User login
Influenza tied to long-term increased risk for Parkinson’s disease
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Art therapy linked to slowed Parkinson’s progression
Adding art therapy to standard drug treatment in Parkinson’s disease (PD) not only improves severity of both motor and nonmotor symptoms, but also slows rates of disease progression, new research suggests.
Fifty PD patients were randomly assigned to receive either art therapy, including sculpting and drawing, plus drug therapy or drug therapy alone, and followed up over 12 months.
Patients receiving combined therapy experienced improvements in symptoms, depression, and cognitive scores, and had reduced tremor and daytime sleepiness. They were also substantially less likely to experience disease progression.
“The use of art therapy can reduce the severity of motor and nonmotor manifestations of Parkinson’s disease,” said study investigator Iryna Khubetova, MD, PhD, head of the neurology department, Odessa (Ukraine) Regional Clinical Hospital.
she added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
A promising approach
Dr. Khubetova told this news organization that offering art therapy to PD patients was “very affordable,” especially as professional artists “provided materials for painting and other art supplies free of charge.”
“We hope this approach is very promising and would be widely adopted.”
She suggested the positive effect of art therapy could be related to “activating the brain’s reward neural network.”
This may be via improved visual attention acting on visuospatial mechanisms and emotional drive, with “activation of the medial orbitofrontal cortex, ventral striatum, and other structures.”
The researchers note PD, a “multisystem progressive neurodegenerative disease,” is among the three most common neurological disorders, with an incidence of 100-150 cases per 100,000 people.
They also note that nonpharmacologic approaches are “widely used” as an adjunct to drug therapy and as part of an “integrated approach” to disease management.
To examine the clinical efficacy of art therapy, the team recruited patients with PD who had preserved facility for independent movement, defined as stages 1-2.5 on the Hoehn and Yahr scale.
Patients were randomly assigned to art therapy sessions alongside standard drug therapy or to standard drug therapy alone. The art therapy included sculpting, free drawing, and coloring patterns.
Multiple benefits
Participants were assessed at baseline and at 6 and 12 months with the Unified Parkinson Disease Rating Scale (UPDRS), the Beck Depression Inventory, the Montreal Cognitive Assessment, and the Pegboard Test of finger dexterity.
Fifty patients were included in the study, with 30 assigned to standard drug therapy alone and 20 to the combined intervention. Participants had a mean age of 57.8 years, and 46% were women.
Over the study period, investigators found patients assigned to art therapy plus drug treatment had improved mood, as well as decreased daytime sleeping, reduced tremor, and a decrease in anxiety and fear intensity.
Between baseline and the 6- and 12-month assessments, patients in the combined therapy group showed improvements in scores on all of the questionnaires, and on the Pegboard Test. In contrast, scores were either stable or worsened in the standard drug therapy–alone group.
The team notes that there was also a marked difference in rates of disease progression, defined as a change on the Hoehn and Yahr scale of at least 0.5 points, between the two groups.
Only two (10%) patients in the combined drug and art therapy progressed over the study period, compared with 10 (33%) in the control group (P = .05).
The findings complement those of a recent study conducted by Alberto Cucca, MD, of the Fresco Institute for Parkinson’s and Movement Disorders, New York University, and colleagues.
Eighteen patients took part in the prospective, open-label trial. They were assessed before and after 20 sessions of art therapy on a range of measures.
Results revealed that following the art therapy, patients had improvements in the Navon Test (which assesses visual neglect, eye tracking, and UPDRS scores), as well as significantly increased functional connectivity levels in the visual cortex on resting-state functional MRI.
Many benefits, no side effects
Rebecca Gilbert, MD, PhD, vice president and chief scientific officer of the American Parkinson Disease Association, who was not involved in either study, told this news organization that the idea of art therapy for patients with Parkinson’s is “very reasonable.”
She highlighted that “people with Parkinson’s have many issues with their visuospatial abilities,” as well as their depth and distance perception, and so “enhancing that aspect could potentially be very beneficial.”
“So I’m hopeful that it’s a really good avenue to explore, and the preliminary data are very exciting.”
