User login
In reply: Essential tremor, beta-blockers, calcium channel blockers
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
In Reply: We agree and thank Dr. Keller for raising this valid point. The two classes of calcium channel blockers are distinct in their actions, and the warning about not combining a calcium channel blocker with a beta-blocker because of the increased risk of developing significant bradycardia applies only to the nondihydropyridine class.
Essential tremor: Choosing the right management plan for your patient
Essential tremor, one of the most common movement disorders, affects about 4% of adults 40 years of age and older.1 It is often referred to as familial tremor in patients with a family history of tremor. It has also been called benign tremor to differentiate it from tremor associated with neurodegenerative diseases, particularly Parkinson disease, but this condition is certainly not benign, as it can cause substantial functional impairment and difficulties with routine activities of daily living. The terms “essential” and “idiopathic” refer to the primary nature of the disorder and differentiate it from tremor that is a feature of a distinct neurologic entity or is secondary to a metabolic disease or drug therapy.
Successful management entails exclusion of secondary causes and careful selection of drug therapy. To date, there is no cure for essential tremor; all currently available treatments are purely symptomatic.
In this review, we outline the major diagnostic and therapeutic principles of managing essential tremor, indications for referral to specialists, and alternative and advanced therapeutic options.
CLINICAL PICTURE
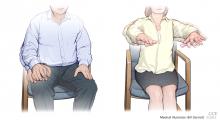
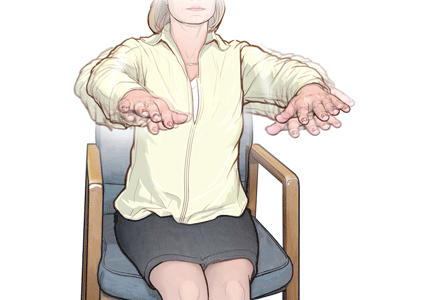
Tremor is defined as rhythmic to-and-fro movement in any body part. It can be slow or fast, and its amplitude can be large and coarse, or small or even “fine.” It can appear at rest, with action, or during a sustained posture. In contrast to parkinsonian tremor (which presents mainly at rest), essential tremor is typically but not exclusively postural, kinetic, or both.
Postural tremor refers to tremor seen when the patient holds the affected limb (commonly the arm) unsupported against gravity. Kinetic tremor refers to tremor that appears with active movements. This is often demonstrated clinically by the finger-nose-finger test. Patients with essential tremor commonly have both postural and kinetic tremor.
The tremor commonly involves the arms, hands, and fingers.2 Less commonly, it involves the head, the lips, the tongue, the legs, and the voice. In contrast to parkinsonian tremor, which typically affects one side of the body first, bilateral involvement is the general rule in essential tremor. However, one side of the body may be affected first, or may be more affected than the other. The frequency of the tremor ranges from 4 to 12 Hz (ie, beats per second).
The tremor usually starts in middle age and progresses slowly over time,3 but onset in old age or childhood is also possible.4 Both sexes are equally affected.
The tremor usually gets worse with anxiety, stress, and caffeine intake. It usually gets temporarily better with the consumption of small amounts of alcohol.
The functional impact of essential tremor is judged by its effect on different daily activities, especially writing, eating, drinking, dressing, manual work, and household chores.
In addition to motor dysfunction, the tremor can also have a significant psychological impact on the patient, because it usually gets worse in social situations.
Although it has long been thought that tremor is the sole neurologic sign of essential tremor, recent studies have shown that many patients have additional subtle findings, such as mild gait difficulty,5 slight incoordination,6 mild cognitive impairment,7 and decreased hearing,8 and are more likely to have anxiety and social phobia.9
Although different studies have varied in their findings, it is generally thought that about 50% of patients with essential tremor have a positive family history, often in a first-degree relative, suggesting autosomal dominant inheritance with variable penetrance.10,11 Polygenetic and sporadic variants are also common.
DIFFERENTIAL DIAGNOSIS
Resting tremor
Resting tremor is typically an extrapyramidal sign and, when accompanied by rigidity and bradykinesia, is often part of a parkinsonian syndrome. It is most pronounced at rest when the affected body part is fully supported and stationary. The tremor tends to improve with action or posture. It usually has a “pill-rolling” character and, as mentioned, is associated with other extrapyramidal signs, such as rigidity, slowness, and, later on, postural instability.
About 20% of patients with essential tremor have resting tremor. These patients usually suffer from severe or long-standing disease.12 However, the resting element in these cases is often milder than the postural and kinetic components, and it is typically not associated with other extrapyramidal signs. Also, some patients may have both essential tremor and Parkinson disease.13
Intentional tremor
Pure intentional tremor is usually seen with cerebellar pathology, which includes tumors, stroke, multiple sclerosis, trauma, and spinocerebellar degeneration. The amplitude of this type of tremor increases as the affected limb approaches the final target. It can best be demonstrated clinically by the finger-nose-finger test. The frequency of intentional tremor is slow (2 to 4 Hz) and is usually associated with other cerebellar signs, such as dysmetria, decomposition, rebound, and dysdiadochokinesia (ie, the inability to perform rapid alternating movements in a smooth and coordinated manner).
About 50% of patients with essential tremor have an intentional component to their tremor,6 or it can be mildly present in the form of a slight gait difficulty. However, in essential tremor, other features of cerebellar dysfunction are either absent or only very slight.
Secondary causes of postural-kinetic tremor
Enhanced physiologic tremor. A very mild postural tremor is present in almost all people and is considered “physiologic” since it has almost no clinical significance. This type of tremor is often invisible, but when “enhanced,” it can be visually demonstrated by placing a piece of paper over the stretched hands and watching the ripple from the paper.
Certain conditions can aggravate this physiologic tremor and can make it symptomatic. Common causes include anxiety, sleep deprivation, hypoglycemia, hyperthyroidism, pheochromocytoma, serotonin syndrome, and carcinoid syndrome.
Metabolic tremor. Hyperammonemia can cause tremor in patients with hepatic encephalopathy, and uremia can cause tremor in patients with renal failure. These metabolic conditions classically result in “flappy” tremor (asterixis), a special form of postural tremor characterized by jerking movements with high amplitude. It is best seen when the patient stretches out the arms and extends the wrists as if trying to stop traffic. But even though it may look like tremor, asterixis is thought to be a form of “negative” myoclonus.
Drug-related tremor. Postural-kinetic tremor can be induced by drugs, including lithium (Lithobid), valproate (Depakote), amiodarone (Cordarone), central nervous system stimulants, beta agonists (including inhalers), and some antidepressants. Tremor can also occur with alcohol or sedative withdrawal.
