User login
Imaging algorithm may improve stroke treatment selection
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.
"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
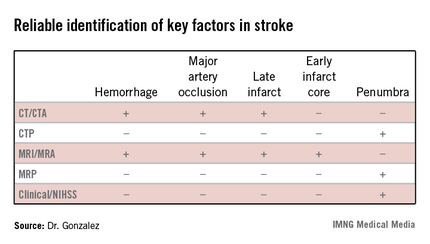
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ASNR ANNUAL MEETING
Transfers may have worse ischemic stroke outcomes
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Significantly fewer patients who were transferred from an outside institution to a comprehensive stroke center had good functional outcomes at 90 days than did nontransferred patients (modified Rankin Scale score of 0-2; 16% vs. 60%, respectively), independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and procedural success/complications.
Data source: A retrospective study of 116 patients with acute ischemic stroke who were eligible for intra-arterial therapy.
Disclosures: Dr. Shaibani said he had no relevant financial disclosures.
The rheumatologist's role in the care of the limping child
NEW YORK – A timely diagnosis of a child presenting with a limp is essential because of the wide variety of possible causes, including benign disease such as a mild injury, a chronic disabling disease such as juvenile idiopathic arthritis, or a life-threatening infection or malignancy. It may be up to the pediatric rheumatologist to discriminate among the possible causes of the limp, distinguish pathology from normal, call for the proper consults and tests, as well as manage any identified rheumatic diseases, Dr. Philip J. Kahn said at a meeting sponsored by New York University.
By the time the rheumatologist sees a child presenting with a limp, the child may have already seen a pediatrician, emergency physician, and even an orthopedist.
Dr. Kahn, a pediatric rheumatologist at New York University Langone Medical Center, said he is concerned when a limp is associated, with fever, rash, weakness, joint swelling, and bearing weight. "If the child has fever and severe musculoskeletal pain, even with a normal platelet count, you should think of malignancy."
One of the first questions to ask is "Does it hurt?" A child with an antalgic gait (a gait that has adapted to counter or avoid pain) may refuse to bear weight. In this case, inquire whether the child has walked into the office or hospital or had to be carried. Nonpainful limp is usually insidious in onset, Dr. Kahn said, and may be suggestive of a rheumatic condition, weakness, stiffness, or deformity. Question the child as well as the parent, even though young patients may not be able to verbalize pain, and older children may deny it.
Other important clues about the limp are the time of onset; association with any known event, injury, or time of day; duration of pain (constant pain is suggestive of infection or malignancy); and location of pain (focal or diffuse, bone or joint). The physician should inquire about fever, anorexia, weight loss, or night sweats, which should raise suspicion of malignancy, infection, or rheumatologic problems. If fever is present, determine whether it is continuous, nocturnal, or quotidian (appearing daily, often at the same time). Delayed motor development or regression of achieved milestones, such as when a child who has walked independently suddenly asks to be carried around, may suggest neurologic or rheumatic disease. While a child may deny joint stiffness, a parent may notice the child cannot move easily in the morning or after long car rides or sitting in a classroom (Best Pract. Res. Clin. Rheumatol. 2009;23:625-42). Age is also an important consideration when evaluating the child with a limp.
Because observation is key during the physical exam, Dr. Kahn said to allow time to watch the child move around freely, looking for aberrations in gait. The child should be unclothed, barefoot, and observed during motion and at rest. While palpating the legs, be alert to areas of tenderness, suggestive of contusion, fracture, malignancy, or infection. Joints should be inspected for effusion, warmth, and tenderness, keeping in mind the possibility of referred pain from the hip or back, and range of motion should be assessed. He noted that the Childhood Myositis Assessment Scale (CMAS) can be helpful for assessing weakness (Arthritis Care Res. 2011;63 [Suppl. 11]:S118-57).
"Laboratory and radiographic evaluation will depend on what is discovered from the history and physical," Dr. Kahn said. Initial work-up may include complete/full blood count with differential, routine serum chemistries (including creatinine and liver and muscle enzymes), acute phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein), and urinalysis. Testing synovial fluid for white blood cells is appropriate if septic arthritis is suspected, although the test is not highly sensitive or specific. "Elevated ESR in the presence of a normal or low platelet count in a child with musculoskeletal pain, especially if the child is febrile, is concerning for malignancy," he said. Dr. Kahn is somewhat reluctant to order antinuclear antibody testing unless there is a compelling reason because he says parents often panic upon hearing that.
The American College of Radiology in 2012 issued Appropriateness Criteria for evaluating limping children aged 0 to 5 years and selecting an imaging study (J. Am. Coll. Radiol. 2012;9:545-53). The criteria are categorized according to three variants: trauma; no trauma and no sign of infection; or possible presence of infection. With possible infection, imaging protocols are described for patients with localized pain to the hip, localized pain to the nonhip and lower extremity, and nonlocalized pain. Each imaging modality is given an appropriateness rating and an assessment of the relative radiation level. In brief, the criteria suggest that localized radiographs or tibial radiographs are appropriate following trauma. With an atraumatic and noninfectious history, hip ultrasound may be the initial study of choice, followed by radiography if the ultrasound is negative. Ultrasound of the hip allows a quick and accurate diagnosis of joint effusion, and aspiration can differentiate septic arthritis – an emergency situation – from transient synovitis. When long-term infection is suspected, MRI is the study of choice to demonstrate osteomyelitis or soft-tissue abscess.
Dr. Kahn presented a series of case studies, illustrating some key differentiating features that can help make the diagnosis:
• Case 1: A 2.5-year-old girl is carried into the emergency department (ED) after limping for 2 days. She began limping after coming home from the playground. She has no fever, rash, or constitutional features, and appears happy and smiling. Her hips, knees, ankles, and feet are not swollen, warm, or tender. There is tenderness at a point along her right tibia. The diagnosis is toddler’s fracture.
• Case 2: A 2.5-year-old girl is carried into the ED after limping for 2 days. There is no history of trauma, but the pain has become so severe that it awakens her at night. She refuses to walk, has constant pain not controlled by NSAIDS, and is cranky, febrile, tachycardic, and appears sick. She holds her right hip in a FABER (flexion, abduction, and external rotation) position. Her labs are C-reactive protein of 100 mg/L and a white blood cell of 30,000. The diagnosis is septic arthritis, with immediate referral to an orthopedist.
• Case 3: A 2.5-year-old girl walks into the ED after limping for 3 months. She denies having any pain, and her mother says she is lazy. She no longer alternates her feet when ascending steps and has fallen once when descending the stairs. When you examine her, she shows edematous and purple eyelids and a rash over her knuckles, as well as proximal weakness. Her muscle enzymes are elevated. The diagnosis is juvenile dermatomyositis.
• Case 4: A 2.5-year-old girl walks into the ED after limping for 3 months. An active girl, she fell off a slide the previous day and developed a large effusion after scraping her knee. She is smiling and running around the ED. She has no fever, malaise, joint swelling, or nocturnal wakening, although her mother says her limp is worse in the morning but lessens after breakfast. Inflammatory markers are normal and rheumatoid factor is absent. The diagnosis is oligoarticular juvenile idiopathic arthritis.
• Case 5: A 2.5-year-old girl walks into the ED after complaining that her legs have bothered her for 3 months. At night, she complains of lower leg pain in both legs and awakes sometimes at night from the pain but seems fine in the morning. No erythema or swollen joints are seen. She has been taken three times to the ED over the last few weeks, but blood tests and x-rays are said to be normal. Fever, rash, or constitutional symptoms are absent. The diagnosis is growing pains.
Dr. Kahn reported having no relevant financial disclosures.
NEW YORK – A timely diagnosis of a child presenting with a limp is essential because of the wide variety of possible causes, including benign disease such as a mild injury, a chronic disabling disease such as juvenile idiopathic arthritis, or a life-threatening infection or malignancy. It may be up to the pediatric rheumatologist to discriminate among the possible causes of the limp, distinguish pathology from normal, call for the proper consults and tests, as well as manage any identified rheumatic diseases, Dr. Philip J. Kahn said at a meeting sponsored by New York University.
By the time the rheumatologist sees a child presenting with a limp, the child may have already seen a pediatrician, emergency physician, and even an orthopedist.
Dr. Kahn, a pediatric rheumatologist at New York University Langone Medical Center, said he is concerned when a limp is associated, with fever, rash, weakness, joint swelling, and bearing weight. "If the child has fever and severe musculoskeletal pain, even with a normal platelet count, you should think of malignancy."
One of the first questions to ask is "Does it hurt?" A child with an antalgic gait (a gait that has adapted to counter or avoid pain) may refuse to bear weight. In this case, inquire whether the child has walked into the office or hospital or had to be carried. Nonpainful limp is usually insidious in onset, Dr. Kahn said, and may be suggestive of a rheumatic condition, weakness, stiffness, or deformity. Question the child as well as the parent, even though young patients may not be able to verbalize pain, and older children may deny it.
Other important clues about the limp are the time of onset; association with any known event, injury, or time of day; duration of pain (constant pain is suggestive of infection or malignancy); and location of pain (focal or diffuse, bone or joint). The physician should inquire about fever, anorexia, weight loss, or night sweats, which should raise suspicion of malignancy, infection, or rheumatologic problems. If fever is present, determine whether it is continuous, nocturnal, or quotidian (appearing daily, often at the same time). Delayed motor development or regression of achieved milestones, such as when a child who has walked independently suddenly asks to be carried around, may suggest neurologic or rheumatic disease. While a child may deny joint stiffness, a parent may notice the child cannot move easily in the morning or after long car rides or sitting in a classroom (Best Pract. Res. Clin. Rheumatol. 2009;23:625-42). Age is also an important consideration when evaluating the child with a limp.
Because observation is key during the physical exam, Dr. Kahn said to allow time to watch the child move around freely, looking for aberrations in gait. The child should be unclothed, barefoot, and observed during motion and at rest. While palpating the legs, be alert to areas of tenderness, suggestive of contusion, fracture, malignancy, or infection. Joints should be inspected for effusion, warmth, and tenderness, keeping in mind the possibility of referred pain from the hip or back, and range of motion should be assessed. He noted that the Childhood Myositis Assessment Scale (CMAS) can be helpful for assessing weakness (Arthritis Care Res. 2011;63 [Suppl. 11]:S118-57).
"Laboratory and radiographic evaluation will depend on what is discovered from the history and physical," Dr. Kahn said. Initial work-up may include complete/full blood count with differential, routine serum chemistries (including creatinine and liver and muscle enzymes), acute phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein), and urinalysis. Testing synovial fluid for white blood cells is appropriate if septic arthritis is suspected, although the test is not highly sensitive or specific. "Elevated ESR in the presence of a normal or low platelet count in a child with musculoskeletal pain, especially if the child is febrile, is concerning for malignancy," he said. Dr. Kahn is somewhat reluctant to order antinuclear antibody testing unless there is a compelling reason because he says parents often panic upon hearing that.
The American College of Radiology in 2012 issued Appropriateness Criteria for evaluating limping children aged 0 to 5 years and selecting an imaging study (J. Am. Coll. Radiol. 2012;9:545-53). The criteria are categorized according to three variants: trauma; no trauma and no sign of infection; or possible presence of infection. With possible infection, imaging protocols are described for patients with localized pain to the hip, localized pain to the nonhip and lower extremity, and nonlocalized pain. Each imaging modality is given an appropriateness rating and an assessment of the relative radiation level. In brief, the criteria suggest that localized radiographs or tibial radiographs are appropriate following trauma. With an atraumatic and noninfectious history, hip ultrasound may be the initial study of choice, followed by radiography if the ultrasound is negative. Ultrasound of the hip allows a quick and accurate diagnosis of joint effusion, and aspiration can differentiate septic arthritis – an emergency situation – from transient synovitis. When long-term infection is suspected, MRI is the study of choice to demonstrate osteomyelitis or soft-tissue abscess.
Dr. Kahn presented a series of case studies, illustrating some key differentiating features that can help make the diagnosis:
• Case 1: A 2.5-year-old girl is carried into the emergency department (ED) after limping for 2 days. She began limping after coming home from the playground. She has no fever, rash, or constitutional features, and appears happy and smiling. Her hips, knees, ankles, and feet are not swollen, warm, or tender. There is tenderness at a point along her right tibia. The diagnosis is toddler’s fracture.
• Case 2: A 2.5-year-old girl is carried into the ED after limping for 2 days. There is no history of trauma, but the pain has become so severe that it awakens her at night. She refuses to walk, has constant pain not controlled by NSAIDS, and is cranky, febrile, tachycardic, and appears sick. She holds her right hip in a FABER (flexion, abduction, and external rotation) position. Her labs are C-reactive protein of 100 mg/L and a white blood cell of 30,000. The diagnosis is septic arthritis, with immediate referral to an orthopedist.
• Case 3: A 2.5-year-old girl walks into the ED after limping for 3 months. She denies having any pain, and her mother says she is lazy. She no longer alternates her feet when ascending steps and has fallen once when descending the stairs. When you examine her, she shows edematous and purple eyelids and a rash over her knuckles, as well as proximal weakness. Her muscle enzymes are elevated. The diagnosis is juvenile dermatomyositis.
• Case 4: A 2.5-year-old girl walks into the ED after limping for 3 months. An active girl, she fell off a slide the previous day and developed a large effusion after scraping her knee. She is smiling and running around the ED. She has no fever, malaise, joint swelling, or nocturnal wakening, although her mother says her limp is worse in the morning but lessens after breakfast. Inflammatory markers are normal and rheumatoid factor is absent. The diagnosis is oligoarticular juvenile idiopathic arthritis.
• Case 5: A 2.5-year-old girl walks into the ED after complaining that her legs have bothered her for 3 months. At night, she complains of lower leg pain in both legs and awakes sometimes at night from the pain but seems fine in the morning. No erythema or swollen joints are seen. She has been taken three times to the ED over the last few weeks, but blood tests and x-rays are said to be normal. Fever, rash, or constitutional symptoms are absent. The diagnosis is growing pains.
Dr. Kahn reported having no relevant financial disclosures.
NEW YORK – A timely diagnosis of a child presenting with a limp is essential because of the wide variety of possible causes, including benign disease such as a mild injury, a chronic disabling disease such as juvenile idiopathic arthritis, or a life-threatening infection or malignancy. It may be up to the pediatric rheumatologist to discriminate among the possible causes of the limp, distinguish pathology from normal, call for the proper consults and tests, as well as manage any identified rheumatic diseases, Dr. Philip J. Kahn said at a meeting sponsored by New York University.
By the time the rheumatologist sees a child presenting with a limp, the child may have already seen a pediatrician, emergency physician, and even an orthopedist.
