User login
‘Concerning’ uptick in pediatric antipsychotic prescribing
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Ecopipam reduces Tourette’s tics without common side effects in phase 2 trial
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
Ecopipam, in development for Tourette syndrome in children and adolescents, has shown in a randomized, controlled trial that, compared with placebo, it reduced tics and reduced the risk for some of the common side effects of other treatments, including weight gain.
Findings of the multicenter, double-blind, trial funded by the drug maker, Emalex Biosciences, were published online in Pediatrics. The trial was conducted at 68 sites in the United States, Canada, Germany, France, and Poland between May 2019 and September 2021.
Donald L. Gilbert, MD, MS, with the division of neurology at Cincinnati Children’s Hospital, and colleagues noted that all Food and Drug Administration–approved medications for Tourette syndrome are antipsychotics. The medications carry a risk of weight gain, electrocardiogram abnormalities, metabolic changes, and drug-induced movement disorders.
First-in-class medication ecopipam, targets the D1 dopamine receptor, while currently approved medications block the D2 receptor. It “may be a safe and effective treatment of Tourette syndrome with advantages over other currently approved therapeutic agents,” the authors wrote.
The study included 153 individuals at least 6 years old up to age 18 with a baseline Yale Global Tic Severity Score Total Tic Score of at least 20.
They were randomly assigned 1:1 to ecopipam or placebo.
Significant reduction in tic severity
Researchers saw a 30% reduction in the tic severity score from baseline to week 12 for the ecopipam group compared with the placebo group.
The data showed a least-squares mean difference of 3.44 (95% confidence interval [CI], 6.09-0.79, P = .01). Researchers also saw improvement in Clinical Global Impression of Tourette Syndrome Severity in the ecopipam group (P = .03).
Sara Pawlowski, MD, division chief for primary care mental health integration at University of Vermont Health Network and assistant professor of psychiatry, University of Vermont, Burlington, said in an interview that several things should be considered with this research.
One is that, though the results show a reduction in tics, the study lasted only 12 weeks and “tics can last a lifetime,” she noted.
“They also can ebb and flow with major life events, stressors, and various other variables. So, I wonder how the effects of improvement can be teased out from the natural ebb and flow of the condition in a 3-month window, which is a snapshot into the course of a known relapsing, remitting, lifetime, and chronically variable condition,” she said.
Headaches, insomnia among side effects
Weight gain was larger in the placebo group than in the ecopipam group: 17.1% in the ecopipam group and 20.3% of those who got a placebo had a weight gain of more than 7% over the study period.
The most common side effects of the study drug were headache (15.8%), insomnia (14.5%), fatigue (7.9%), and somnolence (7.9%).
A limitation of the study was lack of racial and ethnic diversity, as 93.5% of those in the placebo group and 86.8% in the ecopipam group were White.
Guidelines in North America and Europe agree that behavioral treatments should be the first-line therapy.
Dr. Pawlowski said that although effective medications are needed, she urges focusing on better access to nonmedication treatments “that work for children and adolescents” as children who start taking the medications early may take them for the rest of their lives.
Also, while the research didn’t find weight gain in the ecopipam group, the side effects they did find in the group, including headache and insomnia, “do impact a child’s life,” she noted.
“We also can’t be reassured that over the course of chronic treatment there wouldn’t be movement disorders or metabolic disorders that emerge. Those are side effects or disorders that can emerge surreptitiously over time, and more time than 12 weeks,” she said.
The study was funded by Emalex Biosciences. Dr. Gilbert has received consulting fees from Biogen and PTC therapeutics. Study coauthors disclosed ties with Emalex, Alkermes, and Paragon Biosciences. Dr. Pawlowski reports no relevant financial relationships.
