User login
VIDEO: Remote monitoring of cardiac devices cuts hospitalizations
BOSTON – Remote monitoring of cardiovascular implantable electronic devices dramatically improved patient outcomes and cut health care costs by keeping patients out of the hospital and reducing lengths of stays when hospitalization was needed, a retrospective analysis showed.
Remote monitoring “led to improved outcomes, more convenience for patients, and saved money, truly a win-win-win,” in the study of real-world data collected on more than 92,000 U.S. patients during 2008-2013, said Dr. Jonathan P. Piccini Sr. in an interview at the annual scientific sessions of the Heart Rhythm Society.
Using the MarketScan database of U.S. patients covered by commercial insurance or Medicare, Dr. Piccini and his associates analyzed hospitalization records for 92,566 patients who received an implanted pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization device during the study period. Roughly a third of the patients underwent remote monitoring along with their routine clinic visits while the rest were followed exclusively by clinic visits.
The data showed that remotely monitored patients had a statistically significant 18% lower rate of hospitalizations during follow-up and a 35% cut in their average length of stay when hospitalized. This resulted in a 30% drop in hospitalization costs, compared with costs for similar patients who did not undergo remote monitoring of their implanted devices. The cost savings remote monitoring produced meant that every 100,000 patient-years of remote monitoring saved about $370 million in hospital costs.
Coincident with the meeting, an expert panel of the Heart Rhythm Society released a statement on remote monitoring for cardiovascular implantable electronic devices (CIEDs) (Heart Rhythm 2015 [doi: 10.1016/j.hrthm.2015.05.008]. The statement said that “remote monitoring represents the new standard of care for patients with CIEDs.” But Dr. Piccini’s findings showed that U.S. clinicians vastly underused remote monitoring, with two-thirds of U.S. CIED recipients failing to undergo remote monitoring during 2008-2013. “Increased monitoring is a huge opportunity for health care improvement,” said Dr. Piccini, a cardiologist and an electrophysiologist at Duke University in Durham, N.C.
Dr. Piccini has been a consultant to Medtronic and has received research grants from Boston Scientific.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter @mitchelzoler
BOSTON – Remote monitoring of cardiovascular implantable electronic devices dramatically improved patient outcomes and cut health care costs by keeping patients out of the hospital and reducing lengths of stays when hospitalization was needed, a retrospective analysis showed.
Remote monitoring “led to improved outcomes, more convenience for patients, and saved money, truly a win-win-win,” in the study of real-world data collected on more than 92,000 U.S. patients during 2008-2013, said Dr. Jonathan P. Piccini Sr. in an interview at the annual scientific sessions of the Heart Rhythm Society.
Using the MarketScan database of U.S. patients covered by commercial insurance or Medicare, Dr. Piccini and his associates analyzed hospitalization records for 92,566 patients who received an implanted pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization device during the study period. Roughly a third of the patients underwent remote monitoring along with their routine clinic visits while the rest were followed exclusively by clinic visits.
The data showed that remotely monitored patients had a statistically significant 18% lower rate of hospitalizations during follow-up and a 35% cut in their average length of stay when hospitalized. This resulted in a 30% drop in hospitalization costs, compared with costs for similar patients who did not undergo remote monitoring of their implanted devices. The cost savings remote monitoring produced meant that every 100,000 patient-years of remote monitoring saved about $370 million in hospital costs.
Coincident with the meeting, an expert panel of the Heart Rhythm Society released a statement on remote monitoring for cardiovascular implantable electronic devices (CIEDs) (Heart Rhythm 2015 [doi: 10.1016/j.hrthm.2015.05.008]. The statement said that “remote monitoring represents the new standard of care for patients with CIEDs.” But Dr. Piccini’s findings showed that U.S. clinicians vastly underused remote monitoring, with two-thirds of U.S. CIED recipients failing to undergo remote monitoring during 2008-2013. “Increased monitoring is a huge opportunity for health care improvement,” said Dr. Piccini, a cardiologist and an electrophysiologist at Duke University in Durham, N.C.
Dr. Piccini has been a consultant to Medtronic and has received research grants from Boston Scientific.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter @mitchelzoler
BOSTON – Remote monitoring of cardiovascular implantable electronic devices dramatically improved patient outcomes and cut health care costs by keeping patients out of the hospital and reducing lengths of stays when hospitalization was needed, a retrospective analysis showed.
Remote monitoring “led to improved outcomes, more convenience for patients, and saved money, truly a win-win-win,” in the study of real-world data collected on more than 92,000 U.S. patients during 2008-2013, said Dr. Jonathan P. Piccini Sr. in an interview at the annual scientific sessions of the Heart Rhythm Society.
Using the MarketScan database of U.S. patients covered by commercial insurance or Medicare, Dr. Piccini and his associates analyzed hospitalization records for 92,566 patients who received an implanted pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization device during the study period. Roughly a third of the patients underwent remote monitoring along with their routine clinic visits while the rest were followed exclusively by clinic visits.
The data showed that remotely monitored patients had a statistically significant 18% lower rate of hospitalizations during follow-up and a 35% cut in their average length of stay when hospitalized. This resulted in a 30% drop in hospitalization costs, compared with costs for similar patients who did not undergo remote monitoring of their implanted devices. The cost savings remote monitoring produced meant that every 100,000 patient-years of remote monitoring saved about $370 million in hospital costs.
Coincident with the meeting, an expert panel of the Heart Rhythm Society released a statement on remote monitoring for cardiovascular implantable electronic devices (CIEDs) (Heart Rhythm 2015 [doi: 10.1016/j.hrthm.2015.05.008]. The statement said that “remote monitoring represents the new standard of care for patients with CIEDs.” But Dr. Piccini’s findings showed that U.S. clinicians vastly underused remote monitoring, with two-thirds of U.S. CIED recipients failing to undergo remote monitoring during 2008-2013. “Increased monitoring is a huge opportunity for health care improvement,” said Dr. Piccini, a cardiologist and an electrophysiologist at Duke University in Durham, N.C.
Dr. Piccini has been a consultant to Medtronic and has received research grants from Boston Scientific.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Twitter @mitchelzoler
AT HEART RHYTHM 2015
CVD risk persists for 40 years in Hodgkin’s survivors
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Hodgkin’s lymphoma survivors remain at high cardiovascular risk for at least 40 years, which is the longest they have been followed.
Major finding: At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of cardiovascular disease was 50%.
Data source: Retrospective cohort study involved 2,524 Dutch patients who were first treated for Hodgkin’s lymphoma in 1965-1995 and followed for cardiovascular events for up to 47 years.
Disclosures: This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
How to forestall heart failure by 15 years
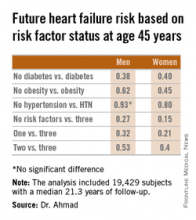
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
AT ACC 15
Key clinical point: Individuals who remain free of hypertension, obesity, and diabetes at age 45 years can expect to enjoy an extra 11-15 years of heart failure–free survival.
Major finding: The lifetime risk of developing heart failure in men without hypertension, obesity, and diabetes at age 45 years was reduced by 73%, compared with the risk in men having all three risk factors at that age. In women free of the three risk factors at age 45 years, the relative risk reduction was 85%.
Data source: This pooled analysis of data from four major studies included 19,429 subjects with 509,650 person-years of follow-up, during which 1,677 participants were newly diagnosed with heart failure.
Disclosures: This analysis was supported by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
2015 class of ‘blockbuster’ drugs has plenty of heart
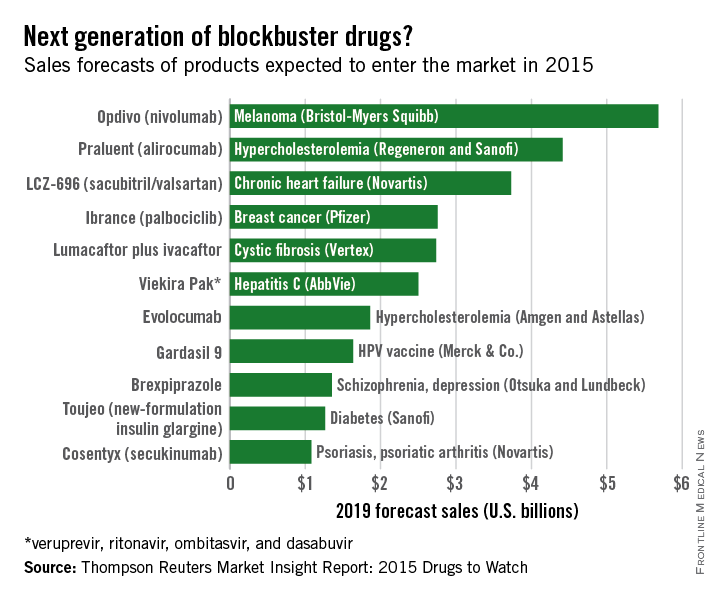
Of the new medications set to be released in 2015, three cardiovascular drugs could achieve “blockbuster” sales of over $1 billion by 2019, according to a report from Thomson Reuters.
