User login
A Dark Horse Diagnosis
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
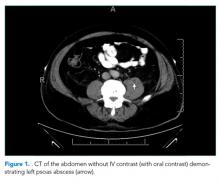
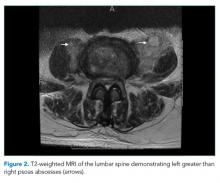
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
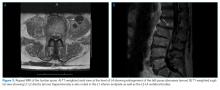
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
A 73-year-old man presented to the emergency department in late winter with fevers, myalgias, fatigue, low back pain, and poor oral intake. Four days earlier, he had fallen and hit his head. His partner also noticed a few episodes of confusion in the days leading up to presentation.
The patient’s symptoms are nonspecific. Fevers prompt the consideration of systemic infection, though fevers can also be seen in a broad range of noninfectious processes, including malignancy, vasculitis, autoimmune conditions, endocrinopathies, and drug reaction. The clinical picture warrants prompt and comprehensive evaluation, beginning with further detailed history (current illnesses, exposures, travel, vaccinations, medications, cancer screenings, weight change) and a careful physical examination, which will help guide laboratory testing and imaging.
His past medical history was notable for coronary artery disease for which he underwent coronary artery bypass grafting five years prior, hypertension, hyperlipidemia, diet-controlled type 2 diabetes mellitus, gastroesophageal reflux disease, osteoarthritis leading to chronic knee and hand pain, and a history of mildly low testosterone levels. His medications included hydrocodone and acetaminophen, metoprolol tartrate, omeprazole, topical testosterone gel (prescribed for daily use, used intermittently), and aspirin. He was retired and lived in rural Michigan with his female partner. He previously worked as a truck driver and used to train racehorses. He had quit smoking five years earlier. He denied alcohol or injection drug use.
The patient has significant underlying medical conditions. Considering infectious causes of his symptoms, it is notable that he has no reported immunodeficiency. It would be relevant to know if he has been tested for HIV. His rural residence and work with horses raise the possibility of zoonotic infections, including plague (Yersinia pestis), brucellosis (Brucella species), Q fever (Coxiella burnetti), Rhodococcus equi, or group C or G Streptococci. Information about tuberculosis risk factors, other geographic exposures, recent dental work, and ill contacts might be helpful to elucidate the causes of this nonspecific febrile illness with a possible CNS component. With regard to malignancy, it would be helpful to ask about recent weight loss, lymphadenopathy, and prior cancer screenings. Considering other etiologies, he does not report a history of autoimmune or endocrine conditions. However, it is important to consider a vasculitis, such as giant cell arteritis or polyarteritis nodosa, autoimmune conditions, and endocrinopathies such as thyrotoxicosis. The differential diagnosis for his clinical syndrome remains broad.
Vital signs were temperature 37.3°C, heart rate 88 beats per minute, respiratory rate 18 breaths per minute, blood pressure 105/64 mmHg, and oxygen saturation 93% on room air. Oral examination revealed poor dentition. The heart had a normal rate and regular rhythm with no murmurs, rubs, or gallops, and lungs were clear to auscultation bilaterally. The abdomen was unremarkable. Examination of the back was notable for mild tenderness to palpation over the sacrum. He was oriented to person, place, and time, with intact cranial nerves and a nonfocal neurologic examination. The remainder of his examination was normal. The white blood cell (WBC) count was 11.1 × 103/μL, with 84% neutrophils and 9% bands, hemoglobin 13.6 g/dL, platelet count 54 × 103/μL, sodium 122 mmol/L, potassium 3.3 mmol/L, chloride 89 mmol/L, bicarbonate 21 mmol/L, creatinine 1.64 mg/dL, albumin 2.7 g/dL, alkaline phosphatase 136 U/L, AST 60 U/L, ALT 37 U/L, and total bilirubin 2.1 mg/dL.