FROM PEDIATRICS
Antiepileptic drugs tied to increased Parkinson’s disease risk
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.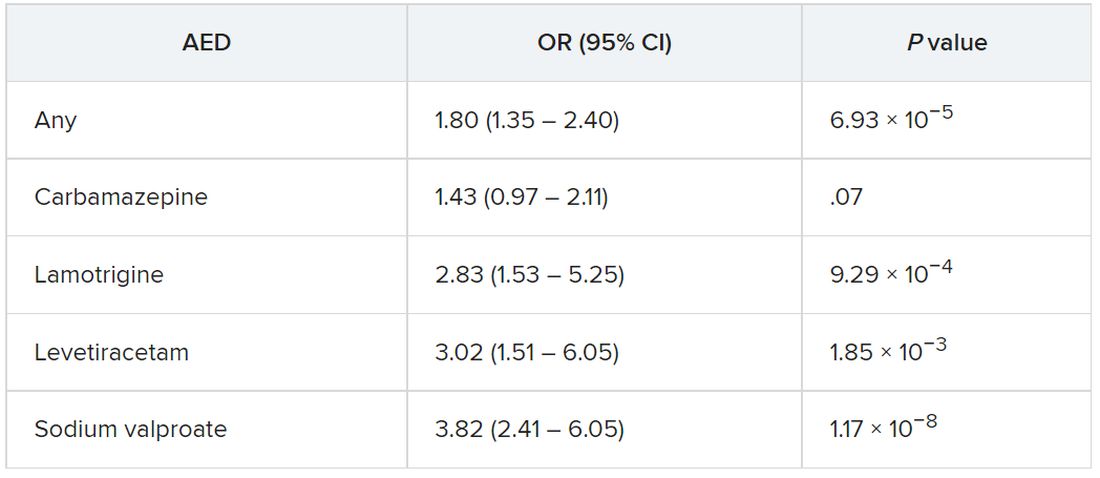
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Prodromal Parkinson’s disease tied to significant functional impairment
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Annual U.S. Parkinson’s disease incidence 50% higher than earlier estimates
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that investigators say highlights the growing strain on clinical services and the need for more research funding.
In an analysis of five databases and more than 15 million people, about 60,000-90,000 individuals older than 45 years are estimated to be diagnosed with Parkinson’s disease each year – which is far more than the previous estimate of around 40,000-60,000 new cases annually.
This is the latest study to update decades-old epidemiologic data on Parkinson’s disease incidence and prevalence. Previous incidence rates came from small, single-population studies that are now more than 25 years old.
“In the advocacy community, we’ve been earnest about the impact of people living with Parkinson’s disease, and what we really lacked was sufficient data to be able to demonstrate the urgency of our need,” said study coinvestigator James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, New York.
“We wanted to revise these numbers, highlight that they are larger than people anticipated, and use it as a call to action to change the approach we have toward Parkinson’s,” Dr. Beck said.
The findings were published online in NPJ Parkinson’s Disease.
Updating an outdated model
The study builds on the Parkinson’s Prevalence Project, a 2018 initiative that used a new model to calculate Parkinson’s disease prevalence. Before then, federal prevalence data was based on a 40-year-old study of just 26 Parkinson’s disease cases in one small county in rural Mississippi.
Dr. Beck and others used a more sophisticated model, using data from five separate cohort studies. They estimated the total number of patients living with Parkinson’s disease in the United States to be 930,000, which is far higher than the 650,000 the old model predicted.
Researchers then moved on to the current project, developing a new method to estimate Parkinson’s disease incidence.
The project included 2012 data on more than 15 million individuals in the United States and Canada. The investigators drew from three large insurance databases (Kaiser Permanente Northern California, Ontario Health Care, and Medicare) and two long-term epidemiologic studies (the Honolulu-Asia Aging Study and the Rochester Epidemiology Project).
On the basis of their analysis, the investigators proposed a working Parkinson’s disease incident rate estimate of 47-77 cases per 100,000 people aged 45 years or older. Limiting the analysis to those aged 65 or older raised the incidence to 108-212 per 100,000 people.
That translates to 60,000-95,000 new cases each year among adults aged 45 years or older. Using the Medicare administrative database alone for this same time period suggests an annual incidence of nearly 90,000 for individuals aged 65 or older.
“The numbers we’re proposing are conservative,” Dr. Beck said. “The true numbers are probably north of 90,000.”
Incidence rates increased with age and were higher in men. The researchers also identified clusters of counties with higher incidence rates in parts of the country called the “Parkinson’s belt.”
That geographic area mirrors the Rust Belt and includes parts of the Northeastern and Midwestern United States with a long history of industrial manufacturing that used heavy metals and industrial solvents, which are environmental factors linked to risk for Parkinson’s disease.
Cases were also higher in southern California, southeastern Texas, and Florida – agricultural regions with high pesticide use, which is also a risk factor for Parkinson’s disease. Central Pennsylvania also had higher incidence rates.
