Article
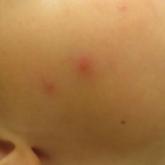
What’s Eating You? Carpet Beetles (Dermestidae)
- Author:
- Amy G. Johnson, MD
- Bethany R. Rohr, MD
Given their ubiquity, dermatologists should be aware of the potential for hypersensitivity reactions to carpet beetles (Dermestidae).
Article

What’s Eating You? Noble False Widow Spider (Steatoda nobilis)
- Author:
- Amy G. Johnson, MD
- Bethany R. Rohr, MD
With evidence of a recent population boom of noble false widow spiders in Europe and spread to California, dermatologists should be aware of these...
