User login
HFpEF: Time for a new approach
SNOWMASS, COLO. – The plethora of comorbidities typically present in patients with heart failure with preserved ejection fraction is increasingly thought to be a key driver of the cardiac structural remodeling and poor clinical outcomes characteristic of this increasingly common condition.
“The new message is that even though HFpHF [heart failure with preserved ejection fraction] is a problem of the heart involving diastolic filling and structural remodeling of the ventricle, it’s also a problem of factors outside the heart. Outcomes are driven not just by the cardiac abnormalities, but by the comorbidities that are so common in this elderly population,” Dr. Akshay S. Desai said at the annual Cardiovascular Conference at Snowmass.
“The evolving theoretical model is one that emphasizes the role these comorbidities play, not just in remodeling of the heart, but also in microvascular inflammation, with its consequences for inflammatory cell migration, transforming growth factor–beta activation, myocardial fibrosis, oxidative stress, endothelial inflammation, and downstream impairment of cyclic guanosine monophosphate signaling,” explained Dr. Desai of Brigham and Women’s Hospital, Boston.
He credited Dr. Walter J. Paulus of the Institute for Cardiovascular Research at VU University Medical Center, Amsterdam, as being the primary developer of the new paradigm, which veers away from the traditional emphasis upon excessive afterload as the primary driver of diastolic dysfunction.
As elaborated in detail by Dr. Paulus, the noncardiac comorbidities that are so highly prevalent in HFpEF – especially obesity, diabetes, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, and anemia – induce a systemic inflammatory state which promotes diastolic left ventricular stiffness, cardiac hypertrophy, and the development of heart failure. Dr. Paulus has buttressed his theoretical framework with endomyocardial biopsy studies that document abnormal myocyte structure and function (J. Am. Coll. Cardiol. 2013;62:263-71).
Dr. Desai said the fresh perspective provided by Dr. Paulus is most welcome because it is easily tested, and also because it points to new pathways for treatment. New therapeutic targets are needed desperately because of the striking lack of progress to date in treatment of HFpEF. No drug has been convincingly shown effective in reducing the morbidity and mortality of HFpEF, although a secondary analysis of the flawed TOPCAT trial did strongly suggest spironolactone may reduce the risks of mortality and heart failure hospitalizations (Circulation 2015;131:34-42).
Clinical trials that are now planned or underway as a consequence of the new HFpEF paradigm are investigating novel treatment strategies targeting low myocardial nitric oxide bioavailability and endothelial dysfunction. Agents under study include statins, interleukin-1 receptor antagonists, oral nitrates aimed at boosting cellular levels of nitric oxide, the oral soluble guanylate cyclase stimulator riociguat (Adempas), and the phosphodiesterase-5 inhibitor sildenafil.
Also, all eyes are on the 4,300 patient, 37-country, phase III, randomized PARAGON HF study which began last summer. PARAGON is evaluating LCZ696, the combined angiotensin receptor neprilysin inhibitor that scored a smashing success in heart failure with reduced ejection fraction in the landmark PARADIGM-HF study (N. Engl. J. Med. 2014;371:993-1004).
The rising prevalence of diabetes, obesity, and other proinflammatory chronic conditions could help explain the increasing proportion of patients with heart failure who have HFpEF.
“Depending on where you draw the cut point for preserved ejection fraction, you could say half or as many as 60% of patients hospitalized for decompensated heart failure do so in the setting of preserved ejection fraction,” Dr. Desai observed.
It’s noteworthy that the trajectory of decline following hospitalization for heart failure is similar in HFpEF and heart failure with reduced ejection fraction.
While awaiting the outcome of clinical trials of novel treatments, and with so little evidence-based therapy available at this point, physicians should redouble their efforts to aggressively manage hypertension and other comorbidities in an effort to prevent HFpEF or slow its progression. The favorable TOPCAT results in the Western Hemisphere are also worthy of consideration, the cardiologist argued.
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – The plethora of comorbidities typically present in patients with heart failure with preserved ejection fraction is increasingly thought to be a key driver of the cardiac structural remodeling and poor clinical outcomes characteristic of this increasingly common condition.
“The new message is that even though HFpHF [heart failure with preserved ejection fraction] is a problem of the heart involving diastolic filling and structural remodeling of the ventricle, it’s also a problem of factors outside the heart. Outcomes are driven not just by the cardiac abnormalities, but by the comorbidities that are so common in this elderly population,” Dr. Akshay S. Desai said at the annual Cardiovascular Conference at Snowmass.
“The evolving theoretical model is one that emphasizes the role these comorbidities play, not just in remodeling of the heart, but also in microvascular inflammation, with its consequences for inflammatory cell migration, transforming growth factor–beta activation, myocardial fibrosis, oxidative stress, endothelial inflammation, and downstream impairment of cyclic guanosine monophosphate signaling,” explained Dr. Desai of Brigham and Women’s Hospital, Boston.
He credited Dr. Walter J. Paulus of the Institute for Cardiovascular Research at VU University Medical Center, Amsterdam, as being the primary developer of the new paradigm, which veers away from the traditional emphasis upon excessive afterload as the primary driver of diastolic dysfunction.
As elaborated in detail by Dr. Paulus, the noncardiac comorbidities that are so highly prevalent in HFpEF – especially obesity, diabetes, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, and anemia – induce a systemic inflammatory state which promotes diastolic left ventricular stiffness, cardiac hypertrophy, and the development of heart failure. Dr. Paulus has buttressed his theoretical framework with endomyocardial biopsy studies that document abnormal myocyte structure and function (J. Am. Coll. Cardiol. 2013;62:263-71).
Dr. Desai said the fresh perspective provided by Dr. Paulus is most welcome because it is easily tested, and also because it points to new pathways for treatment. New therapeutic targets are needed desperately because of the striking lack of progress to date in treatment of HFpEF. No drug has been convincingly shown effective in reducing the morbidity and mortality of HFpEF, although a secondary analysis of the flawed TOPCAT trial did strongly suggest spironolactone may reduce the risks of mortality and heart failure hospitalizations (Circulation 2015;131:34-42).
Clinical trials that are now planned or underway as a consequence of the new HFpEF paradigm are investigating novel treatment strategies targeting low myocardial nitric oxide bioavailability and endothelial dysfunction. Agents under study include statins, interleukin-1 receptor antagonists, oral nitrates aimed at boosting cellular levels of nitric oxide, the oral soluble guanylate cyclase stimulator riociguat (Adempas), and the phosphodiesterase-5 inhibitor sildenafil.
Also, all eyes are on the 4,300 patient, 37-country, phase III, randomized PARAGON HF study which began last summer. PARAGON is evaluating LCZ696, the combined angiotensin receptor neprilysin inhibitor that scored a smashing success in heart failure with reduced ejection fraction in the landmark PARADIGM-HF study (N. Engl. J. Med. 2014;371:993-1004).
The rising prevalence of diabetes, obesity, and other proinflammatory chronic conditions could help explain the increasing proportion of patients with heart failure who have HFpEF.
“Depending on where you draw the cut point for preserved ejection fraction, you could say half or as many as 60% of patients hospitalized for decompensated heart failure do so in the setting of preserved ejection fraction,” Dr. Desai observed.
It’s noteworthy that the trajectory of decline following hospitalization for heart failure is similar in HFpEF and heart failure with reduced ejection fraction.
While awaiting the outcome of clinical trials of novel treatments, and with so little evidence-based therapy available at this point, physicians should redouble their efforts to aggressively manage hypertension and other comorbidities in an effort to prevent HFpEF or slow its progression. The favorable TOPCAT results in the Western Hemisphere are also worthy of consideration, the cardiologist argued.
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – The plethora of comorbidities typically present in patients with heart failure with preserved ejection fraction is increasingly thought to be a key driver of the cardiac structural remodeling and poor clinical outcomes characteristic of this increasingly common condition.
“The new message is that even though HFpHF [heart failure with preserved ejection fraction] is a problem of the heart involving diastolic filling and structural remodeling of the ventricle, it’s also a problem of factors outside the heart. Outcomes are driven not just by the cardiac abnormalities, but by the comorbidities that are so common in this elderly population,” Dr. Akshay S. Desai said at the annual Cardiovascular Conference at Snowmass.
