User login
Impact of Pharmacist-led Discharge Counseling on Hospital Readmission and Emergency Department Visits: A Systematic Review and Meta-analysis
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
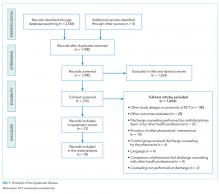
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
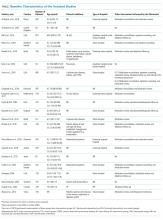
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
© 2020 Society of Hospital Medicine