Of the “Drugs to Watch in 2015,” those for the cardiovascular system are projected to reach nearly $10 billion by 2019.
Two PCSK9 inhibitors, alirocumab and evolocumab, are expected to gain Food and Drug Administration approval as early as this summer and become the next cholesterol-lowering blockbusters. Each has shown unprecedented LDL cholesterol lowering as well as reductions in adverse cardiovascular events, and the report projects sales of $4.4 billion and $1.9 billion, respectively. This $2.5-billion disparity between evolocumab and alirocumab (trade name Praluent) may be explained by alirocumab’s expected arrival on the market a month sooner.
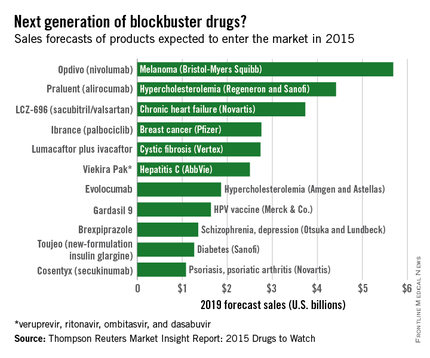
The novel heart failure drug LCZ-696 (sacubitril and valsartan) has projected sales of $3.7 billion through 2019, Thomson Reuters said. The angiotensin receptor neprilysin inhibitor reduced cardiovascular death and heart failure hospitalization by 20%, compared with enalapril, in heart failure patients in the large PARADIGM-HF trial.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. With projected sales of nearly $5.7 billion for 2019, the melanoma drug Opdivo (nivolumab) is at the head of a large 2015 blockbuster class. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of over $10 billion by 2017, far exceeding anything from 2015, the report said.
With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the top five with projected sales of $2.7 billion by 2019.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of the new medications set to be released in 2015, three cardiovascular drugs could achieve “blockbuster” sales of over $1 billion by 2019, according to a report from Thomson Reuters.
Of the “Drugs to Watch in 2015,” those for the cardiovascular system are projected to reach nearly $10 billion by 2019.
Two PCSK9 inhibitors, alirocumab and evolocumab, are expected to gain Food and Drug Administration approval as early as this summer and become the next cholesterol-lowering blockbusters. Each has shown unprecedented LDL cholesterol lowering as well as reductions in adverse cardiovascular events, and the report projects sales of $4.4 billion and $1.9 billion, respectively. This $2.5-billion disparity between evolocumab and alirocumab (trade name Praluent) may be explained by alirocumab’s expected arrival on the market a month sooner.

The novel heart failure drug LCZ-696 (sacubitril and valsartan) has projected sales of $3.7 billion through 2019, Thomson Reuters said. The angiotensin receptor neprilysin inhibitor reduced cardiovascular death and heart failure hospitalization by 20%, compared with enalapril, in heart failure patients in the large PARADIGM-HF trial.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. With projected sales of nearly $5.7 billion for 2019, the melanoma drug Opdivo (nivolumab) is at the head of a large 2015 blockbuster class. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of over $10 billion by 2017, far exceeding anything from 2015, the report said.
With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the top five with projected sales of $2.7 billion by 2019.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of the new medications set to be released in 2015, three cardiovascular drugs could achieve “blockbuster” sales of over $1 billion by 2019, according to a report from Thomson Reuters.
Of the “Drugs to Watch in 2015,” those for the cardiovascular system are projected to reach nearly $10 billion by 2019.
Two PCSK9 inhibitors, alirocumab and evolocumab, are expected to gain Food and Drug Administration approval as early as this summer and become the next cholesterol-lowering blockbusters. Each has shown unprecedented LDL cholesterol lowering as well as reductions in adverse cardiovascular events, and the report projects sales of $4.4 billion and $1.9 billion, respectively. This $2.5-billion disparity between evolocumab and alirocumab (trade name Praluent) may be explained by alirocumab’s expected arrival on the market a month sooner.

The novel heart failure drug LCZ-696 (sacubitril and valsartan) has projected sales of $3.7 billion through 2019, Thomson Reuters said. The angiotensin receptor neprilysin inhibitor reduced cardiovascular death and heart failure hospitalization by 20%, compared with enalapril, in heart failure patients in the large PARADIGM-HF trial.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. With projected sales of nearly $5.7 billion for 2019, the melanoma drug Opdivo (nivolumab) is at the head of a large 2015 blockbuster class. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of over $10 billion by 2017, far exceeding anything from 2015, the report said.
With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the top five with projected sales of $2.7 billion by 2019.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Guidelines for adults with congenital heart disease note changing landscape
New recommendations from the American Heart Association focus on the treatment of people older than age 40 years with congenital heart disease (CHD), a population that was believed to number about 850,000 in the year 2000 and is estimated to increase 5% each year.
“This improved longevity is leading to increased use of the medical system for both routine and episodic care, and caregivers need to be prepared to diagnose, follow-up, and treat the older adult with congenital heart disease,” authors led by Dr. Ami B. Bhatt wrote in a scientific statement published online April 20, 2015 in Circulation. “The predictable natural progression of CHD entities and sequelae of previous interventions must now be treated in the setting of late complications, acquired cardiac disease, multiorgan effects of lifelong processes, and the unrelenting process of aging. Despite the advances in this field, death rates in the population from 20 to [more than] 70 years of age may be twice to 7 times higher for the [adults] with CHD population than for their peers.”
Intended as a complement to the 2008 American College of Cardiology/AHA guidelines for ACHD (Circulation 2008;118:e714-833), the new recommendations cover the diagnosis and management of CHD in adults over the age of 40 years to summarize what is currently known “and to outline areas in which additional knowledge is critical to their care.” The scientific statement is limited to structural CHD, including coronary artery anomalies and aortopathy associated with bicuspid aortic valve disease (Circulation 2015 April 20; doi:10.1161/CIR.0000000000000204).
Working on behalf of the American Heart Association Council on Clinical Cardiology, Dr. Bhatt, who directs the adult congenital heart disease program at Massachusetts General Hospital, Boston, and her coauthors emphasized that the exposure to cardiovascular risk factors among ACHD patients is “no less problematic than with the non-CHD population. The ACHD individual may have abnormal myocardial substrate, abnormal cardiovascular physiology, abnormal anatomy, or any combination of the 3. The adverse impact of superimposed cardiovascular risk factors may well be amplified in this group, who also may already be at risk for systemic ventricular dysfunction, rhythm disturbances, and heart failure.”
In an interview, Dr. Bhatt noted that the ACHD population is distinct from both the pediatric and young adult populations with CHD and has many interactions with the health care system outside of adult congenital cardiac visits. “Therefore, this statement is written to serve as a reference for the many caregivers who will increasingly come across this population in their practice,” she said. “This includes general adult and pediatric cardiologists, electrophysiologists, interventionalists (percutaneous and surgical), cardiac imagers, as well as primary care physicians, hospitalists, and emergency medicine colleagues who need to understand and easily reference the issues and clinical challenges pertinent to this segment of the CHD population.”
The statement addresses diagnosis and management of late presentation of native disease, evolving long-term complications in disease diagnosed and/or intervened upon in childhood, and the additional burden of multiorgan dysfunction and acquired cardiovascular disease with age. Special attention is given to noncardiac involvement, including hepatic and renal disease screening and management, issues of aging including cognitive decline and sexual dysfunction, and challenging populations including those with coronary artery anomalies or superimposed pulmonary hypertension. The statement includes thorough discussions of diagnostic imaging, arrhythmia management, and surgical options in the older adult.
Among the issues addressed in the statement:
• Patient medical records, especially cardiac catheterization reports, should be obtained from primary sources. “This allow[s] comprehensive evaluation of these patients,” the authors wrote. They also emphasized the importance of multidisciplinary care when needed, in a medical center where other illnesses can be managed in a setting that also is knowledgeable about CHD.
• Psychosocial screening should be part of routine care of ACHD patients. This includes a team approach involving physicians, advanced practice nurses, physician assistants, psychologists, and social workers.
• Physical activity is encouraged. Sedentary lifestyle is a risk factor for many older adults with CHD. Cardiopulmonary exercise testing can be used in ACHD patients to help physicians create an individualized exercise plan. Research demonstrates that a structured regimen can improve exercise tolerance in this population.