Dr. Gilbert also highlighted that the “wonderful” aspect of art therapy is that there are “so many benefits and not really any side effects.” Patients can “take the meds … and then enhance that with various therapies, and this would be an additional option.”
Another notable aspect of art therapy is the “social element” and the sense of “camaraderie,” although that has “to be teased out from the benefits you would get from the actual art therapy.”
Finally, Dr. Gilbert pointed out that the difference between the current trial and Dr. Cucca’s trial is the presence of a control group.
“Of course, it’s not blinded, because you know whether you got therapy or not … but that extra element of being able to compare with a group that didn’t get the treatment gives it a little more weight in terms of the field.”
No funding was declared. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding art therapy to standard drug treatment in Parkinson’s disease (PD) not only improves severity of both motor and nonmotor symptoms, but also slows rates of disease progression, new research suggests.
Fifty PD patients were randomly assigned to receive either art therapy, including sculpting and drawing, plus drug therapy or drug therapy alone, and followed up over 12 months.
Patients receiving combined therapy experienced improvements in symptoms, depression, and cognitive scores, and had reduced tremor and daytime sleepiness. They were also substantially less likely to experience disease progression.
“The use of art therapy can reduce the severity of motor and nonmotor manifestations of Parkinson’s disease,” said study investigator Iryna Khubetova, MD, PhD, head of the neurology department, Odessa (Ukraine) Regional Clinical Hospital.
she added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
A promising approach
Dr. Khubetova told this news organization that offering art therapy to PD patients was “very affordable,” especially as professional artists “provided materials for painting and other art supplies free of charge.”
“We hope this approach is very promising and would be widely adopted.”
She suggested the positive effect of art therapy could be related to “activating the brain’s reward neural network.”
This may be via improved visual attention acting on visuospatial mechanisms and emotional drive, with “activation of the medial orbitofrontal cortex, ventral striatum, and other structures.”
The researchers note PD, a “multisystem progressive neurodegenerative disease,” is among the three most common neurological disorders, with an incidence of 100-150 cases per 100,000 people.
They also note that nonpharmacologic approaches are “widely used” as an adjunct to drug therapy and as part of an “integrated approach” to disease management.
To examine the clinical efficacy of art therapy, the team recruited patients with PD who had preserved facility for independent movement, defined as stages 1-2.5 on the Hoehn and Yahr scale.
Patients were randomly assigned to art therapy sessions alongside standard drug therapy or to standard drug therapy alone. The art therapy included sculpting, free drawing, and coloring patterns.
Multiple benefits
Participants were assessed at baseline and at 6 and 12 months with the Unified Parkinson Disease Rating Scale (UPDRS), the Beck Depression Inventory, the Montreal Cognitive Assessment, and the Pegboard Test of finger dexterity.
Fifty patients were included in the study, with 30 assigned to standard drug therapy alone and 20 to the combined intervention. Participants had a mean age of 57.8 years, and 46% were women.
Over the study period, investigators found patients assigned to art therapy plus drug treatment had improved mood, as well as decreased daytime sleeping, reduced tremor, and a decrease in anxiety and fear intensity.
Between baseline and the 6- and 12-month assessments, patients in the combined therapy group showed improvements in scores on all of the questionnaires, and on the Pegboard Test. In contrast, scores were either stable or worsened in the standard drug therapy–alone group.
The team notes that there was also a marked difference in rates of disease progression, defined as a change on the Hoehn and Yahr scale of at least 0.5 points, between the two groups.
Only two (10%) patients in the combined drug and art therapy progressed over the study period, compared with 10 (33%) in the control group (P = .05).
The findings complement those of a recent study conducted by Alberto Cucca, MD, of the Fresco Institute for Parkinson’s and Movement Disorders, New York University, and colleagues.
Eighteen patients took part in the prospective, open-label trial. They were assessed before and after 20 sessions of art therapy on a range of measures.
Results revealed that following the art therapy, patients had improvements in the Navon Test (which assesses visual neglect, eye tracking, and UPDRS scores), as well as significantly increased functional connectivity levels in the visual cortex on resting-state functional MRI.