Psychogenic tremor. Tremor can be seen as part of a somatoform disorder commonly referred to as conversion disorder or conversion reaction. Psychogenic tremor is characterized by acute onset, commonly following a psychosocial stressor; it is often atypical, variable in frequency, amplitude, and body-part involvement, and it can readily be interrupted on examination by distracting the patient.
Neurologic disorders. The postural and kinetic elements of essential tremor may also be seen in the following neurologic conditions:
- Holmes (rubral) tremor, a combination of resting, postural, kinetic, and intentional tremor of low frequency and high amplitude. It usually has a proximal component and is often unilateral. It commonly is due to a lesion that involves the brainstem, eg, red nucleus, inferior olive, cerebellum, or thalamus. Common causes include stroke, prolonged hypoxia, and head trauma (including closed-head trauma with negative imaging). This type of tremor is usually associated with ataxia.14
- Dystonic tremor is predominantly postural and is associated with abnormal dystonic posturing of the affected body part, commonly the head, hands, or feet. Unlike the rhythmic oscillations of essential tremor, dystonic tremor is often irregular in rhythm.
- Multiple sclerosis can present with a combination of postural, kinetic, and intentional tremor. Patients usually have a clear history of recurrent neurologic deficits and show a combination of pyramidal, cerebellar, and sensory signs on examination consistent with multiple sclerosis.15
- Neuropathic tremor is seen in a small proportion of patients with peripheral neuropathy, especially demyelinating neuropathy.16 The tremor is usually posturalkinetic and is associated with signs of neuropathy, such as a glove-and-stocking pattern of hypoesthesia, reduced reflexes, and sensory ataxia (including intentional tremor when the eyes are closed).
- Posttraumatic tremor can occur after severe or even mild head trauma, especially in children. It is commonly rubral, but other types have been reported, including a presentation resembling essential tremor.17
- Monosymptomatic or isolated tremor. A number of conditions related to essential tremor with location-specific or task-specific tremor have been described. These rare conditions historically have been classified as “possible essential tremor” or “essential tremor variants” but are now considered separate entities. These include task-specific tremor (eg, writing tremor), isolated head tremor, isolated voice tremor, and orthostatic tremor (tremor in the legs and trunk upon standing in place, but not when sitting or walking).18,19
DIAGNOSIS IS CLINICAL
Essential tremor is a clinical diagnosis. After a thorough review of the medical history and medication exposures, laboratory and imaging tests may be ordered to rule out a secondary cause. A complete metabolic panel, including blood glucose and thyroid-stimulating hormone levels, is usually sufficient. Brain imaging or other imaging is ordered for patients with an atypical presentation.
TREATMENT IS SYMPTOMATIC
Treatment of essential tremor is symptomatic. Several drugs of different pharmacologic classes can reduce the severity of the tremor and improve function.
Choosing the appropriate treatment depends on the type of tremor and the presence of associated conditions. The response to treatment and the development of side effects guide further adjustments. The following is a brief description of the available antitremor agents.
FIRST-LINE AGENTS
Propranolol
Propranolol (Inderal), a nonselective beta blocker, is the most widely used antitremor drug and the only agent approved by the US Food and Drug Administration for essential tremor. It should be started at a low dose and titrated upward gradually. The usual starting dose is 10 mg three times daily. The average effective dose is 120 mg daily. The dose can be increased up to 320 mg if needed and tolerated.
Sustained-release preparations are equally effective and are given as a single daily dose to improve compliance.20
Propranolol improves tremor in 50% to 70% of patients with essential tremor and achieves an average tremor reduction of 50% to 60%.1,21–25 Side effects include bronchoconstriction, bradycardia, hypotension, depression, impotence, fatigue, and gastrointestinal disturbances.
Other beta-blockers, such as nadolol (Corgard) and timolol, are also effective against tremor but are less potent than propranolol.26,27 The selective beta-1-blocker metoprolol (Lopressor) may be effective and has fewer noncardiac side effects than propranolol.28 It can be used in patients who discontinue propranolol because of adverse effects. Atenolol (Tenormin) and pindolol (Visken) have little or no effect on tremor.29
A good candidate for propranolol therapy in essential tremor is:
- A patient with no known contraindication to propranolol
- A patient with hypertension, coronary heart disease, or tachyarrhythmia
- A patient with anxiety or social phobia.
Absolute contraindications to propranolol are:
- Moderate to severe bronchial asthma
- Significant bradycardia or heart block
- Symptomatic hypotension
- End-stage heart failure
- Concurrent use of a calcium channel blocker.
Relative contraindications are:
- Wheezing (eg, chronic obstructive pulmonary disease)
- Depression
- Diabetes mellitus in a patient more prone to hypoglycemia (propranolol masks the warning signs of hypoglycemia)
- Reduced sexual potency in a male patient.
Primidone
Primidone (Mysoline) is an antiepileptic drug structurally similar to barbiturates. Its antitremor effect is equal to that of propranolol, though some studies suggest it is slightly more efficacious.30,31
It should be started at a low dose, ie, 25 mg once daily at bedtime. The dose should then be increased gradually until satisfactory and tolerable tremor control is achieved. Most patients respond to doses of around 250 mg per day.1,22,24–25 The dose can be increased if needed and tolerated.
Primidone reduces tremor by about 50% to 60%.1,22,24–25 Side effects include sedation, dizziness, fatigue, nausea, and depression, as well as ataxia and confusion in severe cases.
A good candidate for primidone in essential tremor is:
- A patient with no known contraindication to primidone
- A patient with contraindications to propranolol
- A younger patient
- A patient with epilepsy.
Absolute contraindications to primidone include:
- Confusion or dementia
- Oral anticoagulant therapy with difficulty controlling the International Normalized Ratio (primidone is a potent enzyme inducer).
Relative contraindications to primidone in essential tremor are:
- Depression
- Alcohol abuse
- Ongoing therapy with sedating drugs
- Ataxia or vertigo.
SECOND-LINE AGENTS
Other antiepileptics
Topiramate (Topamax) is a broad-spectrum antiepileptic shown to be significantly effective against essential tremor.32 It is usually started at a single daily dose of 25 mg and increased gradually to the most effective dose, usually around 300 mg.
Side effects include reduced appetite, weight loss, cognitive dysfunction, and paresthesia.
Favorable candidates include patients who are epileptic or overweight. Contraindications include cognitive impairment and low body weight. It is also not recommended in children so as to avoid any possible negative effect on cognitive development. In rare cases, topiramate has been reported to cause significant visual disturbances.