• Sexual activity is reasonable for most ACHD patients. Exceptions include those who have decompensated or advanced heart failure, severe and/or significantly symptomatic valvular disease, or uncontrolled arrhythmias. Counseling must be provided by health care providers and “is useful to assist in resumption of sexual activity [especially] after an acute cardiac event, new cardiovascular disease diagnosis, or [implantable cardioverter defibrillator] implantation.”
• Many men with ACHD can take erectile dysfunction drugs as long as they are not taking nitrates and as long as their condition does not preclude sexual activity. However, “the effectiveness of phosphodiesterase type 5 inhibitors has not been established in the presence of severe ventricular outflow tract obstruction.”
• The use of hormone replacement therapy by women with ACHD must consider the risk for thromboembolic disease as well as the severity of menopausal symptoms. “For example, women with Fontan surgery have a high risk of venous thromboembolism and should avoid HRT, whereas women with [tetralogy of Fallot] repair and good RV function have a low risk and could probably receive HRT for symptoms,” the authors wrote.
The statement also includes recommendations for clinicians treating ACHD patients regarding screening for and management of concomitant lung, kidney, or liver disease. For example, it recommends serial evaluation of liver function for all patients with a history of previous palliation with the Fontan procedure and routine assessment of renal function for all adults with moderate-to-complex CHD.
The information provided in the AHA statement is based on scientific research and combined clinical experience from longitudinal care, Dr. Bhatt said in the interview. “The authors engaged in a truly multidisciplinary effort as pediatric and adult cardiologists, cardiac subspecialists, radiologists, and surgeons worked together to create a document to assist caregivers in meeting the needs of this challenging and growing population,” she said. “Importantly, by sharing the clinical trajectory of the older adult with CHD, the authors hope this statement and future versions will inform pediatric and young adult care and research as we strive to together improve lifelong care in congenital heart disease.”
Five of the coauthors disclosed relevant financial relationships. Dr. Michael C. Earing has received honoraria from Actelion Pharmaceuticals. Dr. Elyse Foster has received a research grant from Abbott Vascular and is a consultant or advisory board member for Gilead. Dr. Brian B. Ghoshhajra is a consultant or advisory board member for Siemens Healthcare. Dr. Seema Mital is a consultant or advisory board member for Novartis. Dr. Zian H. Tseng has received honoraria from Biotronik. The remaining authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
Diagnosis and treatment of congenital heart disease has improved dramatically over the last 5 decades, such that there is a growing population of adults with CHD. By some estimates, there are over a million adults with CHD and the population is growing by 5% per year. Despite the significant improvement in outcomes in children with CHD, there are significant sequelae of underlying CHD and necessary repairs that affect adults with CHD (ACHD). Common problems include arrhythmias, heart failure, sudden death, premature mortality, and complications related to other affected organs, such as hepatic or renal dysfunction. While understanding of these issues in ACHD patients as a whole is increasing steadily, most ACHD patients are young adults, thus the understanding of how CHD will impact older adults is less clear. Many of the issues related to CHD would be expected to progressively worsen through the lifespan, such that arrhythmias and heart failure may be even more prevalent in older adults, yet due to the underlying CHD may not respond to treatment in the ways expected in other adults with acquired cardiovascular disease. Additionally, the impact of comorbid diseases commonly encountered in older adults on underlying CHD will add a layer of complexity to both the CHD and the other comorbid diseases.
 |
| Dr. Karen Stout |
The scientific statement from Dr. Bhatt and her colleagues is a comprehensive, detailed discussion of the issues anticipated in older adults with CHD. The statement reviews the breadth of issues in older adults with CHD, beginning with CHD-related complications that occur regardless of the specific type of CHD and followed by a discussion on issues of specific types of CHD, such as transposition of the great arteries and shunt lesions. They discuss both unoperated and operated CHD in the older adult. An important part of the document is the sections reviewing the acquired cardiovascular risks and diseases in the ACHD patient and the noncardiac issues that are important in older adults with CHD.
Throughout the document, there is an overarching theme that ACHD cardiology expertise is needed in the care of these patients. There also is a call to arms that more data are needed to better care for these patients, and that we must develop registries and larger clinical trials to improve outcomes for these patients.
Dr. Karen K. Stout is a cardiologist and professor of medicine at the University of Washington, Seattle.
Diagnosis and treatment of congenital heart disease has improved dramatically over the last 5 decades, such that there is a growing population of adults with CHD. By some estimates, there are over a million adults with CHD and the population is growing by 5% per year. Despite the significant improvement in outcomes in children with CHD, there are significant sequelae of underlying CHD and necessary repairs that affect adults with CHD (ACHD). Common problems include arrhythmias, heart failure, sudden death, premature mortality, and complications related to other affected organs, such as hepatic or renal dysfunction. While understanding of these issues in ACHD patients as a whole is increasing steadily, most ACHD patients are young adults, thus the understanding of how CHD will impact older adults is less clear. Many of the issues related to CHD would be expected to progressively worsen through the lifespan, such that arrhythmias and heart failure may be even more prevalent in older adults, yet due to the underlying CHD may not respond to treatment in the ways expected in other adults with acquired cardiovascular disease. Additionally, the impact of comorbid diseases commonly encountered in older adults on underlying CHD will add a layer of complexity to both the CHD and the other comorbid diseases.
 |
| Dr. Karen Stout |
The scientific statement from Dr. Bhatt and her colleagues is a comprehensive, detailed discussion of the issues anticipated in older adults with CHD. The statement reviews the breadth of issues in older adults with CHD, beginning with CHD-related complications that occur regardless of the specific type of CHD and followed by a discussion on issues of specific types of CHD, such as transposition of the great arteries and shunt lesions. They discuss both unoperated and operated CHD in the older adult. An important part of the document is the sections reviewing the acquired cardiovascular risks and diseases in the ACHD patient and the noncardiac issues that are important in older adults with CHD.
Throughout the document, there is an overarching theme that ACHD cardiology expertise is needed in the care of these patients. There also is a call to arms that more data are needed to better care for these patients, and that we must develop registries and larger clinical trials to improve outcomes for these patients.
Dr. Karen K. Stout is a cardiologist and professor of medicine at the University of Washington, Seattle.
Diagnosis and treatment of congenital heart disease has improved dramatically over the last 5 decades, such that there is a growing population of adults with CHD. By some estimates, there are over a million adults with CHD and the population is growing by 5% per year. Despite the significant improvement in outcomes in children with CHD, there are significant sequelae of underlying CHD and necessary repairs that affect adults with CHD (ACHD). Common problems include arrhythmias, heart failure, sudden death, premature mortality, and complications related to other affected organs, such as hepatic or renal dysfunction. While understanding of these issues in ACHD patients as a whole is increasing steadily, most ACHD patients are young adults, thus the understanding of how CHD will impact older adults is less clear. Many of the issues related to CHD would be expected to progressively worsen through the lifespan, such that arrhythmias and heart failure may be even more prevalent in older adults, yet due to the underlying CHD may not respond to treatment in the ways expected in other adults with acquired cardiovascular disease. Additionally, the impact of comorbid diseases commonly encountered in older adults on underlying CHD will add a layer of complexity to both the CHD and the other comorbid diseases.
 |
| Dr. Karen Stout |
The scientific statement from Dr. Bhatt and her colleagues is a comprehensive, detailed discussion of the issues anticipated in older adults with CHD. The statement reviews the breadth of issues in older adults with CHD, beginning with CHD-related complications that occur regardless of the specific type of CHD and followed by a discussion on issues of specific types of CHD, such as transposition of the great arteries and shunt lesions. They discuss both unoperated and operated CHD in the older adult. An important part of the document is the sections reviewing the acquired cardiovascular risks and diseases in the ACHD patient and the noncardiac issues that are important in older adults with CHD.
Throughout the document, there is an overarching theme that ACHD cardiology expertise is needed in the care of these patients. There also is a call to arms that more data are needed to better care for these patients, and that we must develop registries and larger clinical trials to improve outcomes for these patients.
Dr. Karen K. Stout is a cardiologist and professor of medicine at the University of Washington, Seattle.
New recommendations from the American Heart Association focus on the treatment of people older than age 40 years with congenital heart disease (CHD), a population that was believed to number about 850,000 in the year 2000 and is estimated to increase 5% each year.
“This improved longevity is leading to increased use of the medical system for both routine and episodic care, and caregivers need to be prepared to diagnose, follow-up, and treat the older adult with congenital heart disease,” authors led by Dr. Ami B. Bhatt wrote in a scientific statement published online April 20, 2015 in Circulation. “The predictable natural progression of CHD entities and sequelae of previous interventions must now be treated in the setting of late complications, acquired cardiac disease, multiorgan effects of lifelong processes, and the unrelenting process of aging. Despite the advances in this field, death rates in the population from 20 to [more than] 70 years of age may be twice to 7 times higher for the [adults] with CHD population than for their peers.”