Dr. Kahn, a pediatric rheumatologist at New York University Langone Medical Center, said he is concerned when a limp is associated, with fever, rash, weakness, joint swelling, and bearing weight. "If the child has fever and severe musculoskeletal pain, even with a normal platelet count, you should think of malignancy."
One of the first questions to ask is "Does it hurt?" A child with an antalgic gait (a gait that has adapted to counter or avoid pain) may refuse to bear weight. In this case, inquire whether the child has walked into the office or hospital or had to be carried. Nonpainful limp is usually insidious in onset, Dr. Kahn said, and may be suggestive of a rheumatic condition, weakness, stiffness, or deformity. Question the child as well as the parent, even though young patients may not be able to verbalize pain, and older children may deny it.
Other important clues about the limp are the time of onset; association with any known event, injury, or time of day; duration of pain (constant pain is suggestive of infection or malignancy); and location of pain (focal or diffuse, bone or joint). The physician should inquire about fever, anorexia, weight loss, or night sweats, which should raise suspicion of malignancy, infection, or rheumatologic problems. If fever is present, determine whether it is continuous, nocturnal, or quotidian (appearing daily, often at the same time). Delayed motor development or regression of achieved milestones, such as when a child who has walked independently suddenly asks to be carried around, may suggest neurologic or rheumatic disease. While a child may deny joint stiffness, a parent may notice the child cannot move easily in the morning or after long car rides or sitting in a classroom (Best Pract. Res. Clin. Rheumatol. 2009;23:625-42). Age is also an important consideration when evaluating the child with a limp.
Because observation is key during the physical exam, Dr. Kahn said to allow time to watch the child move around freely, looking for aberrations in gait. The child should be unclothed, barefoot, and observed during motion and at rest. While palpating the legs, be alert to areas of tenderness, suggestive of contusion, fracture, malignancy, or infection. Joints should be inspected for effusion, warmth, and tenderness, keeping in mind the possibility of referred pain from the hip or back, and range of motion should be assessed. He noted that the Childhood Myositis Assessment Scale (CMAS) can be helpful for assessing weakness (Arthritis Care Res. 2011;63 [Suppl. 11]:S118-57).
"Laboratory and radiographic evaluation will depend on what is discovered from the history and physical," Dr. Kahn said. Initial work-up may include complete/full blood count with differential, routine serum chemistries (including creatinine and liver and muscle enzymes), acute phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein), and urinalysis. Testing synovial fluid for white blood cells is appropriate if septic arthritis is suspected, although the test is not highly sensitive or specific. "Elevated ESR in the presence of a normal or low platelet count in a child with musculoskeletal pain, especially if the child is febrile, is concerning for malignancy," he said. Dr. Kahn is somewhat reluctant to order antinuclear antibody testing unless there is a compelling reason because he says parents often panic upon hearing that.
The American College of Radiology in 2012 issued Appropriateness Criteria for evaluating limping children aged 0 to 5 years and selecting an imaging study (J. Am. Coll. Radiol. 2012;9:545-53). The criteria are categorized according to three variants: trauma; no trauma and no sign of infection; or possible presence of infection. With possible infection, imaging protocols are described for patients with localized pain to the hip, localized pain to the nonhip and lower extremity, and nonlocalized pain. Each imaging modality is given an appropriateness rating and an assessment of the relative radiation level. In brief, the criteria suggest that localized radiographs or tibial radiographs are appropriate following trauma. With an atraumatic and noninfectious history, hip ultrasound may be the initial study of choice, followed by radiography if the ultrasound is negative. Ultrasound of the hip allows a quick and accurate diagnosis of joint effusion, and aspiration can differentiate septic arthritis – an emergency situation – from transient synovitis. When long-term infection is suspected, MRI is the study of choice to demonstrate osteomyelitis or soft-tissue abscess.
Dr. Kahn presented a series of case studies, illustrating some key differentiating features that can help make the diagnosis:
• Case 1: A 2.5-year-old girl is carried into the emergency department (ED) after limping for 2 days. She began limping after coming home from the playground. She has no fever, rash, or constitutional features, and appears happy and smiling. Her hips, knees, ankles, and feet are not swollen, warm, or tender. There is tenderness at a point along her right tibia. The diagnosis is toddler’s fracture.
• Case 2: A 2.5-year-old girl is carried into the ED after limping for 2 days. There is no history of trauma, but the pain has become so severe that it awakens her at night. She refuses to walk, has constant pain not controlled by NSAIDS, and is cranky, febrile, tachycardic, and appears sick. She holds her right hip in a FABER (flexion, abduction, and external rotation) position. Her labs are C-reactive protein of 100 mg/L and a white blood cell of 30,000. The diagnosis is septic arthritis, with immediate referral to an orthopedist.
• Case 3: A 2.5-year-old girl walks into the ED after limping for 3 months. She denies having any pain, and her mother says she is lazy. She no longer alternates her feet when ascending steps and has fallen once when descending the stairs. When you examine her, she shows edematous and purple eyelids and a rash over her knuckles, as well as proximal weakness. Her muscle enzymes are elevated. The diagnosis is juvenile dermatomyositis.
• Case 4: A 2.5-year-old girl walks into the ED after limping for 3 months. An active girl, she fell off a slide the previous day and developed a large effusion after scraping her knee. She is smiling and running around the ED. She has no fever, malaise, joint swelling, or nocturnal wakening, although her mother says her limp is worse in the morning but lessens after breakfast. Inflammatory markers are normal and rheumatoid factor is absent. The diagnosis is oligoarticular juvenile idiopathic arthritis.
• Case 5: A 2.5-year-old girl walks into the ED after complaining that her legs have bothered her for 3 months. At night, she complains of lower leg pain in both legs and awakes sometimes at night from the pain but seems fine in the morning. No erythema or swollen joints are seen. She has been taken three times to the ED over the last few weeks, but blood tests and x-rays are said to be normal. Fever, rash, or constitutional symptoms are absent. The diagnosis is growing pains.
Dr. Kahn reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE NYU ANNUAL PEDIATRIC RHEUMATOLOGY UPDATE
Mitral valve bioprosthetic shows 16+ years of durability
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the 2013 Mitral Valve Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the 2013 Mitral Valve Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the 2013 Mitral Valve Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
AT THE 2013 MITRAL VALVE CONCLAVE
Major finding: The mean durability of the Carpentier-Edwards Perimount Pericardial prosthetic was 16.6 years before a structural valve dysfunction occurred.
Data source: Prospective study of 404 patients who underwent mitral valve replacement using the Perimount prosthetic.
Disclosures: Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
IL-1-beta emerges as key molecule for autoinflammatory diseases
NEW YORK – Evidence has accumulated in recent years that IL-1-beta-mediated inflammation and a dysregulation of the innate immune system is common to many autoinflammatory disorders that involve bouts of seemingly unprovoked recurrent inflammation and no indication of malignancy or infection, according to Dr. Jonathan Samuels.
These findings have opened up new therapeutic targets for these disorders, and some recent clinical studies suggest that investigators are on the right track, said Dr. Samuels, a rheumatologist affiliated with the Center for Musculoskeletal Care at NYU Langone Medical Center, New York.
The term "autoinflammation" was coined in 1999 following the discovery of the genes responsible for Familial Mediterranean Fever (FMF), a disease characterized by recurrent episodes of fever and abdominal pain, along with pleurisy, arthritis, and other symptoms. Patients feel normal between bouts and are unresponsive to corticosteroids. Like other patients with autoinflammatory diseases, FMF patients show no evidence of autoantibodies or antigen-specific T-cell reactivity, suggesting instead disordered regulation of the innate immune system, Dr. Samuels said at a meeting sponsored by New York University.
IL-1 has been identified as one of the most potent proinflammatory cytokines of the innate immune system, and is being recognized as a likely therapeutic target. "Many molecular cascades from various autoinflammatory diseases converge at IL-1, with oversecretion (as in the case of neonatal-onset multisystem inflammatory disease or NOMID) or uninhibited signaling (as in the case of deficiency of the interleukin-1-receptor antagonist or DIRA)," Dr. Samuels said.
For example, while colchicine has been shown to prevent acute attacks of FMF by 75% and decrease symptoms in 95%, there are no proven alternative therapies for patients with FMF who are resistant or intolerant to colchicine. Dr. Samuels was a coinvestigator of a recent randomized, double-blind placebo-controlled trial in which 14 patients with FMF who were colchicine resistant or intolerant were treated with the IL-1 antagonist rilonacept (2.2 mg/kg, maximum 160 mg) given by weekly subcutaneous injection for two 3-month courses. Rilonacept resulted in a significant reduction in the number of attacks per month (0.77 vs. 2.00, P = .027) and there were more treatment courses without attacks with rilonacept, compared with placebo. Rilonacept did not affect the duration of attacks (Ann. Intern. Med. 2012;157:533-41).
The cryopyrin-associated periodic syndromes, commonly known as CAPS, are a group of autosomal dominant autoinflammatory diseases that have been linked to mutations in the same gene. All three cryopyrinopathies have an early onset and present with urticarial rash and fever, although they span a spectrum of severity, according to Dr. Samuels. The mildest form is familial cold autoinflammatory syndrome (FCAS), which involves episodes generally lasting less than 24 hours, and is accompanied by headache and polyarthralgia. Muckle-Wells syndrome lasts 24-48 hours, and may cause deafness, optic disk edema, oligoarthritis, and polyarthralgia, as well as abdominal pain. Patients with NOMID are the most severely affected, with almost-continuous episodes, chronic meningitis, mental retardation, deafness, blindness, epiphyseal/patellar overgrowth, and intermittent chronic arthritis.
All three CAPS syndromes have been shown to respond to anti-IL-1 agents, according to Dr. Samuels. For example, in 2012, researchers from the National Institute of Arthritis and Musculoskeletal and Skin Diseases found in an open-label cohort study of 26 patients with NOMID that the IL-1 inhibitor anakinra yielded long-term improvements in measures such as global scores of disease activity, pain, CNS inflammation, and hearing for up to 5 years as long as escalating doses were used (Arthritis Rheum. 2012;64:2375-86).
Treatment with anakinra also has been beneficial for neonates with DIRA, which involves an autosomal recessive mutation on chromosome 2q that leads to uncontrolled IL-1 binding and causes crusting, erythema, pustulosis, and osteolytic lesions.
"This is a very complex group of disorders," Dr. Samuels said. Diagnosis should take into account ethnicity, inheritance pattern, cytokines, attack duration, treatment response, age of onset, triggers, and amyloid frequency. He referred audience members to a helpful diagnostic algorithm (Cleveland Clinic J. Med. 2012;79:569-81).
Dr. Samuels reported no relevant financial disclosures.
NEW YORK – Evidence has accumulated in recent years that IL-1-beta-mediated inflammation and a dysregulation of the innate immune system is common to many autoinflammatory disorders that involve bouts of seemingly unprovoked recurrent inflammation and no indication of malignancy or infection, according to Dr. Jonathan Samuels.
These findings have opened up new therapeutic targets for these disorders, and some recent clinical studies suggest that investigators are on the right track, said Dr. Samuels, a rheumatologist affiliated with the Center for Musculoskeletal Care at NYU Langone Medical Center, New York.
The term "autoinflammation" was coined in 1999 following the discovery of the genes responsible for Familial Mediterranean Fever (FMF), a disease characterized by recurrent episodes of fever and abdominal pain, along with pleurisy, arthritis, and other symptoms. Patients feel normal between bouts and are unresponsive to corticosteroids. Like other patients with autoinflammatory diseases, FMF patients show no evidence of autoantibodies or antigen-specific T-cell reactivity, suggesting instead disordered regulation of the innate immune system, Dr. Samuels said at a meeting sponsored by New York University.
IL-1 has been identified as one of the most potent proinflammatory cytokines of the innate immune system, and is being recognized as a likely therapeutic target. "Many molecular cascades from various autoinflammatory diseases converge at IL-1, with oversecretion (as in the case of neonatal-onset multisystem inflammatory disease or NOMID) or uninhibited signaling (as in the case of deficiency of the interleukin-1-receptor antagonist or DIRA)," Dr. Samuels said.
For example, while colchicine has been shown to prevent acute attacks of FMF by 75% and decrease symptoms in 95%, there are no proven alternative therapies for patients with FMF who are resistant or intolerant to colchicine. Dr. Samuels was a coinvestigator of a recent randomized, double-blind placebo-controlled trial in which 14 patients with FMF who were colchicine resistant or intolerant were treated with the IL-1 antagonist rilonacept (2.2 mg/kg, maximum 160 mg) given by weekly subcutaneous injection for two 3-month courses. Rilonacept resulted in a significant reduction in the number of attacks per month (0.77 vs. 2.00, P = .027) and there were more treatment courses without attacks with rilonacept, compared with placebo. Rilonacept did not affect the duration of attacks (Ann. Intern. Med. 2012;157:533-41).
The cryopyrin-associated periodic syndromes, commonly known as CAPS, are a group of autosomal dominant autoinflammatory diseases that have been linked to mutations in the same gene. All three cryopyrinopathies have an early onset and present with urticarial rash and fever, although they span a spectrum of severity, according to Dr. Samuels. The mildest form is familial cold autoinflammatory syndrome (FCAS), which involves episodes generally lasting less than 24 hours, and is accompanied by headache and polyarthralgia. Muckle-Wells syndrome lasts 24-48 hours, and may cause deafness, optic disk edema, oligoarthritis, and polyarthralgia, as well as abdominal pain. Patients with NOMID are the most severely affected, with almost-continuous episodes, chronic meningitis, mental retardation, deafness, blindness, epiphyseal/patellar overgrowth, and intermittent chronic arthritis.
All three CAPS syndromes have been shown to respond to anti-IL-1 agents, according to Dr. Samuels. For example, in 2012, researchers from the National Institute of Arthritis and Musculoskeletal and Skin Diseases found in an open-label cohort study of 26 patients with NOMID that the IL-1 inhibitor anakinra yielded long-term improvements in measures such as global scores of disease activity, pain, CNS inflammation, and hearing for up to 5 years as long as escalating doses were used (Arthritis Rheum. 2012;64:2375-86).
Treatment with anakinra also has been beneficial for neonates with DIRA, which involves an autosomal recessive mutation on chromosome 2q that leads to uncontrolled IL-1 binding and causes crusting, erythema, pustulosis, and osteolytic lesions.
"This is a very complex group of disorders," Dr. Samuels said. Diagnosis should take into account ethnicity, inheritance pattern, cytokines, attack duration, treatment response, age of onset, triggers, and amyloid frequency. He referred audience members to a helpful diagnostic algorithm (Cleveland Clinic J. Med. 2012;79:569-81).
