediagnosed diabetes than in those with diabetes and confirms the importance of glycemic control during the acute phase of ischemic stroke,” said study researcher Dr. Ming Yao of Peking Union Medical College Hospital at the annual European Stroke Conference.Dr. Yao noted that hyperglycemia after an acute stroke had already been linked to poorer clinical outcomes, with reports of larger infarct volumes, an increased risk for secondary hemorrhagic transformation, and lower recanalization rates after thrombolysis. However, it was not clear how functional outcomes were affected or if there was much of difference based on whether or not a patient had diabetes. Data from the Standard Medical Management in Secondary Prevention of Ischemic Stroke in China (SMART) study were therefore used to see what effect high FBG had on functional outcomes in diabetic versus nondiabetic subjects. SMART was a multicenter, cluster-randomized, controlled trial designed to assess the effectiveness of a guideline-based structured care program versus usual care for the secondary prevention of ischemic stroke (Stroke. 2014;45:515-9) and offered a large population of patients for the subgroup analysis. Of the 3,821 patients enrolled in the study, 2,862 had FBG data available and had complete follow-up data at 6 months.
Potential factors related to functional outcome 6 months after a stroke were first identified in the whole cohort using a binary logistic regression model, which categorized outcome as favorable (mRS ≤2) or poor (mRS >2). Univariate and multivariate analyses were then performed to narrow down the variables that might be the most influential.
For the whole cohort, older age (OR = 1.04, P < .001), hypertension (OR = 1.45, P = .028), baseline National Institutes of Health Stroke Scale (NIHSS) score (OR = 1.28, P < .001), and FBG (OR = 1.07, P = .004) were indicative of a poor functional outcome.
Looking at patients with and without diabetes, older age remained predictive of a poorer functional outcome, with respective odds ratios of 1.04 (P = .011) and 1.04 (P < .001). Baseline NIHSS score was also predictive in both patients with diabetes (OR = 1.33, P < .001) and those without (OR = 1.27, P < .001).
“Our present results demonstrate that a higher FBG following stroke is strongly and independently associated with a poor functional outcome,” Dr. Yao observed, “but this association was found only in patients without prediagnosed diabetes.”
The study was study was sponsored by Peking Union Medical College Hospital. Dr. Yao had no conflicts of interest.
VIENNA – Patients who have had an ischemic stroke and have high blood sugar levels without being diabetic may be more likely to experience functional impairments than those already diagnosed with diabetes, according to results from a large secondary stroke prevention trial performed in China.
In the trial, conducted at 47 hospitals, high levels of fasting blood glucose (FBG) were associated with worse disability at 6 months in the study, but the association only held in patients who did not have a diagnosis of diabetes at the time of their stroke. The odds ratios for poor functional outcome assessed using the modified Rankin scale (mRS) as a score of greater than 2 versus up to 2 were 1.09 (P = .031) in nondiabetics and 0.98 (P = 0.65) in those previously known to have diabetes.
“We think that a high FBG level after stroke might be better for predicting prognosis in patients without prediagnosed diabetes than in those with diabetes and confirms the importance of glycemic control during the acute phase of ischemic stroke,” said study researcher Dr. Ming Yao of Peking Union Medical College Hospital at the annual European Stroke Conference.Dr. Yao noted that hyperglycemia after an acute stroke had already been linked to poorer clinical outcomes, with reports of larger infarct volumes, an increased risk for secondary hemorrhagic transformation, and lower recanalization rates after thrombolysis. However, it was not clear how functional outcomes were affected or if there was much of difference based on whether or not a patient had diabetes. Data from the Standard Medical Management in Secondary Prevention of Ischemic Stroke in China (SMART) study were therefore used to see what effect high FBG had on functional outcomes in diabetic versus nondiabetic subjects. SMART was a multicenter, cluster-randomized, controlled trial designed to assess the effectiveness of a guideline-based structured care program versus usual care for the secondary prevention of ischemic stroke (Stroke. 2014;45:515-9) and offered a large population of patients for the subgroup analysis. Of the 3,821 patients enrolled in the study, 2,862 had FBG data available and had complete follow-up data at 6 months.
Potential factors related to functional outcome 6 months after a stroke were first identified in the whole cohort using a binary logistic regression model, which categorized outcome as favorable (mRS ≤2) or poor (mRS >2). Univariate and multivariate analyses were then performed to narrow down the variables that might be the most influential.
For the whole cohort, older age (OR = 1.04, P < .001), hypertension (OR = 1.45, P = .028), baseline National Institutes of Health Stroke Scale (NIHSS) score (OR = 1.28, P < .001), and FBG (OR = 1.07, P = .004) were indicative of a poor functional outcome.
Looking at patients with and without diabetes, older age remained predictive of a poorer functional outcome, with respective odds ratios of 1.04 (P = .011) and 1.04 (P < .001). Baseline NIHSS score was also predictive in both patients with diabetes (OR = 1.33, P < .001) and those without (OR = 1.27, P < .001).