He had presented to the emergency department five days earlier with fever, flank pain, nausea, vomiting, and weakness. At that time, he had a temperature of 38.2°C, but vital signs otherwise had been normal. Laboratory studies had revealed WBC count 14.0 × 103/μL, hemoglobin 13.7 g/dL, platelet count 175 × 103/μL, sodium 129 mmol/L, chloride 97 mmol/dL, bicarbonate 23 mmol/L, creatinine 1.1 mg/dL, and total bilirubin 1.6 mg/dL. Urinalysis had been negative. He had received one liter of intravenous normal saline and ketorolac for pain and had been discharged with the diagnosis of a viral illness.
A picture of a progressive, subacute illness with multisystem involvement appears to be emerging, and there are several abnormalities consistent with infection, including fever, leukocytosis with bandemia, thrombocytopenia, renal dysfunction, and elevated bilirubin. His borderline hypotension may be due to uninterrupted use of his antihypertensive medication in the setting of poor oral intake or may indicate incipient sepsis. Focal sacral tenderness raises the possibility of vertebral osteomyelitis or epidural abscess, either from a contiguous focus of infection from the surrounding structures, or as a site of seeding from bacteremia. His prior confusion episodes might have been secondary to a systemic process; however, CNS imaging should be done, given the history of confusion and recent fall. Further diagnostic studies are warranted, including: blood cultures; peripheral blood smear; imaging of the spine, chest, abdomen, and pelvis; electrocardiogram; and possibly echocardiogram. Although noninfectious etiologies should not be discounted, the constellation of findings is more compatible with infection.
Two sets of blood cultures and a viral respiratory swab were obtained. Computed tomography (CT) of the head without contrast was negative for acute bleeding or other intracranial pathology. Lumbosacral radiography revealed degenerative changes with intact alignment of the sacrum. The patient was admitted with plans to pursue lumbar puncture if altered mental status recurred. The viral swab was negative. Within 24 hours, one set of blood cultures (both bottles) grew lactose-negative, oxidase-negative, gram-negative rods.
Gram-negative rods (GNRs) rarely are contaminants in blood cultures and should be considered significant until proven otherwise. Prompt empiric therapy and investigation to identify the primary source of bacteremia must be initiated. Although the most common GNRs isolated from blood cultures are enteric coliform organisms such as E. coli, Klebsiella, and Enterobacter, these typically are lactose-positive. Additional possibilities should be considered, including Salmonella species or other organisms comprising the “HACEK” group. This latter group is commonly associated with endocarditis, but the majority are oxidase-positive and have more fastidious growth requirements. Although there are other gram-negative organisms to consider, they have other distinguishing characteristics that have not been indicated in the microbiology results. Broad-spectrum antibiotic therapy is appropriate while awaiting the final identification of the GNR. A thorough search for a primary source and secondary sites of hematogenous seeding should be conducted. His only localizing symptom was tenderness over the sacrum, and this should be further assessed by sensitive imaging such as magnetic resonance imaging (MRI). The identity of the GNR would guide further diagnostic evaluation. For example, a respiratory organism such as Haemophilus influenzae would prompt a CT scan of the chest. Isolation of an enteric or a coliform GNR such as E. coli would prompt abdominal and pelvic imaging to assess for occult abscess. An “HACEK” group organism would prompt echocardiography to evaluate for endocarditis.
He was started on piperacillin–tazobactam. GNR bacteremia without a clear source prompted a CT of the chest, abdomen, and pelvis with and without contrast. The images were unremarkable, with the exception of a signal abnormality in the left psoas muscle concerning for abscess (Figure 1). MRI of the same region revealed L2-4 osteomyelitis and discitis with bilateral psoas abscesses but without epidural abscess (Figure 2).
Because of increasing reports of antibiotic resistance in GNRs, even in community-acquired infections, it is appropriate to initially treat with a broad-spectrum antibiotic such as a fourth-generation cephalosporin or carbapenem while awaiting identification and susceptibility results to guide definitive therapy. In addition to antimicrobial therapy, treatment of psoas abscess usually requires drainage. Vertebral osteomyelitis from a hematogenous source can often be treated with antibiotics alone, as long as there are no associated complications such as epidural abscess and spine instability. Imaging should be reviewed for pathology of the surrounding structures, and surgical consultation should be obtained.