Dr. Samuels reported no relevant financial disclosures.
NEW YORK – Evidence has accumulated in recent years that IL-1-beta-mediated inflammation and a dysregulation of the innate immune system is common to many autoinflammatory disorders that involve bouts of seemingly unprovoked recurrent inflammation and no indication of malignancy or infection, according to Dr. Jonathan Samuels.
These findings have opened up new therapeutic targets for these disorders, and some recent clinical studies suggest that investigators are on the right track, said Dr. Samuels, a rheumatologist affiliated with the Center for Musculoskeletal Care at NYU Langone Medical Center, New York.
The term "autoinflammation" was coined in 1999 following the discovery of the genes responsible for Familial Mediterranean Fever (FMF), a disease characterized by recurrent episodes of fever and abdominal pain, along with pleurisy, arthritis, and other symptoms. Patients feel normal between bouts and are unresponsive to corticosteroids. Like other patients with autoinflammatory diseases, FMF patients show no evidence of autoantibodies or antigen-specific T-cell reactivity, suggesting instead disordered regulation of the innate immune system, Dr. Samuels said at a meeting sponsored by New York University.
IL-1 has been identified as one of the most potent proinflammatory cytokines of the innate immune system, and is being recognized as a likely therapeutic target. "Many molecular cascades from various autoinflammatory diseases converge at IL-1, with oversecretion (as in the case of neonatal-onset multisystem inflammatory disease or NOMID) or uninhibited signaling (as in the case of deficiency of the interleukin-1-receptor antagonist or DIRA)," Dr. Samuels said.
For example, while colchicine has been shown to prevent acute attacks of FMF by 75% and decrease symptoms in 95%, there are no proven alternative therapies for patients with FMF who are resistant or intolerant to colchicine. Dr. Samuels was a coinvestigator of a recent randomized, double-blind placebo-controlled trial in which 14 patients with FMF who were colchicine resistant or intolerant were treated with the IL-1 antagonist rilonacept (2.2 mg/kg, maximum 160 mg) given by weekly subcutaneous injection for two 3-month courses. Rilonacept resulted in a significant reduction in the number of attacks per month (0.77 vs. 2.00, P = .027) and there were more treatment courses without attacks with rilonacept, compared with placebo. Rilonacept did not affect the duration of attacks (Ann. Intern. Med. 2012;157:533-41).
The cryopyrin-associated periodic syndromes, commonly known as CAPS, are a group of autosomal dominant autoinflammatory diseases that have been linked to mutations in the same gene. All three cryopyrinopathies have an early onset and present with urticarial rash and fever, although they span a spectrum of severity, according to Dr. Samuels. The mildest form is familial cold autoinflammatory syndrome (FCAS), which involves episodes generally lasting less than 24 hours, and is accompanied by headache and polyarthralgia. Muckle-Wells syndrome lasts 24-48 hours, and may cause deafness, optic disk edema, oligoarthritis, and polyarthralgia, as well as abdominal pain. Patients with NOMID are the most severely affected, with almost-continuous episodes, chronic meningitis, mental retardation, deafness, blindness, epiphyseal/patellar overgrowth, and intermittent chronic arthritis.
All three CAPS syndromes have been shown to respond to anti-IL-1 agents, according to Dr. Samuels. For example, in 2012, researchers from the National Institute of Arthritis and Musculoskeletal and Skin Diseases found in an open-label cohort study of 26 patients with NOMID that the IL-1 inhibitor anakinra yielded long-term improvements in measures such as global scores of disease activity, pain, CNS inflammation, and hearing for up to 5 years as long as escalating doses were used (Arthritis Rheum. 2012;64:2375-86).
Treatment with anakinra also has been beneficial for neonates with DIRA, which involves an autosomal recessive mutation on chromosome 2q that leads to uncontrolled IL-1 binding and causes crusting, erythema, pustulosis, and osteolytic lesions.
"This is a very complex group of disorders," Dr. Samuels said. Diagnosis should take into account ethnicity, inheritance pattern, cytokines, attack duration, treatment response, age of onset, triggers, and amyloid frequency. He referred audience members to a helpful diagnostic algorithm (Cleveland Clinic J. Med. 2012;79:569-81).
Dr. Samuels reported no relevant financial disclosures.
EXPERT ANALYSIS FROM THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Treat JIA uveitis early, aggressively to avoid vision loss
NEW YORK – Tailoring the frequency of vision screening for children with juvenile idiopathic arthritis is an important step to getting uveitis symptoms treated effectively before permanent morbidity occurs, according to Dr. Sanjay R. Kedhar.
"We know that children with JIA [juvenile idiopathic arthritis] who have chronic inflammation of the eye have a threefold increase in the risk of blindness. We also know that immunosuppressive therapy reduces the risk of blindness by 60%. I believe that physicians should feel the time pressure to identify uveitis in children and treat them aggressively to preserve vision and reduce morbidity," said Dr. Kedhar, an ophthalmologist at The New York (N.Y.) Eye and Ear Infirmary and associate professor of ophthalmology at New York Medical College in Valhalla, N.Y. Uveitis-associated morbidity includes cataracts, glaucoma, band keratopathy, phthisis bulbi, and vision loss.
Uveitis is the third most common cause of blindness in children, and JIA-associated uveitis is the most common cause of uveitis in children. About one in four children with JIA uveitis presents with blindness. Early detection, awareness of risk factors, and prompt and effective treatment can slow or prevent vision loss in children with JIA-associated uveitis, he said at the meeting, sponsored by New York University.
Uveitis associated with JIA most commonly affects the anterior portions of the eye, between the cornea and iris. Symptoms of anterior uveitis include blurred vision, pain, redness, photophobia, floaters, flashing lights, and distorted vision (metamorphopsia). The problem is that symptoms may be absent in children or, alternatively, children may not be able to verbalize what they see. "By the time symptoms are detected in school screening, it may be too late," Dr. Kedhar said.
That is why it is important to make sure children with JIA have regular ophthalmologic examinations as recommended by the American Academy of Pediatrics (Pediatrics 2006;117:1843-5). Screening frequency depends upon the presence of risk factors, such as type of arthritis (oligoarticular), age at onset, antinuclear antibody (ANA) seropositivity, and RF seronegativity. Children with JIA who have oligoarticular and polyarticular joint involvement and who are ANA positive and RF negative generally are at highest risk of developing uveitis, with 33%-70% affected.
"These are the kids we have to worry about the most," Dr. Kedhar said. If the joint symptoms appear before 7 years of age, the children should be seen every 3-4 months. Those children who have oligoarticular and polyarticular joint involvement beginning before age 7 but are ANA negative are considered at intermediate risk and should be seen every 6 months. Those who have a systemic onset of disease require screening every 12 months, reflecting the low risk of uveitis in this group.
Other risk factors for JIA-associated uveitis vision loss include female sex, anterior chamber flare seen on first exam, and abnormal intraocular pressure. The Systemic Immunosuppressive Therapy for Eye Diseases Study, a multicenter, retrospective cohort study of 327 patients with JIA-associated uveitis, showed that posterior synechiae, active uveitis, and intraocular surgery were associated with worse visual acuity outcomes (Ophthalmology 2013;120:186-92).
Another predictor of poor visual outcome is the onset of ocular symptoms before or soon after arthritis symptoms. Generally, having one ocular complication increases the risk of developing another.
Dr. Kedhar strongly suggested having an ophthalmologist be a part of the JIA patient’s medical team, along with the pediatrician and pediatric rheumatologist. A characteristic sign of anterior uveitis is an abnormal, irregular pupil. Ciliary flush is often not present in cases of JIA. In the acute setting, the red reflex may be diminished in the uveitis-affected eye. Keratic precipitates are white blood cells that accumulate on the posterior corneal surface and may indicate long-standing inflammation. Flare is another classic finding, and can be attributed to protein leakage into the aqueous humor. "In some studies, flare is used to monitor response of uveitis to treatment," Dr. Kedhar said. Uveitis is not typically associated with discharge or itching.
While topical corticosteroids are considered the first line of treatment, their use generated quite a bit of discussion from audience members. One of the main concerns is the conundrum that cataracts and glaucoma can occur because of uncontrolled inflammation associated with uveitis or as side effects of topical corticosteroids. Dr. Kedhar said that in his practice, he tries to limit the chronic use of corticosteroid drops to four times a day or less, generally switching patients to immunomodulatory therapy if they are corticosteroid dependent. He says that side effects are related to cumulative dose. He acknowledges that some ophthalmologists avoid topical steroids altogether but he believes they can be used safely for limited times. Because of greater concern for systemic side effects in children, systemic corticosteroids are used for only the short term.
Immunosuppressive therapy has been shown to reduce the risk of blindness in the better eye by 60% (Am. J. Ophthalmol. 2007;143:840-6). The most widely used immunomodulatory agent for children is methotrexate. Anti–TNF-alpha agents may be used in patients who fail to respond to conventional immunosuppressive therapy. A recent study in Italy that compared infliximab and adalimumab for refractory uveitis in 91 patients with JIA found higher remission rates after 1 year with adalimumab (67% vs. 43%, P = .025) (J. Rheumatol. 2013;40:74-9). Adalimumab has the convenience of subcutaneous administration, stable serum concentrations, and a more favorable safety profile, whereas infliximab offers fast onset and potent anti-inflammatory effects (J. Ophthalmic Vis. Res. 2011;6:259-69).
The anti–TNF-alpha agent golimumab was found to be helpful in three cases of refractory JIA uveitis. Golimumab offers the advantage of subcutaneous once-a-month dosing and avoids the expense and time commitment of outpatient infliximab infusions, Dr. Kedhar noted (J. Ophthalmic. Inflamm. Infect. 2012;2:231-3).
"Uveitis may require higher doses of immunomodulatory agents than those used for rheumatologic manifestations," Dr. Kedhar said. "The activity of uveitis associated with JIA may be independent from the rheumatologic disease. Although we used to recommend treating uveitis for 1 year once quiescence is established, many uveitis specialists now recommend treating until 2 years of quiescence to minimize the risk of recurrence."
NEW YORK – Tailoring the frequency of vision screening for children with juvenile idiopathic arthritis is an important step to getting uveitis symptoms treated effectively before permanent morbidity occurs, according to Dr. Sanjay R. Kedhar.
"We know that children with JIA [juvenile idiopathic arthritis] who have chronic inflammation of the eye have a threefold increase in the risk of blindness. We also know that immunosuppressive therapy reduces the risk of blindness by 60%. I believe that physicians should feel the time pressure to identify uveitis in children and treat them aggressively to preserve vision and reduce morbidity," said Dr. Kedhar, an ophthalmologist at The New York (N.Y.) Eye and Ear Infirmary and associate professor of ophthalmology at New York Medical College in Valhalla, N.Y. Uveitis-associated morbidity includes cataracts, glaucoma, band keratopathy, phthisis bulbi, and vision loss.
Uveitis is the third most common cause of blindness in children, and JIA-associated uveitis is the most common cause of uveitis in children. About one in four children with JIA uveitis presents with blindness. Early detection, awareness of risk factors, and prompt and effective treatment can slow or prevent vision loss in children with JIA-associated uveitis, he said at the meeting, sponsored by New York University.
Uveitis associated with JIA most commonly affects the anterior portions of the eye, between the cornea and iris. Symptoms of anterior uveitis include blurred vision, pain, redness, photophobia, floaters, flashing lights, and distorted vision (metamorphopsia). The problem is that symptoms may be absent in children or, alternatively, children may not be able to verbalize what they see. "By the time symptoms are detected in school screening, it may be too late," Dr. Kedhar said.
That is why it is important to make sure children with JIA have regular ophthalmologic examinations as recommended by the American Academy of Pediatrics (Pediatrics 2006;117:1843-5). Screening frequency depends upon the presence of risk factors, such as type of arthritis (oligoarticular), age at onset, antinuclear antibody (ANA) seropositivity, and RF seronegativity. Children with JIA who have oligoarticular and polyarticular joint involvement and who are ANA positive and RF negative generally are at highest risk of developing uveitis, with 33%-70% affected.
"These are the kids we have to worry about the most," Dr. Kedhar said. If the joint symptoms appear before 7 years of age, the children should be seen every 3-4 months. Those children who have oligoarticular and polyarticular joint involvement beginning before age 7 but are ANA negative are considered at intermediate risk and should be seen every 6 months. Those who have a systemic onset of disease require screening every 12 months, reflecting the low risk of uveitis in this group.
Other risk factors for JIA-associated uveitis vision loss include female sex, anterior chamber flare seen on first exam, and abnormal intraocular pressure. The Systemic Immunosuppressive Therapy for Eye Diseases Study, a multicenter, retrospective cohort study of 327 patients with JIA-associated uveitis, showed that posterior synechiae, active uveitis, and intraocular surgery were associated with worse visual acuity outcomes (Ophthalmology 2013;120:186-92).
Another predictor of poor visual outcome is the onset of ocular symptoms before or soon after arthritis symptoms. Generally, having one ocular complication increases the risk of developing another.
Dr. Kedhar strongly suggested having an ophthalmologist be a part of the JIA patient’s medical team, along with the pediatrician and pediatric rheumatologist. A characteristic sign of anterior uveitis is an abnormal, irregular pupil. Ciliary flush is often not present in cases of JIA. In the acute setting, the red reflex may be diminished in the uveitis-affected eye. Keratic precipitates are white blood cells that accumulate on the posterior corneal surface and may indicate long-standing inflammation. Flare is another classic finding, and can be attributed to protein leakage into the aqueous humor. "In some studies, flare is used to monitor response of uveitis to treatment," Dr. Kedhar said. Uveitis is not typically associated with discharge or itching.
While topical corticosteroids are considered the first line of treatment, their use generated quite a bit of discussion from audience members. One of the main concerns is the conundrum that cataracts and glaucoma can occur because of uncontrolled inflammation associated with uveitis or as side effects of topical corticosteroids. Dr. Kedhar said that in his practice, he tries to limit the chronic use of corticosteroid drops to four times a day or less, generally switching patients to immunomodulatory therapy if they are corticosteroid dependent. He says that side effects are related to cumulative dose. He acknowledges that some ophthalmologists avoid topical steroids altogether but he believes they can be used safely for limited times. Because of greater concern for systemic side effects in children, systemic corticosteroids are used for only the short term.
Immunosuppressive therapy has been shown to reduce the risk of blindness in the better eye by 60% (Am. J. Ophthalmol. 2007;143:840-6). The most widely used immunomodulatory agent for children is methotrexate. Anti–TNF-alpha agents may be used in patients who fail to respond to conventional immunosuppressive therapy. A recent study in Italy that compared infliximab and adalimumab for refractory uveitis in 91 patients with JIA found higher remission rates after 1 year with adalimumab (67% vs. 43%, P = .025) (J. Rheumatol. 2013;40:74-9). Adalimumab has the convenience of subcutaneous administration, stable serum concentrations, and a more favorable safety profile, whereas infliximab offers fast onset and potent anti-inflammatory effects (J. Ophthalmic Vis. Res. 2011;6:259-69).