“The evolving theoretical model is one that emphasizes the role these comorbidities play, not just in remodeling of the heart, but also in microvascular inflammation, with its consequences for inflammatory cell migration, transforming growth factor–beta activation, myocardial fibrosis, oxidative stress, endothelial inflammation, and downstream impairment of cyclic guanosine monophosphate signaling,” explained Dr. Desai of Brigham and Women’s Hospital, Boston.
He credited Dr. Walter J. Paulus of the Institute for Cardiovascular Research at VU University Medical Center, Amsterdam, as being the primary developer of the new paradigm, which veers away from the traditional emphasis upon excessive afterload as the primary driver of diastolic dysfunction.
As elaborated in detail by Dr. Paulus, the noncardiac comorbidities that are so highly prevalent in HFpEF – especially obesity, diabetes, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, and anemia – induce a systemic inflammatory state which promotes diastolic left ventricular stiffness, cardiac hypertrophy, and the development of heart failure. Dr. Paulus has buttressed his theoretical framework with endomyocardial biopsy studies that document abnormal myocyte structure and function (J. Am. Coll. Cardiol. 2013;62:263-71).
Dr. Desai said the fresh perspective provided by Dr. Paulus is most welcome because it is easily tested, and also because it points to new pathways for treatment. New therapeutic targets are needed desperately because of the striking lack of progress to date in treatment of HFpEF. No drug has been convincingly shown effective in reducing the morbidity and mortality of HFpEF, although a secondary analysis of the flawed TOPCAT trial did strongly suggest spironolactone may reduce the risks of mortality and heart failure hospitalizations (Circulation 2015;131:34-42).
Clinical trials that are now planned or underway as a consequence of the new HFpEF paradigm are investigating novel treatment strategies targeting low myocardial nitric oxide bioavailability and endothelial dysfunction. Agents under study include statins, interleukin-1 receptor antagonists, oral nitrates aimed at boosting cellular levels of nitric oxide, the oral soluble guanylate cyclase stimulator riociguat (Adempas), and the phosphodiesterase-5 inhibitor sildenafil.
Also, all eyes are on the 4,300 patient, 37-country, phase III, randomized PARAGON HF study which began last summer. PARAGON is evaluating LCZ696, the combined angiotensin receptor neprilysin inhibitor that scored a smashing success in heart failure with reduced ejection fraction in the landmark PARADIGM-HF study (N. Engl. J. Med. 2014;371:993-1004).
The rising prevalence of diabetes, obesity, and other proinflammatory chronic conditions could help explain the increasing proportion of patients with heart failure who have HFpEF.
“Depending on where you draw the cut point for preserved ejection fraction, you could say half or as many as 60% of patients hospitalized for decompensated heart failure do so in the setting of preserved ejection fraction,” Dr. Desai observed.
It’s noteworthy that the trajectory of decline following hospitalization for heart failure is similar in HFpEF and heart failure with reduced ejection fraction.
While awaiting the outcome of clinical trials of novel treatments, and with so little evidence-based therapy available at this point, physicians should redouble their efforts to aggressively manage hypertension and other comorbidities in an effort to prevent HFpEF or slow its progression. The favorable TOPCAT results in the Western Hemisphere are also worthy of consideration, the cardiologist argued.
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
IV fluid use in heart failure patients raises concerns
Intravenous fluids are frequently administered during early hospitalization in patients with acute heart failure who are receiving loop diuretics, and the practice is associated with worse outcomes, according to findings from a retrospective cohort study.
Of 131,430 hospitalizations for heart failure at 346 hospitals from 2009 to 2010, 13,806 (11%) involved administration of at least 500 mL of IV fluids during the first 2 days in patients on diuretics. Normal saline was used most often (80% of cases), followed by half-normal saline (12%), and the median administered volume was 1,000 mL.
Those who received fluids were significantly more likely than were those who did not to have subsequent critical care admission (odds ratio, 1.57), intubation (OR, 1.46), renal replacement therapy (OR, 2.04), and hospital death (OR, 2.02), reported Dr. Behnood Bikdeli of Yale New Haven (Conn.) Hospital, and colleagues. The report was published Feb. 2 in the Journal of the American College of Cardiology.
Wide variation was seen in the proportion of hospitalizations that used fluid treatment (range of 0% to 71%), the researchers noted (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.09.007]).
The use of IV fluids in heart failure patients on diuretics may be inadvertent given that “guidelines generally suggest fluid restriction for patients with heart failure and do not generally recommend intravenous fluid therapy,” the investigators noted, adding that the findings highlight an opportunity for improvement.
The study was funded by two of the National Institutes of Health as well as grants from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Ct. Dr. Bikdeli reported having no disclosures.
The finding by Bikdeli et al. that fluid administration is common in patients admitted to the hospital with heart failure points to a path forward with respect to improving acute care, according to Dr. Larry A. Allen.
“Now that this relatively common practice is revealed, it behooves us to better understand exactly why it is happening; this understanding can then guide efforts to extinguish truly inappropriate care,” he wrote (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.11.001]).
Particular attention should be paid to what matters most with respect to heart failure: a thoughtful approach to the control of fluid status, he said, adding that “good care is grounded in physiology, guided by evidence, and tailored to the patient.
“In the end, we deliver an incredible number and diversity of therapies well in the modern U.S. health care system. The pressing question is whether the totality of therapies makes sense in each individual,” he concluded.
Dr. Allen is with the University of Colorado, Aurora, and the Colorado Cardiovascular Outcomes Research Consortium, Denver. He disclosed funding from the National Heart, Lung, and Blood Institute.
The finding by Bikdeli et al. that fluid administration is common in patients admitted to the hospital with heart failure points to a path forward with respect to improving acute care, according to Dr. Larry A. Allen.
“Now that this relatively common practice is revealed, it behooves us to better understand exactly why it is happening; this understanding can then guide efforts to extinguish truly inappropriate care,” he wrote (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.11.001]).
Particular attention should be paid to what matters most with respect to heart failure: a thoughtful approach to the control of fluid status, he said, adding that “good care is grounded in physiology, guided by evidence, and tailored to the patient.
“In the end, we deliver an incredible number and diversity of therapies well in the modern U.S. health care system. The pressing question is whether the totality of therapies makes sense in each individual,” he concluded.
Dr. Allen is with the University of Colorado, Aurora, and the Colorado Cardiovascular Outcomes Research Consortium, Denver. He disclosed funding from the National Heart, Lung, and Blood Institute.
The finding by Bikdeli et al. that fluid administration is common in patients admitted to the hospital with heart failure points to a path forward with respect to improving acute care, according to Dr. Larry A. Allen.
“Now that this relatively common practice is revealed, it behooves us to better understand exactly why it is happening; this understanding can then guide efforts to extinguish truly inappropriate care,” he wrote (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.11.001]).
Particular attention should be paid to what matters most with respect to heart failure: a thoughtful approach to the control of fluid status, he said, adding that “good care is grounded in physiology, guided by evidence, and tailored to the patient.
“In the end, we deliver an incredible number and diversity of therapies well in the modern U.S. health care system. The pressing question is whether the totality of therapies makes sense in each individual,” he concluded.
Dr. Allen is with the University of Colorado, Aurora, and the Colorado Cardiovascular Outcomes Research Consortium, Denver. He disclosed funding from the National Heart, Lung, and Blood Institute.
Intravenous fluids are frequently administered during early hospitalization in patients with acute heart failure who are receiving loop diuretics, and the practice is associated with worse outcomes, according to findings from a retrospective cohort study.
Of 131,430 hospitalizations for heart failure at 346 hospitals from 2009 to 2010, 13,806 (11%) involved administration of at least 500 mL of IV fluids during the first 2 days in patients on diuretics. Normal saline was used most often (80% of cases), followed by half-normal saline (12%), and the median administered volume was 1,000 mL.
Those who received fluids were significantly more likely than were those who did not to have subsequent critical care admission (odds ratio, 1.57), intubation (OR, 1.46), renal replacement therapy (OR, 2.04), and hospital death (OR, 2.02), reported Dr. Behnood Bikdeli of Yale New Haven (Conn.) Hospital, and colleagues. The report was published Feb. 2 in the Journal of the American College of Cardiology.
Wide variation was seen in the proportion of hospitalizations that used fluid treatment (range of 0% to 71%), the researchers noted (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.09.007]).
