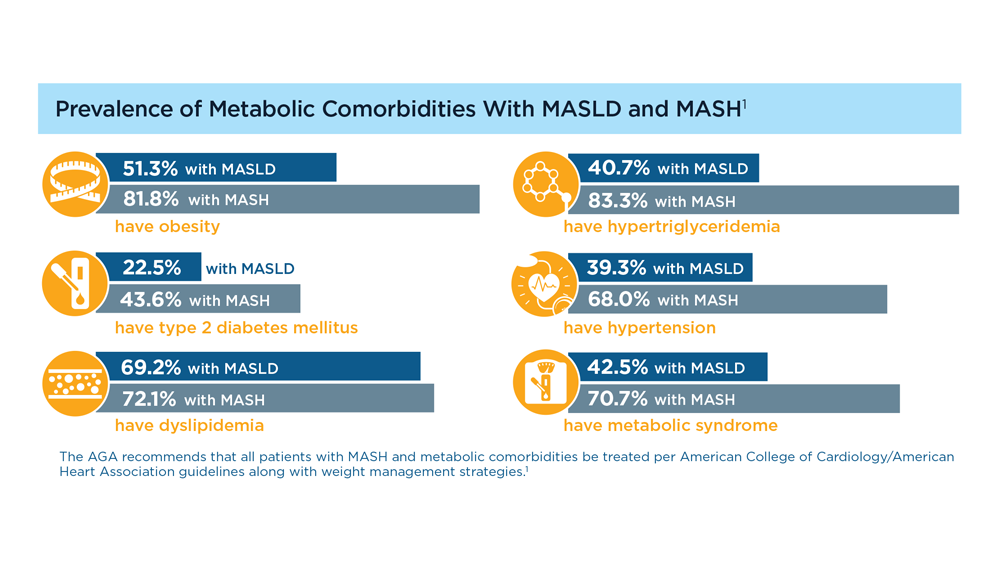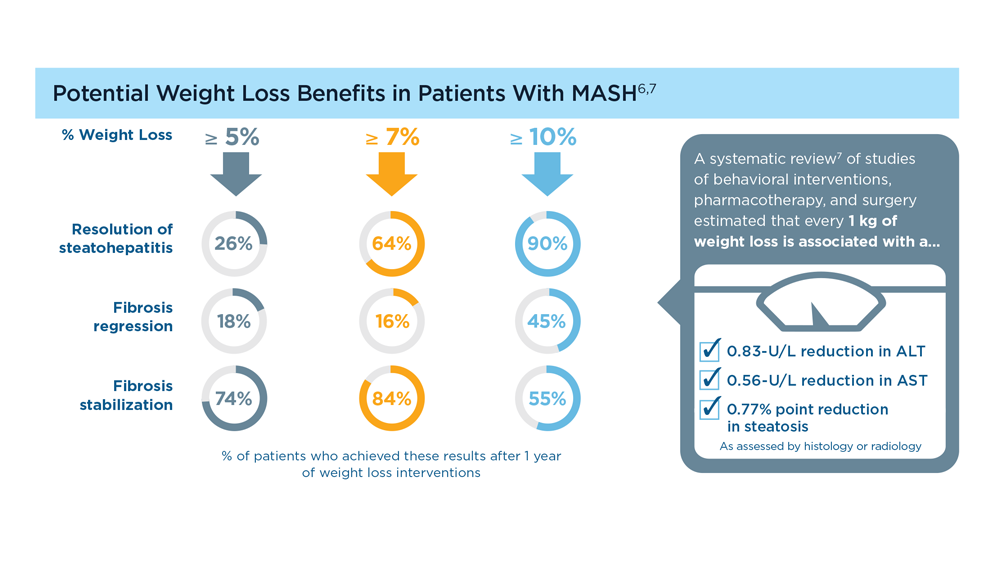User login
Neutrophils may offer therapeutic target for Wilson’s disease
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
The treatment of Wilson disease relies on use of chelators (D-pencilliamine; trientine) that promote urinary copper excretion and zinc, which blocks intestinal absorption.
These drugs, which must be taken continuously, are effective but are associated with significant side effects. Another chelator, bis-choline-tetrathiomolybdate (TTM), promotes biliary, rather than urinary copper excretion.
TTM improved neurological function in clinical trials; however, dose-dependent transaminase elevations were noted.
Thus, there is a need to identify new therapeutic approaches to reduce impact of copper toxicity in hepatocytes.
In the current issue of CMGH, Mi and colleagues utilize zebrafish and mouse models of Wilson disease to generate novel insights into the pathogenesis and molecular basis of liver injury and fibrosis caused by ATP7B mutations. In the zebrafish model, they first showed that fluorescently-labeled neutrophils accumulate in the livers of live, mutant animals, which are transparent, and thus, uniquely suited to these studies. Gene expression analyses showed that the liver neutrophils are metabolically active and sensitize hepatocytes to copper-induced injury, thus providing a therapeutic rational for neutrophil inhibition. Next, the authors confirmed these findings in the mouse model, showing specifically that the N2-neutrophil subtype predominated and correlated with the degree of liver injury. Subsequent gene expression studies in the mouse, combined with in vitro analysis of bone marrow-derived neutrophils, identified a molecular signaling pathway originating in hepatocytes that triggered N2 differentiation. This pathway, which was previously shown to drive N2 differentiation in cancer models, involves TGF-beta induced methylation (and hence repression) of a gene (SOCS3) that itself, blocks expression of STAT3, a gene that drives N2 differentiation. Importantly, liver injury and fibrosis were reduced in the mouse model by drugs that inhibit TGF-beta or DNA methylation, and hence N2 differentiation, or by directly blocking the activity of N2 neutrophils.
In summary, this new study provides novel insights into not only into the pathogenesis and potential treatment of Wilson disease, but also demonstrates how signaling pathways, such as the one involving TGFbeta-SOCS3-STAT3, are reiteratively used in a variety of pathologic contexts. Going forward, it will be important to determine whether this pharmacologically modifiable signaling pathway is activated in Wilson disease patients, and whether it impacts the pathogenesis of more common liver disorders.
Michael Pack, M.D., is professor of medicine at Perelman School of Medicine, University of Pennsylvania. He has no conflicts.
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
Inhibiting neutrophil function via transforming growth factor (TGF-beta 1) inhibition or methylation inhibition reduced parenchymal liver fibrosis and injury while improving liver function in a mouse model of Wilson’s disease, shows new research published in Cellular and Molecular Gastroenterology and Hepatology.
Also called progressive hepatolenticular degeneration, Wilson’s disease is an inherited nervous system disorder that can occur as a result of severe liver disease. It is caused by variants in the ATP7B gene which can lead to abnormalities in copper metabolism that lead to accumulation of the heavy metal in the liver and brain, resulting in damage to both organs. Approximately 60% of patients with Wilson’s disease present with hepatic syndromes, and of those 50%-60% go on to develop liver cirrhosis.
Current treatments aim to address metal deposition, but this approach is poorly tolerated by many patients, wrote investigators who were led by Junping Shi, MD, PhD, of the Institute of Hepatology and Metabolic Diseases, The Affiliated Hospital of Hangzhou Normal University, China.
“Drug interventions (such as copper chelators and zinc salts) reduce pathologic copper deposition, but side effects can be observed in up to 40% of patients during treatment and even after years of treatment, particularly nephropathy, autoimmune conditions, and skin changes,” the investigators wrote. “Liver transplantation is an effective treatment for Wilson’s disease, particularly for patients with end-stage liver disease, but donor shortages and lifelong immunosuppression limit its use. Therefore, alternative treatments with higher specificity in Wilson’s disease patients are urgently needed.”
The present study explored the underlying metabolic abnormalities in Wilson’s disease that result in liver injury and fibrosis, and related therapeutic approaches. Based on previous studies that have shown a relationship between persistent neutrophil infiltration and chronic tissue inflammation and damage, the investigators sought to explore the role of neutrophils in Wilson’s disease, with a focus on the N2 subtype.
First, they analyzed neutrophil populations in the livers of Atp7b–/– mice and atp7b–/– zebrafish, both of which are established animal models of Wilson’s disease. Compared with the wild-type comparison animals, the livers of disease model animals showed increased neutrophil infiltration, in terms of both count and density.
In one of several related experiments, administering a neutrophil agonist in the presence of copper led to significantly greater neutrophil infiltration in mutant versus wild-type fish, as well as greater increases in lipid droplets and disorganized tissue structure, which serve as markers of disease activity.
“Collectively, these data suggested that neutrophils infiltrated the liver and accelerated liver defects in Wilson’s disease,” the investigators wrote.
Additional experiments with the mouse model showed that pharmacologic ablation of N2 neutrophils via two approaches led to reduced liver fibrosis, offering a glimpse at therapeutic potential.
These findings were further supported by experiments involving a cellular model of Wilson’s disease with isolated bone marrow neutrophils. These analyses revealed the role of the TGF1–DNMT3A/STAT3 signaling axis in neutrophil polarization, and resultant liver disease progression, in Wilson’s disease.
“Neutrophil heterogeneity shows therapeutic potential, and pharmacologic modulation of N2-neutrophil activity should be explored as an alternative therapeutic to improve liver function in Wilson’s disease,” the investigators concluded, noting that TGF-beta 1, DNMT3A, or STAT3 could all serve as rational therapeutic targets.
Beyond Wilson’s disease, the findings may offer broader value for understanding the mechanisms driving other neutrophil-related diseases, as well as possible therapeutic approaches for those conditions, the authors added.