The anti–TNF-alpha agent golimumab was found to be helpful in three cases of refractory JIA uveitis. Golimumab offers the advantage of subcutaneous once-a-month dosing and avoids the expense and time commitment of outpatient infliximab infusions, Dr. Kedhar noted (J. Ophthalmic. Inflamm. Infect. 2012;2:231-3).
"Uveitis may require higher doses of immunomodulatory agents than those used for rheumatologic manifestations," Dr. Kedhar said. "The activity of uveitis associated with JIA may be independent from the rheumatologic disease. Although we used to recommend treating uveitis for 1 year once quiescence is established, many uveitis specialists now recommend treating until 2 years of quiescence to minimize the risk of recurrence."
NEW YORK – Tailoring the frequency of vision screening for children with juvenile idiopathic arthritis is an important step to getting uveitis symptoms treated effectively before permanent morbidity occurs, according to Dr. Sanjay R. Kedhar.
"We know that children with JIA [juvenile idiopathic arthritis] who have chronic inflammation of the eye have a threefold increase in the risk of blindness. We also know that immunosuppressive therapy reduces the risk of blindness by 60%. I believe that physicians should feel the time pressure to identify uveitis in children and treat them aggressively to preserve vision and reduce morbidity," said Dr. Kedhar, an ophthalmologist at The New York (N.Y.) Eye and Ear Infirmary and associate professor of ophthalmology at New York Medical College in Valhalla, N.Y. Uveitis-associated morbidity includes cataracts, glaucoma, band keratopathy, phthisis bulbi, and vision loss.
Uveitis is the third most common cause of blindness in children, and JIA-associated uveitis is the most common cause of uveitis in children. About one in four children with JIA uveitis presents with blindness. Early detection, awareness of risk factors, and prompt and effective treatment can slow or prevent vision loss in children with JIA-associated uveitis, he said at the meeting, sponsored by New York University.
Uveitis associated with JIA most commonly affects the anterior portions of the eye, between the cornea and iris. Symptoms of anterior uveitis include blurred vision, pain, redness, photophobia, floaters, flashing lights, and distorted vision (metamorphopsia). The problem is that symptoms may be absent in children or, alternatively, children may not be able to verbalize what they see. "By the time symptoms are detected in school screening, it may be too late," Dr. Kedhar said.
That is why it is important to make sure children with JIA have regular ophthalmologic examinations as recommended by the American Academy of Pediatrics (Pediatrics 2006;117:1843-5). Screening frequency depends upon the presence of risk factors, such as type of arthritis (oligoarticular), age at onset, antinuclear antibody (ANA) seropositivity, and RF seronegativity. Children with JIA who have oligoarticular and polyarticular joint involvement and who are ANA positive and RF negative generally are at highest risk of developing uveitis, with 33%-70% affected.
"These are the kids we have to worry about the most," Dr. Kedhar said. If the joint symptoms appear before 7 years of age, the children should be seen every 3-4 months. Those children who have oligoarticular and polyarticular joint involvement beginning before age 7 but are ANA negative are considered at intermediate risk and should be seen every 6 months. Those who have a systemic onset of disease require screening every 12 months, reflecting the low risk of uveitis in this group.
Other risk factors for JIA-associated uveitis vision loss include female sex, anterior chamber flare seen on first exam, and abnormal intraocular pressure. The Systemic Immunosuppressive Therapy for Eye Diseases Study, a multicenter, retrospective cohort study of 327 patients with JIA-associated uveitis, showed that posterior synechiae, active uveitis, and intraocular surgery were associated with worse visual acuity outcomes (Ophthalmology 2013;120:186-92).
Another predictor of poor visual outcome is the onset of ocular symptoms before or soon after arthritis symptoms. Generally, having one ocular complication increases the risk of developing another.
Dr. Kedhar strongly suggested having an ophthalmologist be a part of the JIA patient’s medical team, along with the pediatrician and pediatric rheumatologist. A characteristic sign of anterior uveitis is an abnormal, irregular pupil. Ciliary flush is often not present in cases of JIA. In the acute setting, the red reflex may be diminished in the uveitis-affected eye. Keratic precipitates are white blood cells that accumulate on the posterior corneal surface and may indicate long-standing inflammation. Flare is another classic finding, and can be attributed to protein leakage into the aqueous humor. "In some studies, flare is used to monitor response of uveitis to treatment," Dr. Kedhar said. Uveitis is not typically associated with discharge or itching.
While topical corticosteroids are considered the first line of treatment, their use generated quite a bit of discussion from audience members. One of the main concerns is the conundrum that cataracts and glaucoma can occur because of uncontrolled inflammation associated with uveitis or as side effects of topical corticosteroids. Dr. Kedhar said that in his practice, he tries to limit the chronic use of corticosteroid drops to four times a day or less, generally switching patients to immunomodulatory therapy if they are corticosteroid dependent. He says that side effects are related to cumulative dose. He acknowledges that some ophthalmologists avoid topical steroids altogether but he believes they can be used safely for limited times. Because of greater concern for systemic side effects in children, systemic corticosteroids are used for only the short term.
Immunosuppressive therapy has been shown to reduce the risk of blindness in the better eye by 60% (Am. J. Ophthalmol. 2007;143:840-6). The most widely used immunomodulatory agent for children is methotrexate. Anti–TNF-alpha agents may be used in patients who fail to respond to conventional immunosuppressive therapy. A recent study in Italy that compared infliximab and adalimumab for refractory uveitis in 91 patients with JIA found higher remission rates after 1 year with adalimumab (67% vs. 43%, P = .025) (J. Rheumatol. 2013;40:74-9). Adalimumab has the convenience of subcutaneous administration, stable serum concentrations, and a more favorable safety profile, whereas infliximab offers fast onset and potent anti-inflammatory effects (J. Ophthalmic Vis. Res. 2011;6:259-69).
The anti–TNF-alpha agent golimumab was found to be helpful in three cases of refractory JIA uveitis. Golimumab offers the advantage of subcutaneous once-a-month dosing and avoids the expense and time commitment of outpatient infliximab infusions, Dr. Kedhar noted (J. Ophthalmic. Inflamm. Infect. 2012;2:231-3).
"Uveitis may require higher doses of immunomodulatory agents than those used for rheumatologic manifestations," Dr. Kedhar said. "The activity of uveitis associated with JIA may be independent from the rheumatologic disease. Although we used to recommend treating uveitis for 1 year once quiescence is established, many uveitis specialists now recommend treating until 2 years of quiescence to minimize the risk of recurrence."
EXPERT ANALYSIS FROM THE NYU ANNUAL PEDIATRIC RHEUMATOLOGY UPDATE
Preimmunosuppresive hepatitis B screening often goes by the wayside
NEW YORK – While nowadays there is less likelihood that the hepatitis B virus causes rheumatic disease, major changes in epidemiology and drug therapy, especially the use of immunosuppressives, have made reactivation of the virus during the course of rheumatic disease treatment a major public health concern, according to Dr. Leonard H. Calabrese.
"HBV reactivation has been well known in cancer and transplantation but is now really coming to the fore in autoimmune and autoinflammatory diseases. It may be mild (asymptomatic viremia) or severe (fulminant liver failure and death) and has been observed in patients with both chronic infection or resolved disease. While it is largely preventable, unfortunately many physicians who regularly prescribe immunosuppressive therapies – including rheumatologists, oncologists, gastroenterologists, and dermatologists – do not recognize this potential," Dr. Calabrese said at a meeting sponsored by New York University.
The major risk of HBV is not during but after immunosuppression, when reactivation leading to severe or fatal hepatitis can occur. Reactivations have been reported rarely with low-dose prednisone and methotrexate, as well as all tumor necrosis factor (TNF) inhibitors, rituximab, abatacept, and infliximab, prompting a black box warning for many biologics, said Dr. Calabrese, director of the Fasenmyer Center for Clinical Immunology at the Cleveland Clinic.
In a retrospective study of 257 patients with positive HBV markers who were treated with TNF-targeted therapies, 39% with chronic infections (as indicated by the presence of HB surface antigens) developed HBV reactivation. Five developed acute liver failure, and four died (Medicine 2011;90:359-71). "This is not a trivial risk," Dr. Calabrese said.
Yet many rheumatologists do not screen for HBV prior to prescribing immunosuppressive therapy, he said. Dr. Calabrese cited a 2010 survey of rheumatologists that found that only 42% routinely screen for HBV before a patient begins nonbiologic disease-modifying antirheumatic drug therapy and 69% screen before prescribing a biologic DMARD. Seven percent of rheumatologists reported witnessing a reactivation event (Arthritis Care Res. 2010;62:704-11).
Of the three serologic markers for HBV (the hepatitis B surface antigen [HBsAg], the total hepatitis B core antibody [anti-HBc]), and the hepatitis B surface antibody (anti-HBsAb), Dr. Calabrese said he prefers HBsAg. "If you remember anything from this talk, remember that all patients with hepatitis B surface antigen need to be tended to before beginning any rheumatic therapy," he said. All positive tests should be followed up with HBV DNA testing.
Although all patients with a history of HBV are at some risk for HBV reactivation, with or without the presence of serologic markers, Dr. Calabrese outlined the relative risks of HBV reactivation as indicated by serologic findings. Those at highest risk are patients with chronic HBV infection (HBsAg positive, high level of HBV DNA), followed by inactive carriers (HBsAg positive, some HBV DNA) and those with resolved HBV (HBsAg negative, anti-HBc positive, anti-HBsAb positive). Those at lowest risk have resolved HBV (anti-HBc alone).
Rheumatologists should also be aware of the increased chance of HBV reactivation in high-risk populations. While in urban areas HBV is known to be associated with drug abuse, there is also a heightened risk in immigrants, especially those from Asia. Asian Americans are 2.7 times more likely to develop hepatocellular carcinoma. According to Dr. Calabrese, in mainland China there are an estimated 1 million people with rheumatologic problems who manifest chronic HBsAg positivity. "This is a global health problem," he noted.
Dr. Calabrese recommended that preemptive therapeutic measures be taken for all HBsAg-positive and HBV DNA–positive patients, including possible lifelong antiviral medication, and then these patients can be treated for their rheumatologic issues. Those who test positive for anti-HBc should be carefully monitored. "There should not be a patient with spondylitis, rheumatoid arthritis, lupus, or vasculitis who is getting less than effective therapy because they have chronic HBV," he said.
Dr. Calabrese reported no relevant financial disclosures.
NEW YORK – While nowadays there is less likelihood that the hepatitis B virus causes rheumatic disease, major changes in epidemiology and drug therapy, especially the use of immunosuppressives, have made reactivation of the virus during the course of rheumatic disease treatment a major public health concern, according to Dr. Leonard H. Calabrese.
"HBV reactivation has been well known in cancer and transplantation but is now really coming to the fore in autoimmune and autoinflammatory diseases. It may be mild (asymptomatic viremia) or severe (fulminant liver failure and death) and has been observed in patients with both chronic infection or resolved disease. While it is largely preventable, unfortunately many physicians who regularly prescribe immunosuppressive therapies – including rheumatologists, oncologists, gastroenterologists, and dermatologists – do not recognize this potential," Dr. Calabrese said at a meeting sponsored by New York University.
The major risk of HBV is not during but after immunosuppression, when reactivation leading to severe or fatal hepatitis can occur. Reactivations have been reported rarely with low-dose prednisone and methotrexate, as well as all tumor necrosis factor (TNF) inhibitors, rituximab, abatacept, and infliximab, prompting a black box warning for many biologics, said Dr. Calabrese, director of the Fasenmyer Center for Clinical Immunology at the Cleveland Clinic.
In a retrospective study of 257 patients with positive HBV markers who were treated with TNF-targeted therapies, 39% with chronic infections (as indicated by the presence of HB surface antigens) developed HBV reactivation. Five developed acute liver failure, and four died (Medicine 2011;90:359-71). "This is not a trivial risk," Dr. Calabrese said.
Yet many rheumatologists do not screen for HBV prior to prescribing immunosuppressive therapy, he said. Dr. Calabrese cited a 2010 survey of rheumatologists that found that only 42% routinely screen for HBV before a patient begins nonbiologic disease-modifying antirheumatic drug therapy and 69% screen before prescribing a biologic DMARD. Seven percent of rheumatologists reported witnessing a reactivation event (Arthritis Care Res. 2010;62:704-11).
Of the three serologic markers for HBV (the hepatitis B surface antigen [HBsAg], the total hepatitis B core antibody [anti-HBc]), and the hepatitis B surface antibody (anti-HBsAb), Dr. Calabrese said he prefers HBsAg. "If you remember anything from this talk, remember that all patients with hepatitis B surface antigen need to be tended to before beginning any rheumatic therapy," he said. All positive tests should be followed up with HBV DNA testing.
Although all patients with a history of HBV are at some risk for HBV reactivation, with or without the presence of serologic markers, Dr. Calabrese outlined the relative risks of HBV reactivation as indicated by serologic findings. Those at highest risk are patients with chronic HBV infection (HBsAg positive, high level of HBV DNA), followed by inactive carriers (HBsAg positive, some HBV DNA) and those with resolved HBV (HBsAg negative, anti-HBc positive, anti-HBsAb positive). Those at lowest risk have resolved HBV (anti-HBc alone).
Rheumatologists should also be aware of the increased chance of HBV reactivation in high-risk populations. While in urban areas HBV is known to be associated with drug abuse, there is also a heightened risk in immigrants, especially those from Asia. Asian Americans are 2.7 times more likely to develop hepatocellular carcinoma. According to Dr. Calabrese, in mainland China there are an estimated 1 million people with rheumatologic problems who manifest chronic HBsAg positivity. "This is a global health problem," he noted.
Dr. Calabrese recommended that preemptive therapeutic measures be taken for all HBsAg-positive and HBV DNA–positive patients, including possible lifelong antiviral medication, and then these patients can be treated for their rheumatologic issues. Those who test positive for anti-HBc should be carefully monitored. "There should not be a patient with spondylitis, rheumatoid arthritis, lupus, or vasculitis who is getting less than effective therapy because they have chronic HBV," he said.
Dr. Calabrese reported no relevant financial disclosures.
NEW YORK – While nowadays there is less likelihood that the hepatitis B virus causes rheumatic disease, major changes in epidemiology and drug therapy, especially the use of immunosuppressives, have made reactivation of the virus during the course of rheumatic disease treatment a major public health concern, according to Dr. Leonard H. Calabrese.