The use of IV fluids in heart failure patients on diuretics may be inadvertent given that “guidelines generally suggest fluid restriction for patients with heart failure and do not generally recommend intravenous fluid therapy,” the investigators noted, adding that the findings highlight an opportunity for improvement.
The study was funded by two of the National Institutes of Health as well as grants from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Ct. Dr. Bikdeli reported having no disclosures.
Intravenous fluids are frequently administered during early hospitalization in patients with acute heart failure who are receiving loop diuretics, and the practice is associated with worse outcomes, according to findings from a retrospective cohort study.
Of 131,430 hospitalizations for heart failure at 346 hospitals from 2009 to 2010, 13,806 (11%) involved administration of at least 500 mL of IV fluids during the first 2 days in patients on diuretics. Normal saline was used most often (80% of cases), followed by half-normal saline (12%), and the median administered volume was 1,000 mL.
Those who received fluids were significantly more likely than were those who did not to have subsequent critical care admission (odds ratio, 1.57), intubation (OR, 1.46), renal replacement therapy (OR, 2.04), and hospital death (OR, 2.02), reported Dr. Behnood Bikdeli of Yale New Haven (Conn.) Hospital, and colleagues. The report was published Feb. 2 in the Journal of the American College of Cardiology.
Wide variation was seen in the proportion of hospitalizations that used fluid treatment (range of 0% to 71%), the researchers noted (J. Am. Coll. Cardiol. HF 2015 Feb. 2 [doi:10.1016/j.jchf.2014.09.007]).
The use of IV fluids in heart failure patients on diuretics may be inadvertent given that “guidelines generally suggest fluid restriction for patients with heart failure and do not generally recommend intravenous fluid therapy,” the investigators noted, adding that the findings highlight an opportunity for improvement.
The study was funded by two of the National Institutes of Health as well as grants from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Ct. Dr. Bikdeli reported having no disclosures.
Key clinical point: Patients hospitalized for heart failure may receive IV fluids inappropriately.
Major finding: Eleven percent of heart failure patients on diuretics received IV fluids during the first 2 inpatient days.
Data source: A retrospective cohort study of 131,430 hospitalizations.
Disclosures: The study was funded by two of the National Institutes of Health as well as grants from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Conn. Dr. Bikdeli reported having no disclosures.
Renal denervation therapy: What’s next
SNOWMASS, COLO. – Reports of the demise of catheter-based renal denervation therapy for resistant hypertension in light of the disappointing SYMPLICITY HTN-3 trial results are greatly exaggerated, according to Dr. Bernard J. Gersh.
“I think what we can say about renal denervation is that the initial enthusiasm has been tempered and the number of unanswered questions is not decreasing. But the concept, I assure you, still maintains its promise,” the cardiologist said at the annual Cardiovascular Conference at Snowmass.
Expectations for SYMPLICITY HTN-3 were sky high in the cardiology and business worlds on the basis of the prior unblinded SYMPLICITY HTN-1 and -2 studies showing renal denervation (RDN) achieved spectacular reductions in systolic blood pressure of 25-30 mm Hg in patients with multidrug-resistant hypertension. As the chair of the SYMPLICITY HTN-3 data safety monitoring board, he knew the outcome prior to release of the results, and he was struck that during that period the talk at cardiology conferences was only of next-generation RDN catheters and expanded indications, such as heart failure and even metabolic syndrome. Absolutely no one seemed to entertain the possibility that the results wouldn’t be positive.
So the general reaction to the SYMPLICITY HTN-3 results was profound, crushing disappointment. The trial, which was the first blinded, controlled study of RDN, showed significant reduction in blood pressure at 6 months in the RDN recipients, but a similar reduction in medically managed controls who underwent a sham RDN procedure, making for a neutral study outcome (N. Engl. J. Med. 2014;370:1393-59).
Yet the physiologic basis remains strong for RDN as a treatment for hypertension, heart failure, and perhaps other cardiovascular disorders in which sympathetic nervous system activation plays a crucial role, according to Dr. Gersh, who chaired the data safety monitoring board for SYMPLICITY HTN-3 and is a professor of medicine at the Mayo Clinic in Rochester, Minn.
He cited a recommended review written by a pioneer in the field, Dr. Murray Esler of Australia, who observed that increased renal sympathetic nervous system activity increases the secretion rate of renin, reduces renal blood flow, and boosts renal tubular sodium reabsorption while reducing urinary sodium excretion, all actions that contribute to hypertension (Exp. Physiol. 2011;96:611-22).
Dr. Gersh ticked off many potential explanations for the neutral outcome in SYMPLICITY HTN-3, including the placebo effect; regression to the mean; operator inexperience; limitations of the current technology; and the likelihood that a fair number of study participants didn’t have true treatment-resistant hypertension, but were merely resistant to taking their medication until they entered a structured, supervised randomized trial setting. The power of a placebo procedure is not to be underestimated, the cardiologist emphasized. He cited a striking illustration from another field of medicine, in which a recent meta-analysis of 137 controlled studies in more than 33,000 patients with knee osteoarthritis showed that intra-articular placebo injections outperformed oral naproxen, celecoxib, and placebo in terms of pain relief (Ann. Intern. Med. 2015;162:46-54).
Regression to the mean definitely occurred in SYMPLICITY HTN-3, as evidenced by the fact that reductions in office systolic blood pressure (SBP) averaged 25.7 and 19.7 mm Hg, respectively, in RDN recipients and controls with a baseline greater than 184 mm Hg, compared with 13.8 and 9.8 mm Hg in those with a lower baseline SBP, of 170-184 mm Hg, Dr. Gersh continued.
Operator inexperience was likely a factor in the study outcomes: Nearly one-third of the operators dad done just one procedure. And while the study protocol called for four to six ablations per side, a recent secondary analysis of SYMPLICITY HTN-3 showed that the greater the number of ablations, the greater the drop in SBP. Patients who received at least seven ablations per side averaged a nearly threefold larger blood pressure reduction at 6 months, compared with those who got five or six per side (Eur. Heart J. 2015;36:219-27).
Some 39% of study participants had a medication change because of side effects during the 6-month trial. This confounds attempts to assess the true impact of RDN. To answer this question, the Food and Drug Administration recently granted Boston Scientific approval to test RDN in a sham-controlled trial in patients with mild to moderate hypertension after a 4-week medication washout period. The 6-month trial is due to start shortly. This is an important study because it will demonstrate whether RDN in its present form actually works.
“Clearly, what we’ve learned in SYMPLICITY HTN-3 is ‘resistant’ hypertension is amenable to expert pharmacologic control. This raises an interesting question: If renal nerve denervation works, could we offer it as an alternative to taking two or three drugs for the next 10 or 20 years? That might be, in certain patient populations, a very attractive option. That’s why we’ve got to show whether this procedure works well,” according to Dr. Gersh.
Now that SYMPLICITY HTN-3 has provided a reality check, the necessary next steps include improving the technology. Current levels of achieved RDN using only proximal ablation are suboptimal. What’s needed are higher-energy, multipolar electrodes that deliver energy both proximally and distally; such equipment is well along in development.
A thornier limitation involves the lack of a method for immediate testing of the completeness of an RDN procedure in a given patient. Unlike in coronary stent placement, where an interventional cardiologist can immediately see angiographically whether the device is properly seated, RDN operators have no way to tell intraprocedurally whether effective RDN has been achieved. Two methods now under investigation are intra-arterial adenosine and urinary biomarkers of nerve degradation.
The pathophysiology of hypertension is variable, so efforts are underway to identify patient subsets in which sympathetic nervous system overactivity is a primary underlying mechanism and RDN should have its greatest impact.
“I think renal denervation is still worthy of investigation, particularly in patients with resistant hypertension and perhaps other disease states characterized by sympathetic overactivity, such as heart failure,” Dr. Gersh concluded. “How do I think it’s going to all turn out? I just don’t know. We’ll see. We need the trials. I don’t think we should close the book on this very exciting technique. I simply don’t know what the trials are going to show.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for trials sponsored by Baxter, Medtronic, and Teva Pharmaceuticals.
SNOWMASS, COLO. – Reports of the demise of catheter-based renal denervation therapy for resistant hypertension in light of the disappointing SYMPLICITY HTN-3 trial results are greatly exaggerated, according to Dr. Bernard J. Gersh.