“Our present results demonstrate that a higher FBG following stroke is strongly and independently associated with a poor functional outcome,” Dr. Yao observed, “but this association was found only in patients without prediagnosed diabetes.”
The study was study was sponsored by Peking Union Medical College Hospital. Dr. Yao had no conflicts of interest.
VIENNA – Patients who have had an ischemic stroke and have high blood sugar levels without being diabetic may be more likely to experience functional impairments than those already diagnosed with diabetes, according to results from a large secondary stroke prevention trial performed in China.
In the trial, conducted at 47 hospitals, high levels of fasting blood glucose (FBG) were associated with worse disability at 6 months in the study, but the association only held in patients who did not have a diagnosis of diabetes at the time of their stroke. The odds ratios for poor functional outcome assessed using the modified Rankin scale (mRS) as a score of greater than 2 versus up to 2 were 1.09 (P = .031) in nondiabetics and 0.98 (P = 0.65) in those previously known to have diabetes.
“We think that a high FBG level after stroke might be better for predicting prognosis in patients without prediagnosed diabetes than in those with diabetes and confirms the importance of glycemic control during the acute phase of ischemic stroke,” said study researcher Dr. Ming Yao of Peking Union Medical College Hospital at the annual European Stroke Conference.Dr. Yao noted that hyperglycemia after an acute stroke had already been linked to poorer clinical outcomes, with reports of larger infarct volumes, an increased risk for secondary hemorrhagic transformation, and lower recanalization rates after thrombolysis. However, it was not clear how functional outcomes were affected or if there was much of difference based on whether or not a patient had diabetes. Data from the Standard Medical Management in Secondary Prevention of Ischemic Stroke in China (SMART) study were therefore used to see what effect high FBG had on functional outcomes in diabetic versus nondiabetic subjects. SMART was a multicenter, cluster-randomized, controlled trial designed to assess the effectiveness of a guideline-based structured care program versus usual care for the secondary prevention of ischemic stroke (Stroke. 2014;45:515-9) and offered a large population of patients for the subgroup analysis. Of the 3,821 patients enrolled in the study, 2,862 had FBG data available and had complete follow-up data at 6 months.
Potential factors related to functional outcome 6 months after a stroke were first identified in the whole cohort using a binary logistic regression model, which categorized outcome as favorable (mRS ≤2) or poor (mRS >2). Univariate and multivariate analyses were then performed to narrow down the variables that might be the most influential.
For the whole cohort, older age (OR = 1.04, P < .001), hypertension (OR = 1.45, P = .028), baseline National Institutes of Health Stroke Scale (NIHSS) score (OR = 1.28, P < .001), and FBG (OR = 1.07, P = .004) were indicative of a poor functional outcome.
Looking at patients with and without diabetes, older age remained predictive of a poorer functional outcome, with respective odds ratios of 1.04 (P = .011) and 1.04 (P < .001). Baseline NIHSS score was also predictive in both patients with diabetes (OR = 1.33, P < .001) and those without (OR = 1.27, P < .001).
“Our present results demonstrate that a higher FBG following stroke is strongly and independently associated with a poor functional outcome,” Dr. Yao observed, “but this association was found only in patients without prediagnosed diabetes.”
The study was study was sponsored by Peking Union Medical College Hospital. Dr. Yao had no conflicts of interest.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: Glycemic control during acute stroke is important, particularly in nondiabetic patients.
Major finding: High fasting plasma glucose was associated with poor functional outcome at 6 months in nondiabetic patients (OR = 1.09, P = .031).
Data source: 2,862 patients from the SMART study, a multicenter, cluster-randomized, controlled, secondary stroke prevention trial conducted in China.
Disclosures: The study was sponsored by Peking Union Medical College Hospital. Dr. Yao had no disclosures.
Carotid artery stenosis guidelines need modernizing
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: Guidelines for carotid stenosis need reviewing and updating in line with modern evidence and practice.
Major finding: Carotid angiography/stenting was recommended for both asymptomatic and symptomatic patients despite current evidence showing that it is more likely to cause harm than provide benefit.
Data source: Systematic review of 33 guidelines for asymptomatic and symptomatic carotid stenosis.
Disclosures: Dr. Abbott had no relevant disclosures.
Statins, fibrates lower stroke risk in elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased the risk of stroke by 30% in a community-dwelling population of elderly people, according to prospective European study published online May 19 in the British Medical Journal.
Participants in almost all the randomized clinical trials assessing cardiovascular drugs are younger than age 70, so the benefits of these agents in older patients – particularly their effectiveness as primary prevention in people who have no known cardiovascular illness – is uncertain. Nevertheless, “in real life, statins are commonly prescribed to older people without clinical evidence of atherosclerosis,” said Dr. Annick Alperovitch of the University of Bordeaux (France) and her associates.