"HBV reactivation has been well known in cancer and transplantation but is now really coming to the fore in autoimmune and autoinflammatory diseases. It may be mild (asymptomatic viremia) or severe (fulminant liver failure and death) and has been observed in patients with both chronic infection or resolved disease. While it is largely preventable, unfortunately many physicians who regularly prescribe immunosuppressive therapies – including rheumatologists, oncologists, gastroenterologists, and dermatologists – do not recognize this potential," Dr. Calabrese said at a meeting sponsored by New York University.
The major risk of HBV is not during but after immunosuppression, when reactivation leading to severe or fatal hepatitis can occur. Reactivations have been reported rarely with low-dose prednisone and methotrexate, as well as all tumor necrosis factor (TNF) inhibitors, rituximab, abatacept, and infliximab, prompting a black box warning for many biologics, said Dr. Calabrese, director of the Fasenmyer Center for Clinical Immunology at the Cleveland Clinic.
In a retrospective study of 257 patients with positive HBV markers who were treated with TNF-targeted therapies, 39% with chronic infections (as indicated by the presence of HB surface antigens) developed HBV reactivation. Five developed acute liver failure, and four died (Medicine 2011;90:359-71). "This is not a trivial risk," Dr. Calabrese said.
Yet many rheumatologists do not screen for HBV prior to prescribing immunosuppressive therapy, he said. Dr. Calabrese cited a 2010 survey of rheumatologists that found that only 42% routinely screen for HBV before a patient begins nonbiologic disease-modifying antirheumatic drug therapy and 69% screen before prescribing a biologic DMARD. Seven percent of rheumatologists reported witnessing a reactivation event (Arthritis Care Res. 2010;62:704-11).
Of the three serologic markers for HBV (the hepatitis B surface antigen [HBsAg], the total hepatitis B core antibody [anti-HBc]), and the hepatitis B surface antibody (anti-HBsAb), Dr. Calabrese said he prefers HBsAg. "If you remember anything from this talk, remember that all patients with hepatitis B surface antigen need to be tended to before beginning any rheumatic therapy," he said. All positive tests should be followed up with HBV DNA testing.
Although all patients with a history of HBV are at some risk for HBV reactivation, with or without the presence of serologic markers, Dr. Calabrese outlined the relative risks of HBV reactivation as indicated by serologic findings. Those at highest risk are patients with chronic HBV infection (HBsAg positive, high level of HBV DNA), followed by inactive carriers (HBsAg positive, some HBV DNA) and those with resolved HBV (HBsAg negative, anti-HBc positive, anti-HBsAb positive). Those at lowest risk have resolved HBV (anti-HBc alone).
Rheumatologists should also be aware of the increased chance of HBV reactivation in high-risk populations. While in urban areas HBV is known to be associated with drug abuse, there is also a heightened risk in immigrants, especially those from Asia. Asian Americans are 2.7 times more likely to develop hepatocellular carcinoma. According to Dr. Calabrese, in mainland China there are an estimated 1 million people with rheumatologic problems who manifest chronic HBsAg positivity. "This is a global health problem," he noted.
Dr. Calabrese recommended that preemptive therapeutic measures be taken for all HBsAg-positive and HBV DNA–positive patients, including possible lifelong antiviral medication, and then these patients can be treated for their rheumatologic issues. Those who test positive for anti-HBc should be carefully monitored. "There should not be a patient with spondylitis, rheumatoid arthritis, lupus, or vasculitis who is getting less than effective therapy because they have chronic HBV," he said.
Dr. Calabrese reported no relevant financial disclosures.
AT THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Push to expand newborn screening for SCID
The Centers for Disease Control and Prevention announced on April 1 the availability of funding opportunities to expand newborn screening for severe combined immunodeficiency, also known as SCID.
Currently, only 12 states regularly test for SCID as part of their newborn screening programs. With the availability of a highly specific and low-cost test that measures a biomarker for a T-cell deficiency associated with SCID that can easily be added to current routine newborn screening, patient advocacy groups as well as state and federal governments are working to spur more widespread adoption of newborn SCID screening.
"Severe combined immunodeficiency is a pediatric emergency," medical geneticist Amy Brower, Ph.D., said at the NYU Annual Pediatric Rheumatology Update. "We need to get the message out to pediatricians and ob.gyns. that talking about newborn screening should begin prenatally. ... Unfortunately, surveys find that ob.gyns. do not have time for that conversation, and parents don’t remember it. We want physicians to know that there is a new test for SCID that has been vetted, is evidence based, and is available that can identify children before they become gravely ill."
Dr. Brower also suggests that parents should be made aware that even if their state does not offer SCID screening, they can request to have a newborn dried blood spot sample sent to a commercial lab for SCID analysis.
SCID is known colloquially as "bubble boy syndrome" because poor immune systems make these children vulnerable to infections. While babies born with SCID appear normal at birth, they stand a high risk of developing life-threatening viral, bacterial, and fungal infections. Right after birth, they are still protected by transplacentally derived maternal IgG antibodies, but soon lose that protection. If the condition is detected early and treated, survival rates are excellent. If treated before 3.5 months of age, 95% of affected babies will have long-term survival, but the survival rate falls below 70% if SCID is diagnosed after 3.5 months, even with intensive care and intervention (Public Health Rep. 2010;125:88-95).
Treatments include hematopoietic cell transplantation, enzyme replacement in cases of adenosine deaminase (ADA) deficiency, and gene therapy.
"Newborn screening for SCID is cost-effective, leads to years of productive life, and prevents the real negative outcomes associated with having one of this group of conditions," said Michael Watson, Ph.D., director of the American College of Medical Genetics and Genomics (ACMG) and principal investigator of the Newborn Screening Translational Research Network (NBSTRN). "Bone marrow transplant costs about $50,000-plus, but when these babies start getting infections and need hospitalization, the costs can be $1 to 1.2 million."
Using an estimated cost of SCID screening of $7.10 per baby, a medical model developed by the Washington State Department of Health reported a potential benefit:cost ratio of 4.93, meaning that almost $5 in benefits would accrue from every dollar of costs to provide SCID screening (Thompson JD, Glass M. Guide to the newborn screening cost-benefit model for adding severe combined immunodeficiency, 2012).
In January 2010, the U.S. Secretary of Health and Human Services approved SCID as the 30th core condition of the Recommended Uniform Screening Panel and the first immunological disorder. In May 2010, HHS Secretary Kathleen Sebelius endorsed newborn screening for SCID as the standard of care. However, there has been a wide disparity of adoption among states. "Currently no states are doing all newborn screening recommended by the secretary of HHS’s Advisory Committee, which now consists of 31 core conditions, including SCID and congenital heart screening," said Dr. Brower, project manager for the NBSTRN.
States that have screening programs in place for SCID are California, Colorado, Connecticut, Delaware, Florida, Massachusetts, Michigan, Minnesota, Mississippi, New York, Texas, and Wisconsin (also Puerto Rico).
Figure 1 from the NBSTRN shows the estimated number of newborns screened throughout the United States up until Dec. 31, 2012. Screening in the small area of New Mexico and Arizona reflects the high rate of SCID in Navajo Native Americans: In 2012, about 45% of all newborns were screened for SCID, and the hope is to reach 65% by 2014, Dr. Brower said at the meeting sponsored by New York University.
The Centers for Disease Control and Prevention (CDC) has been instrumental in trying to expand SCID newborn screening capability to more states, and contributed funding to pilot projects in Massachusetts and Wisconsin. The new CDC program is known as the Program to Support New Implementation of State or Territorial Public Health Laboratory Capacity for Newborn Bloodspot Screening of Severe Combined Immunodeficiency (SCID). Eligibility is restricted to states, special districts, and territorial governments that do not currently conduct SCID newborn screening. The total funds available are $1.8 million and it is anticipated that three awards will be made of $300,000 each year for 2 years. The application deadline is May 28, 2013, for funding that can begin on Aug. 1.
"Implementation of newborn screening for SCID is going slowly," agreed Dr. Watson. One challenge is the disparity of processes necessary for approval. Some states require legislative action, some need approval by the governor, and others require a go-ahead from the state’s department of health, which sometimes requires completion of a pilot program despite evidence collected from other states. Funding to properly outfit labs to carry out the new technology also can be a barrier.
Dr. Watson says that most of the states that initially began offering SCID screening already had the molecular assay technology in place from other programs, such as second-tier testing for cystic fibrosis. But even labs with the technical capability to conduct limited testing were not prepared to handle the high volume necessary for newborn screening. A possible cost-saving solution is that instead of having testing facilities available in every state, Dr. Watson suggests regionalized test centers, as is now done in New England, state partnerships, or even the use of commercial labs. Once a lab becomes equipped to handle SCID newborn screening, it often has the capacity to handle more than its own state’s needs, said Dr. Brower. Right now, Wisconsin and Massachusetts have the capacity to offer screening for other states.
Another limiting factor facing states considering adding SCID newborn screening can be the lack of a proper response mechanism. If a child screens positive for SCID, the child’s pediatrician must be prepared to shepherd the family through the next steps of diagnosis and arrange for immediate treatment, said Dr. Brower. To better inform pediatricians and other primary care physicians about SCID, the American College of Medical Genetics and Genomics offers an ACT sheet on SCID management aimed for the primary care provider. Dr. Watson also advises primary care providers to establish relationships with specialists in immunology, so that if a SCID baby is identified, the primary care provider can reach out for support regarding medical management, as well as for dealing with parental concerns.
The SCID newborn screening test is effective. Wisconsin was the first state to begin newborn screening for SCID. Between January 2008 and December 31, 2012, 5 infants with SCID or other forms of severe T-cell lymphopenia were detected out of almost 208,000 infants screened. No infants with SCID were missed during the screening period. The specificity of the assay used was 99.98%, with a false-positive rate of 0.018%. (Pediatrics 2012;130:S50-1). In California, the test had 99.91% specificity. Of 11 infants identified with SCID, all were able to be treated, with more than 90% alive at 6-21 months. In Colorado, a child with SCID was identified within the first week the SCID screening program began.
As part of the ACMG, the NBSTRN (www.nbstrn.org) maintains several SCID resources for investigators, including links to patient registries, a virtual repository of dried blood spot samples, and a longitudinal pediatric data resource. It also was involved with the National SCID Pilot Study that screened newborns in California, Louisiana, New York, and Puerto Rico. The ACMG operates the NBSTRN as part of funding it receives from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for research into new therapeutics, technologies, and other aspects of newborn screening.
The NBSTRN also holds monthly conference calls for stakeholders, including scientists, clinicians, public health specialists, administrators, and patient advocates, to discuss SCID-related developments. "After decades of living with SCID children, some of whom we have already lost, mothers with children like mine who are older and were late transplants understand the urgency. They push us to think, what else can we be doing?" said Dr. Brower. Those interested in helping to broaden SCID screening may consult the Immune Deficiency Foundation’s SCID Newborn Screening Toolkit for Advocates or attend its national conference in Baltimore on June 27-29.
Screening for SCID
SCID is a group of conditions characterized by blocks in T-cell development, leading to functional deficiencies in both T cells and B cells.
Fortunately, a screening test able to detect low levels of a DNA biomarker of normal T-cell development has proven successful at identifying individuals before symptoms appear. The test, developed by Dr. Jennifer M. Puck of the University of California, San Francisco, measures T-cell receptor excision circles (TRECs) using a polymerase chain reaction (PCR) process in samples of dried blood spots commonly taken from infants that are used for other screening purposes. Infants with SCID or related disorders have very low or undetectable levels of TRECs.
Being able to measure TRECs "was a real game changer for SCID," said Dr. Brower. It fit well into ongoing public health programs that utilized dried blood spot collections. "This was a technology developed at [the National Institutes of Health] that was developed and translated into clinical care with clear public health benefits." At the NBSTRN, Dr. Brower leads similar efforts to take genomic advances from bench to bedside.
The Wisconsin screening program uses a cutoff of 25 TRECs/mcL. Most screening programs have an algorithm in place for dealing with abnormal results. In Wisconsin, the first step is to redo the TREC assay. If the results are still positive, a physician member of the screening program is notified, who then contacts the newborn’s primary care physician. A decision is then made to either do another filter-paper specimen analysis or have a whole-blood specimen sent for T-cell enumeration by flow cytometry. A full workup for SCID is then recommended if results are still abnormal. (Curr. Opin. Pediatr. 2011;23:667-73).
In the first 18 months of the California screening program, 116 babies had samples sent for flow cytometry. Of those, 81 were normal. There were 10 cases of typical SCID, 1 child with Omenn syndrome (leaky SCID), 4 with variants of SCID, 8 with low T cell syndromes, and 7 with secondary causes of low T cells. There were 5 preterm births.
Children with leaky SCID may have a later onset of symptoms, with rash, adenopathy, and poorly functioning T cells. SCID variants include cartilage-hair hypoplasia, CHARGE syndrome (coloboma, heart defect, atresia choanae [also known as choanal atresia], retarded growth and development, genital abnormality, and ear abnormality), Down syndrome, or DiGeorge syndrome.
SCID newborn screening also could be helpful to children with non-SCID, secondary causes of T-cell lymphopenia who might also be vulnerable to serious opportunistic infections. Just recently, abnormal TREC findings led investigators to diagnose ataxia telangiectasia in two infants (J. Clin. Immunol. 2013;33:540-9).
Dr. Brower and Dr. Watson both said that they had no relevant financial disclosures.
The Centers for Disease Control and Prevention announced on April 1 the availability of funding opportunities to expand newborn screening for severe combined immunodeficiency, also known as SCID.
Currently, only 12 states regularly test for SCID as part of their newborn screening programs. With the availability of a highly specific and low-cost test that measures a biomarker for a T-cell deficiency associated with SCID that can easily be added to current routine newborn screening, patient advocacy groups as well as state and federal governments are working to spur more widespread adoption of newborn SCID screening.
"Severe combined immunodeficiency is a pediatric emergency," medical geneticist Amy Brower, Ph.D., said at the NYU Annual Pediatric Rheumatology Update. "We need to get the message out to pediatricians and ob.gyns. that talking about newborn screening should begin prenatally. ... Unfortunately, surveys find that ob.gyns. do not have time for that conversation, and parents don’t remember it. We want physicians to know that there is a new test for SCID that has been vetted, is evidence based, and is available that can identify children before they become gravely ill."
Dr. Brower also suggests that parents should be made aware that even if their state does not offer SCID screening, they can request to have a newborn dried blood spot sample sent to a commercial lab for SCID analysis.