The authors disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
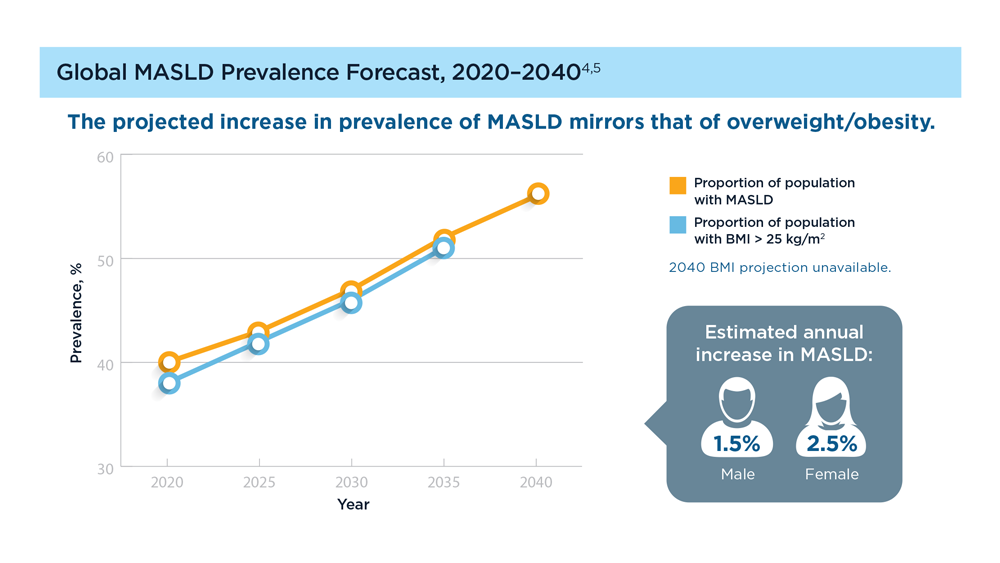
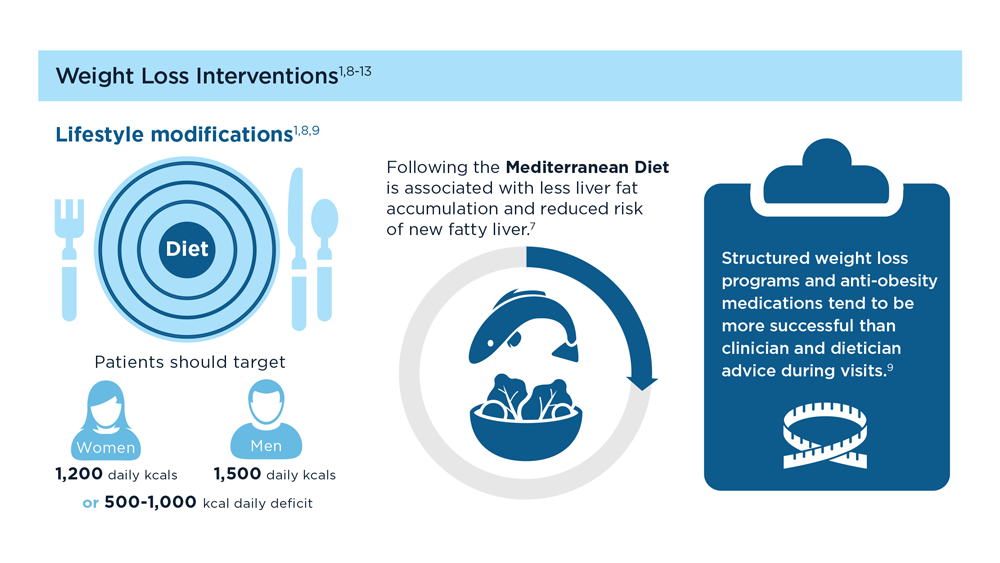
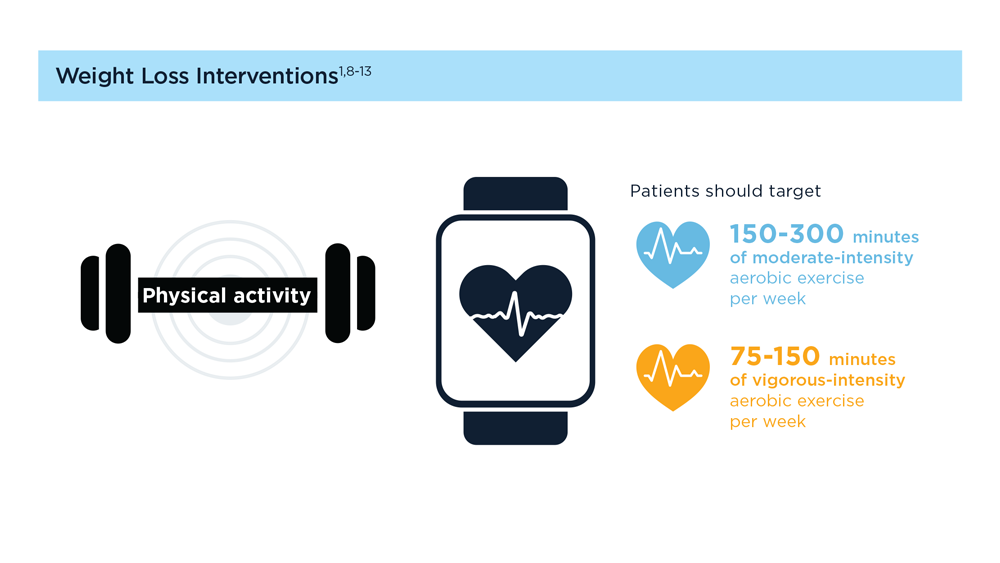
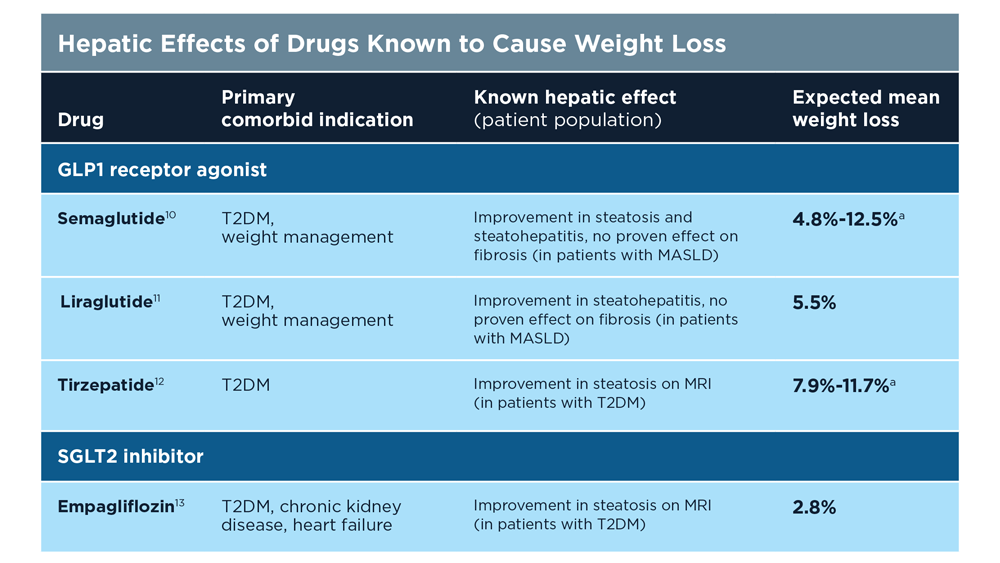
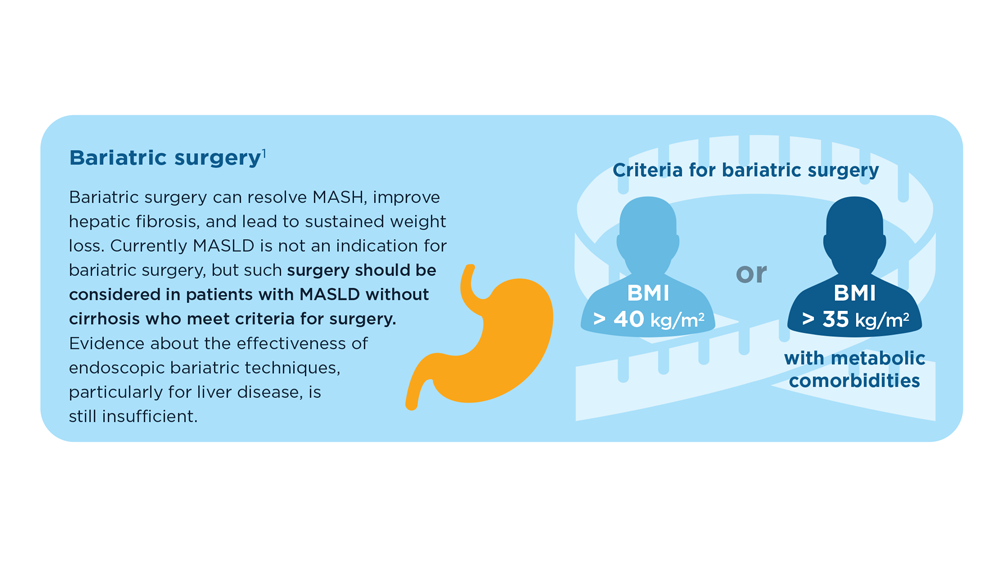
MASLD/MASH and Weight Loss
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
Gastroenterology Data Trends 2023
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
AGA CPU focuses on noninvasive tests in patients with NAFLD
Noninvasive testing allows for routine risk stratification and long-term monitoring of patients with nonalcoholic fatty liver disease (NAFLD), offering a safer, more practical approach than biopsy, according to a recent Clinical Practice Update Expert Review by the American Gastroenterological Association.
The update, published online in Gastroenterology, includes eight best practice advice statements.
“The health care burden of longitudinal management of patients with NAFLD is significant. The emergence and utilization of noninvasive testing (NIT) in gastroenterology practices has the potential to significantly enhance the care of patients with NAFLD by improving detection of patients with advanced fibrosis who are at increased risk for cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC), thereby facilitating timely clinical management,” wrote authors who were led by Julia J. Wattacheril, MD, MPH, of the Columbia University–New York Presbyterian Hospital nonalcoholic fatty liver disease program and center for liver disease and transplantation.
“In this Expert Review, we have provided clinicians with best practice advice for optimal utilization of NITs in patients with NAFLD,” the authors wrote.
Consensus best practice for implementing NITs in practice are scarce, giving rise to the present clinical practice update. The expert panel reviewed available evidence for these tests during longitudinal care of patients with advanced fibrosis as a means of predicting liver-related outcomes and informing treatment decisions.
The first statement encourages use of NITs for risk stratification during the diagnosis of NAFLD, typically in the form of clinical calculators like fibrosis 4 index (FIB-4), vibration controlled transient elastography (VCTE), shear wave
elastography (SWE), or magnetic resonance elastography (MRE), all of which have been validated in NAFLD.
“Ultrasound-based 3-dimensional elastography (Velacur) and iron-corrected T1 magnetic resonance imaging, although used less frequently, are emerging technologies,” the panelists noted.
Second, the update suggests that patients with a FIB-4 less than 1.3 are unlikely to have advanced hepatic fibrosis, based on this threshold’s strong negative predictive value (NPV).
Still, clinicians should remember that this FIB-4 threshold may be less reliable among patients younger than 35 years or older than 65 years, making it necessary to also consider other clinical measurements, according to the update. The third best practice advice encourages use of two or more NITs among patients with a FIB-4 score greater than 1.3.
The fourth piece of best practice advice suggests that clinicians follow manufacturer’s specifications when implementing NITs, as misuse may lead to “discordant results and adverse events.”
Fifth, to increase the positive predictive value (PPV) for detecting advanced fibrosis, NITs are best interpreted in the context of relevant clinical data, such as physical exam and endoscopy findings.
Next, the document encourages use of liver biopsy when NIT findings are discordant or indeterminate, conflict with findings from other test modalities, or if alternative, non–NAFLD etiologies are suspected.
The penultimate best practice advice suggests use of NITs for serial longitudinal disease monitoring, with signs of progression or regression used to guide clinical decisions.
“Additional evidence for longitudinal prediction of fibrosis regression and progression and response to intervention (lifestyle and pharmacologic) is needed in trials and real-world clinical practice,” Dr. Wattacheril and colleagues noted.
Finally, the clinical practice update advises surveillance of liver complications, such as hepatocellular carcinoma, among patients with NIT results that suggest advanced fibrosis (F3) or cirrhosis (F4).
This clinical practice update was commissioned by the AGA Institute. The investigators disclosed relationships with AstraZeneca, BMS, Novo Nordisk, and others.
Noninvasive testing allows for routine risk stratification and long-term monitoring of patients with nonalcoholic fatty liver disease (NAFLD), offering a safer, more practical approach than biopsy, according to a recent Clinical Practice Update Expert Review by the American Gastroenterological Association.
The update, published online in Gastroenterology, includes eight best practice advice statements.
“The health care burden of longitudinal management of patients with NAFLD is significant. The emergence and utilization of noninvasive testing (NIT) in gastroenterology practices has the potential to significantly enhance the care of patients with NAFLD by improving detection of patients with advanced fibrosis who are at increased risk for cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC), thereby facilitating timely clinical management,” wrote authors who were led by Julia J. Wattacheril, MD, MPH, of the Columbia University–New York Presbyterian Hospital nonalcoholic fatty liver disease program and center for liver disease and transplantation.
“In this Expert Review, we have provided clinicians with best practice advice for optimal utilization of NITs in patients with NAFLD,” the authors wrote.
Consensus best practice for implementing NITs in practice are scarce, giving rise to the present clinical practice update. The expert panel reviewed available evidence for these tests during longitudinal care of patients with advanced fibrosis as a means of predicting liver-related outcomes and informing treatment decisions.
The first statement encourages use of NITs for risk stratification during the diagnosis of NAFLD, typically in the form of clinical calculators like fibrosis 4 index (FIB-4), vibration controlled transient elastography (VCTE), shear wave
elastography (SWE), or magnetic resonance elastography (MRE), all of which have been validated in NAFLD.
“Ultrasound-based 3-dimensional elastography (Velacur) and iron-corrected T1 magnetic resonance imaging, although used less frequently, are emerging technologies,” the panelists noted.
Second, the update suggests that patients with a FIB-4 less than 1.3 are unlikely to have advanced hepatic fibrosis, based on this threshold’s strong negative predictive value (NPV).
Still, clinicians should remember that this FIB-4 threshold may be less reliable among patients younger than 35 years or older than 65 years, making it necessary to also consider other clinical measurements, according to the update. The third best practice advice encourages use of two or more NITs among patients with a FIB-4 score greater than 1.3.