To assess the effects of statin and fibrate therapies on incident cardiovascular events in an elderly population, the investigators analyzed data from an ongoing cohort study of vascular disease among elderly residents of Bordeaux, Dijon, and Montpellier. Dr. Alperovitch and her associates examined the medical records of a subset of 7,484 men and women (mean age 74 years) who were followed every 2 years for a mean of 9 years. A total of 27% reported using lipid-lowering medications at baseline; roughly half used statins and half used fibrates. There were 292 strokes during follow-up.
The risk of stroke was cut by roughly 30% among statin and fibrate users, compared with nonusers (hazard ratio, 0.66). This decrease was similar between the two medications. All-cause mortality was slightly lower in people who took statins or fibrates, compared with nonusers (HR 0.87), the investigators said (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2335]).
This is the first observational study to show a significant association between lipid-lowering drugs and decreased stroke risk, they noted.
The overall incidence of stroke in this study was low (0.47 per 100 person-years), so even a 30% decrease produced “a limited number of avoided cases.” That may be attributable in part to the generally healthy lifestyle, high educational achievement, and high economic status of this urban French study population. But if the findings are confirmed in future studies, they could have an important impact on public health in other populations, Dr. Alperovitch and her associates said.
The findings of a single observational study will not change guidelines regarding cholesterol therapy, but they are sufficiently compelling to warrant further research on primary prevention of stroke in elderly people.
This is the first observational study to describe a significant association between lipid-lowering medications and decreased stroke risk. Previous studies have shown only a weak association in early middle age, and no association in the elderly.
Graeme J. Hankey, M.D., is professor of neurology at the University of Western Australia’s Harry Perkins Institute of Medical Research, Perth, and honorary senior research fellow at Western Australian Neuroscience Research Institute, also in Perth. He reported ties to Sanofi Aventis, Bayer Pharmaceuticals, and Medscape. Dr. Hankey made these remarks in an editorial accompanying Dr. Alperovitch’s report (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2568]).
The findings of a single observational study will not change guidelines regarding cholesterol therapy, but they are sufficiently compelling to warrant further research on primary prevention of stroke in elderly people.
This is the first observational study to describe a significant association between lipid-lowering medications and decreased stroke risk. Previous studies have shown only a weak association in early middle age, and no association in the elderly.
Graeme J. Hankey, M.D., is professor of neurology at the University of Western Australia’s Harry Perkins Institute of Medical Research, Perth, and honorary senior research fellow at Western Australian Neuroscience Research Institute, also in Perth. He reported ties to Sanofi Aventis, Bayer Pharmaceuticals, and Medscape. Dr. Hankey made these remarks in an editorial accompanying Dr. Alperovitch’s report (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2568]).
The findings of a single observational study will not change guidelines regarding cholesterol therapy, but they are sufficiently compelling to warrant further research on primary prevention of stroke in elderly people.
This is the first observational study to describe a significant association between lipid-lowering medications and decreased stroke risk. Previous studies have shown only a weak association in early middle age, and no association in the elderly.
Graeme J. Hankey, M.D., is professor of neurology at the University of Western Australia’s Harry Perkins Institute of Medical Research, Perth, and honorary senior research fellow at Western Australian Neuroscience Research Institute, also in Perth. He reported ties to Sanofi Aventis, Bayer Pharmaceuticals, and Medscape. Dr. Hankey made these remarks in an editorial accompanying Dr. Alperovitch’s report (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2568]).
Both statin and fibrate therapies taken to improve lipid profiles decreased the risk of stroke by 30% in a community-dwelling population of elderly people, according to prospective European study published online May 19 in the British Medical Journal.
Participants in almost all the randomized clinical trials assessing cardiovascular drugs are younger than age 70, so the benefits of these agents in older patients – particularly their effectiveness as primary prevention in people who have no known cardiovascular illness – is uncertain. Nevertheless, “in real life, statins are commonly prescribed to older people without clinical evidence of atherosclerosis,” said Dr. Annick Alperovitch of the University of Bordeaux (France) and her associates.
To assess the effects of statin and fibrate therapies on incident cardiovascular events in an elderly population, the investigators analyzed data from an ongoing cohort study of vascular disease among elderly residents of Bordeaux, Dijon, and Montpellier. Dr. Alperovitch and her associates examined the medical records of a subset of 7,484 men and women (mean age 74 years) who were followed every 2 years for a mean of 9 years. A total of 27% reported using lipid-lowering medications at baseline; roughly half used statins and half used fibrates. There were 292 strokes during follow-up.
The risk of stroke was cut by roughly 30% among statin and fibrate users, compared with nonusers (hazard ratio, 0.66). This decrease was similar between the two medications. All-cause mortality was slightly lower in people who took statins or fibrates, compared with nonusers (HR 0.87), the investigators said (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2335]).