Neurosurgery, Interventional Radiology, and Infectious Disease services were consulted. Antibiotic coverage was expanded to vancomycin, cefepime, and metronidazole due to the possibility of polymicrobial infection. No surgical intervention was recommended since the abscesses were too small to drain.
The next day, the GNR was identified as Serratia marcescens.
S. marcescens is a widely distributed organism in the environment, but not a common component of endogenous human flora. Serratia is generally considered as an opportunistic nosocomial pathogen. Community-acquired infection with this organism is unusual and implies exogenous acquisition. A careful re-evaluation of exposures, including injection drug use or other parenteral exposures is important to identify the likely source of infection, as these have been previously linked to outbreaks of environmental organisms. Based on the presumed pathogenesis of infection and the initial microbiology suggesting monomicrobial Serratia infection, antibiotics should be narrowed based on the susceptibility results. There is concern that antibiotics might not adequately penetrate the abscesses and result in a lack of clinical improvement and/or lead to the emergence of antibiotic resistance during therapy. This is an important concern with Serratia, which typically harbors an AmpC beta-lactamase that can mediate resistance to broad-spectrum cephalosporins. If medical therapy alone without drainage is planned, short-interval re-imaging is warranted.
Blood cultures from days two and three of hospitalization also grew S. marcescens. No other organisms grew. Based on culture sensitivity data, antibiotics were narrowed to ceftriaxone.
This surprising culture result prompted the medical team to obtain screening laboratory tests for immunocompromising conditions and to revisit the patient’s history. His type 2 diabetes mellitus was well controlled with a hemoglobin A1c of 6.5%. HIV testing was negative. Further questioning of the patient revealed that he had fallen from a truck onto rocks four months prior, injuring his back and hip, but without puncture of the skin or loss of consciousness; he denied recent falls or other injuries but reported significant chronic knee pain. He had not been hospitalized recently. He had never taken corticosteroids or immunomodulatory medications. He continued to deny injection drug use. He did, however, clarify that his work with racehorses, which was originally understood to be a prior hobby, was ongoing, including recent work of cleaning the stables.
The following morning, he experienced confusion, rigors, and hypoxia, which prompted transfer to the intensive care unit (ICU).
Acute worsening during treatment is worrisome, and could be a potential complication of his infection or treatment – or even a separate process altogether. Knee pain in the setting of bacteremia raises the possibility of septic or crystal-induced arthritis and warrants imaging. Confusion and hypoxia might represent secondary sites of seeding from bacteremia (CNS infection and pneumonia, respectively) or manifestations of endocarditis, the latter being unusual for Serratia. An echocardiogram should be obtained. Other neurologic causes, including seizure, should also be considered. Further evaluation by chest imaging and repeat neurologic examination and imaging should be performed. Emergence of resistance during therapy is a theoretical concern with Serratia as an AmpC beta-lactamase-containing organism. While awaiting additional microbiology data, an empiric change to an AmpC beta-lactamase stable antibiotic such as a carbapenem should be made, especially since he has clinically deteriorated on therapy with a β-lactamase susceptible antibiotic, raising concerns of the emergence of resistance on initial therapy.
Antibiotics were changed to meropenem, vancomycin, and metronidazole given the clinical worsening and concerns that this represented infection unresponsive to prior antibiotics. The acute episode resolved spontaneously after one hour. His neurologic examination remained nonfocal. Chest radiography, urinalysis, urine culture, and right upper quadrant ultrasound were unremarkable. Transesophageal echocardiogram revealed no heart valve vegetations. MRI and bone scan of the lower extremities did not show any evidence of septic arthritis or other infection. He remained stable and was transferred out of the ICU the following day. Antibiotic coverage was switched to cefepime. On discussion with his significant other, this event was found to be similar to the intermittent confusion that occurred in the days prior to admission.
The acute onset and other features of these intermittent periods of deterioration are compatible with infection; intermittent seeding of the blood with microbes or their products (eg, lipopolysaccharides) from an abscess or vascular infection could explain these episodes. Some of the previous hypotheses to explain the episodes, such as a secondary infectious process, have not been supported by diagnostic testing or the clinical course. He needs close clinical monitoring and interval assessment of the known sites of infection.