Intended as a complement to the 2008 American College of Cardiology/AHA guidelines for ACHD (Circulation 2008;118:e714-833), the new recommendations cover the diagnosis and management of CHD in adults over the age of 40 years to summarize what is currently known “and to outline areas in which additional knowledge is critical to their care.” The scientific statement is limited to structural CHD, including coronary artery anomalies and aortopathy associated with bicuspid aortic valve disease (Circulation 2015 April 20; doi:10.1161/CIR.0000000000000204).
Working on behalf of the American Heart Association Council on Clinical Cardiology, Dr. Bhatt, who directs the adult congenital heart disease program at Massachusetts General Hospital, Boston, and her coauthors emphasized that the exposure to cardiovascular risk factors among ACHD patients is “no less problematic than with the non-CHD population. The ACHD individual may have abnormal myocardial substrate, abnormal cardiovascular physiology, abnormal anatomy, or any combination of the 3. The adverse impact of superimposed cardiovascular risk factors may well be amplified in this group, who also may already be at risk for systemic ventricular dysfunction, rhythm disturbances, and heart failure.”
In an interview, Dr. Bhatt noted that the ACHD population is distinct from both the pediatric and young adult populations with CHD and has many interactions with the health care system outside of adult congenital cardiac visits. “Therefore, this statement is written to serve as a reference for the many caregivers who will increasingly come across this population in their practice,” she said. “This includes general adult and pediatric cardiologists, electrophysiologists, interventionalists (percutaneous and surgical), cardiac imagers, as well as primary care physicians, hospitalists, and emergency medicine colleagues who need to understand and easily reference the issues and clinical challenges pertinent to this segment of the CHD population.”
The statement addresses diagnosis and management of late presentation of native disease, evolving long-term complications in disease diagnosed and/or intervened upon in childhood, and the additional burden of multiorgan dysfunction and acquired cardiovascular disease with age. Special attention is given to noncardiac involvement, including hepatic and renal disease screening and management, issues of aging including cognitive decline and sexual dysfunction, and challenging populations including those with coronary artery anomalies or superimposed pulmonary hypertension. The statement includes thorough discussions of diagnostic imaging, arrhythmia management, and surgical options in the older adult.
Among the issues addressed in the statement:
• Patient medical records, especially cardiac catheterization reports, should be obtained from primary sources. “This allow[s] comprehensive evaluation of these patients,” the authors wrote. They also emphasized the importance of multidisciplinary care when needed, in a medical center where other illnesses can be managed in a setting that also is knowledgeable about CHD.
• Psychosocial screening should be part of routine care of ACHD patients. This includes a team approach involving physicians, advanced practice nurses, physician assistants, psychologists, and social workers.
• Physical activity is encouraged. Sedentary lifestyle is a risk factor for many older adults with CHD. Cardiopulmonary exercise testing can be used in ACHD patients to help physicians create an individualized exercise plan. Research demonstrates that a structured regimen can improve exercise tolerance in this population.
• Sexual activity is reasonable for most ACHD patients. Exceptions include those who have decompensated or advanced heart failure, severe and/or significantly symptomatic valvular disease, or uncontrolled arrhythmias. Counseling must be provided by health care providers and “is useful to assist in resumption of sexual activity [especially] after an acute cardiac event, new cardiovascular disease diagnosis, or [implantable cardioverter defibrillator] implantation.”
• Many men with ACHD can take erectile dysfunction drugs as long as they are not taking nitrates and as long as their condition does not preclude sexual activity. However, “the effectiveness of phosphodiesterase type 5 inhibitors has not been established in the presence of severe ventricular outflow tract obstruction.”
• The use of hormone replacement therapy by women with ACHD must consider the risk for thromboembolic disease as well as the severity of menopausal symptoms. “For example, women with Fontan surgery have a high risk of venous thromboembolism and should avoid HRT, whereas women with [tetralogy of Fallot] repair and good RV function have a low risk and could probably receive HRT for symptoms,” the authors wrote.
The statement also includes recommendations for clinicians treating ACHD patients regarding screening for and management of concomitant lung, kidney, or liver disease. For example, it recommends serial evaluation of liver function for all patients with a history of previous palliation with the Fontan procedure and routine assessment of renal function for all adults with moderate-to-complex CHD.
The information provided in the AHA statement is based on scientific research and combined clinical experience from longitudinal care, Dr. Bhatt said in the interview. “The authors engaged in a truly multidisciplinary effort as pediatric and adult cardiologists, cardiac subspecialists, radiologists, and surgeons worked together to create a document to assist caregivers in meeting the needs of this challenging and growing population,” she said. “Importantly, by sharing the clinical trajectory of the older adult with CHD, the authors hope this statement and future versions will inform pediatric and young adult care and research as we strive to together improve lifelong care in congenital heart disease.”
Five of the coauthors disclosed relevant financial relationships. Dr. Michael C. Earing has received honoraria from Actelion Pharmaceuticals. Dr. Elyse Foster has received a research grant from Abbott Vascular and is a consultant or advisory board member for Gilead. Dr. Brian B. Ghoshhajra is a consultant or advisory board member for Siemens Healthcare. Dr. Seema Mital is a consultant or advisory board member for Novartis. Dr. Zian H. Tseng has received honoraria from Biotronik. The remaining authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
New recommendations from the American Heart Association focus on the treatment of people older than age 40 years with congenital heart disease (CHD), a population that was believed to number about 850,000 in the year 2000 and is estimated to increase 5% each year.
“This improved longevity is leading to increased use of the medical system for both routine and episodic care, and caregivers need to be prepared to diagnose, follow-up, and treat the older adult with congenital heart disease,” authors led by Dr. Ami B. Bhatt wrote in a scientific statement published online April 20, 2015 in Circulation. “The predictable natural progression of CHD entities and sequelae of previous interventions must now be treated in the setting of late complications, acquired cardiac disease, multiorgan effects of lifelong processes, and the unrelenting process of aging. Despite the advances in this field, death rates in the population from 20 to [more than] 70 years of age may be twice to 7 times higher for the [adults] with CHD population than for their peers.”
Intended as a complement to the 2008 American College of Cardiology/AHA guidelines for ACHD (Circulation 2008;118:e714-833), the new recommendations cover the diagnosis and management of CHD in adults over the age of 40 years to summarize what is currently known “and to outline areas in which additional knowledge is critical to their care.” The scientific statement is limited to structural CHD, including coronary artery anomalies and aortopathy associated with bicuspid aortic valve disease (Circulation 2015 April 20; doi:10.1161/CIR.0000000000000204).
Working on behalf of the American Heart Association Council on Clinical Cardiology, Dr. Bhatt, who directs the adult congenital heart disease program at Massachusetts General Hospital, Boston, and her coauthors emphasized that the exposure to cardiovascular risk factors among ACHD patients is “no less problematic than with the non-CHD population. The ACHD individual may have abnormal myocardial substrate, abnormal cardiovascular physiology, abnormal anatomy, or any combination of the 3. The adverse impact of superimposed cardiovascular risk factors may well be amplified in this group, who also may already be at risk for systemic ventricular dysfunction, rhythm disturbances, and heart failure.”
In an interview, Dr. Bhatt noted that the ACHD population is distinct from both the pediatric and young adult populations with CHD and has many interactions with the health care system outside of adult congenital cardiac visits. “Therefore, this statement is written to serve as a reference for the many caregivers who will increasingly come across this population in their practice,” she said. “This includes general adult and pediatric cardiologists, electrophysiologists, interventionalists (percutaneous and surgical), cardiac imagers, as well as primary care physicians, hospitalists, and emergency medicine colleagues who need to understand and easily reference the issues and clinical challenges pertinent to this segment of the CHD population.”
The statement addresses diagnosis and management of late presentation of native disease, evolving long-term complications in disease diagnosed and/or intervened upon in childhood, and the additional burden of multiorgan dysfunction and acquired cardiovascular disease with age. Special attention is given to noncardiac involvement, including hepatic and renal disease screening and management, issues of aging including cognitive decline and sexual dysfunction, and challenging populations including those with coronary artery anomalies or superimposed pulmonary hypertension. The statement includes thorough discussions of diagnostic imaging, arrhythmia management, and surgical options in the older adult.
Among the issues addressed in the statement:
• Patient medical records, especially cardiac catheterization reports, should be obtained from primary sources. “This allow[s] comprehensive evaluation of these patients,” the authors wrote. They also emphasized the importance of multidisciplinary care when needed, in a medical center where other illnesses can be managed in a setting that also is knowledgeable about CHD.
• Psychosocial screening should be part of routine care of ACHD patients. This includes a team approach involving physicians, advanced practice nurses, physician assistants, psychologists, and social workers.