This is the first observational study to show a significant association between lipid-lowering drugs and decreased stroke risk, they noted.
The overall incidence of stroke in this study was low (0.47 per 100 person-years), so even a 30% decrease produced “a limited number of avoided cases.” That may be attributable in part to the generally healthy lifestyle, high educational achievement, and high economic status of this urban French study population. But if the findings are confirmed in future studies, they could have an important impact on public health in other populations, Dr. Alperovitch and her associates said.
Both statin and fibrate therapies taken to improve lipid profiles decreased the risk of stroke by 30% in a community-dwelling population of elderly people, according to prospective European study published online May 19 in the British Medical Journal.
Participants in almost all the randomized clinical trials assessing cardiovascular drugs are younger than age 70, so the benefits of these agents in older patients – particularly their effectiveness as primary prevention in people who have no known cardiovascular illness – is uncertain. Nevertheless, “in real life, statins are commonly prescribed to older people without clinical evidence of atherosclerosis,” said Dr. Annick Alperovitch of the University of Bordeaux (France) and her associates.
To assess the effects of statin and fibrate therapies on incident cardiovascular events in an elderly population, the investigators analyzed data from an ongoing cohort study of vascular disease among elderly residents of Bordeaux, Dijon, and Montpellier. Dr. Alperovitch and her associates examined the medical records of a subset of 7,484 men and women (mean age 74 years) who were followed every 2 years for a mean of 9 years. A total of 27% reported using lipid-lowering medications at baseline; roughly half used statins and half used fibrates. There were 292 strokes during follow-up.
The risk of stroke was cut by roughly 30% among statin and fibrate users, compared with nonusers (hazard ratio, 0.66). This decrease was similar between the two medications. All-cause mortality was slightly lower in people who took statins or fibrates, compared with nonusers (HR 0.87), the investigators said (Br. Med. J. 2015 May 19 [doi:10.1136/bmj.h2335]).
This is the first observational study to show a significant association between lipid-lowering drugs and decreased stroke risk, they noted.
The overall incidence of stroke in this study was low (0.47 per 100 person-years), so even a 30% decrease produced “a limited number of avoided cases.” That may be attributable in part to the generally healthy lifestyle, high educational achievement, and high economic status of this urban French study population. But if the findings are confirmed in future studies, they could have an important impact on public health in other populations, Dr. Alperovitch and her associates said.
Key clinical point: Statin and fibrate therapies significantly decreased stroke risk in a large cohort of elderly people.
Major finding: The risk of stroke was cut by 30% among statin and fibrate users, compared with nonusers (HR, 0.66).
Data source: A prospective, population-based cohort study of 7,484 elderly community-dwelling people followed for 9 years.
Disclosures: This study was supported by Institut National de la Sante et de la Recherche Medicale, Victor Segalen-Bordeaux II University, and Sanofi-Aventis. Dr. Alperovitch reported receiving honoraria from the French National Medicines Agency, the Fondation Plan Alzheimer, and Fondation Bettencourt-Schueller.
Asymptomatic carotid stenosis and central sleep apnea linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely have sleep apnea, according to an observational study.
The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea. Obstructive sleep apnea was present in 42% of patients and central sleep apnea in 27%.
Stenosis severity was significantly associated with central sleep apnea, but not with obstructive sleep apnea. Researchers found that central sleep apnea, but not obstructive sleep apnea, was associated with arterial hypertension and diabetes mellitus in those patients with asymptomatic carotid stenosis (CHEST 2015;147:1029-1036 [doi:10.1378/chest.14-1655]).
The patients ranged in age from 39 to 86 years (mean age, 70 years); 64 were men. Of the 96 patients, 21 had mild/moderate stenosis and 75 had severe carotid stenosis. Patients with severe stenosis were older, average age 67 years, than were those with mild/moderate stenosis, average age 61 years. The frequency of arterial hypertension and diabetes mellitus was higher in the severe stenosis group than in the mild/moderate stenosis group.
The prevalence of sleep apnea was 76% in patients with severe stenosis compared with 29% in those with mild/moderate carotid stenosis. Total apnea-hypopnea index was higher in the severe stenosis group compared with the mild/moderate stenosis group (P less than or equal to .009). Increase in sleep apnea severity was based on an increase in central apnea-hypopnea index (P less than or equal to .001) but not in obstructive apnea-hypopnea index, reflecting an augmentation of central sleep apnea and not of obstructive sleep apnea in patients with severe compared with mild/moderate carotid stenosis.
“This vascular risk constellation seems to be more strongly connected with CSA [central sleep apnea] than with OSA [obstructive sleep apnea], possibly attributable to carotid chemoreceptor dysfunction,” wrote Dr. Jens Ehrhardt and colleagues at Jena University Hospital, Germany.
No conflicts of interest were declared.