“I think what we can say about renal denervation is that the initial enthusiasm has been tempered and the number of unanswered questions is not decreasing. But the concept, I assure you, still maintains its promise,” the cardiologist said at the annual Cardiovascular Conference at Snowmass.
Expectations for SYMPLICITY HTN-3 were sky high in the cardiology and business worlds on the basis of the prior unblinded SYMPLICITY HTN-1 and -2 studies showing renal denervation (RDN) achieved spectacular reductions in systolic blood pressure of 25-30 mm Hg in patients with multidrug-resistant hypertension. As the chair of the SYMPLICITY HTN-3 data safety monitoring board, he knew the outcome prior to release of the results, and he was struck that during that period the talk at cardiology conferences was only of next-generation RDN catheters and expanded indications, such as heart failure and even metabolic syndrome. Absolutely no one seemed to entertain the possibility that the results wouldn’t be positive.
So the general reaction to the SYMPLICITY HTN-3 results was profound, crushing disappointment. The trial, which was the first blinded, controlled study of RDN, showed significant reduction in blood pressure at 6 months in the RDN recipients, but a similar reduction in medically managed controls who underwent a sham RDN procedure, making for a neutral study outcome (N. Engl. J. Med. 2014;370:1393-59).
Yet the physiologic basis remains strong for RDN as a treatment for hypertension, heart failure, and perhaps other cardiovascular disorders in which sympathetic nervous system activation plays a crucial role, according to Dr. Gersh, who chaired the data safety monitoring board for SYMPLICITY HTN-3 and is a professor of medicine at the Mayo Clinic in Rochester, Minn.
He cited a recommended review written by a pioneer in the field, Dr. Murray Esler of Australia, who observed that increased renal sympathetic nervous system activity increases the secretion rate of renin, reduces renal blood flow, and boosts renal tubular sodium reabsorption while reducing urinary sodium excretion, all actions that contribute to hypertension (Exp. Physiol. 2011;96:611-22).
Dr. Gersh ticked off many potential explanations for the neutral outcome in SYMPLICITY HTN-3, including the placebo effect; regression to the mean; operator inexperience; limitations of the current technology; and the likelihood that a fair number of study participants didn’t have true treatment-resistant hypertension, but were merely resistant to taking their medication until they entered a structured, supervised randomized trial setting. The power of a placebo procedure is not to be underestimated, the cardiologist emphasized. He cited a striking illustration from another field of medicine, in which a recent meta-analysis of 137 controlled studies in more than 33,000 patients with knee osteoarthritis showed that intra-articular placebo injections outperformed oral naproxen, celecoxib, and placebo in terms of pain relief (Ann. Intern. Med. 2015;162:46-54).
Regression to the mean definitely occurred in SYMPLICITY HTN-3, as evidenced by the fact that reductions in office systolic blood pressure (SBP) averaged 25.7 and 19.7 mm Hg, respectively, in RDN recipients and controls with a baseline greater than 184 mm Hg, compared with 13.8 and 9.8 mm Hg in those with a lower baseline SBP, of 170-184 mm Hg, Dr. Gersh continued.
Operator inexperience was likely a factor in the study outcomes: Nearly one-third of the operators dad done just one procedure. And while the study protocol called for four to six ablations per side, a recent secondary analysis of SYMPLICITY HTN-3 showed that the greater the number of ablations, the greater the drop in SBP. Patients who received at least seven ablations per side averaged a nearly threefold larger blood pressure reduction at 6 months, compared with those who got five or six per side (Eur. Heart J. 2015;36:219-27).
Some 39% of study participants had a medication change because of side effects during the 6-month trial. This confounds attempts to assess the true impact of RDN. To answer this question, the Food and Drug Administration recently granted Boston Scientific approval to test RDN in a sham-controlled trial in patients with mild to moderate hypertension after a 4-week medication washout period. The 6-month trial is due to start shortly. This is an important study because it will demonstrate whether RDN in its present form actually works.
“Clearly, what we’ve learned in SYMPLICITY HTN-3 is ‘resistant’ hypertension is amenable to expert pharmacologic control. This raises an interesting question: If renal nerve denervation works, could we offer it as an alternative to taking two or three drugs for the next 10 or 20 years? That might be, in certain patient populations, a very attractive option. That’s why we’ve got to show whether this procedure works well,” according to Dr. Gersh.
Now that SYMPLICITY HTN-3 has provided a reality check, the necessary next steps include improving the technology. Current levels of achieved RDN using only proximal ablation are suboptimal. What’s needed are higher-energy, multipolar electrodes that deliver energy both proximally and distally; such equipment is well along in development.
A thornier limitation involves the lack of a method for immediate testing of the completeness of an RDN procedure in a given patient. Unlike in coronary stent placement, where an interventional cardiologist can immediately see angiographically whether the device is properly seated, RDN operators have no way to tell intraprocedurally whether effective RDN has been achieved. Two methods now under investigation are intra-arterial adenosine and urinary biomarkers of nerve degradation.
The pathophysiology of hypertension is variable, so efforts are underway to identify patient subsets in which sympathetic nervous system overactivity is a primary underlying mechanism and RDN should have its greatest impact.
“I think renal denervation is still worthy of investigation, particularly in patients with resistant hypertension and perhaps other disease states characterized by sympathetic overactivity, such as heart failure,” Dr. Gersh concluded. “How do I think it’s going to all turn out? I just don’t know. We’ll see. We need the trials. I don’t think we should close the book on this very exciting technique. I simply don’t know what the trials are going to show.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for trials sponsored by Baxter, Medtronic, and Teva Pharmaceuticals.
SNOWMASS, COLO. – Reports of the demise of catheter-based renal denervation therapy for resistant hypertension in light of the disappointing SYMPLICITY HTN-3 trial results are greatly exaggerated, according to Dr. Bernard J. Gersh.
“I think what we can say about renal denervation is that the initial enthusiasm has been tempered and the number of unanswered questions is not decreasing. But the concept, I assure you, still maintains its promise,” the cardiologist said at the annual Cardiovascular Conference at Snowmass.
Expectations for SYMPLICITY HTN-3 were sky high in the cardiology and business worlds on the basis of the prior unblinded SYMPLICITY HTN-1 and -2 studies showing renal denervation (RDN) achieved spectacular reductions in systolic blood pressure of 25-30 mm Hg in patients with multidrug-resistant hypertension. As the chair of the SYMPLICITY HTN-3 data safety monitoring board, he knew the outcome prior to release of the results, and he was struck that during that period the talk at cardiology conferences was only of next-generation RDN catheters and expanded indications, such as heart failure and even metabolic syndrome. Absolutely no one seemed to entertain the possibility that the results wouldn’t be positive.
So the general reaction to the SYMPLICITY HTN-3 results was profound, crushing disappointment. The trial, which was the first blinded, controlled study of RDN, showed significant reduction in blood pressure at 6 months in the RDN recipients, but a similar reduction in medically managed controls who underwent a sham RDN procedure, making for a neutral study outcome (N. Engl. J. Med. 2014;370:1393-59).
Yet the physiologic basis remains strong for RDN as a treatment for hypertension, heart failure, and perhaps other cardiovascular disorders in which sympathetic nervous system activation plays a crucial role, according to Dr. Gersh, who chaired the data safety monitoring board for SYMPLICITY HTN-3 and is a professor of medicine at the Mayo Clinic in Rochester, Minn.
He cited a recommended review written by a pioneer in the field, Dr. Murray Esler of Australia, who observed that increased renal sympathetic nervous system activity increases the secretion rate of renin, reduces renal blood flow, and boosts renal tubular sodium reabsorption while reducing urinary sodium excretion, all actions that contribute to hypertension (Exp. Physiol. 2011;96:611-22).
Dr. Gersh ticked off many potential explanations for the neutral outcome in SYMPLICITY HTN-3, including the placebo effect; regression to the mean; operator inexperience; limitations of the current technology; and the likelihood that a fair number of study participants didn’t have true treatment-resistant hypertension, but were merely resistant to taking their medication until they entered a structured, supervised randomized trial setting. The power of a placebo procedure is not to be underestimated, the cardiologist emphasized. He cited a striking illustration from another field of medicine, in which a recent meta-analysis of 137 controlled studies in more than 33,000 patients with knee osteoarthritis showed that intra-articular placebo injections outperformed oral naproxen, celecoxib, and placebo in terms of pain relief (Ann. Intern. Med. 2015;162:46-54).