SCID is known colloquially as "bubble boy syndrome" because poor immune systems make these children vulnerable to infections. While babies born with SCID appear normal at birth, they stand a high risk of developing life-threatening viral, bacterial, and fungal infections. Right after birth, they are still protected by transplacentally derived maternal IgG antibodies, but soon lose that protection. If the condition is detected early and treated, survival rates are excellent. If treated before 3.5 months of age, 95% of affected babies will have long-term survival, but the survival rate falls below 70% if SCID is diagnosed after 3.5 months, even with intensive care and intervention (Public Health Rep. 2010;125:88-95).
Treatments include hematopoietic cell transplantation, enzyme replacement in cases of adenosine deaminase (ADA) deficiency, and gene therapy.
"Newborn screening for SCID is cost-effective, leads to years of productive life, and prevents the real negative outcomes associated with having one of this group of conditions," said Michael Watson, Ph.D., director of the American College of Medical Genetics and Genomics (ACMG) and principal investigator of the Newborn Screening Translational Research Network (NBSTRN). "Bone marrow transplant costs about $50,000-plus, but when these babies start getting infections and need hospitalization, the costs can be $1 to 1.2 million."
Using an estimated cost of SCID screening of $7.10 per baby, a medical model developed by the Washington State Department of Health reported a potential benefit:cost ratio of 4.93, meaning that almost $5 in benefits would accrue from every dollar of costs to provide SCID screening (Thompson JD, Glass M. Guide to the newborn screening cost-benefit model for adding severe combined immunodeficiency, 2012).
In January 2010, the U.S. Secretary of Health and Human Services approved SCID as the 30th core condition of the Recommended Uniform Screening Panel and the first immunological disorder. In May 2010, HHS Secretary Kathleen Sebelius endorsed newborn screening for SCID as the standard of care. However, there has been a wide disparity of adoption among states. "Currently no states are doing all newborn screening recommended by the secretary of HHS’s Advisory Committee, which now consists of 31 core conditions, including SCID and congenital heart screening," said Dr. Brower, project manager for the NBSTRN.
States that have screening programs in place for SCID are California, Colorado, Connecticut, Delaware, Florida, Massachusetts, Michigan, Minnesota, Mississippi, New York, Texas, and Wisconsin (also Puerto Rico).
Figure 1 from the NBSTRN shows the estimated number of newborns screened throughout the United States up until Dec. 31, 2012. Screening in the small area of New Mexico and Arizona reflects the high rate of SCID in Navajo Native Americans: In 2012, about 45% of all newborns were screened for SCID, and the hope is to reach 65% by 2014, Dr. Brower said at the meeting sponsored by New York University.
The Centers for Disease Control and Prevention (CDC) has been instrumental in trying to expand SCID newborn screening capability to more states, and contributed funding to pilot projects in Massachusetts and Wisconsin. The new CDC program is known as the Program to Support New Implementation of State or Territorial Public Health Laboratory Capacity for Newborn Bloodspot Screening of Severe Combined Immunodeficiency (SCID). Eligibility is restricted to states, special districts, and territorial governments that do not currently conduct SCID newborn screening. The total funds available are $1.8 million and it is anticipated that three awards will be made of $300,000 each year for 2 years. The application deadline is May 28, 2013, for funding that can begin on Aug. 1.
"Implementation of newborn screening for SCID is going slowly," agreed Dr. Watson. One challenge is the disparity of processes necessary for approval. Some states require legislative action, some need approval by the governor, and others require a go-ahead from the state’s department of health, which sometimes requires completion of a pilot program despite evidence collected from other states. Funding to properly outfit labs to carry out the new technology also can be a barrier.
Dr. Watson says that most of the states that initially began offering SCID screening already had the molecular assay technology in place from other programs, such as second-tier testing for cystic fibrosis. But even labs with the technical capability to conduct limited testing were not prepared to handle the high volume necessary for newborn screening. A possible cost-saving solution is that instead of having testing facilities available in every state, Dr. Watson suggests regionalized test centers, as is now done in New England, state partnerships, or even the use of commercial labs. Once a lab becomes equipped to handle SCID newborn screening, it often has the capacity to handle more than its own state’s needs, said Dr. Brower. Right now, Wisconsin and Massachusetts have the capacity to offer screening for other states.
Another limiting factor facing states considering adding SCID newborn screening can be the lack of a proper response mechanism. If a child screens positive for SCID, the child’s pediatrician must be prepared to shepherd the family through the next steps of diagnosis and arrange for immediate treatment, said Dr. Brower. To better inform pediatricians and other primary care physicians about SCID, the American College of Medical Genetics and Genomics offers an ACT sheet on SCID management aimed for the primary care provider. Dr. Watson also advises primary care providers to establish relationships with specialists in immunology, so that if a SCID baby is identified, the primary care provider can reach out for support regarding medical management, as well as for dealing with parental concerns.
The SCID newborn screening test is effective. Wisconsin was the first state to begin newborn screening for SCID. Between January 2008 and December 31, 2012, 5 infants with SCID or other forms of severe T-cell lymphopenia were detected out of almost 208,000 infants screened. No infants with SCID were missed during the screening period. The specificity of the assay used was 99.98%, with a false-positive rate of 0.018%. (Pediatrics 2012;130:S50-1). In California, the test had 99.91% specificity. Of 11 infants identified with SCID, all were able to be treated, with more than 90% alive at 6-21 months. In Colorado, a child with SCID was identified within the first week the SCID screening program began.
As part of the ACMG, the NBSTRN (www.nbstrn.org) maintains several SCID resources for investigators, including links to patient registries, a virtual repository of dried blood spot samples, and a longitudinal pediatric data resource. It also was involved with the National SCID Pilot Study that screened newborns in California, Louisiana, New York, and Puerto Rico. The ACMG operates the NBSTRN as part of funding it receives from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for research into new therapeutics, technologies, and other aspects of newborn screening.
The NBSTRN also holds monthly conference calls for stakeholders, including scientists, clinicians, public health specialists, administrators, and patient advocates, to discuss SCID-related developments. "After decades of living with SCID children, some of whom we have already lost, mothers with children like mine who are older and were late transplants understand the urgency. They push us to think, what else can we be doing?" said Dr. Brower. Those interested in helping to broaden SCID screening may consult the Immune Deficiency Foundation’s SCID Newborn Screening Toolkit for Advocates or attend its national conference in Baltimore on June 27-29.
Screening for SCID
SCID is a group of conditions characterized by blocks in T-cell development, leading to functional deficiencies in both T cells and B cells.
Fortunately, a screening test able to detect low levels of a DNA biomarker of normal T-cell development has proven successful at identifying individuals before symptoms appear. The test, developed by Dr. Jennifer M. Puck of the University of California, San Francisco, measures T-cell receptor excision circles (TRECs) using a polymerase chain reaction (PCR) process in samples of dried blood spots commonly taken from infants that are used for other screening purposes. Infants with SCID or related disorders have very low or undetectable levels of TRECs.
Being able to measure TRECs "was a real game changer for SCID," said Dr. Brower. It fit well into ongoing public health programs that utilized dried blood spot collections. "This was a technology developed at [the National Institutes of Health] that was developed and translated into clinical care with clear public health benefits." At the NBSTRN, Dr. Brower leads similar efforts to take genomic advances from bench to bedside.
The Wisconsin screening program uses a cutoff of 25 TRECs/mcL. Most screening programs have an algorithm in place for dealing with abnormal results. In Wisconsin, the first step is to redo the TREC assay. If the results are still positive, a physician member of the screening program is notified, who then contacts the newborn’s primary care physician. A decision is then made to either do another filter-paper specimen analysis or have a whole-blood specimen sent for T-cell enumeration by flow cytometry. A full workup for SCID is then recommended if results are still abnormal. (Curr. Opin. Pediatr. 2011;23:667-73).
In the first 18 months of the California screening program, 116 babies had samples sent for flow cytometry. Of those, 81 were normal. There were 10 cases of typical SCID, 1 child with Omenn syndrome (leaky SCID), 4 with variants of SCID, 8 with low T cell syndromes, and 7 with secondary causes of low T cells. There were 5 preterm births.
Children with leaky SCID may have a later onset of symptoms, with rash, adenopathy, and poorly functioning T cells. SCID variants include cartilage-hair hypoplasia, CHARGE syndrome (coloboma, heart defect, atresia choanae [also known as choanal atresia], retarded growth and development, genital abnormality, and ear abnormality), Down syndrome, or DiGeorge syndrome.
SCID newborn screening also could be helpful to children with non-SCID, secondary causes of T-cell lymphopenia who might also be vulnerable to serious opportunistic infections. Just recently, abnormal TREC findings led investigators to diagnose ataxia telangiectasia in two infants (J. Clin. Immunol. 2013;33:540-9).
Dr. Brower and Dr. Watson both said that they had no relevant financial disclosures.
The Centers for Disease Control and Prevention announced on April 1 the availability of funding opportunities to expand newborn screening for severe combined immunodeficiency, also known as SCID.
Currently, only 12 states regularly test for SCID as part of their newborn screening programs. With the availability of a highly specific and low-cost test that measures a biomarker for a T-cell deficiency associated with SCID that can easily be added to current routine newborn screening, patient advocacy groups as well as state and federal governments are working to spur more widespread adoption of newborn SCID screening.
"Severe combined immunodeficiency is a pediatric emergency," medical geneticist Amy Brower, Ph.D., said at the NYU Annual Pediatric Rheumatology Update. "We need to get the message out to pediatricians and ob.gyns. that talking about newborn screening should begin prenatally. ... Unfortunately, surveys find that ob.gyns. do not have time for that conversation, and parents don’t remember it. We want physicians to know that there is a new test for SCID that has been vetted, is evidence based, and is available that can identify children before they become gravely ill."
Dr. Brower also suggests that parents should be made aware that even if their state does not offer SCID screening, they can request to have a newborn dried blood spot sample sent to a commercial lab for SCID analysis.
SCID is known colloquially as "bubble boy syndrome" because poor immune systems make these children vulnerable to infections. While babies born with SCID appear normal at birth, they stand a high risk of developing life-threatening viral, bacterial, and fungal infections. Right after birth, they are still protected by transplacentally derived maternal IgG antibodies, but soon lose that protection. If the condition is detected early and treated, survival rates are excellent. If treated before 3.5 months of age, 95% of affected babies will have long-term survival, but the survival rate falls below 70% if SCID is diagnosed after 3.5 months, even with intensive care and intervention (Public Health Rep. 2010;125:88-95).
Treatments include hematopoietic cell transplantation, enzyme replacement in cases of adenosine deaminase (ADA) deficiency, and gene therapy.
"Newborn screening for SCID is cost-effective, leads to years of productive life, and prevents the real negative outcomes associated with having one of this group of conditions," said Michael Watson, Ph.D., director of the American College of Medical Genetics and Genomics (ACMG) and principal investigator of the Newborn Screening Translational Research Network (NBSTRN). "Bone marrow transplant costs about $50,000-plus, but when these babies start getting infections and need hospitalization, the costs can be $1 to 1.2 million."
Using an estimated cost of SCID screening of $7.10 per baby, a medical model developed by the Washington State Department of Health reported a potential benefit:cost ratio of 4.93, meaning that almost $5 in benefits would accrue from every dollar of costs to provide SCID screening (Thompson JD, Glass M. Guide to the newborn screening cost-benefit model for adding severe combined immunodeficiency, 2012).
In January 2010, the U.S. Secretary of Health and Human Services approved SCID as the 30th core condition of the Recommended Uniform Screening Panel and the first immunological disorder. In May 2010, HHS Secretary Kathleen Sebelius endorsed newborn screening for SCID as the standard of care. However, there has been a wide disparity of adoption among states. "Currently no states are doing all newborn screening recommended by the secretary of HHS’s Advisory Committee, which now consists of 31 core conditions, including SCID and congenital heart screening," said Dr. Brower, project manager for the NBSTRN.
States that have screening programs in place for SCID are California, Colorado, Connecticut, Delaware, Florida, Massachusetts, Michigan, Minnesota, Mississippi, New York, Texas, and Wisconsin (also Puerto Rico).
Figure 1 from the NBSTRN shows the estimated number of newborns screened throughout the United States up until Dec. 31, 2012. Screening in the small area of New Mexico and Arizona reflects the high rate of SCID in Navajo Native Americans: In 2012, about 45% of all newborns were screened for SCID, and the hope is to reach 65% by 2014, Dr. Brower said at the meeting sponsored by New York University.
The Centers for Disease Control and Prevention (CDC) has been instrumental in trying to expand SCID newborn screening capability to more states, and contributed funding to pilot projects in Massachusetts and Wisconsin. The new CDC program is known as the Program to Support New Implementation of State or Territorial Public Health Laboratory Capacity for Newborn Bloodspot Screening of Severe Combined Immunodeficiency (SCID). Eligibility is restricted to states, special districts, and territorial governments that do not currently conduct SCID newborn screening. The total funds available are $1.8 million and it is anticipated that three awards will be made of $300,000 each year for 2 years. The application deadline is May 28, 2013, for funding that can begin on Aug. 1.
"Implementation of newborn screening for SCID is going slowly," agreed Dr. Watson. One challenge is the disparity of processes necessary for approval. Some states require legislative action, some need approval by the governor, and others require a go-ahead from the state’s department of health, which sometimes requires completion of a pilot program despite evidence collected from other states. Funding to properly outfit labs to carry out the new technology also can be a barrier.
Dr. Watson says that most of the states that initially began offering SCID screening already had the molecular assay technology in place from other programs, such as second-tier testing for cystic fibrosis. But even labs with the technical capability to conduct limited testing were not prepared to handle the high volume necessary for newborn screening. A possible cost-saving solution is that instead of having testing facilities available in every state, Dr. Watson suggests regionalized test centers, as is now done in New England, state partnerships, or even the use of commercial labs. Once a lab becomes equipped to handle SCID newborn screening, it often has the capacity to handle more than its own state’s needs, said Dr. Brower. Right now, Wisconsin and Massachusetts have the capacity to offer screening for other states.
Another limiting factor facing states considering adding SCID newborn screening can be the lack of a proper response mechanism. If a child screens positive for SCID, the child’s pediatrician must be prepared to shepherd the family through the next steps of diagnosis and arrange for immediate treatment, said Dr. Brower. To better inform pediatricians and other primary care physicians about SCID, the American College of Medical Genetics and Genomics offers an ACT sheet on SCID management aimed for the primary care provider. Dr. Watson also advises primary care providers to establish relationships with specialists in immunology, so that if a SCID baby is identified, the primary care provider can reach out for support regarding medical management, as well as for dealing with parental concerns.