More than two-thirds of patients with asymptomatic carotid stenosis are likely have sleep apnea, according to an observational study.
The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea. Obstructive sleep apnea was present in 42% of patients and central sleep apnea in 27%.
Stenosis severity was significantly associated with central sleep apnea, but not with obstructive sleep apnea. Researchers found that central sleep apnea, but not obstructive sleep apnea, was associated with arterial hypertension and diabetes mellitus in those patients with asymptomatic carotid stenosis (CHEST 2015;147:1029-1036 [doi:10.1378/chest.14-1655]).
The patients ranged in age from 39 to 86 years (mean age, 70 years); 64 were men. Of the 96 patients, 21 had mild/moderate stenosis and 75 had severe carotid stenosis. Patients with severe stenosis were older, average age 67 years, than were those with mild/moderate stenosis, average age 61 years. The frequency of arterial hypertension and diabetes mellitus was higher in the severe stenosis group than in the mild/moderate stenosis group.
The prevalence of sleep apnea was 76% in patients with severe stenosis compared with 29% in those with mild/moderate carotid stenosis. Total apnea-hypopnea index was higher in the severe stenosis group compared with the mild/moderate stenosis group (P less than or equal to .009). Increase in sleep apnea severity was based on an increase in central apnea-hypopnea index (P less than or equal to .001) but not in obstructive apnea-hypopnea index, reflecting an augmentation of central sleep apnea and not of obstructive sleep apnea in patients with severe compared with mild/moderate carotid stenosis.
“This vascular risk constellation seems to be more strongly connected with CSA [central sleep apnea] than with OSA [obstructive sleep apnea], possibly attributable to carotid chemoreceptor dysfunction,” wrote Dr. Jens Ehrhardt and colleagues at Jena University Hospital, Germany.
No conflicts of interest were declared.
More than two-thirds of patients with asymptomatic carotid stenosis are likely have sleep apnea, according to an observational study.
The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea. Obstructive sleep apnea was present in 42% of patients and central sleep apnea in 27%.
Stenosis severity was significantly associated with central sleep apnea, but not with obstructive sleep apnea. Researchers found that central sleep apnea, but not obstructive sleep apnea, was associated with arterial hypertension and diabetes mellitus in those patients with asymptomatic carotid stenosis (CHEST 2015;147:1029-1036 [doi:10.1378/chest.14-1655]).
The patients ranged in age from 39 to 86 years (mean age, 70 years); 64 were men. Of the 96 patients, 21 had mild/moderate stenosis and 75 had severe carotid stenosis. Patients with severe stenosis were older, average age 67 years, than were those with mild/moderate stenosis, average age 61 years. The frequency of arterial hypertension and diabetes mellitus was higher in the severe stenosis group than in the mild/moderate stenosis group.
The prevalence of sleep apnea was 76% in patients with severe stenosis compared with 29% in those with mild/moderate carotid stenosis. Total apnea-hypopnea index was higher in the severe stenosis group compared with the mild/moderate stenosis group (P less than or equal to .009). Increase in sleep apnea severity was based on an increase in central apnea-hypopnea index (P less than or equal to .001) but not in obstructive apnea-hypopnea index, reflecting an augmentation of central sleep apnea and not of obstructive sleep apnea in patients with severe compared with mild/moderate carotid stenosis.
“This vascular risk constellation seems to be more strongly connected with CSA [central sleep apnea] than with OSA [obstructive sleep apnea], possibly attributable to carotid chemoreceptor dysfunction,” wrote Dr. Jens Ehrhardt and colleagues at Jena University Hospital, Germany.
No conflicts of interest were declared.
FROM CHEST
Key clinical point: More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea.
Major finding: The prevalence of sleep apnea was 76% in patients with severe stenosis compared with 29% in those with mild/moderate carotid stenosis.
Data source: Study of 96 patients with asymptomatic extracranial carotid stenosis.
Disclosures: No conflicts of interest were declared.
Hybrid carotid stents eyed positively
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholzsaid at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events From Carotid Endarterectomy (SCAFFOLD) trial, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said. The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections. In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micrometer pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholzsaid at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events From Carotid Endarterectomy (SCAFFOLD) trial, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said. The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections. In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micrometer pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholzsaid at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events From Carotid Endarterectomy (SCAFFOLD) trial, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said. The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections. In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micrometer pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
POINT/COUNTERPOINT: Renal artery occlusive disease – To treat or not to treat? ASTRAL and CORAL trials show no indication to treat percutaneously. There are still indications to treat renal artery occlusive disease.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
Percutaneous treatment of renal artery occlusive disease is unnecessary and should be abandoned, except in pediatric cases.
BY GEORGE HAMILTON, M.D.
This position is supported by findings from both the ASTRAL trial (N. Engl. J. Med. 2009;361:1953-62) and the CORAL trial (N. Engl. J. Med. 2013 Nov. 18 [doi:10.1056/NEJMoa1310753]).