• Physical activity is encouraged. Sedentary lifestyle is a risk factor for many older adults with CHD. Cardiopulmonary exercise testing can be used in ACHD patients to help physicians create an individualized exercise plan. Research demonstrates that a structured regimen can improve exercise tolerance in this population.
• Sexual activity is reasonable for most ACHD patients. Exceptions include those who have decompensated or advanced heart failure, severe and/or significantly symptomatic valvular disease, or uncontrolled arrhythmias. Counseling must be provided by health care providers and “is useful to assist in resumption of sexual activity [especially] after an acute cardiac event, new cardiovascular disease diagnosis, or [implantable cardioverter defibrillator] implantation.”
• Many men with ACHD can take erectile dysfunction drugs as long as they are not taking nitrates and as long as their condition does not preclude sexual activity. However, “the effectiveness of phosphodiesterase type 5 inhibitors has not been established in the presence of severe ventricular outflow tract obstruction.”
• The use of hormone replacement therapy by women with ACHD must consider the risk for thromboembolic disease as well as the severity of menopausal symptoms. “For example, women with Fontan surgery have a high risk of venous thromboembolism and should avoid HRT, whereas women with [tetralogy of Fallot] repair and good RV function have a low risk and could probably receive HRT for symptoms,” the authors wrote.
The statement also includes recommendations for clinicians treating ACHD patients regarding screening for and management of concomitant lung, kidney, or liver disease. For example, it recommends serial evaluation of liver function for all patients with a history of previous palliation with the Fontan procedure and routine assessment of renal function for all adults with moderate-to-complex CHD.
The information provided in the AHA statement is based on scientific research and combined clinical experience from longitudinal care, Dr. Bhatt said in the interview. “The authors engaged in a truly multidisciplinary effort as pediatric and adult cardiologists, cardiac subspecialists, radiologists, and surgeons worked together to create a document to assist caregivers in meeting the needs of this challenging and growing population,” she said. “Importantly, by sharing the clinical trajectory of the older adult with CHD, the authors hope this statement and future versions will inform pediatric and young adult care and research as we strive to together improve lifelong care in congenital heart disease.”
Five of the coauthors disclosed relevant financial relationships. Dr. Michael C. Earing has received honoraria from Actelion Pharmaceuticals. Dr. Elyse Foster has received a research grant from Abbott Vascular and is a consultant or advisory board member for Gilead. Dr. Brian B. Ghoshhajra is a consultant or advisory board member for Siemens Healthcare. Dr. Seema Mital is a consultant or advisory board member for Novartis. Dr. Zian H. Tseng has received honoraria from Biotronik. The remaining authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
FROM CIRCULATION
Novel oral anticoagulants best warfarin for AF in heart failure
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
AT ACC 15
Key clinical point: Patients with nonvalvular atrial fibrillation and heart failure clearly fare better on any of the novel oral anticoagulants than with warfarin for stroke prophylaxis.
Major finding: Dual diagnosis patients randomized to a novel oral anticoagulant had a 14% reduction in stroke/systemic embolism and a 23% decrease in major bleeding compared with those on warfarin.
Data source: This was a meta-analysis of the 26,384 patients with both atrial fibrillation and heart failure who were included in four pivotal Phase 3 clinical trials that led to approval of dabigatran, apixaban, rivaroxaban, and edoxaban.
Disclosures: The presenter reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
Ivabradine approved to reduce heart failure hospitalizations
The heart rate–lowering agent ivabradine was approved by the Food and Drug Administration on April 15 to reduce hospitalizations in patients with worsening heart failure.
In an April 15 statement, the FDA announced that ivabradine, after undergoing a fast-track evaluation process, is indicated in patients with chronic, stable, symptomatic heart failure and left ventricular ejection fractions at or below 35%; resting heart rates of at least 70 beats per minute; and who are on maximum beta-blockers doses or have beta-blocker contraindications.
Ivabradine “is thought to work by decreasing heart rate and represents the first approved product in [its] drug class,” Dr. Norman Stockbridge, director of the FDA’s division of cardiovascular and renal products, said in a written statement.
The drug was given priority review based on the results of SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which involved 6,505 clinically stable patients, all hospitalized for heart failure in the preceding year and all on standard background therapy, including beta-blockers (89%), ACE inhibitors and/or angiotensin II receptor blockers (91%), diuretics (83%), and antialdosterone agents (60%) (Lancet 2010;376:875-85).
There was a 4.7% absolute risk reduction and a 26% relative risk reduction for hospitalizations as a result of deteriorating heart failure in the 3,241 ivabradine patients, but the drug did not reduce mortality, according to a statement from the drug’s manufacturer, Amgen.
The most common adverse events were bradycardia (10% vs. 2.2% with placebo), hypertension or increased blood pressure (8.9% vs. 7.8% with placebo), atrial fibrillation (8.3% vs. 6.6%), and luminous phenomena or visual brightness (2.8% vs. 0.5%).
Ivabradine is a specific inhibitor of the If (“funny”) current in the sinoatrial node, but not other currents. The drug is contraindicated in patients with acute decompensated heart failure, blood pressure below 90/50 mm Hg, sick sinus syndrome, sinoatrial block, third-degree AV block (unless a functioning demand pacemaker is present), resting heart rate below 60 bpm prior to treatment, severe hepatic impairment, pacemaker dependence, and use of strong cytochrome P450 3A4 inhibitors. Ivabradine increases the risk of atrial fibrillation and can cause fetal toxicity. Bradycardia, sinus arrest, and heart block have been reported with its use, Amgen said in its announcement.
Concurrent use of the calcium channel blockers verapamil or diltiazem increases exposure to the drug and should be avoided. Ivabradine also should be avoided in patients with second-degree AV block unless a functioning demand pacemaker is present.
Ivabradine will be available in 5-mg and 7.5-mg tablets, according to the product’s label. The recommended starting dose is a 5-mg tablet twice daily with meals. After 2 weeks of treatment, the dose should be adjusted depending on heart rate. In patients with a history of conduction defects or others in whom bradycardia could lead to hemodynamic compromise, Amgen said to initiate therapy at 2.5 mg twice daily.
Patients should alert their health care professional if they develop an irregular heartbeat, a pounding or racing heart, chest pressure, worse shortness of breath, dizziness, weakness, or fatigue, Amgen said.
Ivabradine will be available within about a week of the approval under the trade name Corlanor, and will come with a patient medication guide. Wholesale acquisition cost will be $4,500 per year, or $375 per month, and patient costs will vary according to insurance coverage, said Amgen spokesman Cuyler Mayer.
Ivabradine has been available in Europe as Procoralan for several years.
The heart rate–lowering agent ivabradine was approved by the Food and Drug Administration on April 15 to reduce hospitalizations in patients with worsening heart failure.
In an April 15 statement, the FDA announced that ivabradine, after undergoing a fast-track evaluation process, is indicated in patients with chronic, stable, symptomatic heart failure and left ventricular ejection fractions at or below 35%; resting heart rates of at least 70 beats per minute; and who are on maximum beta-blockers doses or have beta-blocker contraindications.
Ivabradine “is thought to work by decreasing heart rate and represents the first approved product in [its] drug class,” Dr. Norman Stockbridge, director of the FDA’s division of cardiovascular and renal products, said in a written statement.
The drug was given priority review based on the results of SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which involved 6,505 clinically stable patients, all hospitalized for heart failure in the preceding year and all on standard background therapy, including beta-blockers (89%), ACE inhibitors and/or angiotensin II receptor blockers (91%), diuretics (83%), and antialdosterone agents (60%) (Lancet 2010;376:875-85).
There was a 4.7% absolute risk reduction and a 26% relative risk reduction for hospitalizations as a result of deteriorating heart failure in the 3,241 ivabradine patients, but the drug did not reduce mortality, according to a statement from the drug’s manufacturer, Amgen.
The most common adverse events were bradycardia (10% vs. 2.2% with placebo), hypertension or increased blood pressure (8.9% vs. 7.8% with placebo), atrial fibrillation (8.3% vs. 6.6%), and luminous phenomena or visual brightness (2.8% vs. 0.5%).
Ivabradine is a specific inhibitor of the If (“funny”) current in the sinoatrial node, but not other currents. The drug is contraindicated in patients with acute decompensated heart failure, blood pressure below 90/50 mm Hg, sick sinus syndrome, sinoatrial block, third-degree AV block (unless a functioning demand pacemaker is present), resting heart rate below 60 bpm prior to treatment, severe hepatic impairment, pacemaker dependence, and use of strong cytochrome P450 3A4 inhibitors. Ivabradine increases the risk of atrial fibrillation and can cause fetal toxicity. Bradycardia, sinus arrest, and heart block have been reported with its use, Amgen said in its announcement.