The fourth piece of best practice advice suggests that clinicians follow manufacturer’s specifications when implementing NITs, as misuse may lead to “discordant results and adverse events.”
Fifth, to increase the positive predictive value (PPV) for detecting advanced fibrosis, NITs are best interpreted in the context of relevant clinical data, such as physical exam and endoscopy findings.
Next, the document encourages use of liver biopsy when NIT findings are discordant or indeterminate, conflict with findings from other test modalities, or if alternative, non–NAFLD etiologies are suspected.
The penultimate best practice advice suggests use of NITs for serial longitudinal disease monitoring, with signs of progression or regression used to guide clinical decisions.
“Additional evidence for longitudinal prediction of fibrosis regression and progression and response to intervention (lifestyle and pharmacologic) is needed in trials and real-world clinical practice,” Dr. Wattacheril and colleagues noted.
Finally, the clinical practice update advises surveillance of liver complications, such as hepatocellular carcinoma, among patients with NIT results that suggest advanced fibrosis (F3) or cirrhosis (F4).
This clinical practice update was commissioned by the AGA Institute. The investigators disclosed relationships with AstraZeneca, BMS, Novo Nordisk, and others.
Noninvasive testing allows for routine risk stratification and long-term monitoring of patients with nonalcoholic fatty liver disease (NAFLD), offering a safer, more practical approach than biopsy, according to a recent Clinical Practice Update Expert Review by the American Gastroenterological Association.
The update, published online in Gastroenterology, includes eight best practice advice statements.
“The health care burden of longitudinal management of patients with NAFLD is significant. The emergence and utilization of noninvasive testing (NIT) in gastroenterology practices has the potential to significantly enhance the care of patients with NAFLD by improving detection of patients with advanced fibrosis who are at increased risk for cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC), thereby facilitating timely clinical management,” wrote authors who were led by Julia J. Wattacheril, MD, MPH, of the Columbia University–New York Presbyterian Hospital nonalcoholic fatty liver disease program and center for liver disease and transplantation.
“In this Expert Review, we have provided clinicians with best practice advice for optimal utilization of NITs in patients with NAFLD,” the authors wrote.
Consensus best practice for implementing NITs in practice are scarce, giving rise to the present clinical practice update. The expert panel reviewed available evidence for these tests during longitudinal care of patients with advanced fibrosis as a means of predicting liver-related outcomes and informing treatment decisions.
The first statement encourages use of NITs for risk stratification during the diagnosis of NAFLD, typically in the form of clinical calculators like fibrosis 4 index (FIB-4), vibration controlled transient elastography (VCTE), shear wave
elastography (SWE), or magnetic resonance elastography (MRE), all of which have been validated in NAFLD.
“Ultrasound-based 3-dimensional elastography (Velacur) and iron-corrected T1 magnetic resonance imaging, although used less frequently, are emerging technologies,” the panelists noted.
Second, the update suggests that patients with a FIB-4 less than 1.3 are unlikely to have advanced hepatic fibrosis, based on this threshold’s strong negative predictive value (NPV).
Still, clinicians should remember that this FIB-4 threshold may be less reliable among patients younger than 35 years or older than 65 years, making it necessary to also consider other clinical measurements, according to the update. The third best practice advice encourages use of two or more NITs among patients with a FIB-4 score greater than 1.3.
The fourth piece of best practice advice suggests that clinicians follow manufacturer’s specifications when implementing NITs, as misuse may lead to “discordant results and adverse events.”
Fifth, to increase the positive predictive value (PPV) for detecting advanced fibrosis, NITs are best interpreted in the context of relevant clinical data, such as physical exam and endoscopy findings.
Next, the document encourages use of liver biopsy when NIT findings are discordant or indeterminate, conflict with findings from other test modalities, or if alternative, non–NAFLD etiologies are suspected.
The penultimate best practice advice suggests use of NITs for serial longitudinal disease monitoring, with signs of progression or regression used to guide clinical decisions.
“Additional evidence for longitudinal prediction of fibrosis regression and progression and response to intervention (lifestyle and pharmacologic) is needed in trials and real-world clinical practice,” Dr. Wattacheril and colleagues noted.
Finally, the clinical practice update advises surveillance of liver complications, such as hepatocellular carcinoma, among patients with NIT results that suggest advanced fibrosis (F3) or cirrhosis (F4).
This clinical practice update was commissioned by the AGA Institute. The investigators disclosed relationships with AstraZeneca, BMS, Novo Nordisk, and others.
FROM GASTROENTEROLOGY
Hepatic presentations of celiac disease
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
New guide for acute liver failure urges early treatment, transplant referral
Acute liver failure (ALF), a rare life-threatening condition, is potentially reversible if recognized and treated early, according to the latest guidelines from the American College of Gastroenterology.
The guidelines emphasize the need for timely transfer to a transplant center for patients who are at risk for poor outcomes.
“We wanted to produce an updated set of ALF guidelines for general gastroenterologists,” said lead author Alexandra Shingina, MD, MSc, Vanderbilt University Medical Center, Nashville, Tenn.
The aim was to “provide a comprehensive review of early evaluation and management of these patients,” she added.
The guidelines were published in the American Journal of Gastroenterology.
In 2017, the American Gastroenterological Association issued guidelines specific to the diagnosis and management of acute liver failure.
Siddharth Singh, MD, a gastroenterologist with UC San Diego Health and an author of the AGA guidelines, said the new guidelines will help inform the treatment of ALF. “It is encouraging to see the recent ACG guidelines building on prior guidelines published by the AGA in 2017,” he said.
ALF is typically defined as severe liver impairment and rapid clinical deterioration that, with few exceptions, “occurs in patients with no pre-existing liver disease,” the authors write. It is critical to distinguish ALF from the more common acutely decompensated cirrhosis or acute on chronic liver failure, the guidelines note, because their management differs significantly.
“ALF has a multitude of etiologies and a variety of clinical presentations that can affect virtually every organ system,” the authors write.
The cause of ALF is an essential indicator for prognosis and treatment strategy, especially for liver transplantation. For example, hyperacute ALF is predominantly seen in the setting of viral hepatitis A and E, acetaminophen toxicity, and ischemic injury, they note. Although the hyperacute subtype “carries a high risk for cerebral edema, it has the best prognosis without transplantation,” compared with other forms of ALF.
Before liver transplants, nearly 80% of patients with ALF died from the condition. In the past 20 years, 1- and 5-year survival rates from liver transplants are about 80% and 75%, respectively.
The authors emphasize that it is “imperative for clinicians to recognize ALF early ... because initiation of treatment and transplant considerations could be life-saving.”
Notable new recommendations
To develop the new guidelines, a writing group was assembled that included hepatology experts across a range of practice settings and different stages of their clinical and research careers.
They conducted a literature search of the MEDLINE, EMBASE, and Cochrane Library databases for relevant studies published in English up to January 2022, focusing on the highest quality of evidence, where available. Owing to a lack of solid data, the recommendations are based predominantly on expert opinion, the authors note.
ALF “is a rare entity. Literature reporting on outcomes is sparse and limited to retrospective cases series, with almost no randomized controlled trials available,” Dr. Shingina said.
She and her colleagues developed the recommendations to cover all aspects of ALF management, from initial diagnosis through to system- and etiology-specific management of ALF and liver transplantation.
“One of the new recommendations is the early use of CRRT [continuous renal replacement therapy] in patients with ALF and grade 2 encephalopathy, even in the absence of conventional RRT indications,” Dr. Shingina said.
“Although the evidence is limited, we felt that it was an important point in the multidisciplinary management of complex ALF patients, which can potentially save lives by reducing cerebral edema and allowing for more time if a liver transplant is not readily available,” she said.
She also highlighted a recommendation supporting intravenous N-acetylcysteine use in patients with acetaminophen-induced ALF and pointed out that the routine use of intracranial pressure monitors is no longer recommended “given the lack of literature on improved outcomes.”
Dr. Shingina emphasized that living donor liver transplantation can be considered in patients with ALF who are listed as status 1A priority for transplantation in experienced centers, when deceased donor liver transplantation is not readily available, as can ABO-incompatible grafts in patients who are rapidly declining.
The authors also present a timeline of ALF presentation and investigations.
During the first 2-4 hours after presentation at the emergency department, the patient should undergo initial stabilization and investigations, with a transfer to the ICU for those with grade 2 or higher hepatic encephalopathy. The transplant center should also be contacted during this period, the authors write.
After transfer to the ICU or a transplant center and during hours 4-12 After the initial presentation, patients should undergo intensive monitoring.
Psychiatry, social work, and hepatobiliary surgery consults should also be undertaken to determine the patient’s transplant eligibility, and if eligible, they should be put on a list.
Those who are ineligible for transplant or who show improvements should subsequently receive supportive management.
Overall, Dr. Shingina said that risk stratification and contact with a transplant center for potential transfer is of “utmost importance” for general gastroenterologists working in the community.
She said that either the Kings College Criteria or Model for End-Stage Liver Disease score can be used for prognostication, with a MELD score of 25 indicating worse outcomes.
“These are the patients who would benefit from early transfer to the nearest transplant center,” Dr. Shingina said.
Guidelines valuable, offer ‘concrete advice’
Approached for comment, Michael P. Curry, MD, Beth Israel Deaconess Medical Center, Boston, welcomed the guidelines, saying they are “very well written.”
He said there have been “a lot of changes in the field” since the 2011 guidelines. The current recommendations “provide concrete advice to all physicians on the appropriate assessment of patients with ALF,” he said.
Dr. Curry singled out the new recommendation on the early use of CRRT in patients with encephalopathy. He agreed on the need for gastroenterologists outside of transplant centers to make contact for potential transfer early.
“These are not patients who should, or could, be managed in a small community hospital or in a program that does not have a transplant center with which they work in close collaboration,” he said.
“So, identifying patients who are at highest risk of progressing is really important,” he said.
Dr. Curry hopes the guidelines will be shared widely by colleagues, but he is concerned that they are “not going to make it to some of these intensive care units in community, non-tertiary care centers.”
Nikolaos Pyrsopoulos, MD, PhD, MBA, Rutgers New Jersey Medical School, Newark, said the guidelines offer a “very comprehensive review of the literature.”
He said they are also a “very thorough evaluation of the quality of the evidence-based publications.”
It was “about time” that there was a set of guidelines of this quality, he added.
As for the recommendations, Dr. Pyrsopoulos believes that they will be “really valuable for the general gastroenterologist practicing in the community,” as well as for pathologists, to help them evaluate patients with ALF “as soon as possible, and in a standardized manner.”
He also emphasized the need for the rapid transfer of patients for transplant “when they are still lucid ... so we have the opportunity to discuss with and evaluate the patient.” This can be problematic in those who have been intubated and in patients with hepatic encephalopathy because they “become really confused.”
“The window of opportunity is closing very rapidly in some of these patients ... and morbidity and mortality is really pretty high” he said, so the transplant centers “appreciate when the referral is made to them earlier.”
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Acute liver failure (ALF), a rare life-threatening condition, is potentially reversible if recognized and treated early, according to the latest guidelines from the American College of Gastroenterology.
The guidelines emphasize the need for timely transfer to a transplant center for patients who are at risk for poor outcomes.
“We wanted to produce an updated set of ALF guidelines for general gastroenterologists,” said lead author Alexandra Shingina, MD, MSc, Vanderbilt University Medical Center, Nashville, Tenn.
The aim was to “provide a comprehensive review of early evaluation and management of these patients,” she added.
The guidelines were published in the American Journal of Gastroenterology.
In 2017, the American Gastroenterological Association issued guidelines specific to the diagnosis and management of acute liver failure.
Siddharth Singh, MD, a gastroenterologist with UC San Diego Health and an author of the AGA guidelines, said the new guidelines will help inform the treatment of ALF. “It is encouraging to see the recent ACG guidelines building on prior guidelines published by the AGA in 2017,” he said.
ALF is typically defined as severe liver impairment and rapid clinical deterioration that, with few exceptions, “occurs in patients with no pre-existing liver disease,” the authors write. It is critical to distinguish ALF from the more common acutely decompensated cirrhosis or acute on chronic liver failure, the guidelines note, because their management differs significantly.