The SCID newborn screening test is effective. Wisconsin was the first state to begin newborn screening for SCID. Between January 2008 and December 31, 2012, 5 infants with SCID or other forms of severe T-cell lymphopenia were detected out of almost 208,000 infants screened. No infants with SCID were missed during the screening period. The specificity of the assay used was 99.98%, with a false-positive rate of 0.018%. (Pediatrics 2012;130:S50-1). In California, the test had 99.91% specificity. Of 11 infants identified with SCID, all were able to be treated, with more than 90% alive at 6-21 months. In Colorado, a child with SCID was identified within the first week the SCID screening program began.
As part of the ACMG, the NBSTRN (www.nbstrn.org) maintains several SCID resources for investigators, including links to patient registries, a virtual repository of dried blood spot samples, and a longitudinal pediatric data resource. It also was involved with the National SCID Pilot Study that screened newborns in California, Louisiana, New York, and Puerto Rico. The ACMG operates the NBSTRN as part of funding it receives from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for research into new therapeutics, technologies, and other aspects of newborn screening.
The NBSTRN also holds monthly conference calls for stakeholders, including scientists, clinicians, public health specialists, administrators, and patient advocates, to discuss SCID-related developments. "After decades of living with SCID children, some of whom we have already lost, mothers with children like mine who are older and were late transplants understand the urgency. They push us to think, what else can we be doing?" said Dr. Brower. Those interested in helping to broaden SCID screening may consult the Immune Deficiency Foundation’s SCID Newborn Screening Toolkit for Advocates or attend its national conference in Baltimore on June 27-29.
Screening for SCID
SCID is a group of conditions characterized by blocks in T-cell development, leading to functional deficiencies in both T cells and B cells.
Fortunately, a screening test able to detect low levels of a DNA biomarker of normal T-cell development has proven successful at identifying individuals before symptoms appear. The test, developed by Dr. Jennifer M. Puck of the University of California, San Francisco, measures T-cell receptor excision circles (TRECs) using a polymerase chain reaction (PCR) process in samples of dried blood spots commonly taken from infants that are used for other screening purposes. Infants with SCID or related disorders have very low or undetectable levels of TRECs.
Being able to measure TRECs "was a real game changer for SCID," said Dr. Brower. It fit well into ongoing public health programs that utilized dried blood spot collections. "This was a technology developed at [the National Institutes of Health] that was developed and translated into clinical care with clear public health benefits." At the NBSTRN, Dr. Brower leads similar efforts to take genomic advances from bench to bedside.
The Wisconsin screening program uses a cutoff of 25 TRECs/mcL. Most screening programs have an algorithm in place for dealing with abnormal results. In Wisconsin, the first step is to redo the TREC assay. If the results are still positive, a physician member of the screening program is notified, who then contacts the newborn’s primary care physician. A decision is then made to either do another filter-paper specimen analysis or have a whole-blood specimen sent for T-cell enumeration by flow cytometry. A full workup for SCID is then recommended if results are still abnormal. (Curr. Opin. Pediatr. 2011;23:667-73).
In the first 18 months of the California screening program, 116 babies had samples sent for flow cytometry. Of those, 81 were normal. There were 10 cases of typical SCID, 1 child with Omenn syndrome (leaky SCID), 4 with variants of SCID, 8 with low T cell syndromes, and 7 with secondary causes of low T cells. There were 5 preterm births.
Children with leaky SCID may have a later onset of symptoms, with rash, adenopathy, and poorly functioning T cells. SCID variants include cartilage-hair hypoplasia, CHARGE syndrome (coloboma, heart defect, atresia choanae [also known as choanal atresia], retarded growth and development, genital abnormality, and ear abnormality), Down syndrome, or DiGeorge syndrome.
SCID newborn screening also could be helpful to children with non-SCID, secondary causes of T-cell lymphopenia who might also be vulnerable to serious opportunistic infections. Just recently, abnormal TREC findings led investigators to diagnose ataxia telangiectasia in two infants (J. Clin. Immunol. 2013;33:540-9).
Dr. Brower and Dr. Watson both said that they had no relevant financial disclosures.
Hydroxychloroquine under scrutiny for cardiac neonatal lupus prevention
NEW YORK – Positive signs from the nearly completed first stage of a prospective prevention trial for cardiac neonatal lupus are giving investigators hope that hydroxychloroquine can reduce the risk of fetal heart block if given during pregnancy to women with systemic lupus erythematosus who have previously had a child with the condition.
The first stage of the PATCH (Preventive Approach to Congenital Heart Block With Hydroxychloroquine) study has enrolled all of its intended 19 pregnant women with systemic lupus erythematosus (SLE) and anti-SSA/Ro and anti-SSB/La antibodies who had a previous child with cardiac neonatal lupus (CNL). So far, cardiac abnormalities have been found in only 1 of 17 completed pregnancies in women who began taking 400-mg hydroxychloroquine (HCQ) before 10 weeks’ gestation. The study will go on to a second stage if fewer than three cases of second- or third-degree heart block occur. Enrollment of an additional 35 women is planned for the second stage, Dr. Jill P. Buyon said at the New York University Seminar in Advanced Rheumatology. She is director of the New York University Lupus Center, a professor in the division of rheumatology at New York University, and the principal investigator of the open-label PATCH study.
HCQ treatment will be considered efficacious if fewer than 6 mothers of the 54 total have a child with advanced congenital heart block. With this design, the study has 90% power to conclude that HCQ is preventive if the true recurrence rate with the treatment is 5%. In addition, the probability of rejecting the treatment for further study is 95% if the true recurrence rate is 18%, according to Dr. Buyon and her colleagues.
Women in the trial are given weekly serial fetal echocardiograms and blood tests for potential biomarkers of efficacy and compliance. The results of the study are expected to become an integral part of the counseling of women with anti-Ro/La antibodies who are considering pregnancy, according to the investigators.
"We need prophylactic treatment for autoimmune-associated congenital heart block because of the significant morbidity and mortality. The fibrosis is immutable, and there is no sustained reversal of complete heart block," Dr. Buyon said at the meeting sponsored by NYU. In previous work, Dr. Buyon reported a 17.5% mortality rate of neonates with cardiac neonatal lupus (CNL), 30% in utero (Circulation 2011;124:1927-35).
Congenital atrioventricular block and cardiomyopathy are the two major manifestations of CNL. The risk of CNL is approximately 2% in women with SLE who have given birth to an unaffected child, but the recurrence rate is 17.4% in those who have previously given birth to a baby with CNL. Mechanistically, HCQ’s inhibitory effect on toll-like receptors is thought to interfere with the pathophysiologic cascade underlying CNL.
Research leading up to the PATCH study indicated that HCQ might reduce the risk of CNL by 65% when pregnant mothers with SLE took HCQ throughout their pregnancy, Dr. Buyon said.
She and her colleagues analyzed a historical cohort gathered from three international databases (the Research Registry for Neonatal Lupus, the PRIDE study, and the PROMISSE study). In total, 257 pregnancies of anti-SSA/Ro–positive mothers with SLE who had previously given birth to a child with CNL were identified. Forty of the women were exposed throughout their pregnancies (starting before 10 weeks of gestation) to HCQ and 217 were not exposed to HCQ during pregnancy (Circulation 2012;126:76-82).
Significantly more fetuses in the unexposed group developed CNL (46 [21%] of 217) than in the exposed group (3 [8%] of 40 fetuses) (P = .05). The overall case fatality rate of the CNL fetuses in the unexposed group was 22%, compared with no deaths in the exposed group. No reduction in CNL recurrence was found for prednisone, dexamethasone, or fluorinated steroids.
Dr. Buyon reported having no relevant financial disclosures.
NEW YORK – Positive signs from the nearly completed first stage of a prospective prevention trial for cardiac neonatal lupus are giving investigators hope that hydroxychloroquine can reduce the risk of fetal heart block if given during pregnancy to women with systemic lupus erythematosus who have previously had a child with the condition.
The first stage of the PATCH (Preventive Approach to Congenital Heart Block With Hydroxychloroquine) study has enrolled all of its intended 19 pregnant women with systemic lupus erythematosus (SLE) and anti-SSA/Ro and anti-SSB/La antibodies who had a previous child with cardiac neonatal lupus (CNL). So far, cardiac abnormalities have been found in only 1 of 17 completed pregnancies in women who began taking 400-mg hydroxychloroquine (HCQ) before 10 weeks’ gestation. The study will go on to a second stage if fewer than three cases of second- or third-degree heart block occur. Enrollment of an additional 35 women is planned for the second stage, Dr. Jill P. Buyon said at the New York University Seminar in Advanced Rheumatology. She is director of the New York University Lupus Center, a professor in the division of rheumatology at New York University, and the principal investigator of the open-label PATCH study.
HCQ treatment will be considered efficacious if fewer than 6 mothers of the 54 total have a child with advanced congenital heart block. With this design, the study has 90% power to conclude that HCQ is preventive if the true recurrence rate with the treatment is 5%. In addition, the probability of rejecting the treatment for further study is 95% if the true recurrence rate is 18%, according to Dr. Buyon and her colleagues.
Women in the trial are given weekly serial fetal echocardiograms and blood tests for potential biomarkers of efficacy and compliance. The results of the study are expected to become an integral part of the counseling of women with anti-Ro/La antibodies who are considering pregnancy, according to the investigators.
"We need prophylactic treatment for autoimmune-associated congenital heart block because of the significant morbidity and mortality. The fibrosis is immutable, and there is no sustained reversal of complete heart block," Dr. Buyon said at the meeting sponsored by NYU. In previous work, Dr. Buyon reported a 17.5% mortality rate of neonates with cardiac neonatal lupus (CNL), 30% in utero (Circulation 2011;124:1927-35).
Congenital atrioventricular block and cardiomyopathy are the two major manifestations of CNL. The risk of CNL is approximately 2% in women with SLE who have given birth to an unaffected child, but the recurrence rate is 17.4% in those who have previously given birth to a baby with CNL. Mechanistically, HCQ’s inhibitory effect on toll-like receptors is thought to interfere with the pathophysiologic cascade underlying CNL.
Research leading up to the PATCH study indicated that HCQ might reduce the risk of CNL by 65% when pregnant mothers with SLE took HCQ throughout their pregnancy, Dr. Buyon said.
She and her colleagues analyzed a historical cohort gathered from three international databases (the Research Registry for Neonatal Lupus, the PRIDE study, and the PROMISSE study). In total, 257 pregnancies of anti-SSA/Ro–positive mothers with SLE who had previously given birth to a child with CNL were identified. Forty of the women were exposed throughout their pregnancies (starting before 10 weeks of gestation) to HCQ and 217 were not exposed to HCQ during pregnancy (Circulation 2012;126:76-82).
Significantly more fetuses in the unexposed group developed CNL (46 [21%] of 217) than in the exposed group (3 [8%] of 40 fetuses) (P = .05). The overall case fatality rate of the CNL fetuses in the unexposed group was 22%, compared with no deaths in the exposed group. No reduction in CNL recurrence was found for prednisone, dexamethasone, or fluorinated steroids.
Dr. Buyon reported having no relevant financial disclosures.
NEW YORK – Positive signs from the nearly completed first stage of a prospective prevention trial for cardiac neonatal lupus are giving investigators hope that hydroxychloroquine can reduce the risk of fetal heart block if given during pregnancy to women with systemic lupus erythematosus who have previously had a child with the condition.
The first stage of the PATCH (Preventive Approach to Congenital Heart Block With Hydroxychloroquine) study has enrolled all of its intended 19 pregnant women with systemic lupus erythematosus (SLE) and anti-SSA/Ro and anti-SSB/La antibodies who had a previous child with cardiac neonatal lupus (CNL). So far, cardiac abnormalities have been found in only 1 of 17 completed pregnancies in women who began taking 400-mg hydroxychloroquine (HCQ) before 10 weeks’ gestation. The study will go on to a second stage if fewer than three cases of second- or third-degree heart block occur. Enrollment of an additional 35 women is planned for the second stage, Dr. Jill P. Buyon said at the New York University Seminar in Advanced Rheumatology. She is director of the New York University Lupus Center, a professor in the division of rheumatology at New York University, and the principal investigator of the open-label PATCH study.
HCQ treatment will be considered efficacious if fewer than 6 mothers of the 54 total have a child with advanced congenital heart block. With this design, the study has 90% power to conclude that HCQ is preventive if the true recurrence rate with the treatment is 5%. In addition, the probability of rejecting the treatment for further study is 95% if the true recurrence rate is 18%, according to Dr. Buyon and her colleagues.
Women in the trial are given weekly serial fetal echocardiograms and blood tests for potential biomarkers of efficacy and compliance. The results of the study are expected to become an integral part of the counseling of women with anti-Ro/La antibodies who are considering pregnancy, according to the investigators.
"We need prophylactic treatment for autoimmune-associated congenital heart block because of the significant morbidity and mortality. The fibrosis is immutable, and there is no sustained reversal of complete heart block," Dr. Buyon said at the meeting sponsored by NYU. In previous work, Dr. Buyon reported a 17.5% mortality rate of neonates with cardiac neonatal lupus (CNL), 30% in utero (Circulation 2011;124:1927-35).
Congenital atrioventricular block and cardiomyopathy are the two major manifestations of CNL. The risk of CNL is approximately 2% in women with SLE who have given birth to an unaffected child, but the recurrence rate is 17.4% in those who have previously given birth to a baby with CNL. Mechanistically, HCQ’s inhibitory effect on toll-like receptors is thought to interfere with the pathophysiologic cascade underlying CNL.
Research leading up to the PATCH study indicated that HCQ might reduce the risk of CNL by 65% when pregnant mothers with SLE took HCQ throughout their pregnancy, Dr. Buyon said.
She and her colleagues analyzed a historical cohort gathered from three international databases (the Research Registry for Neonatal Lupus, the PRIDE study, and the PROMISSE study). In total, 257 pregnancies of anti-SSA/Ro–positive mothers with SLE who had previously given birth to a child with CNL were identified. Forty of the women were exposed throughout their pregnancies (starting before 10 weeks of gestation) to HCQ and 217 were not exposed to HCQ during pregnancy (Circulation 2012;126:76-82).
Significantly more fetuses in the unexposed group developed CNL (46 [21%] of 217) than in the exposed group (3 [8%] of 40 fetuses) (P = .05). The overall case fatality rate of the CNL fetuses in the unexposed group was 22%, compared with no deaths in the exposed group. No reduction in CNL recurrence was found for prednisone, dexamethasone, or fluorinated steroids.
Dr. Buyon reported having no relevant financial disclosures.
AT THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Fine-tune screening for adverse pregnancy outcome biomarkers in antiphospholipid syndrome
NEW YORK – What you screen and where you send your samples can have a tremendous impact on the usefulness of biomarkers to predict adverse pregnancy outcomes in patients with antiphospholipid syndrome, according to Dr. Michael D. Lockshin.
