User login
What’s Eating You? Clinical Manifestations of Dermacentor Tick Bites
Background and Distribution
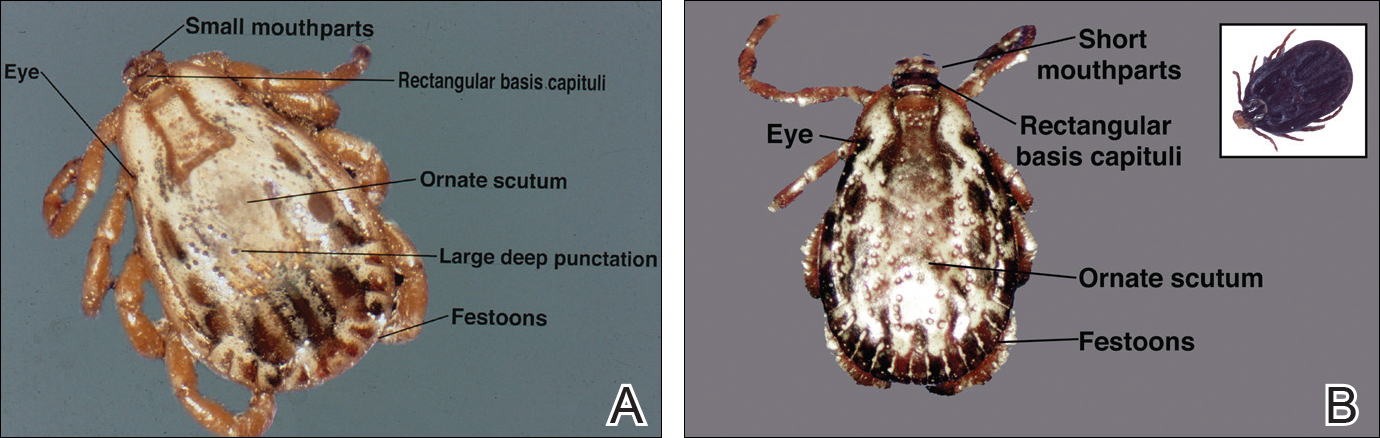
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.
Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.