Gabapentin (Neurontin) is an antiepileptic that is now more often used as a symptomatic treatment for neuropathic pain. Studies have suggested a beneficial effect on essential tremor,33,34 but some investigators have questioned its efficacy.35
Like other antitremor agents, it should be started at a low dose, ie, around 300 mg, and escalated gradually until the tremor is controlled. The usual effective dose is 1,200 mg.
Gabapentin is generally well tolerated, and side effects such as dizziness, drowsiness, sedation, and unsteadiness are rare and usually mild.
The favorable candidate is a patient with associated neuropathy or multiple comorbidities. Gabapentin has also been reported to alleviate neuropathic tremor.
Contraindications are minimal and include intolerability or hypersensitivity to the drug. It also should be avoided in patients at a high risk of falling.
Levetiracetam (Keppra) is a novel antiepileptic effective against partial seizures. Studies have shown contradictory results regarding its antitremor effect. One double-blind, placebo-controlled study demonstrated significant reduction in essential tremor with 1,000 mg of levetiracetam.36 However, its effect on tremor is believed to be short-lived, and some studies argue against its efficacy.37 It has a favorable side-effect profile and is generally very well tolerated. It can be used as an adjunct to other antitremor agents and is preferred for patients with coexisting partial seizures or myoclonus.
Benzodiazepines. Minor tranquilizers are often used to control tremor, especially in coexisting anxiety or insomnia. Alprazolam (Xanax) is the one most widely used for this indication.38 It can be started in a dose of 0.25 mg once at bedtime and increased gradually up to 0.75 to 2 mg. Clonazepam (Klonopin) is particularly useful for orthostatic tremor, a variant of essential tremor characterized by tremor of the legs and trunk upon standing.39
Common side effects of benzodiazepines include sedation, cognitive dysfunction, hypotension, respiratory inhibition, and addiction after prolonged use. In the elderly, they can lead to confusion and disinhibition and can increase the risk of falling. They should be avoided in the elderly and in alcoholic patients and those with a high risk of substance abuse.
Stopping benzodiazepines should be done gradually to avoid withdrawal symptoms, including aggravation of tremor.
THIRD-LINE AGENTS
Clozapine
Clozapine (Clozaril) is a novel antipsychotic drug with no extrapyramidal side effects. It has been reported effective in essential tremor and drug-induced tremor,40,41 but the results of these early studies have not been confirmed.
Clozapine is started as a single daily dose of 12.5 mg and is increased up to 75 mg or 100 mg. It is an attractive option for patients with coexisting psychosis, bipolar disorder, or chorea. Its main side effects are sedation, salivation, weight gain, hypertension, diabetes, and seizures.
One especially serious side effect is agranulocytosis. This potentially fatal effect is rare, occurring in about 1.3% of patients receiving this drug. Weekly monitoring of the white blood cell count is mandated during treatment with clozapine, and this has made clozapine a less attractive option for the routine treatment of essential tremor.
Mirtazapine
Mirtazapine (Remeron) is a novel antidepressant widely used in Parkinson disease as both an antidepressant and a sleeping aid. Case studies have reported efficacy in both essential tremor and parkinsonian tremor,42 but controlled studies have not confirmed this.43 Mirtazapine is a reasonable option in patients with coexisting depression or insomnia. It is usually given as a single bedtime dose of 15 to 30 mg.
Other drugs
Studies of other agents for the treatment of essential tremor—eg, carbonic anhydrase enzyme inhibitors, calcium channel blockers, isoniazid (Tubizid), clonidine (Catapres), phenobarbital, and theophylline—have yielded highly contradictory results. Thus, they are not recommended as first- or second-line agents for essential tremor.
SPECIALTY-LEVEL CARE
When essential tremor does not respond to drug therapy or the patient cannot tolerate drug therapy, the patient should be referred to a center specializing in movement disorders for more advanced treatment options, ie, botulinum toxin injection and deep brain stimulation surgery.
Botulinum toxin
Botulinum toxin type A has been studied for the treatment of essential tremor with variable degrees of success. It has been effective in reducing hand tremor in essential tremor, but without a concomitant improvement in functional disability.44 This limited functional improvement has been attributed to the development of muscle weakness after injection of the neurotoxin. This has also raised questions about unintentional unblinding when interpreting study results. Therefore, most clinicians restrict its use to focal forms of tremor such as voice tremor,45 head tremor, and task-specific tremor.
Side effects are limited and temporary and include muscle weakness, pain at the injection site, dysphagia (when injected for head or voice tremor), and a breathy vocal quality (when injected for voice tremor). Botulinum toxin injection is the treatment of choice for focal dystonia, and therefore would be a good option for dystonic tremor.
Thalamic deep brain stimulation
This technique involves stereotactic implantation of a stimulation lead in the ventral intermediate nucleus of the thalamus. The lead connects via a subcutaneous wire to an intermittent pulse generator, implanted subcutaneously in the infraclavicular region. The stimulation lead produces continuous stimulation of the ventralis intermedius nucleus that is functionally equal to lesional surgery, thus antagonizing the relay of tremor signals at the thalamus.
The battery of the pulse generator must be replaced every 4 to 7 years depending on usage and stimulation parameters. Battery replacement can be performed with minor surgery at the infraclavicular region.
Thalamic deep brain stimulation is indicated for patients with severe, disabling essential tremor who have tremor resistant to drug therapy or who cannot tolerate drug therapy.
The procedure has been shown to provide benefit in 90% of patients, with more than an 80% improvement in tremor severity and functional impact.46–49 Deep brain stimulation is effective against tremor affecting parts of the body other than the limbs, including the head; an exception to this is voice tremor, which usually does not improve dramatically. The procedure can be done unilaterally or bilaterally, depending on symptoms. Patients with asymmetrical tremor and those at risk of side effects can undergo unilateral surgery. Bilateral treatment is recommended for patients with symmetric tremor or significant head tremor, or who are young and healthy.
Surgical risks include brain hemorrhage and infection. Side effects of the stimulation include paresthesias, paresis, imbalance, dysarthria, and, in rare cases, dysphagia.
CHOOSING THE BEST MANAGEMENT PLAN FOR YOUR PATIENT
The choice of treatment may be challenging, given the multiple treatment options and the variability of tremor severity from one patient to another. The following guidelines can be used to help make this decision.
All patients should be advised to reduce caffeine intake, to have sufficient hours of sleep, and to avoid stressful situations.
Patients with minor, nondisabling tremor can be left untreated if the tremors are not bothersome or if the patient prefers not to pursue active treatment.