Concurrent use of the calcium channel blockers verapamil or diltiazem increases exposure to the drug and should be avoided. Ivabradine also should be avoided in patients with second-degree AV block unless a functioning demand pacemaker is present.
Ivabradine will be available in 5-mg and 7.5-mg tablets, according to the product’s label. The recommended starting dose is a 5-mg tablet twice daily with meals. After 2 weeks of treatment, the dose should be adjusted depending on heart rate. In patients with a history of conduction defects or others in whom bradycardia could lead to hemodynamic compromise, Amgen said to initiate therapy at 2.5 mg twice daily.
Patients should alert their health care professional if they develop an irregular heartbeat, a pounding or racing heart, chest pressure, worse shortness of breath, dizziness, weakness, or fatigue, Amgen said.
Ivabradine will be available within about a week of the approval under the trade name Corlanor, and will come with a patient medication guide. Wholesale acquisition cost will be $4,500 per year, or $375 per month, and patient costs will vary according to insurance coverage, said Amgen spokesman Cuyler Mayer.
Ivabradine has been available in Europe as Procoralan for several years.
The heart rate–lowering agent ivabradine was approved by the Food and Drug Administration on April 15 to reduce hospitalizations in patients with worsening heart failure.
In an April 15 statement, the FDA announced that ivabradine, after undergoing a fast-track evaluation process, is indicated in patients with chronic, stable, symptomatic heart failure and left ventricular ejection fractions at or below 35%; resting heart rates of at least 70 beats per minute; and who are on maximum beta-blockers doses or have beta-blocker contraindications.
Ivabradine “is thought to work by decreasing heart rate and represents the first approved product in [its] drug class,” Dr. Norman Stockbridge, director of the FDA’s division of cardiovascular and renal products, said in a written statement.
The drug was given priority review based on the results of SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which involved 6,505 clinically stable patients, all hospitalized for heart failure in the preceding year and all on standard background therapy, including beta-blockers (89%), ACE inhibitors and/or angiotensin II receptor blockers (91%), diuretics (83%), and antialdosterone agents (60%) (Lancet 2010;376:875-85).
There was a 4.7% absolute risk reduction and a 26% relative risk reduction for hospitalizations as a result of deteriorating heart failure in the 3,241 ivabradine patients, but the drug did not reduce mortality, according to a statement from the drug’s manufacturer, Amgen.
The most common adverse events were bradycardia (10% vs. 2.2% with placebo), hypertension or increased blood pressure (8.9% vs. 7.8% with placebo), atrial fibrillation (8.3% vs. 6.6%), and luminous phenomena or visual brightness (2.8% vs. 0.5%).
Ivabradine is a specific inhibitor of the If (“funny”) current in the sinoatrial node, but not other currents. The drug is contraindicated in patients with acute decompensated heart failure, blood pressure below 90/50 mm Hg, sick sinus syndrome, sinoatrial block, third-degree AV block (unless a functioning demand pacemaker is present), resting heart rate below 60 bpm prior to treatment, severe hepatic impairment, pacemaker dependence, and use of strong cytochrome P450 3A4 inhibitors. Ivabradine increases the risk of atrial fibrillation and can cause fetal toxicity. Bradycardia, sinus arrest, and heart block have been reported with its use, Amgen said in its announcement.
Concurrent use of the calcium channel blockers verapamil or diltiazem increases exposure to the drug and should be avoided. Ivabradine also should be avoided in patients with second-degree AV block unless a functioning demand pacemaker is present.
Ivabradine will be available in 5-mg and 7.5-mg tablets, according to the product’s label. The recommended starting dose is a 5-mg tablet twice daily with meals. After 2 weeks of treatment, the dose should be adjusted depending on heart rate. In patients with a history of conduction defects or others in whom bradycardia could lead to hemodynamic compromise, Amgen said to initiate therapy at 2.5 mg twice daily.
Patients should alert their health care professional if they develop an irregular heartbeat, a pounding or racing heart, chest pressure, worse shortness of breath, dizziness, weakness, or fatigue, Amgen said.
Ivabradine will be available within about a week of the approval under the trade name Corlanor, and will come with a patient medication guide. Wholesale acquisition cost will be $4,500 per year, or $375 per month, and patient costs will vary according to insurance coverage, said Amgen spokesman Cuyler Mayer.
Ivabradine has been available in Europe as Procoralan for several years.
Alogliptin CV risk acceptable, FDA panel agrees
SILVER SPRING, MD.– Alogliptin’s cardiovascular risk profile is acceptably safe for high-risk patients with type 2 diabetes, a Food and Drug Administration advisory panel has unanimously agreed.
At a meeting of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee on April 14, panelists reviewed the results of the results of a large cardiovascular outcomes study of the dipeptidyl peptidase-4 (DPP-4) inhibitor, the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study, an international, randomized, double-blind, placebo-controlled trial of about 5,400 people with type 2 diabetes and established cardiovascular disease.
The FDA is requiring CV outcomes trials for all type 2 diabetes drugs, including those in development, and issued a guidance for industry for these trials in December 2008, which states that the trials should evaluate the major adverse cardiovascular event (MACE) incidence in patients at increased risk for CVD and that a 30% or greater excess CV risk over placebo should be excluded to meet the criteria for acceptable CV safety.
Alogliptin, marketed as Nesina by Takeda Pharmaceutical USA, was approved in January 2013. It also is combined with metformin (Kazano) and with pioglitazone (Oseni). The EXAMINE study, which was underway at the time of approval, enrolled people who had an acute coronary syndrome event (acute MI or unstable angina requiring hospitalization) within 15-90 days of enrollment.
Over a median follow-up of 1.5 years, there was no increase in the MACE composite endpoint of CV death, nonfatal MI, and nonfatal stroke among those on alogliptin (11.3%), compared with those on placebo (11.8%), for a hazard ratio of 0.96 – meeting the FDA criteria for CV safety (N. Engl. J. Med. 2013;369:1327-35).
Some of the issues raised with the study included a lower-than-planned proportion of patients enrolled in the United States and Canada – less than 16% of the total – and a subgroup analysis showing that, in the North American patients, the MACE rate among those on alogliptin was elevated compared with placebo (HR, 1.28). The FDA reviewers said that the North American population had some characteristics that might explain this difference.
Another finding discussed was the higher number of hospitalizations for heart failure in patients on alogliptin (106) than for those on placebo (89), for a rate of 2.6 vs. 2.3 cases per 100 patient-years (HR, 1.19), according to the FDA. In the CV outcomes study of another DPP-4 inhibitor, saxagliptin, which the panel reviewed earlier in the day, there was a similar signal for an increased risk of heart failure hospitalizations associated with the drug.
Panelists, however, said they were not convinced that the geographic differences represented a real effect, which they said could be due to chance and did not affect the overall results – although they would have preferred to have more people from the United States enrolled in the study. Panelists also were not overly concerned about the risk of heart failure, but they pointed out that the risk of heart failure risk should be monitored in patients on the drug because of the signal seen with saxagliptin. Several panelists said that they suspected that heart failure may turn out to be a class effect. CV outcomes trials for the two other approved DPP-4 inhibitors – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing.
“It’s clear that the trial achieved its primary objective in a manner consistent with the 2008 guidelines,” said one of the panelists, Dr. Thomas Wang, director of the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn. Although there were some limitations of the study, such as the low proportion of U.S. participants and the relatively short duration of follow-up, “these limitations do not come close to negating the conclusion” regarding the cardiovascular risk profile, he commented.
In another vote, 13 of the 16 panelists said that the new safety information should be added to the alogliptin prescribing information. “This trial represented a huge effort and we know a lot about the outcomes related to this drug now,” and that information should be included in the label, said one of the panelists, Dr. Susan Heckbert, professor in the department of epidemiology, University of Washington, Seattle.
Three panelists voted not to add any information to the label. The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest
SILVER SPRING, MD.– Alogliptin’s cardiovascular risk profile is acceptably safe for high-risk patients with type 2 diabetes, a Food and Drug Administration advisory panel has unanimously agreed.
At a meeting of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee on April 14, panelists reviewed the results of the results of a large cardiovascular outcomes study of the dipeptidyl peptidase-4 (DPP-4) inhibitor, the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study, an international, randomized, double-blind, placebo-controlled trial of about 5,400 people with type 2 diabetes and established cardiovascular disease.
The FDA is requiring CV outcomes trials for all type 2 diabetes drugs, including those in development, and issued a guidance for industry for these trials in December 2008, which states that the trials should evaluate the major adverse cardiovascular event (MACE) incidence in patients at increased risk for CVD and that a 30% or greater excess CV risk over placebo should be excluded to meet the criteria for acceptable CV safety.