Ten days after osteomyelitis and discitis were diagnosed, the patient developed worsening low back pain, prompting repeat spine MRI. This was significant for bilateral psoas abscess enlargement and extension of osteomyelitis and discitis (Figure 3). He was re-evaluated by Neurosurgery and Interventional Radiology and underwent psoas abscess drainage; abscess cultures grew S. marcescens.
He slowly improved over several weeks and was discharged to a subacute rehabilitation facility. He completed a 3.5-week course of intravenous antibiotics before leaving against medical advice. He completed eight weeks of oral trimethoprim-sulfamethoxazole and remains without long-term sequelae from the infection.
DISCUSSION
S. marcescens is a gram-negative rod in the Enterobacteriaceae family known for its red pigment. Primarily, S. marcescens causes nosocomial infections, most commonly of the respiratory and urinary tracts. However, a wide range of manifestations has been documented, including meningitis, ocular infections (conjunctivitis, keratitis, endophthalmitis), endocarditis, skin infections (cellulitis, necrotizing fasciitis), and osteomyelitis.1, 2 S. marcescens is often reported as the cause of outbreaks in ICUs;3-6 infection is thought to occur via contamination of water pipes, hospital equipment, and disinfectants.3, 7 Its natural environment includes soil, water, and GI tracts of animals,4 and there are published reports of S. marcescens infection in horses.8, 9 This patient was most likely exposed to S. marcescens through his work with horses and their environment.
S. marcescens has wide-ranging target organs, and successful treatment can be difficult. S. marcescens can infect the renal, respiratory, gastrointestinal, ocular, cardiovascular, and musculoskeletal systems. S. marcescens, like other “SPACE” organisms (Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter), expresses inducible AmpC beta-lactamase.10 At baseline, AmpC beta-lactamase expression is repressed.11 Mutants with stably de-repressed (constitutively expressed) AmpC can be selected during therapy and lead to clinical failure, as has been best described during therapy for Enterobacter infections.12 Infectious Disease consultation may be helpful when caring for patients with S. marcescens bacteremia given these complexities.
This was an unusual case of S. marcescens infection. It most commonly infects immunocompromised hosts. Reported risk factors include solid organ or hematopoietic stem cell transplant, malignancy, HIV/AIDS, and receipt of immunosuppressive agents. The patient did not have these risk factors, but did have well-controlled type 2 diabetes mellitus. Although diabetes is associated with an increased risk of infection and more severe infections,13, 14 there is no evidence in the literature that well-controlled type 2 diabetes mellitus compromises the immune system. A few case reports document cutaneous S. marcescens infection in immunocompetent adults.15,16 A case report of S. marcescens septic arthritis and adjacent osteomyelitis has also been published, but the patient had poorly controlled diabetes.17 This case provides a report of systemic S. marcescens infection in an individual without clear risk factors.
S. marcescens osteomyelitis is rare, and there have been only a few prior case reports.2,18 The presentation of osteomyelitis, regardless of the causative organism, is subtle, often insidious, and can easily be missed. Hospitalists should have a high index of suspicion for the diagnosis as it requires prompt evaluation and treatment for complications, including epidural abscess. Risk factors include diabetes mellitus, rheumatoid arthritis, injection drug use, and other immunocompromising illnesses.19 Degenerative changes in the spine such as osteoarthritis may be risk factors as well,20 though not well studied or quantified. A hypothesized mechanism involves local inflammation and joint damage, leaving the area susceptible to bacterial seeding. Osteoarthritis and degenerative disc disease, along with exposure to racehorses, likely put this patient at risk for bacterial seeding in the vertebrae, ultimately leading to a “dark horse” diagnosis.
TEACHING POINTS
- Serratia marcescens is a gram-negative rod bacterium that most commonly infects immunocompromised individuals in hospital settings. This report demonstrates that S. marcescens can cause serious infection in immunocompetent, nonhospitalized adults.
- S. marcescens bacteremia or infection of organs outside of the urinary or respiratory systems is uncommon, and therapy can be complicated by emergence of resistance.