In patients who have bothersome tremor only when anxious or in certain social situations, give propranolol or alprazolam (or both) to be taken as needed. Relaxation techniques and meditation are also useful for these patients.
Patients with constant bothersome tremor should be started on either propranolol or primidone based on the patient’s profile and propensity to develop side effects from each of these drugs. The dosing should be optimized gradually according to the patient’s response and the drug’s tolerability.
If essential tremor is not sufficiently controlled with one first-line agent (propranolol or primidone), try combining the two first-line agents if the patient finds it tolerable.
A second-line agent can be added to either of the first-line agents or to the combination of both if tremor control is not yet sufficient. A second-line or third-line agent can also be used as the primary treatment if both first-line agents are contraindicated or intolerable. Combining two or more second- and third-line agents is another option. The choice of second- or third-line agent should be guided by the patient’s characteristics and comorbidities in relation to the agent’s side effects and contraindications as detailed in the above section.
Patients should be referred to a movement disorders specialist in cases of resistant tremor, intolerance to oral medications, severe disability, and atypical presentation. Types of tremor known to be poorly responsive to oral medications (eg, head tremor, voice tremor) deserve a specialist evaluation if they contribute significantly to the patient’s morbidity.
The usual specialist treatment of severe voice tremor and head tremor is botulinum toxin injection. Patients with resistant and disabling hand tremor are evaluated for thalamic deep brain stimulation.
Patients with residual disability despite medical and surgical treatment should be referred for occupational therapy. Occupational therapy can improve quality of life through the use of special utensils, pens, computer gadgets, and arm weights, among other devices.
- Zesiewicz TA, Chari A, Jahan I, et al. Overview of essential tremor. Neuropsychiatr Dis Treat 2010; 6:401–408.
- Elble RJ. Essential tremor frequency decreases with time. Neurology 2000; 55:1547–1551.
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998; 13:5–10.
- Louis ED, Dure LS, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord 2001; 16:921–923.
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord 1994; 9:193–196.
- Deuschl G, Wenzelburger R, Löffler K, et al. Essential tremor and cerebellar dysfunction. Clinical and kinematic analysis of intention tremor. Brain 2000; 123:1568–1580.
- Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord 2010; 25:481–485.
- Ondo WG, Sutton L, Dat Vuong K, et al. Hearing impairment in essential tremor. Neurology 2003; 61:1093–1097.
- Schneier FR, Barnes LF, Albert SM, et al. Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001; 62:367–372.
- Whaley NR, Putzke JD, Baba Y, et al. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord 2007; 13:333–339.
- Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain 2007; 130:1456–1464.
- Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol 2003; 60:405–410.
- Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord 2007; 13:67–76.
- Yang YW, Chang FC, Tsai CH, et al. Clinical and magnetic resonance imaging manifestations of Holmes tremor. Acta Neurol Taiwan 2005; 14:9–15.
- Alusi SH, Worthington J, Glickman S, et al. A study of tremor in multiple sclerosis. Brain 2001; 124:720–730.
- Breit S, Wächter T, Schöls L, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. J Neurol Neurosurg Psychiatry 2009; 80:235–236.
- Koller WC, Wong GF, Lang A. Posttraumatic movement disorders: a review. Mov Disord 1989; 4:20–36.
- Jankovic J. Essential tremor: a heterogenous disorder. Mov Disord 2002; 17:638–644.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13(suppl 3):2–23.
- Calzetti S, Findley LJ, Gresty MA, et al. Effect of a single oral dose of propranolol on essential tremor: a double-blind controlled study. Ann Neurol 1983; 13:165–171.
- Larsen TA, Teräväinen H, Calne DB. Atenolol vs propranolol in essential tremor. A controlled, quantitative study. Acta Neurol Scand 1982; 66:547–554.
- Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2005; 64:2008–2020.
- Lyons KE, Pahwa R, Comella CL, et al.Benefits and risks of pharmacological treatments for essential tremor. Drug Saf 2003; 26:461–481.
- Pahwa R, Lyons KE. Essential tremor: differential diagnosis and current therapy. Am J Med 2003; 115:134–142.
- Louis ED. Clinical practice. Essential tremor. N Engl J Med 2001; 345:887–891.
- Koller WC. Nadolol in essential tremor. Neurology 1983; 33:1076–1077.
- Dietrichson P, Espen E. Effects of timolol and atenolol on benign essential tremor: placebo-controlled studies based on quantitative tremor recording. J Neurol Neurosurg Psychiatry 1981; 44:677–683.
- Calzetti S, Findley LJ, Gresty MA, et al. Metoprolol and propranolol in essential tremor: a double-blind, controlled study. J Neurol Neurosurg Psychiatry 1981; 44:814–819.
- Teravainen H, Larsen A, Fogelholm R. Comparison between the effects of pindolol and propranolol on essential tremor. Neurology 1977; 27:439–442.
- Gorman WP, Cooper R, Pocock P, et al. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry 1986; 49:64–68.
- Koller WC, Royse VL. Efficacy of primidone in essential tremor. Neurology 1986; 36:121–124.
- Connor GS. A double-blind placebo-controlled trial of topiramate treatment for essential tremor. Neurology 2002; 59:132–134.
- Gironell A, Kulisevsky J, Barbanoj M, et al. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol 1999; 56:475–480.
- Ondo W, Hunter C, Vuong KD, et al. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov Disord 2000; 15:678–682.
- Pahwa R, Lyons K, Hubble JP, et al. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord 1998; 13:465–467.
- Bushara KO, Malik T, Exconde RE. The effect of levetiracetam on essential tremor. Neurology 2005; 64:1078–1080.
- Sullivan KL, Hauser RA, Zesiewicz TA. Levetiracetam for the treatment of essential tremor. Mov Disord 2005; 20:640.
- Huber SJ, Paulson GW. Efficacy of alprazolam for essential tremor. Neurology 1988; 38:241–243.
- McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve 1993; 16:1254–1260.
- Ceravolo R, Salvetti S, Piccini P, et al. Acute and chronic effects of clozapine in essential tremor. Mov Disord 1999; 14:468–472.
- Pakkenberg H, Pakkenberg B. Clozapine in the treatment of tremor. Acta Neurol Scand 1986; 73:295–297.
- Pact V, Giduz T. Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 1999; 53:1154.
- Lyons KE, Pahwa R. A double-blind, placebo-controlled, pilot study of mirtazapine in essential tremor. Presented at the 54th Annual Meeting of the American Academy of Neurology, Denver, Colorado. Neurology 2002; 58(suppl 3):A254.
- Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001; 56:1523–1528.