“ALF has a multitude of etiologies and a variety of clinical presentations that can affect virtually every organ system,” the authors write.
The cause of ALF is an essential indicator for prognosis and treatment strategy, especially for liver transplantation. For example, hyperacute ALF is predominantly seen in the setting of viral hepatitis A and E, acetaminophen toxicity, and ischemic injury, they note. Although the hyperacute subtype “carries a high risk for cerebral edema, it has the best prognosis without transplantation,” compared with other forms of ALF.
Before liver transplants, nearly 80% of patients with ALF died from the condition. In the past 20 years, 1- and 5-year survival rates from liver transplants are about 80% and 75%, respectively.
The authors emphasize that it is “imperative for clinicians to recognize ALF early ... because initiation of treatment and transplant considerations could be life-saving.”
Notable new recommendations
To develop the new guidelines, a writing group was assembled that included hepatology experts across a range of practice settings and different stages of their clinical and research careers.
They conducted a literature search of the MEDLINE, EMBASE, and Cochrane Library databases for relevant studies published in English up to January 2022, focusing on the highest quality of evidence, where available. Owing to a lack of solid data, the recommendations are based predominantly on expert opinion, the authors note.
ALF “is a rare entity. Literature reporting on outcomes is sparse and limited to retrospective cases series, with almost no randomized controlled trials available,” Dr. Shingina said.
She and her colleagues developed the recommendations to cover all aspects of ALF management, from initial diagnosis through to system- and etiology-specific management of ALF and liver transplantation.
“One of the new recommendations is the early use of CRRT [continuous renal replacement therapy] in patients with ALF and grade 2 encephalopathy, even in the absence of conventional RRT indications,” Dr. Shingina said.
“Although the evidence is limited, we felt that it was an important point in the multidisciplinary management of complex ALF patients, which can potentially save lives by reducing cerebral edema and allowing for more time if a liver transplant is not readily available,” she said.
She also highlighted a recommendation supporting intravenous N-acetylcysteine use in patients with acetaminophen-induced ALF and pointed out that the routine use of intracranial pressure monitors is no longer recommended “given the lack of literature on improved outcomes.”
Dr. Shingina emphasized that living donor liver transplantation can be considered in patients with ALF who are listed as status 1A priority for transplantation in experienced centers, when deceased donor liver transplantation is not readily available, as can ABO-incompatible grafts in patients who are rapidly declining.
The authors also present a timeline of ALF presentation and investigations.
During the first 2-4 hours after presentation at the emergency department, the patient should undergo initial stabilization and investigations, with a transfer to the ICU for those with grade 2 or higher hepatic encephalopathy. The transplant center should also be contacted during this period, the authors write.
After transfer to the ICU or a transplant center and during hours 4-12 After the initial presentation, patients should undergo intensive monitoring.
Psychiatry, social work, and hepatobiliary surgery consults should also be undertaken to determine the patient’s transplant eligibility, and if eligible, they should be put on a list.
Those who are ineligible for transplant or who show improvements should subsequently receive supportive management.
Overall, Dr. Shingina said that risk stratification and contact with a transplant center for potential transfer is of “utmost importance” for general gastroenterologists working in the community.
She said that either the Kings College Criteria or Model for End-Stage Liver Disease score can be used for prognostication, with a MELD score of 25 indicating worse outcomes.
“These are the patients who would benefit from early transfer to the nearest transplant center,” Dr. Shingina said.
Guidelines valuable, offer ‘concrete advice’
Approached for comment, Michael P. Curry, MD, Beth Israel Deaconess Medical Center, Boston, welcomed the guidelines, saying they are “very well written.”
He said there have been “a lot of changes in the field” since the 2011 guidelines. The current recommendations “provide concrete advice to all physicians on the appropriate assessment of patients with ALF,” he said.
Dr. Curry singled out the new recommendation on the early use of CRRT in patients with encephalopathy. He agreed on the need for gastroenterologists outside of transplant centers to make contact for potential transfer early.
“These are not patients who should, or could, be managed in a small community hospital or in a program that does not have a transplant center with which they work in close collaboration,” he said.
“So, identifying patients who are at highest risk of progressing is really important,” he said.
Dr. Curry hopes the guidelines will be shared widely by colleagues, but he is concerned that they are “not going to make it to some of these intensive care units in community, non-tertiary care centers.”
Nikolaos Pyrsopoulos, MD, PhD, MBA, Rutgers New Jersey Medical School, Newark, said the guidelines offer a “very comprehensive review of the literature.”
He said they are also a “very thorough evaluation of the quality of the evidence-based publications.”
It was “about time” that there was a set of guidelines of this quality, he added.
As for the recommendations, Dr. Pyrsopoulos believes that they will be “really valuable for the general gastroenterologist practicing in the community,” as well as for pathologists, to help them evaluate patients with ALF “as soon as possible, and in a standardized manner.”
He also emphasized the need for the rapid transfer of patients for transplant “when they are still lucid ... so we have the opportunity to discuss with and evaluate the patient.” This can be problematic in those who have been intubated and in patients with hepatic encephalopathy because they “become really confused.”
“The window of opportunity is closing very rapidly in some of these patients ... and morbidity and mortality is really pretty high” he said, so the transplant centers “appreciate when the referral is made to them earlier.”
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Acute liver failure (ALF), a rare life-threatening condition, is potentially reversible if recognized and treated early, according to the latest guidelines from the American College of Gastroenterology.
The guidelines emphasize the need for timely transfer to a transplant center for patients who are at risk for poor outcomes.
“We wanted to produce an updated set of ALF guidelines for general gastroenterologists,” said lead author Alexandra Shingina, MD, MSc, Vanderbilt University Medical Center, Nashville, Tenn.
The aim was to “provide a comprehensive review of early evaluation and management of these patients,” she added.
The guidelines were published in the American Journal of Gastroenterology.
In 2017, the American Gastroenterological Association issued guidelines specific to the diagnosis and management of acute liver failure.
Siddharth Singh, MD, a gastroenterologist with UC San Diego Health and an author of the AGA guidelines, said the new guidelines will help inform the treatment of ALF. “It is encouraging to see the recent ACG guidelines building on prior guidelines published by the AGA in 2017,” he said.
ALF is typically defined as severe liver impairment and rapid clinical deterioration that, with few exceptions, “occurs in patients with no pre-existing liver disease,” the authors write. It is critical to distinguish ALF from the more common acutely decompensated cirrhosis or acute on chronic liver failure, the guidelines note, because their management differs significantly.
“ALF has a multitude of etiologies and a variety of clinical presentations that can affect virtually every organ system,” the authors write.
The cause of ALF is an essential indicator for prognosis and treatment strategy, especially for liver transplantation. For example, hyperacute ALF is predominantly seen in the setting of viral hepatitis A and E, acetaminophen toxicity, and ischemic injury, they note. Although the hyperacute subtype “carries a high risk for cerebral edema, it has the best prognosis without transplantation,” compared with other forms of ALF.
Before liver transplants, nearly 80% of patients with ALF died from the condition. In the past 20 years, 1- and 5-year survival rates from liver transplants are about 80% and 75%, respectively.
The authors emphasize that it is “imperative for clinicians to recognize ALF early ... because initiation of treatment and transplant considerations could be life-saving.”
Notable new recommendations
To develop the new guidelines, a writing group was assembled that included hepatology experts across a range of practice settings and different stages of their clinical and research careers.
They conducted a literature search of the MEDLINE, EMBASE, and Cochrane Library databases for relevant studies published in English up to January 2022, focusing on the highest quality of evidence, where available. Owing to a lack of solid data, the recommendations are based predominantly on expert opinion, the authors note.
ALF “is a rare entity. Literature reporting on outcomes is sparse and limited to retrospective cases series, with almost no randomized controlled trials available,” Dr. Shingina said.
She and her colleagues developed the recommendations to cover all aspects of ALF management, from initial diagnosis through to system- and etiology-specific management of ALF and liver transplantation.
“One of the new recommendations is the early use of CRRT [continuous renal replacement therapy] in patients with ALF and grade 2 encephalopathy, even in the absence of conventional RRT indications,” Dr. Shingina said.
“Although the evidence is limited, we felt that it was an important point in the multidisciplinary management of complex ALF patients, which can potentially save lives by reducing cerebral edema and allowing for more time if a liver transplant is not readily available,” she said.
She also highlighted a recommendation supporting intravenous N-acetylcysteine use in patients with acetaminophen-induced ALF and pointed out that the routine use of intracranial pressure monitors is no longer recommended “given the lack of literature on improved outcomes.”
Dr. Shingina emphasized that living donor liver transplantation can be considered in patients with ALF who are listed as status 1A priority for transplantation in experienced centers, when deceased donor liver transplantation is not readily available, as can ABO-incompatible grafts in patients who are rapidly declining.
The authors also present a timeline of ALF presentation and investigations.
During the first 2-4 hours after presentation at the emergency department, the patient should undergo initial stabilization and investigations, with a transfer to the ICU for those with grade 2 or higher hepatic encephalopathy. The transplant center should also be contacted during this period, the authors write.
After transfer to the ICU or a transplant center and during hours 4-12 After the initial presentation, patients should undergo intensive monitoring.
Psychiatry, social work, and hepatobiliary surgery consults should also be undertaken to determine the patient’s transplant eligibility, and if eligible, they should be put on a list.
Those who are ineligible for transplant or who show improvements should subsequently receive supportive management.
Overall, Dr. Shingina said that risk stratification and contact with a transplant center for potential transfer is of “utmost importance” for general gastroenterologists working in the community.
She said that either the Kings College Criteria or Model for End-Stage Liver Disease score can be used for prognostication, with a MELD score of 25 indicating worse outcomes.
“These are the patients who would benefit from early transfer to the nearest transplant center,” Dr. Shingina said.
Guidelines valuable, offer ‘concrete advice’
Approached for comment, Michael P. Curry, MD, Beth Israel Deaconess Medical Center, Boston, welcomed the guidelines, saying they are “very well written.”
He said there have been “a lot of changes in the field” since the 2011 guidelines. The current recommendations “provide concrete advice to all physicians on the appropriate assessment of patients with ALF,” he said.
Dr. Curry singled out the new recommendation on the early use of CRRT in patients with encephalopathy. He agreed on the need for gastroenterologists outside of transplant centers to make contact for potential transfer early.
“These are not patients who should, or could, be managed in a small community hospital or in a program that does not have a transplant center with which they work in close collaboration,” he said.
“So, identifying patients who are at highest risk of progressing is really important,” he said.
Dr. Curry hopes the guidelines will be shared widely by colleagues, but he is concerned that they are “not going to make it to some of these intensive care units in community, non-tertiary care centers.”
Nikolaos Pyrsopoulos, MD, PhD, MBA, Rutgers New Jersey Medical School, Newark, said the guidelines offer a “very comprehensive review of the literature.”
He said they are also a “very thorough evaluation of the quality of the evidence-based publications.”
It was “about time” that there was a set of guidelines of this quality, he added.
As for the recommendations, Dr. Pyrsopoulos believes that they will be “really valuable for the general gastroenterologist practicing in the community,” as well as for pathologists, to help them evaluate patients with ALF “as soon as possible, and in a standardized manner.”
He also emphasized the need for the rapid transfer of patients for transplant “when they are still lucid ... so we have the opportunity to discuss with and evaluate the patient.” This can be problematic in those who have been intubated and in patients with hepatic encephalopathy because they “become really confused.”
“The window of opportunity is closing very rapidly in some of these patients ... and morbidity and mortality is really pretty high” he said, so the transplant centers “appreciate when the referral is made to them earlier.”
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Bulevirtide shows promise in chronic hepatitis D
shows an ongoing phase 3 study conducted in the United States and four other countries.
The findings were published in the New England Journal of Medicine.
Led by Heiner Wedemeyer, MD, of Hannover Medical School in Germany, the study included 150 patients with HDV, with and without compensated cirrhosis (mean age, 42 years; 57% male; 83% White). They were randomly assigned to receive 2 mg or 10 mg of bulevirtide subcutaneously daily for 144 weeks or, as a control group, receive no treatment for 48 weeks, followed by 10 mg of bulevirtide daily for 96 weeks. All patients were followed for 96 weeks after treatment ends.