Alogliptin, marketed as Nesina by Takeda Pharmaceutical USA, was approved in January 2013. It also is combined with metformin (Kazano) and with pioglitazone (Oseni). The EXAMINE study, which was underway at the time of approval, enrolled people who had an acute coronary syndrome event (acute MI or unstable angina requiring hospitalization) within 15-90 days of enrollment.
Over a median follow-up of 1.5 years, there was no increase in the MACE composite endpoint of CV death, nonfatal MI, and nonfatal stroke among those on alogliptin (11.3%), compared with those on placebo (11.8%), for a hazard ratio of 0.96 – meeting the FDA criteria for CV safety (N. Engl. J. Med. 2013;369:1327-35).
Some of the issues raised with the study included a lower-than-planned proportion of patients enrolled in the United States and Canada – less than 16% of the total – and a subgroup analysis showing that, in the North American patients, the MACE rate among those on alogliptin was elevated compared with placebo (HR, 1.28). The FDA reviewers said that the North American population had some characteristics that might explain this difference.
Another finding discussed was the higher number of hospitalizations for heart failure in patients on alogliptin (106) than for those on placebo (89), for a rate of 2.6 vs. 2.3 cases per 100 patient-years (HR, 1.19), according to the FDA. In the CV outcomes study of another DPP-4 inhibitor, saxagliptin, which the panel reviewed earlier in the day, there was a similar signal for an increased risk of heart failure hospitalizations associated with the drug.
Panelists, however, said they were not convinced that the geographic differences represented a real effect, which they said could be due to chance and did not affect the overall results – although they would have preferred to have more people from the United States enrolled in the study. Panelists also were not overly concerned about the risk of heart failure, but they pointed out that the risk of heart failure risk should be monitored in patients on the drug because of the signal seen with saxagliptin. Several panelists said that they suspected that heart failure may turn out to be a class effect. CV outcomes trials for the two other approved DPP-4 inhibitors – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing.
“It’s clear that the trial achieved its primary objective in a manner consistent with the 2008 guidelines,” said one of the panelists, Dr. Thomas Wang, director of the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn. Although there were some limitations of the study, such as the low proportion of U.S. participants and the relatively short duration of follow-up, “these limitations do not come close to negating the conclusion” regarding the cardiovascular risk profile, he commented.
In another vote, 13 of the 16 panelists said that the new safety information should be added to the alogliptin prescribing information. “This trial represented a huge effort and we know a lot about the outcomes related to this drug now,” and that information should be included in the label, said one of the panelists, Dr. Susan Heckbert, professor in the department of epidemiology, University of Washington, Seattle.
Three panelists voted not to add any information to the label. The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest
SILVER SPRING, MD.– Alogliptin’s cardiovascular risk profile is acceptably safe for high-risk patients with type 2 diabetes, a Food and Drug Administration advisory panel has unanimously agreed.
At a meeting of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee on April 14, panelists reviewed the results of the results of a large cardiovascular outcomes study of the dipeptidyl peptidase-4 (DPP-4) inhibitor, the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study, an international, randomized, double-blind, placebo-controlled trial of about 5,400 people with type 2 diabetes and established cardiovascular disease.
The FDA is requiring CV outcomes trials for all type 2 diabetes drugs, including those in development, and issued a guidance for industry for these trials in December 2008, which states that the trials should evaluate the major adverse cardiovascular event (MACE) incidence in patients at increased risk for CVD and that a 30% or greater excess CV risk over placebo should be excluded to meet the criteria for acceptable CV safety.
Alogliptin, marketed as Nesina by Takeda Pharmaceutical USA, was approved in January 2013. It also is combined with metformin (Kazano) and with pioglitazone (Oseni). The EXAMINE study, which was underway at the time of approval, enrolled people who had an acute coronary syndrome event (acute MI or unstable angina requiring hospitalization) within 15-90 days of enrollment.
Over a median follow-up of 1.5 years, there was no increase in the MACE composite endpoint of CV death, nonfatal MI, and nonfatal stroke among those on alogliptin (11.3%), compared with those on placebo (11.8%), for a hazard ratio of 0.96 – meeting the FDA criteria for CV safety (N. Engl. J. Med. 2013;369:1327-35).
Some of the issues raised with the study included a lower-than-planned proportion of patients enrolled in the United States and Canada – less than 16% of the total – and a subgroup analysis showing that, in the North American patients, the MACE rate among those on alogliptin was elevated compared with placebo (HR, 1.28). The FDA reviewers said that the North American population had some characteristics that might explain this difference.
Another finding discussed was the higher number of hospitalizations for heart failure in patients on alogliptin (106) than for those on placebo (89), for a rate of 2.6 vs. 2.3 cases per 100 patient-years (HR, 1.19), according to the FDA. In the CV outcomes study of another DPP-4 inhibitor, saxagliptin, which the panel reviewed earlier in the day, there was a similar signal for an increased risk of heart failure hospitalizations associated with the drug.
Panelists, however, said they were not convinced that the geographic differences represented a real effect, which they said could be due to chance and did not affect the overall results – although they would have preferred to have more people from the United States enrolled in the study. Panelists also were not overly concerned about the risk of heart failure, but they pointed out that the risk of heart failure risk should be monitored in patients on the drug because of the signal seen with saxagliptin. Several panelists said that they suspected that heart failure may turn out to be a class effect. CV outcomes trials for the two other approved DPP-4 inhibitors – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing.
“It’s clear that the trial achieved its primary objective in a manner consistent with the 2008 guidelines,” said one of the panelists, Dr. Thomas Wang, director of the division of cardiovascular medicine at Vanderbilt University, Nashville, Tenn. Although there were some limitations of the study, such as the low proportion of U.S. participants and the relatively short duration of follow-up, “these limitations do not come close to negating the conclusion” regarding the cardiovascular risk profile, he commented.
In another vote, 13 of the 16 panelists said that the new safety information should be added to the alogliptin prescribing information. “This trial represented a huge effort and we know a lot about the outcomes related to this drug now,” and that information should be included in the label, said one of the panelists, Dr. Susan Heckbert, professor in the department of epidemiology, University of Washington, Seattle.
Three panelists voted not to add any information to the label. The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest
AT AN FDA ADVISORY COMMITTEE MEETING
VIDEO: Treating heart failure congestion improved hyperglycemia
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM ACC 15
FDA panel reassured by saxagliptin’s CV safety data
SILVER SPRING, MD.– Results of a large postmarketing study evaluating the cardiovascular safety of saxagliptin in a high-risk population provided reassuring evidence about the drug’s overall cardiovascular risk, although a signal for heart failure hospitalizations associated with the drug was a concern, according to a Food and Drug Administration advisory panel.
At a meeting on April 14, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 13-1, with one abstention, that the results of the SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus) trial showed that the use of saxagliptin in patients with type 2 diabetes had an acceptable cardiovascular risk profile. However, the panelists were concerned about the signal for an increased risk of hospitalizations for heart failure associated with saxagliptin in the study, which they agreed should be studied further. Nearly all of the panelists also voted that the safety data, including the heart failure finding and an imbalance in all-cause mortality among those on saxagliptin arm of the study, be added to the drug’s prescribing information.
Approved in 2009, saxagliptin, a dipeptidyl peptidase–4 (DPP4) inhibitor, is indicated “as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings” and is marketed as Onglyza by AstraZeneca. A fixed-dose combination of saxagliptin with extended-release metformin (Kombiglyze XR) was approved in 2010. The FDA panel votes apply to both products.
The FDA is requiring that manufacturers conduct CV outcomes trials for all type 2 diabetes drugs in patients at high risk for cardiovascular disease (CVD), and in December 2008, issued a guidance document for industry, outlining the requirements for these studies, which included showing that the CVD risk, based on a major adverse cardiovascular event (MACE) endpoint, is not increased by more than 30% compared to placebo.
AstraZeneca conducted SAVOR, a prospective, randomized, double-blind, placebo-controlled study of nearly 16,500 people with type 2 diabetes, who had or were at risk of CVD, comparing saxagliptin to placebo, on top of standard treatment (N. Engl. J. Med. 2013;369:1317-26). After a median follow-up of about 2 years, the incidence of MACE (a composite of cardiovascular death, nonfatal MI, and nonfatal ischemic stroke) was identical in both groups, at 7.4%, with a hazard ratio of 1.0, meeting the FDA criteria for cardiovascular safety as outlined in the guidance, according to the company. Saxagliptin was not superior to placebo in reducing the MACE events, another primary objective of the study.
Unexpectedly, hospitalization for heart failure (HF), a component of the secondary composite endpoint, was increased among those treated with saxagliptin (hazard ratio, 1.27). There was also a “numerical imbalance” in all-cause mortality between groups, with 17 deaths in the saxagliptin patients vs. 11 in the placebo group (HR, 1.11).