- Blitzer A, Brin MF, Stewart C, et al. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope 1992; 102:163–167.
- Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000; 342:461–468.
- Flora ED, Perera CL, Cameron AL, et al. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 2010; 25:1550–1559.
- Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg 1986; 65:296–302.
- Zirh A, Reich SG, Dougherty PM, et al. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry 1999; 66:772–775.
Essential tremor, one of the most common movement disorders, affects about 4% of adults 40 years of age and older.1 It is often referred to as familial tremor in patients with a family history of tremor. It has also been called benign tremor to differentiate it from tremor associated with neurodegenerative diseases, particularly Parkinson disease, but this condition is certainly not benign, as it can cause substantial functional impairment and difficulties with routine activities of daily living. The terms “essential” and “idiopathic” refer to the primary nature of the disorder and differentiate it from tremor that is a feature of a distinct neurologic entity or is secondary to a metabolic disease or drug therapy.
Successful management entails exclusion of secondary causes and careful selection of drug therapy. To date, there is no cure for essential tremor; all currently available treatments are purely symptomatic.
In this review, we outline the major diagnostic and therapeutic principles of managing essential tremor, indications for referral to specialists, and alternative and advanced therapeutic options.
CLINICAL PICTURE
Tremor is defined as rhythmic to-and-fro movement in any body part. It can be slow or fast, and its amplitude can be large and coarse, or small or even “fine.” It can appear at rest, with action, or during a sustained posture. In contrast to parkinsonian tremor (which presents mainly at rest), essential tremor is typically but not exclusively postural, kinetic, or both.
Postural tremor refers to tremor seen when the patient holds the affected limb (commonly the arm) unsupported against gravity. Kinetic tremor refers to tremor that appears with active movements. This is often demonstrated clinically by the finger-nose-finger test. Patients with essential tremor commonly have both postural and kinetic tremor.
The tremor commonly involves the arms, hands, and fingers.2 Less commonly, it involves the head, the lips, the tongue, the legs, and the voice. In contrast to parkinsonian tremor, which typically affects one side of the body first, bilateral involvement is the general rule in essential tremor. However, one side of the body may be affected first, or may be more affected than the other. The frequency of the tremor ranges from 4 to 12 Hz (ie, beats per second).
The tremor usually starts in middle age and progresses slowly over time,3 but onset in old age or childhood is also possible.4 Both sexes are equally affected.
The tremor usually gets worse with anxiety, stress, and caffeine intake. It usually gets temporarily better with the consumption of small amounts of alcohol.
The functional impact of essential tremor is judged by its effect on different daily activities, especially writing, eating, drinking, dressing, manual work, and household chores.
In addition to motor dysfunction, the tremor can also have a significant psychological impact on the patient, because it usually gets worse in social situations.
Although it has long been thought that tremor is the sole neurologic sign of essential tremor, recent studies have shown that many patients have additional subtle findings, such as mild gait difficulty,5 slight incoordination,6 mild cognitive impairment,7 and decreased hearing,8 and are more likely to have anxiety and social phobia.9
Although different studies have varied in their findings, it is generally thought that about 50% of patients with essential tremor have a positive family history, often in a first-degree relative, suggesting autosomal dominant inheritance with variable penetrance.10,11 Polygenetic and sporadic variants are also common.
DIFFERENTIAL DIAGNOSIS
Resting tremor
Resting tremor is typically an extrapyramidal sign and, when accompanied by rigidity and bradykinesia, is often part of a parkinsonian syndrome. It is most pronounced at rest when the affected body part is fully supported and stationary. The tremor tends to improve with action or posture. It usually has a “pill-rolling” character and, as mentioned, is associated with other extrapyramidal signs, such as rigidity, slowness, and, later on, postural instability.
About 20% of patients with essential tremor have resting tremor. These patients usually suffer from severe or long-standing disease.12 However, the resting element in these cases is often milder than the postural and kinetic components, and it is typically not associated with other extrapyramidal signs. Also, some patients may have both essential tremor and Parkinson disease.13
Intentional tremor
Pure intentional tremor is usually seen with cerebellar pathology, which includes tumors, stroke, multiple sclerosis, trauma, and spinocerebellar degeneration. The amplitude of this type of tremor increases as the affected limb approaches the final target. It can best be demonstrated clinically by the finger-nose-finger test. The frequency of intentional tremor is slow (2 to 4 Hz) and is usually associated with other cerebellar signs, such as dysmetria, decomposition, rebound, and dysdiadochokinesia (ie, the inability to perform rapid alternating movements in a smooth and coordinated manner).
About 50% of patients with essential tremor have an intentional component to their tremor,6 or it can be mildly present in the form of a slight gait difficulty. However, in essential tremor, other features of cerebellar dysfunction are either absent or only very slight.
Secondary causes of postural-kinetic tremor
Enhanced physiologic tremor. A very mild postural tremor is present in almost all people and is considered “physiologic” since it has almost no clinical significance. This type of tremor is often invisible, but when “enhanced,” it can be visually demonstrated by placing a piece of paper over the stretched hands and watching the ripple from the paper.
Certain conditions can aggravate this physiologic tremor and can make it symptomatic. Common causes include anxiety, sleep deprivation, hypoglycemia, hyperthyroidism, pheochromocytoma, serotonin syndrome, and carcinoid syndrome.
Metabolic tremor. Hyperammonemia can cause tremor in patients with hepatic encephalopathy, and uremia can cause tremor in patients with renal failure. These metabolic conditions classically result in “flappy” tremor (asterixis), a special form of postural tremor characterized by jerking movements with high amplitude. It is best seen when the patient stretches out the arms and extends the wrists as if trying to stop traffic. But even though it may look like tremor, asterixis is thought to be a form of “negative” myoclonus.
Drug-related tremor. Postural-kinetic tremor can be induced by drugs, including lithium (Lithobid), valproate (Depakote), amiodarone (Cordarone), central nervous system stimulants, beta agonists (including inhalers), and some antidepressants. Tremor can also occur with alcohol or sedative withdrawal.
Psychogenic tremor. Tremor can be seen as part of a somatoform disorder commonly referred to as conversion disorder or conversion reaction. Psychogenic tremor is characterized by acute onset, commonly following a psychosocial stressor; it is often atypical, variable in frequency, amplitude, and body-part involvement, and it can readily be interrupted on examination by distracting the patient.