For the primary endpoint, the combined viral and ALT response at week 48 was similar in the 2-mg (45%) and 10-mg (48%) groups, compared with 2% in the control group (one patient). Twelve percent of patients in the 2-mg group and 20% of patients in the 10-mg group had a clinical benefit, compared with none of the patients in the control group.
Among those with a combined response, normalization of the ALT level occurred in most patients by week 24, while the HDV RNA level continued to decline between week 24 and week 48, the authors wrote.
“This surrogate end point is considered to be a reasonably likely predictor of improved clinical outcomes in patients with HDV; however, longer-term data are needed to confirm the clinical benefit of bulevirtide,” the investigators wrote.
The results offer a glimmer of hope, Marc Ghany, MD, MHSc, of the National Institute of Diabetes and Digestive and Kidney Diseases wrote in an accompanying editorial. “The goal of HDV therapy is to improve patient survival by preventing progression to cirrhosis, liver failure, and liver cancer,” he wrote.
In safety results, headache, pruritus, fatigue, and eosinophilia were more common in the bulevirtide groups than in the control group. All adverse events were mild to moderate.
HDV infects about 5% of people with chronic HBV and relies on HBV surface antigen (HBsAg) for transmission and infectivity. Bulevirtide is derived from a region of the large envelope protein of HBsAg and irreversibly binds to the hepatocyte entry receptor for both HDV and HBV.
Bulevirtide has received conditional approval in the European Union. In 2022, the Food and Drug Administration declined to approve bulevirtide over concerns about production and delivery of the drug. There are no approved treatments for HDV in the United States.
The study was supported by Gilead Sciences. Dr. Wedemeyer disclosed research funding, acting as a consultant to, and giving paid lectures on behalf of Gilead Sciences. He and other coauthors disclosed financial relationships with Gilead and other pharmaceutical companies.
shows an ongoing phase 3 study conducted in the United States and four other countries.
The findings were published in the New England Journal of Medicine.
Led by Heiner Wedemeyer, MD, of Hannover Medical School in Germany, the study included 150 patients with HDV, with and without compensated cirrhosis (mean age, 42 years; 57% male; 83% White). They were randomly assigned to receive 2 mg or 10 mg of bulevirtide subcutaneously daily for 144 weeks or, as a control group, receive no treatment for 48 weeks, followed by 10 mg of bulevirtide daily for 96 weeks. All patients were followed for 96 weeks after treatment ends.
For the primary endpoint, the combined viral and ALT response at week 48 was similar in the 2-mg (45%) and 10-mg (48%) groups, compared with 2% in the control group (one patient). Twelve percent of patients in the 2-mg group and 20% of patients in the 10-mg group had a clinical benefit, compared with none of the patients in the control group.
Among those with a combined response, normalization of the ALT level occurred in most patients by week 24, while the HDV RNA level continued to decline between week 24 and week 48, the authors wrote.
“This surrogate end point is considered to be a reasonably likely predictor of improved clinical outcomes in patients with HDV; however, longer-term data are needed to confirm the clinical benefit of bulevirtide,” the investigators wrote.
The results offer a glimmer of hope, Marc Ghany, MD, MHSc, of the National Institute of Diabetes and Digestive and Kidney Diseases wrote in an accompanying editorial. “The goal of HDV therapy is to improve patient survival by preventing progression to cirrhosis, liver failure, and liver cancer,” he wrote.
In safety results, headache, pruritus, fatigue, and eosinophilia were more common in the bulevirtide groups than in the control group. All adverse events were mild to moderate.
HDV infects about 5% of people with chronic HBV and relies on HBV surface antigen (HBsAg) for transmission and infectivity. Bulevirtide is derived from a region of the large envelope protein of HBsAg and irreversibly binds to the hepatocyte entry receptor for both HDV and HBV.
Bulevirtide has received conditional approval in the European Union. In 2022, the Food and Drug Administration declined to approve bulevirtide over concerns about production and delivery of the drug. There are no approved treatments for HDV in the United States.
The study was supported by Gilead Sciences. Dr. Wedemeyer disclosed research funding, acting as a consultant to, and giving paid lectures on behalf of Gilead Sciences. He and other coauthors disclosed financial relationships with Gilead and other pharmaceutical companies.
shows an ongoing phase 3 study conducted in the United States and four other countries.
The findings were published in the New England Journal of Medicine.
Led by Heiner Wedemeyer, MD, of Hannover Medical School in Germany, the study included 150 patients with HDV, with and without compensated cirrhosis (mean age, 42 years; 57% male; 83% White). They were randomly assigned to receive 2 mg or 10 mg of bulevirtide subcutaneously daily for 144 weeks or, as a control group, receive no treatment for 48 weeks, followed by 10 mg of bulevirtide daily for 96 weeks. All patients were followed for 96 weeks after treatment ends.
For the primary endpoint, the combined viral and ALT response at week 48 was similar in the 2-mg (45%) and 10-mg (48%) groups, compared with 2% in the control group (one patient). Twelve percent of patients in the 2-mg group and 20% of patients in the 10-mg group had a clinical benefit, compared with none of the patients in the control group.
Among those with a combined response, normalization of the ALT level occurred in most patients by week 24, while the HDV RNA level continued to decline between week 24 and week 48, the authors wrote.
“This surrogate end point is considered to be a reasonably likely predictor of improved clinical outcomes in patients with HDV; however, longer-term data are needed to confirm the clinical benefit of bulevirtide,” the investigators wrote.
The results offer a glimmer of hope, Marc Ghany, MD, MHSc, of the National Institute of Diabetes and Digestive and Kidney Diseases wrote in an accompanying editorial. “The goal of HDV therapy is to improve patient survival by preventing progression to cirrhosis, liver failure, and liver cancer,” he wrote.
In safety results, headache, pruritus, fatigue, and eosinophilia were more common in the bulevirtide groups than in the control group. All adverse events were mild to moderate.
HDV infects about 5% of people with chronic HBV and relies on HBV surface antigen (HBsAg) for transmission and infectivity. Bulevirtide is derived from a region of the large envelope protein of HBsAg and irreversibly binds to the hepatocyte entry receptor for both HDV and HBV.
Bulevirtide has received conditional approval in the European Union. In 2022, the Food and Drug Administration declined to approve bulevirtide over concerns about production and delivery of the drug. There are no approved treatments for HDV in the United States.
The study was supported by Gilead Sciences. Dr. Wedemeyer disclosed research funding, acting as a consultant to, and giving paid lectures on behalf of Gilead Sciences. He and other coauthors disclosed financial relationships with Gilead and other pharmaceutical companies.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Two-pronged approach needed in alcohol-associated hepatitis
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), concludes a review discussing care for patients recently hospitalized.
“Probably the biggest thing I would want providers to take away from the review is to remember that these patients are likely to carry a dual diagnosis,” said lead author Akshay Shetty, MD, Pfleger Liver Institute, UCLA Medical Center.
“It is important to address the liver disease, because it probably carries the biggest mortality and morbidity risk in the short term, but we have to remember to treat their alcohol use disorder simultaneously,” Dr. Shetty said.
The guidance by Dr. Shetty and coauthors was published online in the Journal of Clinical Gastroenterology.
More alcohol misuse means more liver disease
AH is a “unique, severe form of alcohol-associated steatohepatitis that is seen in the background of recent heavy alcohol use,” the team writes. Patients with severe AH have faced mortality rates as high as 20%-50%. A recent study reported a drop in 30-day mortality rates to 17%, which the authors credit to improved supportive medical management.
Alcohol misuse has surged over the past two decades, which experts believe will lead to a rise in alcohol-related liver disease, including AH hospitalization, the authors note. Rates of high-risk drinking in the United States (four or more drinks daily for women, five or more for men) increased by almost 30% between 2002 and 2012, particularly among women and ethnic minorities.
At the same time, rates of AUD rose 25% among young adults. In 2019, a U.S. survey found 14.5 million people aged 12 years and older in the United States carried an AUD diagnosis.
Meanwhile, the U.S. National Inpatient Sample revealed a 28.3% rise in AH-related hospitalizations between 2007 and 2014.
“AH patients carry a high short-term mortality [and] require close outpatient monitoring and significant care coordination,” write the authors. Despite the rising rates of severe AH, there is a lack of standardized guidance on post-discharge management, which motivated their clinical care review.
Liver disease shapes short-term outcomes
The management of patients with a recent episode of severe AH requires a two-pronged approach and shared patient management between gastroenterologists/hepatologists and addiction specialists. The multidisciplinary management both improves outcomes and is linked to reduced health care costs, the authors write.
While abstinence from alcohol remains essential to recovery, the authors note, it is the “severity of hepatic decompensation that has been shown to dictate short-term mortality in the initial 6 months” following discharge.
The team created an outpatient algorithm that divides patient care into two main areas: hepatic decompensation and AUD.
For the risk of hepatic decompensation, patients should undergo close monitoring for infections and frequent laboratory tests in the months following discharge.
Moreover, the “majority of patients with severe AH usually have background cirrhosis and are at risk of portal hypertensive decompensations similar to cirrhosis,” the authors write, and so patients should be assessed for hepatic encephalopathy, as well as for ascites and variceal bleeding.
For HE, the authors recommend a low threshold for treatment initiation with lactulose (a colonic acidifier) and the antibiotic rifaximin, but they suggest that ascites management “should be conservative ... with strict adherence to a low-sodium diet as the first-line approach.”
A key problem among severe AH patients post-discharge is malnutrition, which reaches 100% prevalence and is associated with the severity of liver disease, including decompensation and mortality, they note.
Patients with malnutrition are at risk of entering a catabolic starvation state. The authors recommend avoiding long fasting periods with multiple small meals and late evening snacks.
Long-term, severe AH patients should be assessed for advanced fibrosis, although early diagnosis is often challenging, as the clinical and laboratory results typically mimic findings of liver cirrhosis, the authors write.
Crucially, patients should be considered for early referral for liver transplantation, because early liver transplantation is associated with “excellent transplant outcomes and is noninferior when compared with other etiologies of chronic liver disease,” they write.
Long-term risk rests on preventing alcohol relapse
Turning to AUD, the team notes that long-term outcomes among AH patients depend on the prevention of alcohol relapse, because alcohol use among these patients is directly linked to higher rates of mortality and decompensation.
The authors concede that the “definition of relapse remains a matter of contention, especially in the post-liver transplant population,” but they recommend complete abstinence for patients recovering from AH and define relapse as any use of alcohol.
Dr. Shetty explained that “often, the focus tends to be on the acute threats to a patient’s life, so their liver disease tends to be emphasized, and we often forget why patients present with the liver disease in the first place.”
He continued: “So we do our best to address the liver disease and not a lot gets done for the alcohol-use disorder that the patient may have in the background. The expectation is that, if the doctors help patients with their liver disease, the patients will learn that lesson on their own and stop drinking.”
Instead, Dr. Shetty and his colleagues advise, all patients should be screened for AUD and undergo surveillance with alcohol biomarkers monthly at first. Patients should also be referred to an addiction specialist, where some combination of psychotherapy, mutual support groups, and pharmacotherapy can be tailored to individual patient needs and access.
Multidisciplinary management, comprising hepatology, psychiatry, psychologist, nurse, and social worker consults, has shown “promising results in the management of AUD, improvement in liver disease, and decrease in health care burden,” the authors write, although “multidisciplinary clinics often carry financial and administrative barriers to broad application.”
Moreover, these interventions require a commitment from the patient, at least in the short term, to allow the establishment of a therapeutic relationship between the clinician and the patient and aid compliance over the longer term.
“Patients with AUD remain reluctant to pursue treatment,” the authors write, “and a large-scale effort to improve knowledge gaps in regard to AUD treatment and its success is needed, both from patients’ primary care providers and their consultants.”
Dr. Shetty explained that patient engagement is “probably the most challenging aspect of the disease, especially the alcohol use disorder part.”