Why the increase?
The increase in cases could be the result of the more comprehensive estimation model used, the researchers noted. Or it could be improved detection, the aging population, a rise in sedentary lifestyles, increased exposure to environmental risk factors, or even the sharp decline in smoking in the United States, as some studies have shown that smokers have a lower Parkinson’s disease risk.
“The short answer is, we don’t know; and the long answer is, it’s all the above,” Dr. Beck said.
Although about 15% of Parkinson’s disease cases have a genetic basis, the cause is unknown in the majority of cases. In addition, diagnosis is difficult because there is no blood test or scan that detects the disease.
“Diagnosis requires a skilled clinician with real familiarity with Parkinson’s. And we have a real shortage of neurologists in this country to not only be able to diagnose but also to treat the condition,” Dr. Beck said.
That was one motivation for doing the study: to highlight what experts say is a pending clinical crisis for patients with Parkinson’s disease, he added.
The investigators also wanted to raise awareness about the scope of the disorder – not just about prevalence and incidence but also what those data mean for the health care industry, research aims, drug development and health care coverage, and policies.
In a 2020 study, the same researchers calculated a cost of $52 billion per year for medical and nonmedical costs related to Parkinson’s disease, which works out to about $26,000 per year per patient. That figure is expected to surpass $79 billion by 2030.
“This is an urgent condition for many people who live with the disease. And to the extent we can get our country to recognize that and really make the investment now, this is an area where a stitch in time saves nine,” Dr. Beck said.
“If we can invest some money now, we have a chance to really make a difference in the future,” he added.
‘Groundbreaking’ findings
Commenting on the findings, Jori Fleisher, MD, MSCE, associate professor of neurological sciences at Rush University Medical Center, Chicago, called the results “groundbreaking” and said that they validate what clinicians have been seeing in real-world practice.
“The findings reflect what a lot of us in practice have been appreciating anecdotally, which is that it seems that Parkinson’s is being diagnosed more frequently and that the incidence has been rising,” said Dr. Fleisher, who was not involved with the study.
She noted that the use of multiple datasets is one element of the methodology that makes the data so significant.
“There has been great work out of individual centers; but no matter how good your study methods are within that one population, you’re drawing conclusions based on that one population,” Dr. Fleisher said.
This research, together with the previous work by the group on prevalence data, could go a long way toward raising awareness about the scope of Parkinson’s disease in the United States – which could lead to earlier diagnosis, more research funding, and increased attention on the need for more clinicians who specialize in movement disorders, she added.
“This should increase research funding across the spectrum, including everything from the basic science to translational research, clinical research and implementation, and health services research,” Dr. Fleisher said.
The study was supported by the Parkinson’s Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the Institute for Clinical Evaluative Sciences. Dr. Beck and Dr. Fleisher reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NPJ PARKINSON’S DISEASE
High drug costs exclude most neurology patients from cutting-edge treatment
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Advancing health equity in neurology is essential to patient care
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
FROM ANA 2022
AAP issues clinical update to cerebral palsy guidelines
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
FROM PEDIATRICS
Children with autism show distinct brain features related to motor impairment
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
Previous research suggests that individuals with ASD overlap in motor impairment with those with DCD. But these two conditions may differ significantly in some areas, as children with ASD tend to show weaker skills in social motor tasks such as imitation, wrote Emil Kilroy, PhD, of the University of Southern California, Los Angeles, and colleagues.
The neurobiological basis of autism remains unknown, despite many research efforts, in part because of the heterogeneity of the disease, said corresponding author Lisa Aziz-Zadeh, PhD, also of the University of Southern California, in an interview.
Comorbidity with other disorders is a strong contributing factor to heterogeneity, and approximately 80% of autistic individuals have motor impairments and meet criteria for a diagnosis of DCD, said Dr. Aziz-Zadeh. “Controlling for other comorbidities, such as developmental coordination disorder, when trying to understand the neural basis of autism is important, so that we can understand which neural circuits are related to [core symptoms of autism] and which ones are related to motor impairments that are comorbid with autism, but not necessarily part of the core symptomology,” she explained. “We focused on white matter pathways here because many researchers now think the underlying basis of autism, besides genetics, is brain connectivity differences.”