Neurologic disorders. The postural and kinetic elements of essential tremor may also be seen in the following neurologic conditions:
- Holmes (rubral) tremor, a combination of resting, postural, kinetic, and intentional tremor of low frequency and high amplitude. It usually has a proximal component and is often unilateral. It commonly is due to a lesion that involves the brainstem, eg, red nucleus, inferior olive, cerebellum, or thalamus. Common causes include stroke, prolonged hypoxia, and head trauma (including closed-head trauma with negative imaging). This type of tremor is usually associated with ataxia.14
- Dystonic tremor is predominantly postural and is associated with abnormal dystonic posturing of the affected body part, commonly the head, hands, or feet. Unlike the rhythmic oscillations of essential tremor, dystonic tremor is often irregular in rhythm.
- Multiple sclerosis can present with a combination of postural, kinetic, and intentional tremor. Patients usually have a clear history of recurrent neurologic deficits and show a combination of pyramidal, cerebellar, and sensory signs on examination consistent with multiple sclerosis.15
- Neuropathic tremor is seen in a small proportion of patients with peripheral neuropathy, especially demyelinating neuropathy.16 The tremor is usually posturalkinetic and is associated with signs of neuropathy, such as a glove-and-stocking pattern of hypoesthesia, reduced reflexes, and sensory ataxia (including intentional tremor when the eyes are closed).
- Posttraumatic tremor can occur after severe or even mild head trauma, especially in children. It is commonly rubral, but other types have been reported, including a presentation resembling essential tremor.17
- Monosymptomatic or isolated tremor. A number of conditions related to essential tremor with location-specific or task-specific tremor have been described. These rare conditions historically have been classified as “possible essential tremor” or “essential tremor variants” but are now considered separate entities. These include task-specific tremor (eg, writing tremor), isolated head tremor, isolated voice tremor, and orthostatic tremor (tremor in the legs and trunk upon standing in place, but not when sitting or walking).18,19
DIAGNOSIS IS CLINICAL
Essential tremor is a clinical diagnosis. After a thorough review of the medical history and medication exposures, laboratory and imaging tests may be ordered to rule out a secondary cause. A complete metabolic panel, including blood glucose and thyroid-stimulating hormone levels, is usually sufficient. Brain imaging or other imaging is ordered for patients with an atypical presentation.
TREATMENT IS SYMPTOMATIC
Treatment of essential tremor is symptomatic. Several drugs of different pharmacologic classes can reduce the severity of the tremor and improve function.
Choosing the appropriate treatment depends on the type of tremor and the presence of associated conditions. The response to treatment and the development of side effects guide further adjustments. The following is a brief description of the available antitremor agents.
FIRST-LINE AGENTS
Propranolol
Propranolol (Inderal), a nonselective beta blocker, is the most widely used antitremor drug and the only agent approved by the US Food and Drug Administration for essential tremor. It should be started at a low dose and titrated upward gradually. The usual starting dose is 10 mg three times daily. The average effective dose is 120 mg daily. The dose can be increased up to 320 mg if needed and tolerated.
Sustained-release preparations are equally effective and are given as a single daily dose to improve compliance.20
Propranolol improves tremor in 50% to 70% of patients with essential tremor and achieves an average tremor reduction of 50% to 60%.1,21–25 Side effects include bronchoconstriction, bradycardia, hypotension, depression, impotence, fatigue, and gastrointestinal disturbances.
Other beta-blockers, such as nadolol (Corgard) and timolol, are also effective against tremor but are less potent than propranolol.26,27 The selective beta-1-blocker metoprolol (Lopressor) may be effective and has fewer noncardiac side effects than propranolol.28 It can be used in patients who discontinue propranolol because of adverse effects. Atenolol (Tenormin) and pindolol (Visken) have little or no effect on tremor.29
A good candidate for propranolol therapy in essential tremor is:
- A patient with no known contraindication to propranolol
- A patient with hypertension, coronary heart disease, or tachyarrhythmia
- A patient with anxiety or social phobia.
Absolute contraindications to propranolol are:
- Moderate to severe bronchial asthma
- Significant bradycardia or heart block
- Symptomatic hypotension
- End-stage heart failure
- Concurrent use of a calcium channel blocker.
Relative contraindications are:
- Wheezing (eg, chronic obstructive pulmonary disease)
- Depression
- Diabetes mellitus in a patient more prone to hypoglycemia (propranolol masks the warning signs of hypoglycemia)
- Reduced sexual potency in a male patient.
Primidone
Primidone (Mysoline) is an antiepileptic drug structurally similar to barbiturates. Its antitremor effect is equal to that of propranolol, though some studies suggest it is slightly more efficacious.30,31
It should be started at a low dose, ie, 25 mg once daily at bedtime. The dose should then be increased gradually until satisfactory and tolerable tremor control is achieved. Most patients respond to doses of around 250 mg per day.1,22,24–25 The dose can be increased if needed and tolerated.
Primidone reduces tremor by about 50% to 60%.1,22,24–25 Side effects include sedation, dizziness, fatigue, nausea, and depression, as well as ataxia and confusion in severe cases.
A good candidate for primidone in essential tremor is:
- A patient with no known contraindication to primidone
- A patient with contraindications to propranolol
- A younger patient
- A patient with epilepsy.
Absolute contraindications to primidone include:
- Confusion or dementia
- Oral anticoagulant therapy with difficulty controlling the International Normalized Ratio (primidone is a potent enzyme inducer).
Relative contraindications to primidone in essential tremor are:
- Depression
- Alcohol abuse
- Ongoing therapy with sedating drugs
- Ataxia or vertigo.
SECOND-LINE AGENTS
Other antiepileptics
Topiramate (Topamax) is a broad-spectrum antiepileptic shown to be significantly effective against essential tremor.32 It is usually started at a single daily dose of 25 mg and increased gradually to the most effective dose, usually around 300 mg.
Side effects include reduced appetite, weight loss, cognitive dysfunction, and paresthesia.
Favorable candidates include patients who are epileptic or overweight. Contraindications include cognitive impairment and low body weight. It is also not recommended in children so as to avoid any possible negative effect on cognitive development. In rare cases, topiramate has been reported to cause significant visual disturbances.
Gabapentin (Neurontin) is an antiepileptic that is now more often used as a symptomatic treatment for neuropathic pain. Studies have suggested a beneficial effect on essential tremor,33,34 but some investigators have questioned its efficacy.35
Like other antitremor agents, it should be started at a low dose, ie, around 300 mg, and escalated gradually until the tremor is controlled. The usual effective dose is 1,200 mg.
Gabapentin is generally well tolerated, and side effects such as dizziness, drowsiness, sedation, and unsteadiness are rare and usually mild.
The favorable candidate is a patient with associated neuropathy or multiple comorbidities. Gabapentin has also been reported to alleviate neuropathic tremor.