This is partly because patients often lack insight, and alcohol addiction carries stigma and shame, as well as self-blame, he said, and so patients will “often delay pursuing any therapy ... even when they are sick.”
Dr. Shetty believes that reducing the stigma around alcohol addiction will require better education of patients and health care providers. To that end, he noted that the scientific literature now avoids the pejorative “alcoholic” and instead describes alcohol use as a disorder rather than having it define the patient.
“But this educational aspect is going to take a long time to really take effect, so from a provider perspective ... it is important to be open-minded when seeing these patients,” he said. This means not focusing on “the medical aspect alone but trying to really see the person who’s come to you for help and understand their motivations for pursing medical care.”
“Despite all these things, some patients may still find it very challenging and awkward. It takes several visits to really establish a rapport with them and get a sense of how to get them to share the challenging aspects of the disease,” Dr. Shetty added.
Multidisciplinary management for optimal outcomes
In a comment, Nancy S. Reau, MD, chair of hepatology, Rush Medical College, Chicago, agreed with the need to address both the risk for hepatic decompensation and AUD, the benefits of multidisciplinary management of patients, and the importance of patient engagement to successful outcomes.
“As hepatologists, we are often best at managing liver disease, but if you don’t also address the alcohol use disorder, the patient will not have the optimal outcome,” she said in an interview. “Most patients with severe AH have cirrhosis, [which] makes longitudinal follow-up imperative.”
“They are at risk for liver complications but also need aggressive nutritional support and management of their addiction,” she said. “As they improve, they can usually continue intensive treatment.”
Akhil Anand, MD, an addiction psychiatrist and co-director of the Multidisciplinary Alcohol Program at the Cleveland Clinic, also noted the increase in cases of alcohol-associated hepatitis from rising alcohol use.
The review “provides a timely, comprehensive, and impartial overview” of how to manage the condition, he said, as well as “how to treat co-occurring alcohol use disorder in this life-threatening situation.”
No funding was declared. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Navigating NAFLD: Unveiling the approach to mitigate the impact of NAFLD
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15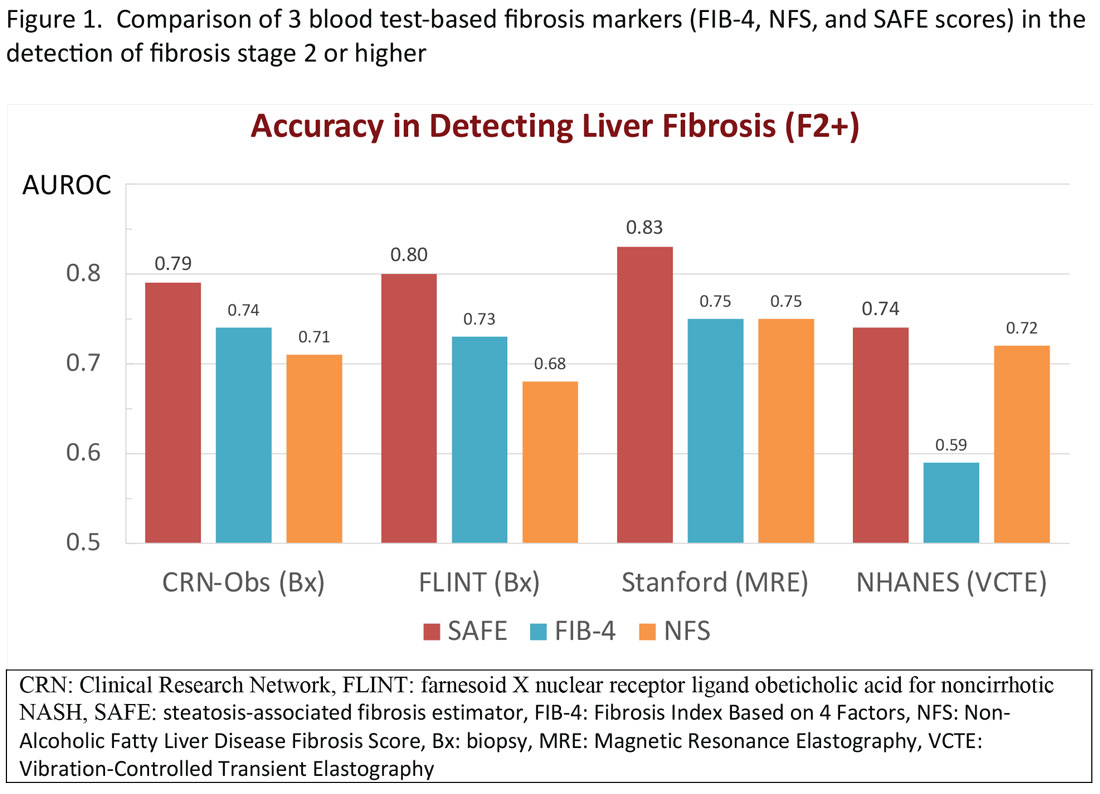
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16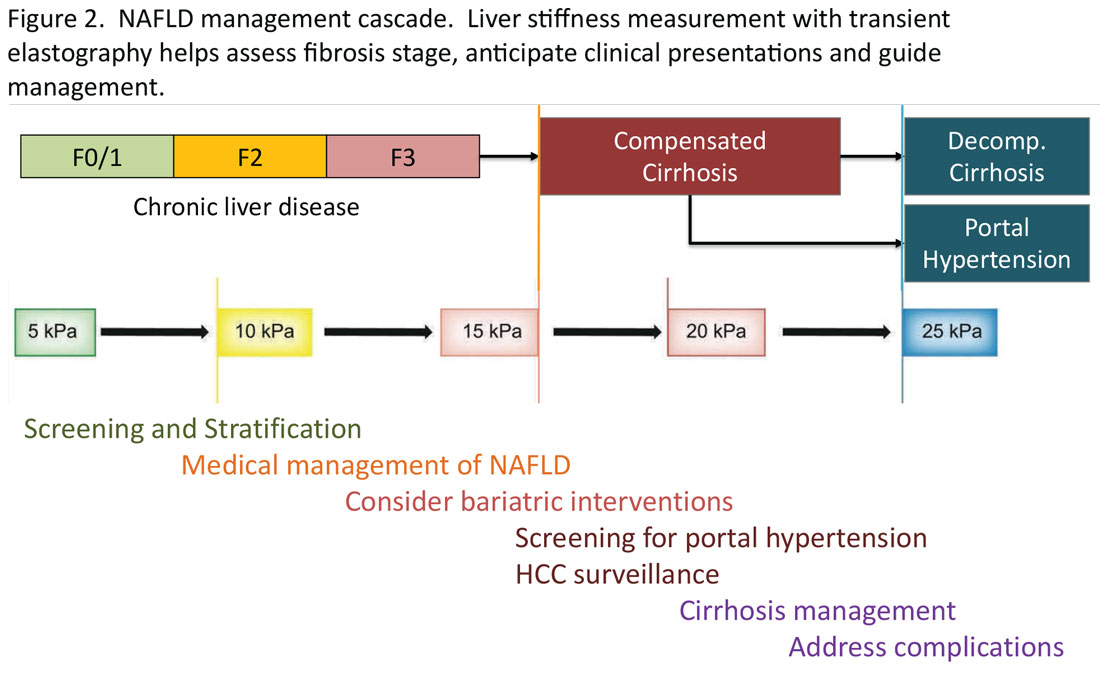
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Burden of NAFLD in the U.S.
NAFLD is a manifestation of systemic metabolic abnormalities, including insulin resistance, dyslipidemia, central obesity, and hypertension. In this short review, we summarize data on the burden of NAFLD in the U.S. and its prognostic determinants and review what clinical and public health approaches may be needed to mitigating its impact.
Epidemiology of NAFLD
Worldwide, the prevalence of NAFLD is estimated at 6% to 35%, with biopsy-based studies reporting NASH in 3% to 5%.1 U.S. estimates for the prevalence of NAFLD range from 10% to 46%.2 In our own analysis of the National Health and Nutrition Examination Survey (NHANES) data, transient elastography-detected steatosis was found in 36%, which projected to a minimum of 73 million American adults.3
NAFLD represents a spectrum of disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), the latter leading, in some cases, to progressive hepatic fibrosis and cirrhosis.4 Out of a large number of subjects with NAFLD, the proportions of NASH patients that develop severe liver problems such as end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) are progressively smaller. For example, we recently reported that less than 2,000 liver-related deaths are attributable to NAFLD in the U.S. per annum, which corresponds to a crude case fatality rate of < 0.005% per year.5
According to the Centers for Disease Control and Prevention (CDC), there have been substantial increases in liver-related deaths over the last 2 decades. Mortality from liver disease including hepatobiliary cancers more than doubled from 41,966 deaths (including 15,321 women and 26,645 men) in 2000 to 85,884 deaths (33,000 women and 52,884 men) in 2020. The proportion of deaths specifically attributed to NAFLD among liver-related deaths was miniscule in 2000, accounting for 1.1% in women and 0.7% in men. By 2020, the proportions increased several folds in both sexes (7.4% in women and 2.7% in men).6 Moreover, it is likely that a substantial portion of deaths from chronic liver disease from unknown causes (“cryptogenic”) are likely end-stage NAFLD, making these figures underestimates of the true impact of NAFLD in the U.S.
From a comparative epidemiologic perspective, there are significant racial and ethnic and socioeconomic disparities in NAFLD prevalence, wherein Hispanic persons and individuals experiencing food insecurity – independent of poverty status, education level, race and ethnicity – are disproportionately more affected by NAFLD.7,8 Furthermore, these disparities persist when examining long-term complications of NAFLD, such as developing HCC.
Prognosis in NAFLD: NASH versus fibrosis
Given the enormous prevalence and increasing public health burden of NAFLD, systematic interventions to mitigate its impact are urgently needed. Clearly, patients who already have developed advanced liver disease need to be directed to specialty care so the disease progression may be halted and complications of ESLD may be prevented or managed. On the other hand, in order to mitigate the future impact of ESLD, prompt identification of at-risk patients and proactive interventions to improve liver health are needed.
In the assessment of disease progression, prior data have shown that the presence of NASH and increasing stages of liver fibrosis are important predictors of disease progression. Fibrosis is a component of NASH, while NASH is thought to be a prerequisite for fibrosis. In a prospective, multicenter follow-up study of NAFLD evaluated by liver biopsies (n = 1,773), over a median follow-up of 4 years, 37 (2%) developed hepatic decompensation, while 47 (3%) died from any cause, which included ESLD (n = 12), cardiovascular complications (n = 4), and malignancies (n = 12), including HCC (n = 9).9 It is not entirely surprising that advanced fibrosis and cirrhosis was highly associated with the development of hepatic decompensation. In their multivariable analysis, patients with F3-4 had a 13.8-fold (95% confidence interval [CI]: 4.6, 41.0) increase in the hazard of reaching a MELD score of 15 compared to those with F0-2. In addition, all-cause mortality was 17.2-fold (95% CI: 5.2, 56.6) higher with F3-4 compared to F0-2.
These data have been borne out by a larger body of literature on the topic. In a recent meta-analysis assessing the relation between liver fibrosis and future mortality, which included 17,301 subjects with NAFLD, patients with at least stage 2 fibrosis experience a significantly increased risk of liver-related and overall mortality, a trend that accelerates at higher fibrosis stages.10 These point to liver fibrosis as the singular determinant of long-term prognosis, in comparison, for example, with the diagnosis of NASH. Hagström conducted a retrospective cohort study of patients with biopsy-proven NAFLD in Sweden. When fibrosis stage and histological diagnosis of NASH were considered together, NASH did not have an impact on overall mortality (hazard ratio [HR] = 0.83, P = .29) or liver morbidity (HR = 0.62, P = .25).11
On an individual level, factors that affect fibrosis progression are not as well studied. It is commonly believed that demographic factors (e.g., age, sex and race), genetic polymorphisms (e.g., PNPLA3, TM6SF2), clinical comorbidities (e.g., obesity, DM, and sleep apnea), and environmental factors (e.g., smoking) may accelerate fibrosis and disease outcomes, although prospective data are sparse to estimate the extent these individual variables affect progression.12 Recent guidelines remain silent about whether and how these data may be incorporated in screening for NAFLD in the population.