Regression to the mean definitely occurred in SYMPLICITY HTN-3, as evidenced by the fact that reductions in office systolic blood pressure (SBP) averaged 25.7 and 19.7 mm Hg, respectively, in RDN recipients and controls with a baseline greater than 184 mm Hg, compared with 13.8 and 9.8 mm Hg in those with a lower baseline SBP, of 170-184 mm Hg, Dr. Gersh continued.
Operator inexperience was likely a factor in the study outcomes: Nearly one-third of the operators dad done just one procedure. And while the study protocol called for four to six ablations per side, a recent secondary analysis of SYMPLICITY HTN-3 showed that the greater the number of ablations, the greater the drop in SBP. Patients who received at least seven ablations per side averaged a nearly threefold larger blood pressure reduction at 6 months, compared with those who got five or six per side (Eur. Heart J. 2015;36:219-27).
Some 39% of study participants had a medication change because of side effects during the 6-month trial. This confounds attempts to assess the true impact of RDN. To answer this question, the Food and Drug Administration recently granted Boston Scientific approval to test RDN in a sham-controlled trial in patients with mild to moderate hypertension after a 4-week medication washout period. The 6-month trial is due to start shortly. This is an important study because it will demonstrate whether RDN in its present form actually works.
“Clearly, what we’ve learned in SYMPLICITY HTN-3 is ‘resistant’ hypertension is amenable to expert pharmacologic control. This raises an interesting question: If renal nerve denervation works, could we offer it as an alternative to taking two or three drugs for the next 10 or 20 years? That might be, in certain patient populations, a very attractive option. That’s why we’ve got to show whether this procedure works well,” according to Dr. Gersh.
Now that SYMPLICITY HTN-3 has provided a reality check, the necessary next steps include improving the technology. Current levels of achieved RDN using only proximal ablation are suboptimal. What’s needed are higher-energy, multipolar electrodes that deliver energy both proximally and distally; such equipment is well along in development.
A thornier limitation involves the lack of a method for immediate testing of the completeness of an RDN procedure in a given patient. Unlike in coronary stent placement, where an interventional cardiologist can immediately see angiographically whether the device is properly seated, RDN operators have no way to tell intraprocedurally whether effective RDN has been achieved. Two methods now under investigation are intra-arterial adenosine and urinary biomarkers of nerve degradation.
The pathophysiology of hypertension is variable, so efforts are underway to identify patient subsets in which sympathetic nervous system overactivity is a primary underlying mechanism and RDN should have its greatest impact.
“I think renal denervation is still worthy of investigation, particularly in patients with resistant hypertension and perhaps other disease states characterized by sympathetic overactivity, such as heart failure,” Dr. Gersh concluded. “How do I think it’s going to all turn out? I just don’t know. We’ll see. We need the trials. I don’t think we should close the book on this very exciting technique. I simply don’t know what the trials are going to show.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Gersh reported serving as a consultant to Merck and Ortho-McNeil-Janssen and on data safety monitoring boards for trials sponsored by Baxter, Medtronic, and Teva Pharmaceuticals.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Moderate alcohol consumption can decrease heart failure risk
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
FROM THE EUROPEAN HEART JOURNAL
Key clinical point: Consumption of up to seven alcoholic beverages per week in early-middle age can lower risk for future heart failure.
Major finding: Among those who consumed up to seven drinks weekly, there were 281 heart failure incidents for men and 191 for women, with rates per 100 person-years of 0.77 and 0.53, respectively (P = .05).
Data source: The biracial, community-based ARIC study, comprising 14,629 adults.
Disclosures: ARIC is funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
First expression of cardiovascular disease differs by gender
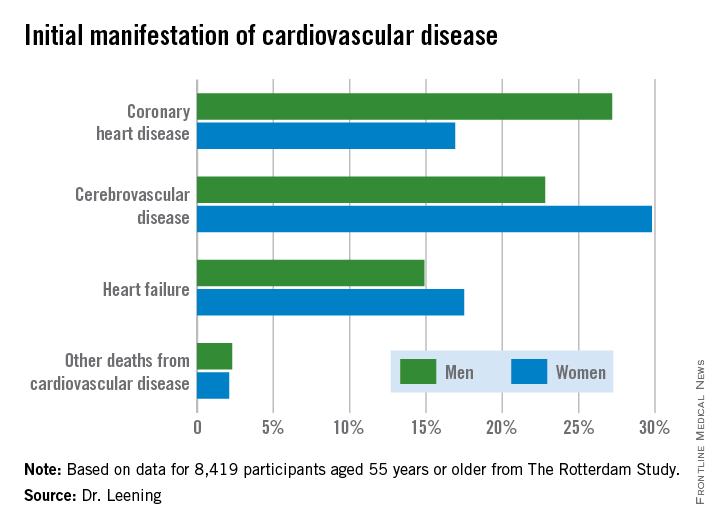
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.
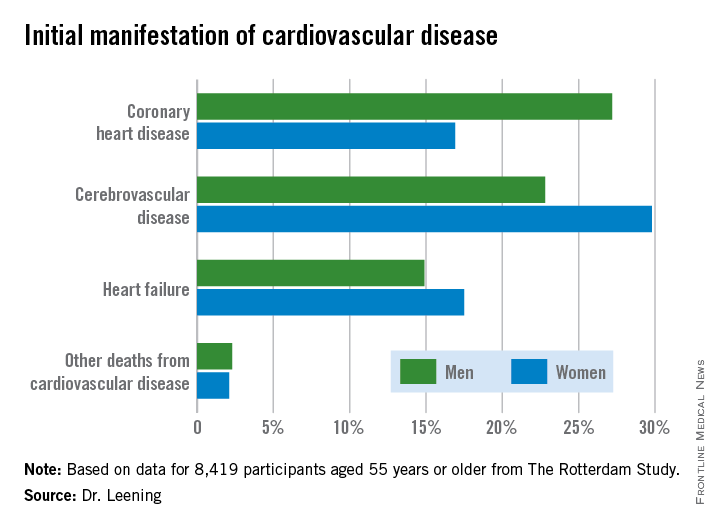
Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.

Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.

Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Women are more likely to have cerebrovascular disease or heart failure as their first cardiovascular disease event.
Major finding: Among 1,000 women free of cardiovascular disease at age 55, there were 102 fewer cases of coronary heart disease as the initial manifestation of cardiovascular disease during their remaining lifetime than in an equal number of 55-year-old men.
Data source: An analysis from the ongoing, prospective, population-based Rotterdam Study of 8,419 subjects.
Disclosures: The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. The presenter reported having no financial conflicts.
Emergency cardiac echocardiography accepted by Europeans
VIENNA – Rapid echocardiographic assessment has become routine for many patients who arrive at an emergency department with suspected acute heart failure, and experts consider these examinations critical for quickly getting patients on the right treatment.
Growing use and the important role for emergency echo exams prompted the European echocardiography community to issue in 2014 both recommendations and a position statement on the practice.
With their actions, European echocardiographers joined their U.S. colleagues who had earlier endorsed rapid, focused echocardiography exams. The European position also highlighted the limitations and pitfalls of emergency echo and the need for proper training.
Use of limited, directed, ultrasound heart examinations on an emergency basis by physicians who are not cardiologists is “an irreversible process, but without appropriate training it may become dangerous,” Dr. Nuno Cardim said at the annual meeting of the European Association of Cardiovascular Imaging (EACVI).
A focused cardiac ultrasound (FoCUS) examination for patients with an emergency cardiac condition such as acute heart failure is not a new concept. In 2010, the American Society of Echocardiography and the American College of Emergency Physicians jointly issued a consensus statement on emergency FoCUS (J. Am. Soc. Echocardiogr. 2010;23:1225-30), and the American Society of Echocardiography followed with additional recommendations in 2013 that also dealt with nonemergency uses for FoCUS (J. Am. Soc. Echocardiogr. 2013;26:567-81).