Contraindications are minimal and include intolerability or hypersensitivity to the drug. It also should be avoided in patients at a high risk of falling.
Levetiracetam (Keppra) is a novel antiepileptic effective against partial seizures. Studies have shown contradictory results regarding its antitremor effect. One double-blind, placebo-controlled study demonstrated significant reduction in essential tremor with 1,000 mg of levetiracetam.36 However, its effect on tremor is believed to be short-lived, and some studies argue against its efficacy.37 It has a favorable side-effect profile and is generally very well tolerated. It can be used as an adjunct to other antitremor agents and is preferred for patients with coexisting partial seizures or myoclonus.
Benzodiazepines. Minor tranquilizers are often used to control tremor, especially in coexisting anxiety or insomnia. Alprazolam (Xanax) is the one most widely used for this indication.38 It can be started in a dose of 0.25 mg once at bedtime and increased gradually up to 0.75 to 2 mg. Clonazepam (Klonopin) is particularly useful for orthostatic tremor, a variant of essential tremor characterized by tremor of the legs and trunk upon standing.39
Common side effects of benzodiazepines include sedation, cognitive dysfunction, hypotension, respiratory inhibition, and addiction after prolonged use. In the elderly, they can lead to confusion and disinhibition and can increase the risk of falling. They should be avoided in the elderly and in alcoholic patients and those with a high risk of substance abuse.
Stopping benzodiazepines should be done gradually to avoid withdrawal symptoms, including aggravation of tremor.
THIRD-LINE AGENTS
Clozapine
Clozapine (Clozaril) is a novel antipsychotic drug with no extrapyramidal side effects. It has been reported effective in essential tremor and drug-induced tremor,40,41 but the results of these early studies have not been confirmed.
Clozapine is started as a single daily dose of 12.5 mg and is increased up to 75 mg or 100 mg. It is an attractive option for patients with coexisting psychosis, bipolar disorder, or chorea. Its main side effects are sedation, salivation, weight gain, hypertension, diabetes, and seizures.
One especially serious side effect is agranulocytosis. This potentially fatal effect is rare, occurring in about 1.3% of patients receiving this drug. Weekly monitoring of the white blood cell count is mandated during treatment with clozapine, and this has made clozapine a less attractive option for the routine treatment of essential tremor.
Mirtazapine
Mirtazapine (Remeron) is a novel antidepressant widely used in Parkinson disease as both an antidepressant and a sleeping aid. Case studies have reported efficacy in both essential tremor and parkinsonian tremor,42 but controlled studies have not confirmed this.43 Mirtazapine is a reasonable option in patients with coexisting depression or insomnia. It is usually given as a single bedtime dose of 15 to 30 mg.
Other drugs
Studies of other agents for the treatment of essential tremor—eg, carbonic anhydrase enzyme inhibitors, calcium channel blockers, isoniazid (Tubizid), clonidine (Catapres), phenobarbital, and theophylline—have yielded highly contradictory results. Thus, they are not recommended as first- or second-line agents for essential tremor.
SPECIALTY-LEVEL CARE
When essential tremor does not respond to drug therapy or the patient cannot tolerate drug therapy, the patient should be referred to a center specializing in movement disorders for more advanced treatment options, ie, botulinum toxin injection and deep brain stimulation surgery.
Botulinum toxin
Botulinum toxin type A has been studied for the treatment of essential tremor with variable degrees of success. It has been effective in reducing hand tremor in essential tremor, but without a concomitant improvement in functional disability.44 This limited functional improvement has been attributed to the development of muscle weakness after injection of the neurotoxin. This has also raised questions about unintentional unblinding when interpreting study results. Therefore, most clinicians restrict its use to focal forms of tremor such as voice tremor,45 head tremor, and task-specific tremor.
Side effects are limited and temporary and include muscle weakness, pain at the injection site, dysphagia (when injected for head or voice tremor), and a breathy vocal quality (when injected for voice tremor). Botulinum toxin injection is the treatment of choice for focal dystonia, and therefore would be a good option for dystonic tremor.
Thalamic deep brain stimulation
This technique involves stereotactic implantation of a stimulation lead in the ventral intermediate nucleus of the thalamus. The lead connects via a subcutaneous wire to an intermittent pulse generator, implanted subcutaneously in the infraclavicular region. The stimulation lead produces continuous stimulation of the ventralis intermedius nucleus that is functionally equal to lesional surgery, thus antagonizing the relay of tremor signals at the thalamus.
The battery of the pulse generator must be replaced every 4 to 7 years depending on usage and stimulation parameters. Battery replacement can be performed with minor surgery at the infraclavicular region.
Thalamic deep brain stimulation is indicated for patients with severe, disabling essential tremor who have tremor resistant to drug therapy or who cannot tolerate drug therapy.
The procedure has been shown to provide benefit in 90% of patients, with more than an 80% improvement in tremor severity and functional impact.46–49 Deep brain stimulation is effective against tremor affecting parts of the body other than the limbs, including the head; an exception to this is voice tremor, which usually does not improve dramatically. The procedure can be done unilaterally or bilaterally, depending on symptoms. Patients with asymmetrical tremor and those at risk of side effects can undergo unilateral surgery. Bilateral treatment is recommended for patients with symmetric tremor or significant head tremor, or who are young and healthy.
Surgical risks include brain hemorrhage and infection. Side effects of the stimulation include paresthesias, paresis, imbalance, dysarthria, and, in rare cases, dysphagia.
CHOOSING THE BEST MANAGEMENT PLAN FOR YOUR PATIENT
The choice of treatment may be challenging, given the multiple treatment options and the variability of tremor severity from one patient to another. The following guidelines can be used to help make this decision.
All patients should be advised to reduce caffeine intake, to have sufficient hours of sleep, and to avoid stressful situations.
Patients with minor, nondisabling tremor can be left untreated if the tremors are not bothersome or if the patient prefers not to pursue active treatment.
In patients who have bothersome tremor only when anxious or in certain social situations, give propranolol or alprazolam (or both) to be taken as needed. Relaxation techniques and meditation are also useful for these patients.
Patients with constant bothersome tremor should be started on either propranolol or primidone based on the patient’s profile and propensity to develop side effects from each of these drugs. The dosing should be optimized gradually according to the patient’s response and the drug’s tolerability.
If essential tremor is not sufficiently controlled with one first-line agent (propranolol or primidone), try combining the two first-line agents if the patient finds it tolerable.