Assessment of liver fibrosis
The traditional means to detect liver fibrosis is liver histology, which also assesses steatosis, individual components of NASH and, often importantly, other concomitant liver pathology. In reality, however, liver biopsies have several limitations including the risk of complications, patient discomfort, economic costs, and sampling variability. Increasingly, “noninvasive” methods have been used to estimate liver fibrosis in patients with NAFLD. Liver elastography estimates the physical stiffness of the organ, which may be measured by MRI or ultrasound. Among ultrasound-based technologies, vibration-controlled transient elastography (VCTE) is more widely accepted and affordable although it may not be as accurate as MR elastography.13
In general, these elastographic tests are not readily accessible to most physicians outside hepatology specialty practices. Instead, blood test-based markers have been developed and widely recommended as the initial modality to assess liver fibrosis. Figure 1 represents a partial list of blood test-based markers. Traditionally, FIB-4 and NFS have been considered the most widely recommended by society guidelines. The AGA Pathway for evaluation of patients with NAFLD recommends first to apply the FIB-4 score and, in patients considered to be at intermediate risk of fibrosis for advanced fibrosis (stage 3 or 4, FIB-4 = 1.3-2.67), to assess liver stiffness by VCTE.14
More recently, the accumulating natural history data have highlighted the inflection in the risk of future outcomes coinciding with F2 and therapeutic trials that target patients with “at risk NASH,” thus more attention has been paid to the identification of patients with stage 2 (or higher). The steatosis-associated fibrosis estimator (SAFE) was developed for this specific purpose. The score has been validated in multiple data sets, in all of which SAFE outperformed FIB-4 and NFS (Figure 1). When the score was applied to assess overall survival in participants of the NHANES, patients with NAFLD deemed to be high risk (SAFE > 100) had significantly lower survival (37% Kaplan-Meier survival at 20 years), compared to those with intermediate (SAFE 0-100, 61% survival) and low (SAFE < 0, 86% survival). In comparison, the 20-year survival of subjects without NAFLD survival was 79%.15
Regardless of the modality for initial stratification, it is widely accepted that mechanical elastography constitutes the next step in prognosticating the patient. In the AGA Pathway, liver stiffness of < 8 kPa is considered low risk, which corresponds in most analysis with lack of stage 2 fibrosis, whereas stiffness of > 12 kPa may be indicative of stage 3 or 4. These recommendations are consistent with those from the latest Baveno Consensus Conference (“Baveno 7”). Figure 2 expands on the so-called “rule of 5” from the consensus document and correlates liver stiffness (by VCTE) with progression of liver fibrosis as well as clinical presentation. For example, liver stiffness < 15 kPa is associated with a low risk of clinically significant portal hypertension (CSPH). Similarly, in patients with a normal platelet count (>150,000/mm3) and liver stiffness < 20 kPa, the probability of gastroesophageal varices is sufficiently low that a screening endoscopy may be avoided. On the other hand, liver stiffness > 25 kPa is associated with increasing risk of decompensated cirrhosis and portal hypertension.16
Partnership between primary care and specialty
The insights expressed in Figure 2 can be utilized to guide management decisions. In patients without evidence of liver fibrosis, emphasis may primarily be on screening, stratification and management of metabolic syndrome. For patients with evidence of incipient liver fibrosis, medical management of NAFLD needs to be implemented including lifestyle changes and pharmacological interventions as appropriate. For patients unresponsive to medical therapy, an endoscopic or surgical bariatric procedure should be considered. Management of patients with evidence of cirrhosis includes screening for portal hypertension, surveillance for HCC, medical management of cirrhosis, and finally, in suitable cases, referral for liver transplant evaluation. The reader is referred to the latest treatment guidelines for detailed discussion of these individual management modalities [ref, AGA and AASLD guidelines].14,17
Given the spectrum of management modalities needed to successfully manage patients with NAFLD, it is unrealistic to expect that hepatologists and gastroenterologists are able to manage the large number of patients with NAFLD. In general, clinical activities on the left side of the figure are in the domain of primary care providers, whereas management of patients with progressive liver fibrosis is conducted by the specialist. An important aspect of the overall management of these patients is risk management in terms of the metabolic syndrome, including cardiovascular risk reduction and diabetes management, as appropriate. Many patients with NAFLD are burdened with several comorbidities and likely to benefit from a multidisciplinary team consisting of primary care, endocrinology, preventive cardiology, pharmacy, nutrition/dietetics, social services, and addiction specialists, as well as hepatology and gastroenterology. Prospective, high-quality data to define these teams and their function are yet to be generated.
Conclusion
NAFLD is an important and increasing public health concern in the U.S. Once diagnosed, assessing liver fibrosis and evaluating the presence of the components of metabolic syndrome in these patients, constitute the key components in the care in terms of risk stratification, medical management, and referral decisions. Noninvasive tests have been increasingly utilized including liver stiffness measurements and various blood test-based indicators. For patients in specialty GI/hepatology care, transient elastography is a widely accepted tool, with which standardized recommendations may be made for screening, stratification, and medical and surgical interventions in patients with NAFLD.
Mai Sedki, MD, MPH, is a doctoral candidate at the University of California, San Francisco. W. Ray Kim, MD, is professor of medicine (gastroenterology and hepatology) at Stanford (Calif.) University. Address correspondence to: wrkim@stanford.edu. The authors disclosed no conflicts of interest. Twitter: @SedkiMD and @WRayKimMD.
References
1. Younossi ZM et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020 Mar;69(3):564-8.
2. Lazo M et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013 Jul 1;178(1):38-45.
3. Kim D et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013 Apr;57:1357-65.
4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346:1221-31.
5. Kim D et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-63.e3.
6. FastStats. Chronic Liver Disease and Cirrhosis. Centers for Disease Control and Prevention.
7. Rich NE et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210. e2.
8. Coleman-Jensen A et al. Household food security in the United States in 2020 (ERR-298). Washington, DC: U.S. Department of Agriculture; Sep 2021.
9. Sanyal AJ et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021 Oct 21;385(17):1559-69.
10. Ng CH et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023 Apr;21(4):931-9.e5.
11. Hagström H et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-73.
12. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
13. Singh S et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015 Mar;13(3):440-51.e6.
14. Kanwal F et al. Clinical Care Pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021 Nov;161(5):1657-69.
15. Sripongpun P et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. .
16. de Franchis R et al. Baveno VII: Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
17. Rinella ME et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
Naltrexone is safe and beneficial in AUD with cirrhosis
VIENNA – , results of the first randomized controlled trial (RCT) show.
After 3 months, 64% of patients who received naltrexone were abstinent from alcohol, compared with 22% of patients who received placebo, Manasa Alla, MD, a hepatologist from the Institute of Liver and Biliary Sciences (ILBS), New Delhi, said at the European Association for the Study of the Liver (EASL) 2023, where she presented the study findings.
Importantly, naltrexone was found to be safe for patients with compensated cirrhosis. “This fragile population of patients has limited drugs to help them quit alcohol. Naltrexone can be a valuable addition to their measures to reduce craving and on their journey to reach de-addiction and abstinence,” Dr. Alla said.
Hepatotoxicity with naltrexone is rare and data are limited. The Food and Drug Administration previously placed a warning on its use for patients with alcoholic liver disease and underlying cirrhosis.
As a clinician constantly challenged with treating patients with AUD and cirrhosis, Dr. Alla wanted to explore the safety of naltrexone and to test its suitability for these patients who struggle to quit alcohol.
“Here we aimed to primarily test the safety of naltrexone in achieving abstinence and reducing alcohol cravings in patients with alcohol-related cirrhosis,” she said, adding, “The FDA black box warning has been removed, but it has never been tested in an RCT in patients with cirrhosis, so this is exactly what we did here.
“Naltrexone is a very good anti-alcohol-craving drug. If we can establish its safety in cirrhotic patients, it may have very good potential in reducing AUD and reducing the related complications of continued alcohol intake,” Dr. Alla said.
Safety, abstinence, lapse, and relapse assessed
The prospective, double-blind, single-center study at the ILBS in New Delhi, enrolled 100 patients with alcohol dependence and cirrhosis between 2020 and 2022. Participants were randomly assigned in a 1:1 ratio to receive naltrexone (50 mg/d) or placebo for 12 weeks. All participants attended regular counseling sessions with the resident psychiatrist. At baseline, the biochemical and drinking-related assessment scores between active and placebo groups of patients with compensated cirrhosis were matched.
Abstinence from alcohol was assessed through self-reported mean number of standard drinks (12 g alcohol per day). Findings were corroborated through an interview with a family member. Serum ethyl glucuronide levels were measured in cases of discrepancy. A relapse was considered to be consumption of over four standard alcoholic drinks/month; a lapse was considered any other alcohol drinking event not classified as relapse.
The primary outcome was the proportion of patients who achieved and maintained alcohol abstinence at 12 weeks; secondary outcomes were the proportion of patients who took naltrexone without a liver-related adverse effect compared with placebo at 12 weeks, the number of relapses and lapses, the difference in craving scores on the Obsessive Compulsive Drinking Scale (OCDS) between groups at 4, 8, and 12 weeks and at 6 months and 12 months, and the proportion of patients who achieved and maintained alcohol abstinence at 6 months.
Abstinence at 3 months
After 3 months, abstinence was noted in 64% of the study population who received naltrexone, compared to 22% of those who received placebo (P < .001). At 6 months, a higher proportion of patients in the naltrexone group achieved abstinence (22% vs. 8% with placebo; P = .09).
“We still need to look at the longer-term effects of naltrexone,” Dr. Alla said. “Here we gave the drug plus counseling for 3 months only, so despite encouraging findings, we need further studies to understand more.”
The researchers analyzed the predictors of abstinence at 3 months. They found that patients who consumed fewer than 17 drinks per month at baseline were more likely to achieve abstinence (sensitivity, 81%).
“Our study showed that patients who are consuming less alcohol at baseline can quit alcohol if adequately motivated. We need the motivation, as well as the drug,” she said.
Patient counseling was also very important and was provided for the 3 months of the study. “Even in the placebo arm, we had some patients who became abstinent [11/50 patients], but this dropped at 6 months [to 4/50],” Dr. Alla said.
At 12 weeks, 28% in the naltrexone group experienced relapse, vs. 72% in the placebo group (P < .001). Regarding the secondary outcome of craving scores and how they were affected by naltrexone, the mean OCDS-O (obsessive element) scores were 6.63, compared with 9.29 in naltrexone and placebo, respectively (P < .01). The mean OCDS-C (compulsive element) scores were 6.34 and 9.02, respectively (P < .01).
“Most important, was the safety of naltrexone in this study,” she said. There were no significant adverse events in either arm, and only one patient discontinued the drug in the naltrexone arm. Three patients in the naltrexone group who continued alcohol consumption developed jaundice, “so the jaundice can be attributed to continuous alcohol intake and may not be secondary to the naltrexone per se. We concluded that naltrexone is safe in a compensated cirrhotic patient,” Dr. Alla said.
Regarding other adverse events, 13.7% of patients experienced gastritis with naltrexone, vs. 3.7% among patients who received placebo. Nausea was more common in the placebo group, at 11.1% compared with 6.8% among patients who received naltrexone. Vomiting was more common in the naltrexone arm, at 10.3% vs. 7.4% with placebo. None of these differences reached statistical significance.
A longer-term study and comparisons to other drugs would provide valuable insights going forward.
Moderator Aleksander Krag, MD, professor and head of hepatology at the University of Southern Denmark and Odense University Hospital, Denmark, said: “Any intervention that can reduce or stop alcohol use in patients with cirrhosis and more advanced cirrhosis will improve outcome as well as reduce complications and mortality.
“In some cases, alcohol rehabilitation can completely revert the damaged liver. We have lots of data that show that continuous alcohol use at the more advanced stages can be devastating and reduction [in alcohol use] improves outcome. Therefore, any intervention that can help us to achieve this on behalf of all patients is most welcome,” he said.