Many benefits, no side effects
Rebecca Gilbert, MD, PhD, vice president and chief scientific officer of the American Parkinson Disease Association, who was not involved in either study, told this news organization that the idea of art therapy for patients with Parkinson’s is “very reasonable.”
She highlighted that “people with Parkinson’s have many issues with their visuospatial abilities,” as well as their depth and distance perception, and so “enhancing that aspect could potentially be very beneficial.”
“So I’m hopeful that it’s a really good avenue to explore, and the preliminary data are very exciting.”
Dr. Gilbert also highlighted that the “wonderful” aspect of art therapy is that there are “so many benefits and not really any side effects.” Patients can “take the meds … and then enhance that with various therapies, and this would be an additional option.”
Another notable aspect of art therapy is the “social element” and the sense of “camaraderie,” although that has “to be teased out from the benefits you would get from the actual art therapy.”
Finally, Dr. Gilbert pointed out that the difference between the current trial and Dr. Cucca’s trial is the presence of a control group.
“Of course, it’s not blinded, because you know whether you got therapy or not … but that extra element of being able to compare with a group that didn’t get the treatment gives it a little more weight in terms of the field.”
No funding was declared. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding art therapy to standard drug treatment in Parkinson’s disease (PD) not only improves severity of both motor and nonmotor symptoms, but also slows rates of disease progression, new research suggests.
Fifty PD patients were randomly assigned to receive either art therapy, including sculpting and drawing, plus drug therapy or drug therapy alone, and followed up over 12 months.
Patients receiving combined therapy experienced improvements in symptoms, depression, and cognitive scores, and had reduced tremor and daytime sleepiness. They were also substantially less likely to experience disease progression.
“The use of art therapy can reduce the severity of motor and nonmotor manifestations of Parkinson’s disease,” said study investigator Iryna Khubetova, MD, PhD, head of the neurology department, Odessa (Ukraine) Regional Clinical Hospital.
she added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
A promising approach
Dr. Khubetova told this news organization that offering art therapy to PD patients was “very affordable,” especially as professional artists “provided materials for painting and other art supplies free of charge.”
“We hope this approach is very promising and would be widely adopted.”
She suggested the positive effect of art therapy could be related to “activating the brain’s reward neural network.”
This may be via improved visual attention acting on visuospatial mechanisms and emotional drive, with “activation of the medial orbitofrontal cortex, ventral striatum, and other structures.”
The researchers note PD, a “multisystem progressive neurodegenerative disease,” is among the three most common neurological disorders, with an incidence of 100-150 cases per 100,000 people.
They also note that nonpharmacologic approaches are “widely used” as an adjunct to drug therapy and as part of an “integrated approach” to disease management.
To examine the clinical efficacy of art therapy, the team recruited patients with PD who had preserved facility for independent movement, defined as stages 1-2.5 on the Hoehn and Yahr scale.
Patients were randomly assigned to art therapy sessions alongside standard drug therapy or to standard drug therapy alone. The art therapy included sculpting, free drawing, and coloring patterns.
Multiple benefits
Participants were assessed at baseline and at 6 and 12 months with the Unified Parkinson Disease Rating Scale (UPDRS), the Beck Depression Inventory, the Montreal Cognitive Assessment, and the Pegboard Test of finger dexterity.
Fifty patients were included in the study, with 30 assigned to standard drug therapy alone and 20 to the combined intervention. Participants had a mean age of 57.8 years, and 46% were women.
Over the study period, investigators found patients assigned to art therapy plus drug treatment had improved mood, as well as decreased daytime sleeping, reduced tremor, and a decrease in anxiety and fear intensity.
Between baseline and the 6- and 12-month assessments, patients in the combined therapy group showed improvements in scores on all of the questionnaires, and on the Pegboard Test. In contrast, scores were either stable or worsened in the standard drug therapy–alone group.
The team notes that there was also a marked difference in rates of disease progression, defined as a change on the Hoehn and Yahr scale of at least 0.5 points, between the two groups.
Only two (10%) patients in the combined drug and art therapy progressed over the study period, compared with 10 (33%) in the control group (P = .05).