The ASTRAL trial, a prospective, randomized comparison of best medical therapy with and without stent angioplasty in more than 800 patients, was the largest trial to date when it began back in the 1990s. The well-known results showed no difference in time to first renal event, first vascular and cardiovascular events, and overall survival. Furthermore, there was no difference in these outcomes among patients with greater than 90% stenosis, with the exception of a possible difference in mortality, which trended toward improvement among those with high-grade stenosis.
We concluded that revascularization in the vast majority of patients is unlikely to improve hypertension control or renal function, and that renal artery stenosis is not pathophysiologically important. We also concluded that there is no point in screening for asymptomatic disease; this was back when every patient was getting screened, and treated primarily on the basis of finding a renal arterial stenosis.
Finally, we concluded that properly applied best medical therapy alone was an extremely good treatment.
Several flaws in the trial garnered extensive criticism, however, and the more rigidly designed CORAL trial was expected to address them. The findings confirmed those of the ASTRAL trial. In more than 900 patients from 88 centers, there was absolutely no benefit of intervention with respect to primary and secondary outcomes, including among those with high-grade stenosis.
We can now see on the basis of extensive level 1 evidence that when added to comprehensive, multifactorial medical therapy, intervention yielded no benefit.
So are there certain patient groups who might benefit more from intervention? Among listed indications are high-grade stenosis (which doesn’t apply any longer); short history of progressive failure (which is quite rare); ACE-induced renal failure (which is also quite rare); difficult-to-control hypertension (there really is no such thing now, except in a tiny percentage of patients); and – the least challenged indication – flash pulmonary edema. These remaining indications move our interventions into a very high risk group of patients.
The current debate is focused almost entirely on endovascular intervention, but a systematic review showed that there is long-term benefit in terms of renal function and hypertension with open procedures. Although overall there is increased mortality, this risk is minimized – and not significantly different from endovascular procedures – in those having only renal revascularization vs. those having concomitant aortic procedures. So open surgery remains a possible treatment option, indeed a recent level 1 study comparing stenting and open surgery, showed better long-term results with open surgery (J. Vasc. Surg. 2009;49:667-75). The authors concluded that surgical reconstruction remains the gold standard in treating renal artery stenosis. Although national data suggest an overall mortality of about 10%, it is much lower at specialist, high-volume centers with mortality rates similar to those of stent angioplasty.
Renal stenting is not a low-risk procedure. In all-comers the complication rates, serious complication rates, and mortality rates are significant with short-term equivalence between focused renal arterial surgery and percutaneous intervention.
Returning to the debate, are either methods of revascularization appropriate? Probably not.
Even in flash pulmonary edema, there is little evidence to support revascularization. Few papers exist suggesting a benefit of revascularization in reduction of flash pulmonary edema, but the patient numbers were small, and there was no benefit in terms of preservation of renal function.
The history of evolution and evaluation of the role of renal revascularization is remarkably similar to that of renal denervation, initially and with considerable conviction thought to be a cure for hypertension. However, when properly assessed by prospective randomized comparison there was found to be absolutely no benefit.
So, given the considerable objective evidence from two major trials and revisiting the basics of the pathophysiology of atherosclerotic renovascular disease, to expect benefit from treating the osteal component of renal artery occlusive disease is at best naive, in my opinion. There remains little clinical evidence of benefit for any indication, with the possible exceptions of ACE-induced renal failure and possibly flash pulmonary edema in the presence of bilateral renal arterial stenoses.
Dr. Hamilton is a professor at the Royal Free London Hospital, University College London, United Kingdom.
There are still indications to treat renal artery occlusive disease
BY MATTHEW A. CORRIERE, M.D.
Although renal artery revascularization has been grossly overutilized and is not indicated in the majority of patients with renal artery stenosis, I perform renal artery revascularization as part of my routine clinical practice and believe that there are many instances where revascularization should be considered, particularly when patients have severe symptoms despite aggressive medical therapy. While neither ASTRAL nor CORAL observed any benefit associated with revascularization, both have important limitations that should be kept in mind when interpreting the results of these trials.
These limitations can be broadly categorized as mismatch between indications for revascularization and clinical endpoints, selection biases favoring enrollment of patients with relatively mild symptoms, and inconsistencies between study protocols and contemporary decision-making strategies.
Given that ASTRAL’s primary outcome was change in renal function (defined by a 20% or greater reduction in the mean slope of the reciprocal of serum creatinine), it is important to remember that the inclusion criteria were renal artery stenosis with unexplained renal dysfunction or poorly controlled hypertension. Patients who had hypertension in the absence of significant renal dysfunction were therefore eligible, and 40% of the randomized participants had preserved baseline renal function (based on a serum creatinine of < 150 micromol/liter). Unlike patients with baseline renal dysfunction (which, in theory, might improve with revascularization), these patients with normal renal function who were treated with revascularization risked decline in renal function resulting from procedure-related adverse events without any real chance of renal function improvement. It would certainly be difficult to justify revascularization for the sake of renal function salvage in these patients, and their inclusion within a randomized trial with change in renal function as its primary outcome is problematic for the same reason.