In their study published in Scientific Reports, the researchers reviewed data from whole-brain correlational tractography for 22 individuals with autism spectrum disorder, 16 with developmental coordination disorder, and 21 normally developing individuals, who served as the control group. The mean age of the participants was approximately 11 years; the age range was 8-17 years.
Overall, patterns of brain diffusion (movement of fluid, mainly water molecules, in the brain) were significantly different in ASD children, compared with typically developing children.
The ASD group showed significantly reduced diffusivity in the bilateral fronto-parietal cingulum and the left parolfactory cingulum. This finding reflects previous studies suggesting an association between brain patterns in the cingulum area and ASD. But the current study is “the first to identify the fronto-parietal and the parolfactory portions of the cingulum as well as the anterior caudal u-fibers as specific to core ASD symptomatology and not related to motor-related comorbidity,” the researchers wrote.
Differences in brain diffusivity were associated with worse performance on motor skills and behavioral measures for children with ASD and children with DCD, compared with controls.
Motor development was assessed using the Total Movement Assessment Battery for Children-2 (MABC-2) and the Florida Apraxia Battery modified for children (FAB-M). The MABC-2 is among the most common tools for measuring motor skills and identifying clinically relevant motor deficits in children and teens aged 3-16 years. The test includes three subtest scores (manual dexterity, gross-motor aiming and catching, and balance) and a total score. Scores are based on a child’s best performance on each component, and higher scores indicate better functioning. In the new study, The MABC-2 total scores averaged 10.57 for controls, compared with 5.76 in the ASD group, and 4.31 in the DCD group.
Children with ASD differed from the other groups in social measures. Social skills were measured using several tools, including the Social Responsivity Scale (SRS Total), which is a parent-completed survey that includes a total score designed to reflect the severity of social deficits in ASD. It is divided into five subscales for parents to assess a child’s social skill impairment: social awareness, social cognition, social communication, social motivation, and mannerisms. Scores for the SRS are calculated in T-scores, in which a score of 50 represents the mean. T-scores of 59 and below are generally not associated with ASD, and patients with these scores are considered to have low to no symptomatology. Scores on the SRS Total in the new study were 45.95, 77.45, and 55.81 for the controls, ASD group, and DCD group, respectively.
Results should raise awareness
“The results were largely predicted in our hypotheses – that we would find specific white matter pathways in autism that would differ from [what we saw in typically developing patients and those with DCD], and that diffusivity in ASD would be related to socioemotional differences,” Dr. Aziz-Zadeh said, in an interview.
“What was surprising was that some pathways that had previously been thought to be different in autism were also compromised in DCD, indicating that they were common to motor deficits which both groups shared, not to core autism symptomology,” she noted.
A message for clinicians from the study is that a dual diagnosis of DCD is often missing in ASD practice, said Dr. Aziz-Zadeh. “Given that approximately 80% of children with ASD have DCD, testing for DCD and addressing potential motor issues should be more common practice,” she said.
Dr. Aziz-Zadeh and colleagues are now investigating relationships between the brain, behavior, and the gut microbiome. “We think that understanding autism from a full-body perspective, examining interactions between the brain and the body, will be an important step in this field,” she emphasized.
The study was limited by several factors, including the small sample size, the use of only right-handed participants, and the use of self-reports by children and parents, the researchers noted. Additionally, they noted that white matter develops at different rates in different age groups, and future studies might consider age as a factor, as well as further behavioral assessments, they said.
Small sample size limits conclusions
“Understanding the neuroanatomic differences that may contribute to the core symptoms of ASD is a very important goal for the field, particularly how they relate to other comorbid symptoms and neurodevelopmental disorders,” said Michael Gandal, MD, of the department of psychiatry at the University of Pennsylvania, Philadelphia, and a member of the Lifespan Brain Institute at the Children’s Hospital of Philadelphia, in an interview.
“While this study provides some clues into how structural connectivity may relate to motor coordination in ASD, it will be important to replicate these findings in a much larger sample before we can really appreciate how robust these findings are and how well they generalize to the broader ASD population,” Dr. Gandal emphasized.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Gandal had no financial conflicts to disclose.
FROM SCIENTIFIC REPORTS
Research fails to justify post-COVID-19 wave of new-onset parkinsonism
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
FROM MDS 2022