The findings complement those of a recent study conducted by Alberto Cucca, MD, of the Fresco Institute for Parkinson’s and Movement Disorders, New York University, and colleagues.
Eighteen patients took part in the prospective, open-label trial. They were assessed before and after 20 sessions of art therapy on a range of measures.
Results revealed that following the art therapy, patients had improvements in the Navon Test (which assesses visual neglect, eye tracking, and UPDRS scores), as well as significantly increased functional connectivity levels in the visual cortex on resting-state functional MRI.
Many benefits, no side effects
Rebecca Gilbert, MD, PhD, vice president and chief scientific officer of the American Parkinson Disease Association, who was not involved in either study, told this news organization that the idea of art therapy for patients with Parkinson’s is “very reasonable.”
She highlighted that “people with Parkinson’s have many issues with their visuospatial abilities,” as well as their depth and distance perception, and so “enhancing that aspect could potentially be very beneficial.”
“So I’m hopeful that it’s a really good avenue to explore, and the preliminary data are very exciting.”
Dr. Gilbert also highlighted that the “wonderful” aspect of art therapy is that there are “so many benefits and not really any side effects.” Patients can “take the meds … and then enhance that with various therapies, and this would be an additional option.”
Another notable aspect of art therapy is the “social element” and the sense of “camaraderie,” although that has “to be teased out from the benefits you would get from the actual art therapy.”
Finally, Dr. Gilbert pointed out that the difference between the current trial and Dr. Cucca’s trial is the presence of a control group.
“Of course, it’s not blinded, because you know whether you got therapy or not … but that extra element of being able to compare with a group that didn’t get the treatment gives it a little more weight in terms of the field.”
No funding was declared. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Customized brain stimulation: New hope for severe depression
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Worsening motor function tied to post COVID syndrome in Parkinson’s disease
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Apple devices identify early Parkinson’s disease
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.
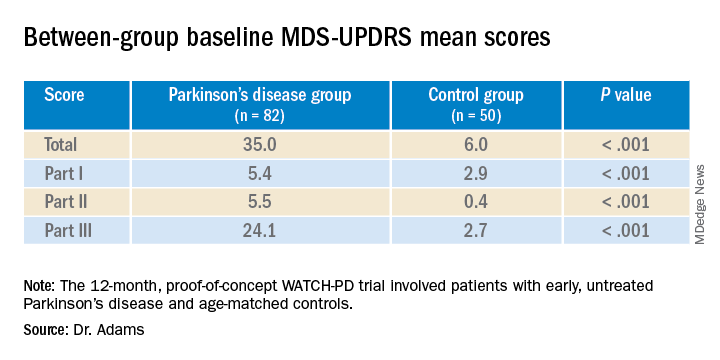
Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.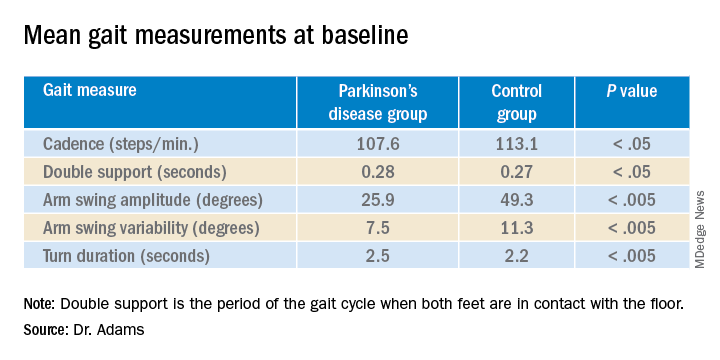
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Study identifies pandemic-related stressor in Parkinson’s disease
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Efforts target underrepresented populations in Parkinson’s disease genetic studies
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
FROM MDS VIRTUAL CONGRESS 2021
Sublingual film well tolerated for Parkinson ‘off’ episodes
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Survey identifies clinicians’ unease with genetic testing
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Toward ‘superhuman cognition’: The future of brain-computer interfaces
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.