In its 2014 position statement released last May, the EACVI directly addressed FoCUS for the first time (Eur. Heart J. Cardiovasc. Imaging 2014:15;956-60). The statement acknowledged the important role for a circumscribed, point-of-care ultrasound exam in patients undergoing cardiopulmonary resuscitation and in other critical cardiac conditions, but highlighted that a FoCUS exam does not substitute for a comprehensive echocardiographic exam, and that FoCUS should only be done by properly trained clinicians who appreciate the limits of a FoCUS exam.
The EASVI recommendations, which came out a few months later in collaboration with the Acute Cardiovascular Care Association, said that “echocardiography is now recommended (where appropriately trained practitioners are available) in the management of cardiac arrest. However, FoCUS should always be used and interpreted thoughtfully, since this fundamentally limited approach may lead to missing/misinterpretation of important findings unless the practitioner is aware of its (and their) limitations” (Eur. Heart J. Cardiovasc. Imaging 2014 [doi:10.1093/ehjci/jeu210]).
“Of course all patients with suspected acute heart failure in the emergency department should undergo an echo exam. The question is, who will do it? These are patients who are the most difficult to assess,” said Dr. Susanna Price, a member of the EACVI recommendations panel and a specialist in critical care cardiology at Royal Brompton Hospital in London.
“Without proper training, the person doing FoCUS could make a false positive diagnosis, or might miss something and make a false negative diagnosis,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon, and another member of the EACVI panel.
To avoid this, emergency-medicine physicians and others who often triage patients with acute heart disorders should be trained in echocardiography and especially the FoCUS exam, which aims to quickly evaluate several important abnormalities of cardiac function: pericardial effusion, cardiac tamponade, left and right ventricular size and function, and intravascular volume status. A FoCUS exam also screens for pulmonary embolism. FoCUS assesses each of these in a yes-or-no or present-or-absent way, information critical for guiding emergency management but lacking the quantitative and detailed information available with a comprehensive echocardiography exam.
“FoCUS must never substitute” for the comprehensive exam, which should always also be done, he said. FoCUS “should be used wisely and cautiously because of its limitations.”
The FoCUS exam also has equipment specifications. Ideally, clinicians should use a portable, hand-held ultrasound machine, which is larger than “pocket-sized” ultrasound devices and hence gives much better image quality compared with pocket-sized devices, Dr. Cardim said in an interview.
Dr. Cardim and Dr. Price had no disclosures.
On Twitter @mitchelzoler
VIENNA – Rapid echocardiographic assessment has become routine for many patients who arrive at an emergency department with suspected acute heart failure, and experts consider these examinations critical for quickly getting patients on the right treatment.
Growing use and the important role for emergency echo exams prompted the European echocardiography community to issue in 2014 both recommendations and a position statement on the practice.
With their actions, European echocardiographers joined their U.S. colleagues who had earlier endorsed rapid, focused echocardiography exams. The European position also highlighted the limitations and pitfalls of emergency echo and the need for proper training.
Use of limited, directed, ultrasound heart examinations on an emergency basis by physicians who are not cardiologists is “an irreversible process, but without appropriate training it may become dangerous,” Dr. Nuno Cardim said at the annual meeting of the European Association of Cardiovascular Imaging (EACVI).
A focused cardiac ultrasound (FoCUS) examination for patients with an emergency cardiac condition such as acute heart failure is not a new concept. In 2010, the American Society of Echocardiography and the American College of Emergency Physicians jointly issued a consensus statement on emergency FoCUS (J. Am. Soc. Echocardiogr. 2010;23:1225-30), and the American Society of Echocardiography followed with additional recommendations in 2013 that also dealt with nonemergency uses for FoCUS (J. Am. Soc. Echocardiogr. 2013;26:567-81).
In its 2014 position statement released last May, the EACVI directly addressed FoCUS for the first time (Eur. Heart J. Cardiovasc. Imaging 2014:15;956-60). The statement acknowledged the important role for a circumscribed, point-of-care ultrasound exam in patients undergoing cardiopulmonary resuscitation and in other critical cardiac conditions, but highlighted that a FoCUS exam does not substitute for a comprehensive echocardiographic exam, and that FoCUS should only be done by properly trained clinicians who appreciate the limits of a FoCUS exam.
The EASVI recommendations, which came out a few months later in collaboration with the Acute Cardiovascular Care Association, said that “echocardiography is now recommended (where appropriately trained practitioners are available) in the management of cardiac arrest. However, FoCUS should always be used and interpreted thoughtfully, since this fundamentally limited approach may lead to missing/misinterpretation of important findings unless the practitioner is aware of its (and their) limitations” (Eur. Heart J. Cardiovasc. Imaging 2014 [doi:10.1093/ehjci/jeu210]).
“Of course all patients with suspected acute heart failure in the emergency department should undergo an echo exam. The question is, who will do it? These are patients who are the most difficult to assess,” said Dr. Susanna Price, a member of the EACVI recommendations panel and a specialist in critical care cardiology at Royal Brompton Hospital in London.
“Without proper training, the person doing FoCUS could make a false positive diagnosis, or might miss something and make a false negative diagnosis,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon, and another member of the EACVI panel.
To avoid this, emergency-medicine physicians and others who often triage patients with acute heart disorders should be trained in echocardiography and especially the FoCUS exam, which aims to quickly evaluate several important abnormalities of cardiac function: pericardial effusion, cardiac tamponade, left and right ventricular size and function, and intravascular volume status. A FoCUS exam also screens for pulmonary embolism. FoCUS assesses each of these in a yes-or-no or present-or-absent way, information critical for guiding emergency management but lacking the quantitative and detailed information available with a comprehensive echocardiography exam.
“FoCUS must never substitute” for the comprehensive exam, which should always also be done, he said. FoCUS “should be used wisely and cautiously because of its limitations.”
The FoCUS exam also has equipment specifications. Ideally, clinicians should use a portable, hand-held ultrasound machine, which is larger than “pocket-sized” ultrasound devices and hence gives much better image quality compared with pocket-sized devices, Dr. Cardim said in an interview.
Dr. Cardim and Dr. Price had no disclosures.
On Twitter @mitchelzoler
VIENNA – Rapid echocardiographic assessment has become routine for many patients who arrive at an emergency department with suspected acute heart failure, and experts consider these examinations critical for quickly getting patients on the right treatment.
Growing use and the important role for emergency echo exams prompted the European echocardiography community to issue in 2014 both recommendations and a position statement on the practice.
With their actions, European echocardiographers joined their U.S. colleagues who had earlier endorsed rapid, focused echocardiography exams. The European position also highlighted the limitations and pitfalls of emergency echo and the need for proper training.
Use of limited, directed, ultrasound heart examinations on an emergency basis by physicians who are not cardiologists is “an irreversible process, but without appropriate training it may become dangerous,” Dr. Nuno Cardim said at the annual meeting of the European Association of Cardiovascular Imaging (EACVI).
A focused cardiac ultrasound (FoCUS) examination for patients with an emergency cardiac condition such as acute heart failure is not a new concept. In 2010, the American Society of Echocardiography and the American College of Emergency Physicians jointly issued a consensus statement on emergency FoCUS (J. Am. Soc. Echocardiogr. 2010;23:1225-30), and the American Society of Echocardiography followed with additional recommendations in 2013 that also dealt with nonemergency uses for FoCUS (J. Am. Soc. Echocardiogr. 2013;26:567-81).
In its 2014 position statement released last May, the EACVI directly addressed FoCUS for the first time (Eur. Heart J. Cardiovasc. Imaging 2014:15;956-60). The statement acknowledged the important role for a circumscribed, point-of-care ultrasound exam in patients undergoing cardiopulmonary resuscitation and in other critical cardiac conditions, but highlighted that a FoCUS exam does not substitute for a comprehensive echocardiographic exam, and that FoCUS should only be done by properly trained clinicians who appreciate the limits of a FoCUS exam.
The EASVI recommendations, which came out a few months later in collaboration with the Acute Cardiovascular Care Association, said that “echocardiography is now recommended (where appropriately trained practitioners are available) in the management of cardiac arrest. However, FoCUS should always be used and interpreted thoughtfully, since this fundamentally limited approach may lead to missing/misinterpretation of important findings unless the practitioner is aware of its (and their) limitations” (Eur. Heart J. Cardiovasc. Imaging 2014 [doi:10.1093/ehjci/jeu210]).