Naltrexone (ADDTREX) and identical placebos were supplied by Rusan Pharma. Dr. Alla has disclosed no relevant financial relationships. Dr. Krag has served as speaker for Norgine, Siemens, and Nordic Bioscience and has participated in advisory boards for Norgine and Siemens outside the submitted work. He receives royalties from Gyldendal and Echosens.
A version of this article appeared on Medscape.com.
VIENNA – , results of the first randomized controlled trial (RCT) show.
After 3 months, 64% of patients who received naltrexone were abstinent from alcohol, compared with 22% of patients who received placebo, Manasa Alla, MD, a hepatologist from the Institute of Liver and Biliary Sciences (ILBS), New Delhi, said at the European Association for the Study of the Liver (EASL) 2023, where she presented the study findings.
Importantly, naltrexone was found to be safe for patients with compensated cirrhosis. “This fragile population of patients has limited drugs to help them quit alcohol. Naltrexone can be a valuable addition to their measures to reduce craving and on their journey to reach de-addiction and abstinence,” Dr. Alla said.
Hepatotoxicity with naltrexone is rare and data are limited. The Food and Drug Administration previously placed a warning on its use for patients with alcoholic liver disease and underlying cirrhosis.
As a clinician constantly challenged with treating patients with AUD and cirrhosis, Dr. Alla wanted to explore the safety of naltrexone and to test its suitability for these patients who struggle to quit alcohol.
“Here we aimed to primarily test the safety of naltrexone in achieving abstinence and reducing alcohol cravings in patients with alcohol-related cirrhosis,” she said, adding, “The FDA black box warning has been removed, but it has never been tested in an RCT in patients with cirrhosis, so this is exactly what we did here.
“Naltrexone is a very good anti-alcohol-craving drug. If we can establish its safety in cirrhotic patients, it may have very good potential in reducing AUD and reducing the related complications of continued alcohol intake,” Dr. Alla said.
Safety, abstinence, lapse, and relapse assessed
The prospective, double-blind, single-center study at the ILBS in New Delhi, enrolled 100 patients with alcohol dependence and cirrhosis between 2020 and 2022. Participants were randomly assigned in a 1:1 ratio to receive naltrexone (50 mg/d) or placebo for 12 weeks. All participants attended regular counseling sessions with the resident psychiatrist. At baseline, the biochemical and drinking-related assessment scores between active and placebo groups of patients with compensated cirrhosis were matched.
Abstinence from alcohol was assessed through self-reported mean number of standard drinks (12 g alcohol per day). Findings were corroborated through an interview with a family member. Serum ethyl glucuronide levels were measured in cases of discrepancy. A relapse was considered to be consumption of over four standard alcoholic drinks/month; a lapse was considered any other alcohol drinking event not classified as relapse.
The primary outcome was the proportion of patients who achieved and maintained alcohol abstinence at 12 weeks; secondary outcomes were the proportion of patients who took naltrexone without a liver-related adverse effect compared with placebo at 12 weeks, the number of relapses and lapses, the difference in craving scores on the Obsessive Compulsive Drinking Scale (OCDS) between groups at 4, 8, and 12 weeks and at 6 months and 12 months, and the proportion of patients who achieved and maintained alcohol abstinence at 6 months.
Abstinence at 3 months
After 3 months, abstinence was noted in 64% of the study population who received naltrexone, compared to 22% of those who received placebo (P < .001). At 6 months, a higher proportion of patients in the naltrexone group achieved abstinence (22% vs. 8% with placebo; P = .09).
“We still need to look at the longer-term effects of naltrexone,” Dr. Alla said. “Here we gave the drug plus counseling for 3 months only, so despite encouraging findings, we need further studies to understand more.”
The researchers analyzed the predictors of abstinence at 3 months. They found that patients who consumed fewer than 17 drinks per month at baseline were more likely to achieve abstinence (sensitivity, 81%).
“Our study showed that patients who are consuming less alcohol at baseline can quit alcohol if adequately motivated. We need the motivation, as well as the drug,” she said.
Patient counseling was also very important and was provided for the 3 months of the study. “Even in the placebo arm, we had some patients who became abstinent [11/50 patients], but this dropped at 6 months [to 4/50],” Dr. Alla said.
At 12 weeks, 28% in the naltrexone group experienced relapse, vs. 72% in the placebo group (P < .001). Regarding the secondary outcome of craving scores and how they were affected by naltrexone, the mean OCDS-O (obsessive element) scores were 6.63, compared with 9.29 in naltrexone and placebo, respectively (P < .01). The mean OCDS-C (compulsive element) scores were 6.34 and 9.02, respectively (P < .01).
“Most important, was the safety of naltrexone in this study,” she said. There were no significant adverse events in either arm, and only one patient discontinued the drug in the naltrexone arm. Three patients in the naltrexone group who continued alcohol consumption developed jaundice, “so the jaundice can be attributed to continuous alcohol intake and may not be secondary to the naltrexone per se. We concluded that naltrexone is safe in a compensated cirrhotic patient,” Dr. Alla said.
Regarding other adverse events, 13.7% of patients experienced gastritis with naltrexone, vs. 3.7% among patients who received placebo. Nausea was more common in the placebo group, at 11.1% compared with 6.8% among patients who received naltrexone. Vomiting was more common in the naltrexone arm, at 10.3% vs. 7.4% with placebo. None of these differences reached statistical significance.
A longer-term study and comparisons to other drugs would provide valuable insights going forward.
Moderator Aleksander Krag, MD, professor and head of hepatology at the University of Southern Denmark and Odense University Hospital, Denmark, said: “Any intervention that can reduce or stop alcohol use in patients with cirrhosis and more advanced cirrhosis will improve outcome as well as reduce complications and mortality.
“In some cases, alcohol rehabilitation can completely revert the damaged liver. We have lots of data that show that continuous alcohol use at the more advanced stages can be devastating and reduction [in alcohol use] improves outcome. Therefore, any intervention that can help us to achieve this on behalf of all patients is most welcome,” he said.
Naltrexone (ADDTREX) and identical placebos were supplied by Rusan Pharma. Dr. Alla has disclosed no relevant financial relationships. Dr. Krag has served as speaker for Norgine, Siemens, and Nordic Bioscience and has participated in advisory boards for Norgine and Siemens outside the submitted work. He receives royalties from Gyldendal and Echosens.
A version of this article appeared on Medscape.com.
VIENNA – , results of the first randomized controlled trial (RCT) show.
After 3 months, 64% of patients who received naltrexone were abstinent from alcohol, compared with 22% of patients who received placebo, Manasa Alla, MD, a hepatologist from the Institute of Liver and Biliary Sciences (ILBS), New Delhi, said at the European Association for the Study of the Liver (EASL) 2023, where she presented the study findings.
Importantly, naltrexone was found to be safe for patients with compensated cirrhosis. “This fragile population of patients has limited drugs to help them quit alcohol. Naltrexone can be a valuable addition to their measures to reduce craving and on their journey to reach de-addiction and abstinence,” Dr. Alla said.
Hepatotoxicity with naltrexone is rare and data are limited. The Food and Drug Administration previously placed a warning on its use for patients with alcoholic liver disease and underlying cirrhosis.
As a clinician constantly challenged with treating patients with AUD and cirrhosis, Dr. Alla wanted to explore the safety of naltrexone and to test its suitability for these patients who struggle to quit alcohol.
“Here we aimed to primarily test the safety of naltrexone in achieving abstinence and reducing alcohol cravings in patients with alcohol-related cirrhosis,” she said, adding, “The FDA black box warning has been removed, but it has never been tested in an RCT in patients with cirrhosis, so this is exactly what we did here.
“Naltrexone is a very good anti-alcohol-craving drug. If we can establish its safety in cirrhotic patients, it may have very good potential in reducing AUD and reducing the related complications of continued alcohol intake,” Dr. Alla said.
Safety, abstinence, lapse, and relapse assessed
The prospective, double-blind, single-center study at the ILBS in New Delhi, enrolled 100 patients with alcohol dependence and cirrhosis between 2020 and 2022. Participants were randomly assigned in a 1:1 ratio to receive naltrexone (50 mg/d) or placebo for 12 weeks. All participants attended regular counseling sessions with the resident psychiatrist. At baseline, the biochemical and drinking-related assessment scores between active and placebo groups of patients with compensated cirrhosis were matched.
Abstinence from alcohol was assessed through self-reported mean number of standard drinks (12 g alcohol per day). Findings were corroborated through an interview with a family member. Serum ethyl glucuronide levels were measured in cases of discrepancy. A relapse was considered to be consumption of over four standard alcoholic drinks/month; a lapse was considered any other alcohol drinking event not classified as relapse.
The primary outcome was the proportion of patients who achieved and maintained alcohol abstinence at 12 weeks; secondary outcomes were the proportion of patients who took naltrexone without a liver-related adverse effect compared with placebo at 12 weeks, the number of relapses and lapses, the difference in craving scores on the Obsessive Compulsive Drinking Scale (OCDS) between groups at 4, 8, and 12 weeks and at 6 months and 12 months, and the proportion of patients who achieved and maintained alcohol abstinence at 6 months.
Abstinence at 3 months
After 3 months, abstinence was noted in 64% of the study population who received naltrexone, compared to 22% of those who received placebo (P < .001). At 6 months, a higher proportion of patients in the naltrexone group achieved abstinence (22% vs. 8% with placebo; P = .09).
“We still need to look at the longer-term effects of naltrexone,” Dr. Alla said. “Here we gave the drug plus counseling for 3 months only, so despite encouraging findings, we need further studies to understand more.”
The researchers analyzed the predictors of abstinence at 3 months. They found that patients who consumed fewer than 17 drinks per month at baseline were more likely to achieve abstinence (sensitivity, 81%).
“Our study showed that patients who are consuming less alcohol at baseline can quit alcohol if adequately motivated. We need the motivation, as well as the drug,” she said.
Patient counseling was also very important and was provided for the 3 months of the study. “Even in the placebo arm, we had some patients who became abstinent [11/50 patients], but this dropped at 6 months [to 4/50],” Dr. Alla said.
At 12 weeks, 28% in the naltrexone group experienced relapse, vs. 72% in the placebo group (P < .001). Regarding the secondary outcome of craving scores and how they were affected by naltrexone, the mean OCDS-O (obsessive element) scores were 6.63, compared with 9.29 in naltrexone and placebo, respectively (P < .01). The mean OCDS-C (compulsive element) scores were 6.34 and 9.02, respectively (P < .01).
“Most important, was the safety of naltrexone in this study,” she said. There were no significant adverse events in either arm, and only one patient discontinued the drug in the naltrexone arm. Three patients in the naltrexone group who continued alcohol consumption developed jaundice, “so the jaundice can be attributed to continuous alcohol intake and may not be secondary to the naltrexone per se. We concluded that naltrexone is safe in a compensated cirrhotic patient,” Dr. Alla said.
Regarding other adverse events, 13.7% of patients experienced gastritis with naltrexone, vs. 3.7% among patients who received placebo. Nausea was more common in the placebo group, at 11.1% compared with 6.8% among patients who received naltrexone. Vomiting was more common in the naltrexone arm, at 10.3% vs. 7.4% with placebo. None of these differences reached statistical significance.
A longer-term study and comparisons to other drugs would provide valuable insights going forward.
Moderator Aleksander Krag, MD, professor and head of hepatology at the University of Southern Denmark and Odense University Hospital, Denmark, said: “Any intervention that can reduce or stop alcohol use in patients with cirrhosis and more advanced cirrhosis will improve outcome as well as reduce complications and mortality.
“In some cases, alcohol rehabilitation can completely revert the damaged liver. We have lots of data that show that continuous alcohol use at the more advanced stages can be devastating and reduction [in alcohol use] improves outcome. Therefore, any intervention that can help us to achieve this on behalf of all patients is most welcome,” he said.
Naltrexone (ADDTREX) and identical placebos were supplied by Rusan Pharma. Dr. Alla has disclosed no relevant financial relationships. Dr. Krag has served as speaker for Norgine, Siemens, and Nordic Bioscience and has participated in advisory boards for Norgine and Siemens outside the submitted work. He receives royalties from Gyldendal and Echosens.
A version of this article appeared on Medscape.com.
AT EASL CONGRESS 2023