Women who have antiphospholipid antibodies are at high risk of developing adverse pregnancy outcomes (APOs), including preeclampsia, restricted fetal growth, prematurity, and fetal neonatal death. Antiphospholipid syndrome (APS) is defined by a clinical event (either thrombosis and/or recurrent pregnancy loss) plus the presence of an antiphospholipid antibody (either IgG or IgM anticardiolipin [aCL] and/or beta-2-glycoprotein I [beta-2 GPI]).
To see which serologic variables associated with APS may best identify women at highest risk of APO, researchers enrolled 144 pregnant patients with antiphospholipid antibodies (aPL) between 2003 and 2011 in the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus) study. Twenty-eight (19%) had an APO. The results showed that 39% of the patients with lupus anticoagulant (LAC) had an APO, compared with 3% who did not have LAC (P = .0001).
A small percentage (5%) of women who were LAC negative but had high titers of IgG aCL antibody (at least 40 units/mL) had APOs. IgM aCL, IgG anti-beta-2 GPI, and IgM anti-beta-2 GPI were not predictive of APOs, according to Dr. Lockshin and his colleagues (Arthritis Rheum. 2012;64:2311-8).
Among women with high titers of IgG aCL, 8% of those who were LAC negative had an APO, compared with 43% who were LAC positive (P = .002).
"The only tests that made a difference in predicting APOs were lupus anticoagulant and IgG aCL," said Dr. Lockshin, director of the Barbara Volcker Center for Women and Rheumatic Disease and professor of medicine and of obstetrics and gynecology at Weill Cornell Medical College, New York.
Similar patterns were seen in women with lupus who also had APS. In that population, 55% of those who were LAC positive and 12% who were LAC negative/high titer IgG aCL had an APO.
Dr. Lockshin then asked whether LAC screening methods differed in predicting the risk of APOs. He compared the positive and negative predictive values of the dilute prothrombin time test (dPT), the dilute Russell viper venom test (dRVVT), and the activated partial thromboplastin time test (APTT), as well as all tests combined, and found that using the results of all three tests together yielded the highest positive predictive value (all, 0.926; dPT, 0.808; dRVVT, 0.560; aPTT, 0.630). The test with the best negative predictive value was the dRVVT (0.910).
Making appropriate risk assessments for women with APS depends on accurate serological measurement. Dr. Lockshin reported that in a group of 610 women who were referred for SLE and/or aPL, 279 were found to be aPL positive by a referral laboratory. Upon reanalysis of the samples by a core laboratory, 147 of those deemed aPL positive by the referral laboratory were deemed to be aPL negative, yielding a 52% false positive rate. In a group of 196 referred as healthy controls, 2 were found to be aPL positive by the core lab, meaning that 2% of the controls were false negative for aPL. "Don’t just casually accept a commercial laboratory’s determination," Dr. Lockshin said at the meeting, sponsored by New York University.
In multivariate analysis, having SLE significantly increased the risk of having an APO (23% vs. 17%). Patients with prior thrombosis also were more likely to have an APO than were those without prior thrombosis (52% vs. 13%, P = .00005). Age, race, or prior pregnancy loss were not determinants of pregnancy problems.
Dr. Lockshin also addressed the question as to whether heparin mitigates the risk of an APO. He found that in patients who were LAC positive, there was no difference in the number of APOs in those given heparin (49%), compared with those not treated with heparin (50%). The rate of APOs was actually higher in women who were LAC negative but treated with heparin, compared with those who did not receive heparin (29% vs. 18%, no significant difference). Aspirin did reduce the occurrence of APOs (14% vs. 30%, P = .04), but neither hydroxychloroquine nor steroids had any protective effects.
At the meeting, Dr. Lockshin also reported the results of a pilot 12-month open-label phase II trial of rituximab for the noncriteria manifestations of APS (thrombocytopenia, cardiac valve disease, skin ulcers, aPL nephropathy, and/or cognitive dysfunction). After two doses of rituximab (1,000 mg) on days 1 and 15, dramatic improvements in leg ulcers were seen, and, for the first time ever, possible improvement in cognitive function, said Dr. Lockshin. No changes were noted in serology, cardiac valves, or abnormal imaging findings (Arthritis Rheum. 2013;65:464-471).
To encourage further investigation of APS, an international research network has been launched to organize well-designed, large-scale, multicenter clinical trials in aPL-positive patients, said Dr. Lockshin. The AntiPhospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) has initiated two collaborative projects, one looking into the use of hydroxychloroquine for primary thrombosis prevention and the other a web-based registry of aPL-positive patients with or without systemic autoimmune diseases. For more information, go to www.apsaction.org.
Dr. Lockshin reported no financial disclosures.
NEW YORK – What you screen and where you send your samples can have a tremendous impact on the usefulness of biomarkers to predict adverse pregnancy outcomes in patients with antiphospholipid syndrome, according to Dr. Michael D. Lockshin.
Women who have antiphospholipid antibodies are at high risk of developing adverse pregnancy outcomes (APOs), including preeclampsia, restricted fetal growth, prematurity, and fetal neonatal death. Antiphospholipid syndrome (APS) is defined by a clinical event (either thrombosis and/or recurrent pregnancy loss) plus the presence of an antiphospholipid antibody (either IgG or IgM anticardiolipin [aCL] and/or beta-2-glycoprotein I [beta-2 GPI]).
To see which serologic variables associated with APS may best identify women at highest risk of APO, researchers enrolled 144 pregnant patients with antiphospholipid antibodies (aPL) between 2003 and 2011 in the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus) study. Twenty-eight (19%) had an APO. The results showed that 39% of the patients with lupus anticoagulant (LAC) had an APO, compared with 3% who did not have LAC (P = .0001).
A small percentage (5%) of women who were LAC negative but had high titers of IgG aCL antibody (at least 40 units/mL) had APOs. IgM aCL, IgG anti-beta-2 GPI, and IgM anti-beta-2 GPI were not predictive of APOs, according to Dr. Lockshin and his colleagues (Arthritis Rheum. 2012;64:2311-8).
Among women with high titers of IgG aCL, 8% of those who were LAC negative had an APO, compared with 43% who were LAC positive (P = .002).
"The only tests that made a difference in predicting APOs were lupus anticoagulant and IgG aCL," said Dr. Lockshin, director of the Barbara Volcker Center for Women and Rheumatic Disease and professor of medicine and of obstetrics and gynecology at Weill Cornell Medical College, New York.
Similar patterns were seen in women with lupus who also had APS. In that population, 55% of those who were LAC positive and 12% who were LAC negative/high titer IgG aCL had an APO.
Dr. Lockshin then asked whether LAC screening methods differed in predicting the risk of APOs. He compared the positive and negative predictive values of the dilute prothrombin time test (dPT), the dilute Russell viper venom test (dRVVT), and the activated partial thromboplastin time test (APTT), as well as all tests combined, and found that using the results of all three tests together yielded the highest positive predictive value (all, 0.926; dPT, 0.808; dRVVT, 0.560; aPTT, 0.630). The test with the best negative predictive value was the dRVVT (0.910).
Making appropriate risk assessments for women with APS depends on accurate serological measurement. Dr. Lockshin reported that in a group of 610 women who were referred for SLE and/or aPL, 279 were found to be aPL positive by a referral laboratory. Upon reanalysis of the samples by a core laboratory, 147 of those deemed aPL positive by the referral laboratory were deemed to be aPL negative, yielding a 52% false positive rate. In a group of 196 referred as healthy controls, 2 were found to be aPL positive by the core lab, meaning that 2% of the controls were false negative for aPL. "Don’t just casually accept a commercial laboratory’s determination," Dr. Lockshin said at the meeting, sponsored by New York University.
In multivariate analysis, having SLE significantly increased the risk of having an APO (23% vs. 17%). Patients with prior thrombosis also were more likely to have an APO than were those without prior thrombosis (52% vs. 13%, P = .00005). Age, race, or prior pregnancy loss were not determinants of pregnancy problems.
Dr. Lockshin also addressed the question as to whether heparin mitigates the risk of an APO. He found that in patients who were LAC positive, there was no difference in the number of APOs in those given heparin (49%), compared with those not treated with heparin (50%). The rate of APOs was actually higher in women who were LAC negative but treated with heparin, compared with those who did not receive heparin (29% vs. 18%, no significant difference). Aspirin did reduce the occurrence of APOs (14% vs. 30%, P = .04), but neither hydroxychloroquine nor steroids had any protective effects.
At the meeting, Dr. Lockshin also reported the results of a pilot 12-month open-label phase II trial of rituximab for the noncriteria manifestations of APS (thrombocytopenia, cardiac valve disease, skin ulcers, aPL nephropathy, and/or cognitive dysfunction). After two doses of rituximab (1,000 mg) on days 1 and 15, dramatic improvements in leg ulcers were seen, and, for the first time ever, possible improvement in cognitive function, said Dr. Lockshin. No changes were noted in serology, cardiac valves, or abnormal imaging findings (Arthritis Rheum. 2013;65:464-471).
To encourage further investigation of APS, an international research network has been launched to organize well-designed, large-scale, multicenter clinical trials in aPL-positive patients, said Dr. Lockshin. The AntiPhospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) has initiated two collaborative projects, one looking into the use of hydroxychloroquine for primary thrombosis prevention and the other a web-based registry of aPL-positive patients with or without systemic autoimmune diseases. For more information, go to www.apsaction.org.
Dr. Lockshin reported no financial disclosures.
NEW YORK – What you screen and where you send your samples can have a tremendous impact on the usefulness of biomarkers to predict adverse pregnancy outcomes in patients with antiphospholipid syndrome, according to Dr. Michael D. Lockshin.
Women who have antiphospholipid antibodies are at high risk of developing adverse pregnancy outcomes (APOs), including preeclampsia, restricted fetal growth, prematurity, and fetal neonatal death. Antiphospholipid syndrome (APS) is defined by a clinical event (either thrombosis and/or recurrent pregnancy loss) plus the presence of an antiphospholipid antibody (either IgG or IgM anticardiolipin [aCL] and/or beta-2-glycoprotein I [beta-2 GPI]).
To see which serologic variables associated with APS may best identify women at highest risk of APO, researchers enrolled 144 pregnant patients with antiphospholipid antibodies (aPL) between 2003 and 2011 in the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus) study. Twenty-eight (19%) had an APO. The results showed that 39% of the patients with lupus anticoagulant (LAC) had an APO, compared with 3% who did not have LAC (P = .0001).
A small percentage (5%) of women who were LAC negative but had high titers of IgG aCL antibody (at least 40 units/mL) had APOs. IgM aCL, IgG anti-beta-2 GPI, and IgM anti-beta-2 GPI were not predictive of APOs, according to Dr. Lockshin and his colleagues (Arthritis Rheum. 2012;64:2311-8).
Among women with high titers of IgG aCL, 8% of those who were LAC negative had an APO, compared with 43% who were LAC positive (P = .002).
"The only tests that made a difference in predicting APOs were lupus anticoagulant and IgG aCL," said Dr. Lockshin, director of the Barbara Volcker Center for Women and Rheumatic Disease and professor of medicine and of obstetrics and gynecology at Weill Cornell Medical College, New York.
Similar patterns were seen in women with lupus who also had APS. In that population, 55% of those who were LAC positive and 12% who were LAC negative/high titer IgG aCL had an APO.
Dr. Lockshin then asked whether LAC screening methods differed in predicting the risk of APOs. He compared the positive and negative predictive values of the dilute prothrombin time test (dPT), the dilute Russell viper venom test (dRVVT), and the activated partial thromboplastin time test (APTT), as well as all tests combined, and found that using the results of all three tests together yielded the highest positive predictive value (all, 0.926; dPT, 0.808; dRVVT, 0.560; aPTT, 0.630). The test with the best negative predictive value was the dRVVT (0.910).
Making appropriate risk assessments for women with APS depends on accurate serological measurement. Dr. Lockshin reported that in a group of 610 women who were referred for SLE and/or aPL, 279 were found to be aPL positive by a referral laboratory. Upon reanalysis of the samples by a core laboratory, 147 of those deemed aPL positive by the referral laboratory were deemed to be aPL negative, yielding a 52% false positive rate. In a group of 196 referred as healthy controls, 2 were found to be aPL positive by the core lab, meaning that 2% of the controls were false negative for aPL. "Don’t just casually accept a commercial laboratory’s determination," Dr. Lockshin said at the meeting, sponsored by New York University.
In multivariate analysis, having SLE significantly increased the risk of having an APO (23% vs. 17%). Patients with prior thrombosis also were more likely to have an APO than were those without prior thrombosis (52% vs. 13%, P = .00005). Age, race, or prior pregnancy loss were not determinants of pregnancy problems.
Dr. Lockshin also addressed the question as to whether heparin mitigates the risk of an APO. He found that in patients who were LAC positive, there was no difference in the number of APOs in those given heparin (49%), compared with those not treated with heparin (50%). The rate of APOs was actually higher in women who were LAC negative but treated with heparin, compared with those who did not receive heparin (29% vs. 18%, no significant difference). Aspirin did reduce the occurrence of APOs (14% vs. 30%, P = .04), but neither hydroxychloroquine nor steroids had any protective effects.
At the meeting, Dr. Lockshin also reported the results of a pilot 12-month open-label phase II trial of rituximab for the noncriteria manifestations of APS (thrombocytopenia, cardiac valve disease, skin ulcers, aPL nephropathy, and/or cognitive dysfunction). After two doses of rituximab (1,000 mg) on days 1 and 15, dramatic improvements in leg ulcers were seen, and, for the first time ever, possible improvement in cognitive function, said Dr. Lockshin. No changes were noted in serology, cardiac valves, or abnormal imaging findings (Arthritis Rheum. 2013;65:464-471).
To encourage further investigation of APS, an international research network has been launched to organize well-designed, large-scale, multicenter clinical trials in aPL-positive patients, said Dr. Lockshin. The AntiPhospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) has initiated two collaborative projects, one looking into the use of hydroxychloroquine for primary thrombosis prevention and the other a web-based registry of aPL-positive patients with or without systemic autoimmune diseases. For more information, go to www.apsaction.org.
Dr. Lockshin reported no financial disclosures.
EXPERT ANALYSIS FROM THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Major finding: Thirty-nine percent of pregnant patients with lupus anticoagulant had an adverse pregnancy outcome, compared with 3% who did not have LAC.
Data source: Multicenter prospective observational study of 144 patients (PROMISSE).
Disclosures: Dr. Lockshin reported no financial disclosures.