ASTRAL also had an additional, somewhat unorthodox inclusion criterion: uncertainty on the part of the treating physician that the patient “definitely would have a worthwhile clinical benefit from revascularization.” Exclusion of patients considered likely to benefit from revascularization would seem to ensure a selection bias favoring the null hypothesis; this approach may also explain the large proportion of participants with relatively mild occlusive disease (40% had stenotic lesions that were < 70% in severity).
A high rate of both technical failure (12%) and adverse events (20%) associated with revascularization, asymmetric crossover between treatment groups (86 of the 110 patients who did not receive their randomized intervention were in the revascularization group), and lack of standardized protocol for medical therapy further limit the conclusions that can be drawn from the ASTRAL results.
Although this trial does not provide us with compelling evidence that renal revascularization should be abandoned for patients failing appropriate medical therapy, ASTRAL demonstrated that no benefit should be expected from nonselective use of revascularization, which can be associated with significant rates of both technical failure and major adverse events.
The CORAL trial overcame many of the design limitations for which ASTRAL drew criticism. CORAL’s primary endpoint (freedom from major adverse cardiovascular or renal events) allowed potential benefit for participants with either systolic hypertension or chronic kidney disease as their indication for treatment. Although participants with systolic hypertension as their inclusion criterion had to be on at least two antihypertensive medications, it is important to acknowledge the growing number of indications for these medications related to cardiovascular risk reduction in the setting of diabetes, heart disease, and other diagnoses that may be unrelated to any specific blood pressure target. Number of antihypertensive medications is therefore often a crude and potentially invalid indicator of hypertension severity or control.
In CORAL, the initial hypertension inclusion criterion of 155 mm Hg was subsequently abandoned during the trial, suggesting that hypertension in many of these patients may have been mild and/or well controlled. Although medical therapy in CORAL was standardized, it also is notable that all patients had their medical therapy adjusted prior to randomization during a roll-in phase to achieve target blood pressure goals of 130/80 in patients with CKD and/or diabetes or 140/90 otherwise. I would suggest that achievement of these blood pressure targets on the study medications (candesartan ± hydrochlorothiazide plus amlodipine-atorvastatin) might be appropriately considered success of medical therapy for patients with hypertension in the absence of renal dysfunction, making it challenging to defend proceeding with revascularization in this scenario.
The study protocol, although well designed from the perspective of attempting to isolate the effect of renal artery angioplasty and stenting, therefore did not uniformly reflect what would be considered responsible utilization of renal revascularization in a real-world environment.
Patient enrollment in CORAL was also very selective; only 947 of the 5,322 patients who were screened went on to be enrolled and randomized. It is likely that at least some of those patients who were not enrolled (especially those who declined to participate or were withdrawn by their physicians) were failing aggressive medical therapy and therefore unwilling to being excluded from angioplasty and stenting through randomization. These limitations aside, however, CORAL does provide some very useful observations that should inform treatment decisions. The results demonstrate the efficacy of contemporary medical therapy for many patients, and show that revascularization offers no additional benefit when medical therapy achieves an acceptable clinical response (defined by stable renal function and reasonable blood pressure control). Additional subgroup analyses of the CORAL data are anticipated, but will likely be underpowered to draw conclusions in the absence of identified revascularization effects.
So when should revascularization be considered for patients with atherosclerotic renal artery stenosis? In general, medical therapy is adequate for most patients and should be implemented prior to any consideration of procedural intervention. Revascularization should be considered only for patients who have failed appropriate, aggressive medical therapy; the medications used in CORAL can certainly be regarded as adequate initial therapy for symptomatic renal artery stenosis, but many providers (including myself) would argue that additional agents should be considered before proceeding with revascularization.
When decline in renal function is the indication for considering revascularization, alternative causes (such as intrinsic renal disease) should diminish enthusiasm for proceeding with angioplasty and stenting, particularly when the anatomic disease distribution does not affect the entire renal mass (as in patients with two kidneys and unilateral stenosis). Appropriate candidates for revascularization include patients with severely impaired renal function (particularly in the setting of a precipitous functional decline) or severe acute blood pressure elevation associated with hypertensive emergency (such as acute congestive heart failure, encephalopathy, acute coronary syndrome, or other signs and symptoms of target organ damage resulting from hypertension and/or volume overload). Continuation of failed medical therapy is often unacceptable to these “no-options” patients as well as their providers, both of whom presumably would be unlikely to accept randomization to ongoing medical management.