“Of course all patients with suspected acute heart failure in the emergency department should undergo an echo exam. The question is, who will do it? These are patients who are the most difficult to assess,” said Dr. Susanna Price, a member of the EACVI recommendations panel and a specialist in critical care cardiology at Royal Brompton Hospital in London.
“Without proper training, the person doing FoCUS could make a false positive diagnosis, or might miss something and make a false negative diagnosis,” said Dr. Cardim, professor and director of echocardiography and cardiac imaging at Hospital da Luz in Lisbon, and another member of the EACVI panel.
To avoid this, emergency-medicine physicians and others who often triage patients with acute heart disorders should be trained in echocardiography and especially the FoCUS exam, which aims to quickly evaluate several important abnormalities of cardiac function: pericardial effusion, cardiac tamponade, left and right ventricular size and function, and intravascular volume status. A FoCUS exam also screens for pulmonary embolism. FoCUS assesses each of these in a yes-or-no or present-or-absent way, information critical for guiding emergency management but lacking the quantitative and detailed information available with a comprehensive echocardiography exam.
“FoCUS must never substitute” for the comprehensive exam, which should always also be done, he said. FoCUS “should be used wisely and cautiously because of its limitations.”
The FoCUS exam also has equipment specifications. Ideally, clinicians should use a portable, hand-held ultrasound machine, which is larger than “pocket-sized” ultrasound devices and hence gives much better image quality compared with pocket-sized devices, Dr. Cardim said in an interview.
Dr. Cardim and Dr. Price had no disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM EUROECHO-IMAGING 2014
HF: Glucose at ED presentation may predict mortality
Among adults who present to the emergency department with acute heart failure, blood glucose levels may predict 30-day mortality, regardless of whether or not the patients have preexisting diabetes.
At ED presentation, patients with acute heart failure syndrome “demonstrate a wide spectrum of physiological and metabolic perturbations.” Hyperglycemia occurs in up to 40% of them, irrespective of their diabetes status. “If blood glucose measurement is prognostically useful, it may be of broad potential utility because it is a rapid, readily available, and inexpensive test that can be used in the acute setting to allow rapid risk stratification for a wide range of potential outcomes,” according to Dr. Maneesh Sud of the University of Toronto and Toronto General Hospital and his associates.
To examine whether initial glucose level correlated with later outcomes, the investigators analyzed data from two large population-based cohorts of patients hospitalized for acute HF during a 3-year period. A total of 9,275 of the 16,524 patients (median age, 79 years) did not have preexisting diabetes (56%), and the remaining 44% did (Eur. Heart J. 2015 [doi:10.1093/eurheartj/ehu462]).
Among patients with diabetes, a blood glucose level exceeding 11.1 mmol/L was associated with significantly increased all-cause 30-day mortality, compared with normal glucose levels, with a hazard ratio (HR) of 1.48. Among patients without diabetes, a blood glucose level exceeding 6.1 mmol/L was associated with significantly increased all-cause 30-day mortality, with an HR of 1.26, and that risk rose with increasing glucose levels to 1.50 at the maximum level of 11.1 mmol/L.
The risk for cardiovascular death within 30 days increased as blood glucose levels rose for both groups of patients, as did the risk for cardiovascular hospitalization. In addition, both patients who had preexisting diabetes and those who did not were at significantly increased risk for diabetes-related hospitalization if their blood glucose level exceeded the normal range at presentation to the ED. And, in patients without diabetes who had elevated blood glucose levels, the risk of developing diabetes was significantly increased, in a dose-dependent fashion. These findings suggest that determining blood glucose levels at ED presentation “may serve as a screen to identify high-risk patients who warrant formal testing for diabetes, allowing for prompt referral to prevent further morbidity and mortality,” Dr. Sud and his associates said.
Among adults who present to the emergency department with acute heart failure, blood glucose levels may predict 30-day mortality, regardless of whether or not the patients have preexisting diabetes.
At ED presentation, patients with acute heart failure syndrome “demonstrate a wide spectrum of physiological and metabolic perturbations.” Hyperglycemia occurs in up to 40% of them, irrespective of their diabetes status. “If blood glucose measurement is prognostically useful, it may be of broad potential utility because it is a rapid, readily available, and inexpensive test that can be used in the acute setting to allow rapid risk stratification for a wide range of potential outcomes,” according to Dr. Maneesh Sud of the University of Toronto and Toronto General Hospital and his associates.
To examine whether initial glucose level correlated with later outcomes, the investigators analyzed data from two large population-based cohorts of patients hospitalized for acute HF during a 3-year period. A total of 9,275 of the 16,524 patients (median age, 79 years) did not have preexisting diabetes (56%), and the remaining 44% did (Eur. Heart J. 2015 [doi:10.1093/eurheartj/ehu462]).
Among patients with diabetes, a blood glucose level exceeding 11.1 mmol/L was associated with significantly increased all-cause 30-day mortality, compared with normal glucose levels, with a hazard ratio (HR) of 1.48. Among patients without diabetes, a blood glucose level exceeding 6.1 mmol/L was associated with significantly increased all-cause 30-day mortality, with an HR of 1.26, and that risk rose with increasing glucose levels to 1.50 at the maximum level of 11.1 mmol/L.
The risk for cardiovascular death within 30 days increased as blood glucose levels rose for both groups of patients, as did the risk for cardiovascular hospitalization. In addition, both patients who had preexisting diabetes and those who did not were at significantly increased risk for diabetes-related hospitalization if their blood glucose level exceeded the normal range at presentation to the ED. And, in patients without diabetes who had elevated blood glucose levels, the risk of developing diabetes was significantly increased, in a dose-dependent fashion. These findings suggest that determining blood glucose levels at ED presentation “may serve as a screen to identify high-risk patients who warrant formal testing for diabetes, allowing for prompt referral to prevent further morbidity and mortality,” Dr. Sud and his associates said.
Among adults who present to the emergency department with acute heart failure, blood glucose levels may predict 30-day mortality, regardless of whether or not the patients have preexisting diabetes.
At ED presentation, patients with acute heart failure syndrome “demonstrate a wide spectrum of physiological and metabolic perturbations.” Hyperglycemia occurs in up to 40% of them, irrespective of their diabetes status. “If blood glucose measurement is prognostically useful, it may be of broad potential utility because it is a rapid, readily available, and inexpensive test that can be used in the acute setting to allow rapid risk stratification for a wide range of potential outcomes,” according to Dr. Maneesh Sud of the University of Toronto and Toronto General Hospital and his associates.
To examine whether initial glucose level correlated with later outcomes, the investigators analyzed data from two large population-based cohorts of patients hospitalized for acute HF during a 3-year period. A total of 9,275 of the 16,524 patients (median age, 79 years) did not have preexisting diabetes (56%), and the remaining 44% did (Eur. Heart J. 2015 [doi:10.1093/eurheartj/ehu462]).
Among patients with diabetes, a blood glucose level exceeding 11.1 mmol/L was associated with significantly increased all-cause 30-day mortality, compared with normal glucose levels, with a hazard ratio (HR) of 1.48. Among patients without diabetes, a blood glucose level exceeding 6.1 mmol/L was associated with significantly increased all-cause 30-day mortality, with an HR of 1.26, and that risk rose with increasing glucose levels to 1.50 at the maximum level of 11.1 mmol/L.
The risk for cardiovascular death within 30 days increased as blood glucose levels rose for both groups of patients, as did the risk for cardiovascular hospitalization. In addition, both patients who had preexisting diabetes and those who did not were at significantly increased risk for diabetes-related hospitalization if their blood glucose level exceeded the normal range at presentation to the ED. And, in patients without diabetes who had elevated blood glucose levels, the risk of developing diabetes was significantly increased, in a dose-dependent fashion. These findings suggest that determining blood glucose levels at ED presentation “may serve as a screen to identify high-risk patients who warrant formal testing for diabetes, allowing for prompt referral to prevent further morbidity and mortality,” Dr. Sud and his associates said.
FROM EUROPEAN HEART JOURNAL
Key clinical point: Among patients who present to the ED with acute HF, blood glucose levels predict 30-day mortality and other outcomes.
Major finding: Patients with diabetes who had elevated blood glucose levels showed increased 30-day mortality with a hazard ratio of up to 1.48, while those without diabetes showed increased 30-day mortality with a hazard ratio of up to 1.50.