- The clinical presentation of vertebral osteomyelitis and discitis and psoas abscess can be subtle and may present without typical signs and symptoms of infection.
ACKNOWLEDGEMENTS
The authors thank the patient and his partner for their willingness to have his story published, Laura Petersen, MHSA, for providing assistance with references and manuscript editing, and Shadi Azar, MBBS, for assistance in selecting the cross-sectional images.
Disclosures
The authors have no conflicts of interest to disclose.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
1. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903-912. doi: 10.1099/00222615-46-11-903. PubMed
2. Lau JX, Li JY, Yong TY. Non-contiguous multifocal vertebral osteomyelitis caused by erratia marcescens. Mod Rheumatol. 2015;25(2):303-306. doi: 10.3109/14397595.2013.874754. PubMed
3. Dessi A, Puddu M, Testa M, Marcialis MA, Pintus MC, Fanos V. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493-499. doi: 10.1179/joc.2009.21.5.493. PubMed
4. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-791. doi: 10.1128/CMR.00017-11. PubMed
5. Montagnani C, Cocchi P, Lega L, et al. Serratia marcescens outbreak in a neonatal intensive care unit: crucial role of implementing hand hygiene among external consultants. BMC Infect Dis. 2015;15:11. doi: 10.1186/s12879-014-0734-6. PubMed
6. van Ogtrop ML, van Zoeren-Grobben D, Verbakel-Salomons EM, van Boven CP. Serratia marcescens infections in neonatal departments: description of an outbreak and review of the literature. J Hosp Infect. 1997;36(2):95-103. doi: 10.1016/S0195-6701(97)90115-8. PubMed
7. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224. doi: 10.1128/AAC.00138-07. PubMed
8. Ewart S, Brown C, Derksen F, Kufuor-Mensa E. Serratia marcescens endocarditis in a horse. J Am Vet Med Assoc. 1992;200(7):961-963. PubMed
9. Jores J, Beutner G, Hirth-Schmidt I, Borchers K, Pitt TL, Lubke-Becker A. Isolation of Serratia marcescens from an equine abortion in Germany. Vet Rec. 2004;154(8):242-244. doi: 10.1136/vr.154.8.242. PubMed
10. Herra C, Falkiner FR. Serratia marcescens. http://www.antimicrobe.org/b26.asp. Accessed August 22, 2017.
11. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182, Table of Contents. doi: 10.1128/CMR.00036-08. PubMed
12. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585-590. doi: 10.7326/0003-4819-115-8-585. PubMed
13. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35(38):5095-5101. doi: 10.1016/j.vaccine.2017.07.095. PubMed
14. Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617-638, vii. doi: 10.1016/j.idc.2007.07.003. PubMed
15. Carlesimo M, Pennica A, Muscianese M, et al. Multiple skin ulcers due to Serratia marcescens in a immunocompetent patient. G Ital Dermatol Venereol. 2014;149(3):367-370. PubMed
16. Rallis E, Karanikola E, Papadakis P. Severe facial infection caused by Serratia marcescens in an immunocompetent soldier. J Am Acad Dermatol. 2008;58(5 Suppl 1):S109-S110. doi: 10.1016/j.jaad.2007.04.010. PubMed
17. Hadid H, Usman M, Thapa S. Severe osteomyelitis and septic arthritis due to Serratia marcescens in an immunocompetent patient. Case Rep Infect Dis. 2015;2015:347652. doi: 10.1155/2015/347652. PubMed
18. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi: 10.1093/cid/civ482. PubMed
19. Vertebral Osteomyelitis Guideline Team (Team Leader: Chenoweth CE; Team Members: Bassin BS HS, Mack MR, Kunapuli A, Park P, Quint DJ, Seagull FJ, Wesorick DH; Consultants: Patel RD, Riddell IV J, Lanava KM). Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults. University of Michigan Guidelines for Clinical Care 2013; http://www.med.umich.edu/1info/FHP/practiceguides/vertebral/VO.pdf. Accessed October 26, 2017.
20. McDonald M. Vertebral osteomyelitis and discitis in adults. 2017; Available at: https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed October 26, 2017.
© 2018 Society of Hospital Medicine