Other populations that are not represented within these trials include patients with renal artery restenosis and those with nonatherosclerotic disease; it is therefore important to exercise caution when generalizing these study results to these distinct groups of patients. Enrolling patients with severe symptoms who have failed medical therapy will likely remain challenging for future randomized studies in the absence of alternative treatment options. Although the benefits of renal angioplasty and stenting for these “no-options” patients remain to be proved, the uncertainty of response to revascularization is often easier to accept than the ongoing morbidity and mortality associated with staying the course when medical therapy has failed.
Dr. Matthew A. Corriere is a vascular surgeon at Wake Forest University School of Medicine, Winston-Salem, N.C.
This article developed from a debate held at the 2014 Vascular Annual Meeting.
Poor response to statins predicts growth in plaque
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
For about one in five patients with known atherosclerotic coronary artery disease, standard-dose therapy with statins did not result in significant lowering of LDL cholesterol.
Furthermore, the results of this large pooled data sample showed that for statin hyporesponders, statin therapy did not prevent progression of intravascular plaque volume as measured by grayscale intravascular ultrasound.
Patients exhibit a wide range of response to standard statin dosing, and the effect of minimal LDL-C lowering on atherosclerotic disease progression had not previously been determined, according to Dr. Yu Kataoka of the University of Adelaide, Australia, and his colleagues (Arterioscler. Thromb. Vasc. Biol. 2015 [doi:10.1161/ATVBAHA.114.304477]).
Investigators pooled data from seven clinical trials that examined 647 total patients with angiographically confirmed CAD who were initiated on statins and followed by serial intravascular ultrasound. The present study analyzed baseline characteristics, serial lipid profile, and atheroma burden for the group.
In all, 130 patients of the 647 (20%) had minimal LDL-C lowering with statin therapy, showing nonsignificant lowering or even an increase in LDL-C levels during the study period. This group of hyporesponders differed in being slightly younger, more obese, less likely to have hypertension and dyslipidemia, and less likely to be receiving beta-blockers than were the statin responders. Other patient characteristics were similar between the two groups. A variety of agents were used, including atorvastatin, rosuvastatin, simvastatin, and pravastatin. Concurrent administration of other antiatherosclerotic agents was permitted and was similar between the groups. Atheroma burden at baseline was also similar between the two groups.
Measuring serial changes in atheroma burden showed a significant difference between statin responders and hyporesponders. The adjusted change in atheroma volume was –0.21% for the responders, compared with +0.83% for the hyporesponders (P = .006). Lumen volume decreased 11.64 mm3 for the responders, while the reduction was 16.54 mm3 for the hyporesponders (P = .006). Of those who responded to lipid therapy with LDL-C lowering, 29.8% had substantial atheroma regression, while 25.9% had substantial plaque progression; among hyporesponders, however, just 13.8% experienced significant plaque regression, while 37.7% had significant atheroma progression, both significant differences.
Dr. Kataoka and his colleagues emphasized that the factors contributing to poor statin response are not well understood. They noted that for this study, the pooled trials all showed adherence rates over 90%, eliminating patient compliance as a variable. Rigorous statistical techniques were used to control for comorbidities and coadministered medications. There are known genetic polymorphisms and phenotypic variations in statin metabolism, though these were not reported here. Although the results were not statistically significant, C-reactive protein levels were higher for the hyporesponse group, suggesting that another factor may be individual response to the anti-inflammatory effect that is among the known pleiotropic effects of this drug class.
In an interview, lead author Stephen Nicholls noted that many clinicians are still reluctant to treat to full effect. Citing the concept of “clinical inertia,” Dr. Nicholls pointed out that “Even when statins are prescribed, they are often at lower doses than ideal. That translated to more plaque growth, which leads directly to more heart attacks and more revascularization procedures.”
Study limitations included the potential residual confounding effects of pooling data from seven discrete clinical trials, though mixed modeling techniques attempted to correct for this effect. The present study also reported atheroma burden, but not actual clinical events. The study authors noted, however, that they had previously reported a direct relationship between atheroma progression and the occurrence of cardiovascular events.
Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Patients on statins who had minimal LDL-C lowering also showed increased atheroma progression.
Major finding: Of 647 patients with CAD, 20% were hyporesponders to statin therapy and experienced greater progression of atheroma volume than statin responders (adjusted +0.83% vs. –0.21%, P = .006).
Data source: Pooled data from seven clinical trials, yielding 647 patients with angiographically confirmed CAD who were initiated on standard lipid dosing and followed by baseline and serial grayscale intravascular ultrasounds.
Disclosures: Dr. Nicholls has received speaking honoraria and research support from many pharmaceutical companies, and from Infraredx. Dr. Steven E. Nissen of the Cleveland Clinic was a coinvestigator and has received research support from and is a consultant/adviser to numerous pharmaceutical companies; all honoraria or consulting fees go directly to charity so that he receives neither income nor a tax deduction. The other authors report no conflicts.