Data source: A secondary analysis of data from two population-based cohorts comprising 16,524 patients who presented to the ED with acute HF during a 3-year period.
Disclosures: This study was supported by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario. Dr. Sud and his associates reported having no relevant financial disclosures.
WOSCOPS 20-year follow-up shows impressive statin ‘legacy effect’
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
CHICAGO – Five years of statin therapy for primary prevention provided an impressive lifetime benefit expressed as reduced risks of a range of cardiovascular disease outcomes in a 20-year follow-up of the landmark WOSCOPS trial.
“There is a rather remarkable persistence of benefit in terms of risk reduction over a long period. You’ve changed the natural history of the disease in some way by lowering LDL,” Dr. Chris J. Packard said in presenting the 20-year WOSCOPS (West of Scotland Coronary Prevention Study) follow-up at the American Heart Association scientific sessions.
The primary prevention study randomized 6,595 middle-aged Scotsmen with an average baseline LDL cholesterol of 190 mg/dL to 4 years of pravastatin at 40 mg/day or placebo. This was one of the early large statin trials, and publication of the 5-year outcomes showing a 31% reduction in the relative risk of cardiovascular death or MI (N. Engl. J. Med. 1995;333:1301-8) caused a great stir.
At that point, WOSCOPS leaders advised study participants’ primary care physicians to seriously consider putting their patients on long-term statin therapy. However, only an identically low 31% of subjects in each of the two study arms did so.
Because the Scottish national health care system effectively captures all utilization of medical services, Dr. Packard and coinvestigators were able to analyze 20-year outcomes in the former study participants. No other major statin trial has come close in terms of length of reported follow-up.

At 20 years, with the only treatment difference between the two original study arms being that the pravastatin group had been on statin therapy for an additional 5 years, the 20-year coronary heart disease mortality rate in the original statin group was reduced by 27%, compared with the original controls. All-cause mortality was reduced by 13%. And numerous other benefits were noted at 20 years with 5 years of statin therapy.
Indeed, patients treated with pravastatin for 5 years during the trial gained an average of 5 extra years free of nonfatal MI or cardiovascular death at the 20-year mark, he said.
“The average age of the men was 55 years during the trial and 20 years on they’re now 75 years old. This covers the entire period of premature cardiovascular morbidity and mortality. We would argue that this is a good picture of the lifetime benefit, which is different from lifetime risk. This is real events happening to real people, not predictions,” observed Dr. Packard, professor of cardiovascular and medical sciences at the University of Glasgow, Scotland.
The group given pravastatin for 5 years collectively spent 24,038 days in the hospital for any cardiovascular event, compared with 30,342 days for controls.
Particularly noteworthy was the divergence in the risk of heart failure, with 96 cases being diagnosed during 20 years in patients who received 5 years of pravastatin, compared with 128 in controls.
“Heart failure was our biggest surprise,” he said. “We got no result for heart failure at all at 5 years; these were middle-aged men with just high cholesterol, so we didn’t see much in the way of incident heart failure. But extrapolating 20 years, there is a 31% risk reduction in the incidence of hospitalization for heart failure in the statin-treated group, compared to the placebo-treated group. This is a remarkable finding.”
For WOSCOPS participants who got a 20% drop in LDL during 5 years of pravastatin from a baseline of 190 mg/dL, as was typical, the number of patients who needed to be treated (NNT) for 5 years to prevent one cardiovascular hospital admission over a 20-year period was six. Moreover, for every 10 patients on statin therapy for 5 years, an average of 19 hospital days were avoided over the course of 20 years. For patients with a baseline LDL of 120 mg/dL, the NNT was 10, and 12 hospital days were saved over a 20-year period for every 10 patients treated for 5 years.
“This is a real savings to the health service and the health care providers to offset the cost of drugs,” Dr. Packard continued.
He urged physicians to look beyond the initial reports of primary outcomes of the statin clinical trials and take a long-term view.
“If you take a lifetime approach to benefit so you’re looking not only at the first event, but the second event, the third, at heart failure, and at death, you can see tremendous benefits, whereas usually we only focus on the first event. And that’s not the full cost evaluation that you need to do,” according to Dr. Packard.
The overall incidence of cancer during 20 years of follow-up was 24.8% in the initial placebo arm and 24.5% in the pravastatin group, with no differences between the two groups in any type of cancer. Nor did the original pravastatin group show any increase in noncardiovascular mortality.
“This is a very important study – the first study to show a legacy effect, with reduced mortality and a gain of 5 event-free years over 20 years attributable to a 5-year treatment allocation,” said discussant Harvey White of Auckland (New Zealand) City Hospital.
This legacy effect, he added, can be viewed as an ongoing carryover effect related to statin-induced slowing and/or stabilization of existing coronary artery plaque. The mechanism is unknown, Dr. White said, but the key to why the legacy effect was seen in WOSCOPS despite the use of pravastatin – a less potent statin – but not to date in other statin trials may lie in the fact that WOSCOPS was a primary prevention study and its participants had the youngest mean age of all the major statin trials.
“Their plaques may not have been calcified yet and therefore were more able to be modified and stabilized. If you treat very early you might get a bigger effect,” said to Dr. White.
Undercutting that argument, however, was the WOSCOPS finding that the long-term benefits of 5 years of pravastatin were independent of age at treatment, Dr. Packard said.
He believes based upon other studies that statins’ coronary disease prevention benefits are expressed within the first 12 months after starting therapy.
“It suggests that whatever is happening to the pathobiology of atherosclerosis happens within a year, and somehow a statis is introduced into plaque. That’s my guess, that an unstable plaque is reduced to a stable one. People then form a new trajectory going forward and they never catch up. We should think of atherosclerosis as a rate effect rather than something that either happens or doesn’t happen. It’s a rate of happening,” Dr. Packard asserted.
Asked why in the aftermath of the strongly positive 5-year results of WOSCOPS only 31% of patients in each treatment arm were on long-term statin therapy, he replied that 20 years ago in Scotland there really was no push for primary prevention.
“The 4S trial had come out the year before [Lancet 1994;344:1383-9] and placed the focus on secondary prevention. Statins were relatively expensive and everybody was putting their money into secondary prevention. Our health care system, which is socialized, had not put any emphasis at all on primary prevention. We were actually amazed that even 31% got treated,” the physician explained.
Dr. Packard reported serving as a consultant to Merck, Roche, and AstraZeneca.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Five years of statin therapy during midlife for primary prevention may reset the clock for atherosclerotic progression, providing persistently lower cardiovascular event rates 20 years later.
Major finding: The number of patients who needed to be treated with pravastatin for 5 years to prevent one cardiovascular hospitalization over a 20-year period was six.
Data source: A 20-year follow-up of the West of Scotland Coronary Prevention Study, in which 6,595 middle-aged men with high cholesterol were randomized to 5 years of pravastatin or placebo.
Disclosures: WOSCOPS was sponsored by Bristol-Myers Squibb. The presenter reported serving as a consultant to Merck, Roche, and AstraZeneca.
Elevated troponin present in 40% with T2D and stable heart disease
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Forty percent of patients with type 2 diabetes and stable ischemic heart disease are walking around with abnormal levels of hsTnT, placing them at substantial risk of a major cardiovascular event within 5 years.
Major finding: Prompt coronary revascularization does not reduce this risk, nor does it reduce the elevated troponin T level.
Data source: A secondary analysis of the randomized, prospective BARI 2D study involving 2,285 participants with type 2 diabetes and stable ischemic heart disease.
Disclosures: The BARI 2D study was funded by the National Institutes of Health. This new secondary analysis was funded by a research grant from Roche Diagnostics.
Insulin glargine shows cardiac safety in ORIGIN-ECHO
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The cardiac safety of insulin glargine in dysglycemic patients at high cardiovascular risk has received strong support from a 3-year echocardiographic study.
Major finding: Left ventricular mass over height was 116 g/m at baseline and 115 g/m after 3 years on insulin glargine.
Data source: The ORIGIN-ECHO substudy included 564 dysglycemic patients at high cardiovascular risk who were randomized to 3 years of insulin glargine or standard therapy.
Disclosures: The ORIGIN trial was sponsored by Sanofi. The presenter reported having no financial conflicts.