However, deaths caused by heart failure were balanced between those on saxagliptin and those on placebo, and no possible mechanism for the HF finding could be identified, according to AstraZeneca, which is planning a mechanistic study to evaluate the effects of saxagliptin on volume, neurohormonal changes, and cardiac function. The company has proposed that the HF finding be addressed by adding this information to the prescribing information.
The cardiovascular safety results were reassuring, said Dr. Morris Schambelan, a panelist and professor emeritus of medicine in the division of endocrinology at the University of California, San Francisco, but like others on the committee, added that he believes the heart failure signal was real and that providing clinicians with information in the drug’s label to help predict those at higher risk in would be helpful.
Several panelists were somewhat concerned about the all-cause mortality finding, which is a strong endpoint, and therefore cannot be ruled out, they said. But they also pointed out that follow-up was relatively short for a drug that is used long term and that the results should be applied cautiously to patients at lower risk than were those enrolled in the trial.
The FDA usually follows the recommendations of its advisory panel members. Panelists had no relevant disclosures.
CV outcomes trials for other approved DPP4 inhibitors approved for type 2 diabetes – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing. The panel review of the CV outcomes trial for another DPP4 inhibitor approved, alogliptin (Nesina), followed the meeting on saxagliptin.
SILVER SPRING, MD.– Results of a large postmarketing study evaluating the cardiovascular safety of saxagliptin in a high-risk population provided reassuring evidence about the drug’s overall cardiovascular risk, although a signal for heart failure hospitalizations associated with the drug was a concern, according to a Food and Drug Administration advisory panel.
At a meeting on April 14, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 13-1, with one abstention, that the results of the SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus) trial showed that the use of saxagliptin in patients with type 2 diabetes had an acceptable cardiovascular risk profile. However, the panelists were concerned about the signal for an increased risk of hospitalizations for heart failure associated with saxagliptin in the study, which they agreed should be studied further. Nearly all of the panelists also voted that the safety data, including the heart failure finding and an imbalance in all-cause mortality among those on saxagliptin arm of the study, be added to the drug’s prescribing information.
Approved in 2009, saxagliptin, a dipeptidyl peptidase–4 (DPP4) inhibitor, is indicated “as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings” and is marketed as Onglyza by AstraZeneca. A fixed-dose combination of saxagliptin with extended-release metformin (Kombiglyze XR) was approved in 2010. The FDA panel votes apply to both products.
The FDA is requiring that manufacturers conduct CV outcomes trials for all type 2 diabetes drugs in patients at high risk for cardiovascular disease (CVD), and in December 2008, issued a guidance document for industry, outlining the requirements for these studies, which included showing that the CVD risk, based on a major adverse cardiovascular event (MACE) endpoint, is not increased by more than 30% compared to placebo.
AstraZeneca conducted SAVOR, a prospective, randomized, double-blind, placebo-controlled study of nearly 16,500 people with type 2 diabetes, who had or were at risk of CVD, comparing saxagliptin to placebo, on top of standard treatment (N. Engl. J. Med. 2013;369:1317-26). After a median follow-up of about 2 years, the incidence of MACE (a composite of cardiovascular death, nonfatal MI, and nonfatal ischemic stroke) was identical in both groups, at 7.4%, with a hazard ratio of 1.0, meeting the FDA criteria for cardiovascular safety as outlined in the guidance, according to the company. Saxagliptin was not superior to placebo in reducing the MACE events, another primary objective of the study.
Unexpectedly, hospitalization for heart failure (HF), a component of the secondary composite endpoint, was increased among those treated with saxagliptin (hazard ratio, 1.27). There was also a “numerical imbalance” in all-cause mortality between groups, with 17 deaths in the saxagliptin patients vs. 11 in the placebo group (HR, 1.11).
However, deaths caused by heart failure were balanced between those on saxagliptin and those on placebo, and no possible mechanism for the HF finding could be identified, according to AstraZeneca, which is planning a mechanistic study to evaluate the effects of saxagliptin on volume, neurohormonal changes, and cardiac function. The company has proposed that the HF finding be addressed by adding this information to the prescribing information.
The cardiovascular safety results were reassuring, said Dr. Morris Schambelan, a panelist and professor emeritus of medicine in the division of endocrinology at the University of California, San Francisco, but like others on the committee, added that he believes the heart failure signal was real and that providing clinicians with information in the drug’s label to help predict those at higher risk in would be helpful.
Several panelists were somewhat concerned about the all-cause mortality finding, which is a strong endpoint, and therefore cannot be ruled out, they said. But they also pointed out that follow-up was relatively short for a drug that is used long term and that the results should be applied cautiously to patients at lower risk than were those enrolled in the trial.
The FDA usually follows the recommendations of its advisory panel members. Panelists had no relevant disclosures.
CV outcomes trials for other approved DPP4 inhibitors approved for type 2 diabetes – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing. The panel review of the CV outcomes trial for another DPP4 inhibitor approved, alogliptin (Nesina), followed the meeting on saxagliptin.
SILVER SPRING, MD.– Results of a large postmarketing study evaluating the cardiovascular safety of saxagliptin in a high-risk population provided reassuring evidence about the drug’s overall cardiovascular risk, although a signal for heart failure hospitalizations associated with the drug was a concern, according to a Food and Drug Administration advisory panel.
At a meeting on April 14, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 13-1, with one abstention, that the results of the SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus) trial showed that the use of saxagliptin in patients with type 2 diabetes had an acceptable cardiovascular risk profile. However, the panelists were concerned about the signal for an increased risk of hospitalizations for heart failure associated with saxagliptin in the study, which they agreed should be studied further. Nearly all of the panelists also voted that the safety data, including the heart failure finding and an imbalance in all-cause mortality among those on saxagliptin arm of the study, be added to the drug’s prescribing information.
Approved in 2009, saxagliptin, a dipeptidyl peptidase–4 (DPP4) inhibitor, is indicated “as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings” and is marketed as Onglyza by AstraZeneca. A fixed-dose combination of saxagliptin with extended-release metformin (Kombiglyze XR) was approved in 2010. The FDA panel votes apply to both products.
The FDA is requiring that manufacturers conduct CV outcomes trials for all type 2 diabetes drugs in patients at high risk for cardiovascular disease (CVD), and in December 2008, issued a guidance document for industry, outlining the requirements for these studies, which included showing that the CVD risk, based on a major adverse cardiovascular event (MACE) endpoint, is not increased by more than 30% compared to placebo.
AstraZeneca conducted SAVOR, a prospective, randomized, double-blind, placebo-controlled study of nearly 16,500 people with type 2 diabetes, who had or were at risk of CVD, comparing saxagliptin to placebo, on top of standard treatment (N. Engl. J. Med. 2013;369:1317-26). After a median follow-up of about 2 years, the incidence of MACE (a composite of cardiovascular death, nonfatal MI, and nonfatal ischemic stroke) was identical in both groups, at 7.4%, with a hazard ratio of 1.0, meeting the FDA criteria for cardiovascular safety as outlined in the guidance, according to the company. Saxagliptin was not superior to placebo in reducing the MACE events, another primary objective of the study.
Unexpectedly, hospitalization for heart failure (HF), a component of the secondary composite endpoint, was increased among those treated with saxagliptin (hazard ratio, 1.27). There was also a “numerical imbalance” in all-cause mortality between groups, with 17 deaths in the saxagliptin patients vs. 11 in the placebo group (HR, 1.11).
However, deaths caused by heart failure were balanced between those on saxagliptin and those on placebo, and no possible mechanism for the HF finding could be identified, according to AstraZeneca, which is planning a mechanistic study to evaluate the effects of saxagliptin on volume, neurohormonal changes, and cardiac function. The company has proposed that the HF finding be addressed by adding this information to the prescribing information.
The cardiovascular safety results were reassuring, said Dr. Morris Schambelan, a panelist and professor emeritus of medicine in the division of endocrinology at the University of California, San Francisco, but like others on the committee, added that he believes the heart failure signal was real and that providing clinicians with information in the drug’s label to help predict those at higher risk in would be helpful.
Several panelists were somewhat concerned about the all-cause mortality finding, which is a strong endpoint, and therefore cannot be ruled out, they said. But they also pointed out that follow-up was relatively short for a drug that is used long term and that the results should be applied cautiously to patients at lower risk than were those enrolled in the trial.
The FDA usually follows the recommendations of its advisory panel members. Panelists had no relevant disclosures.
CV outcomes trials for other approved DPP4 inhibitors approved for type 2 diabetes – sitagliptin (Januvia) and linagliptin (Tradjenta) – are ongoing. The panel review of the CV outcomes trial for another DPP4 inhibitor approved, alogliptin (Nesina), followed the meeting on saxagliptin.
AT AN FDA ADVISORY COMMITTEE MEETING