A second-line agent can be added to either of the first-line agents or to the combination of both if tremor control is not yet sufficient. A second-line or third-line agent can also be used as the primary treatment if both first-line agents are contraindicated or intolerable. Combining two or more second- and third-line agents is another option. The choice of second- or third-line agent should be guided by the patient’s characteristics and comorbidities in relation to the agent’s side effects and contraindications as detailed in the above section.
Patients should be referred to a movement disorders specialist in cases of resistant tremor, intolerance to oral medications, severe disability, and atypical presentation. Types of tremor known to be poorly responsive to oral medications (eg, head tremor, voice tremor) deserve a specialist evaluation if they contribute significantly to the patient’s morbidity.
The usual specialist treatment of severe voice tremor and head tremor is botulinum toxin injection. Patients with resistant and disabling hand tremor are evaluated for thalamic deep brain stimulation.
Patients with residual disability despite medical and surgical treatment should be referred for occupational therapy. Occupational therapy can improve quality of life through the use of special utensils, pens, computer gadgets, and arm weights, among other devices.
Essential tremor, one of the most common movement disorders, affects about 4% of adults 40 years of age and older.1 It is often referred to as familial tremor in patients with a family history of tremor. It has also been called benign tremor to differentiate it from tremor associated with neurodegenerative diseases, particularly Parkinson disease, but this condition is certainly not benign, as it can cause substantial functional impairment and difficulties with routine activities of daily living. The terms “essential” and “idiopathic” refer to the primary nature of the disorder and differentiate it from tremor that is a feature of a distinct neurologic entity or is secondary to a metabolic disease or drug therapy.
Successful management entails exclusion of secondary causes and careful selection of drug therapy. To date, there is no cure for essential tremor; all currently available treatments are purely symptomatic.
In this review, we outline the major diagnostic and therapeutic principles of managing essential tremor, indications for referral to specialists, and alternative and advanced therapeutic options.
CLINICAL PICTURE
Tremor is defined as rhythmic to-and-fro movement in any body part. It can be slow or fast, and its amplitude can be large and coarse, or small or even “fine.” It can appear at rest, with action, or during a sustained posture. In contrast to parkinsonian tremor (which presents mainly at rest), essential tremor is typically but not exclusively postural, kinetic, or both.
Postural tremor refers to tremor seen when the patient holds the affected limb (commonly the arm) unsupported against gravity. Kinetic tremor refers to tremor that appears with active movements. This is often demonstrated clinically by the finger-nose-finger test. Patients with essential tremor commonly have both postural and kinetic tremor.
The tremor commonly involves the arms, hands, and fingers.2 Less commonly, it involves the head, the lips, the tongue, the legs, and the voice. In contrast to parkinsonian tremor, which typically affects one side of the body first, bilateral involvement is the general rule in essential tremor. However, one side of the body may be affected first, or may be more affected than the other. The frequency of the tremor ranges from 4 to 12 Hz (ie, beats per second).
The tremor usually starts in middle age and progresses slowly over time,3 but onset in old age or childhood is also possible.4 Both sexes are equally affected.
The tremor usually gets worse with anxiety, stress, and caffeine intake. It usually gets temporarily better with the consumption of small amounts of alcohol.
The functional impact of essential tremor is judged by its effect on different daily activities, especially writing, eating, drinking, dressing, manual work, and household chores.
In addition to motor dysfunction, the tremor can also have a significant psychological impact on the patient, because it usually gets worse in social situations.
Although it has long been thought that tremor is the sole neurologic sign of essential tremor, recent studies have shown that many patients have additional subtle findings, such as mild gait difficulty,5 slight incoordination,6 mild cognitive impairment,7 and decreased hearing,8 and are more likely to have anxiety and social phobia.9
Although different studies have varied in their findings, it is generally thought that about 50% of patients with essential tremor have a positive family history, often in a first-degree relative, suggesting autosomal dominant inheritance with variable penetrance.10,11 Polygenetic and sporadic variants are also common.
DIFFERENTIAL DIAGNOSIS
Resting tremor
Resting tremor is typically an extrapyramidal sign and, when accompanied by rigidity and bradykinesia, is often part of a parkinsonian syndrome. It is most pronounced at rest when the affected body part is fully supported and stationary. The tremor tends to improve with action or posture. It usually has a “pill-rolling” character and, as mentioned, is associated with other extrapyramidal signs, such as rigidity, slowness, and, later on, postural instability.
About 20% of patients with essential tremor have resting tremor. These patients usually suffer from severe or long-standing disease.12 However, the resting element in these cases is often milder than the postural and kinetic components, and it is typically not associated with other extrapyramidal signs. Also, some patients may have both essential tremor and Parkinson disease.13
Intentional tremor
Pure intentional tremor is usually seen with cerebellar pathology, which includes tumors, stroke, multiple sclerosis, trauma, and spinocerebellar degeneration. The amplitude of this type of tremor increases as the affected limb approaches the final target. It can best be demonstrated clinically by the finger-nose-finger test. The frequency of intentional tremor is slow (2 to 4 Hz) and is usually associated with other cerebellar signs, such as dysmetria, decomposition, rebound, and dysdiadochokinesia (ie, the inability to perform rapid alternating movements in a smooth and coordinated manner).
About 50% of patients with essential tremor have an intentional component to their tremor,6 or it can be mildly present in the form of a slight gait difficulty. However, in essential tremor, other features of cerebellar dysfunction are either absent or only very slight.
Secondary causes of postural-kinetic tremor
Enhanced physiologic tremor. A very mild postural tremor is present in almost all people and is considered “physiologic” since it has almost no clinical significance. This type of tremor is often invisible, but when “enhanced,” it can be visually demonstrated by placing a piece of paper over the stretched hands and watching the ripple from the paper.
Certain conditions can aggravate this physiologic tremor and can make it symptomatic. Common causes include anxiety, sleep deprivation, hypoglycemia, hyperthyroidism, pheochromocytoma, serotonin syndrome, and carcinoid syndrome.
Metabolic tremor. Hyperammonemia can cause tremor in patients with hepatic encephalopathy, and uremia can cause tremor in patients with renal failure. These metabolic conditions classically result in “flappy” tremor (asterixis), a special form of postural tremor characterized by jerking movements with high amplitude. It is best seen when the patient stretches out the arms and extends the wrists as if trying to stop traffic. But even though it may look like tremor, asterixis is thought to be a form of “negative” myoclonus.
Drug-related tremor. Postural-kinetic tremor can be induced by drugs, including lithium (Lithobid), valproate (Depakote), amiodarone (Cordarone), central nervous system stimulants, beta agonists (including inhalers), and some antidepressants. Tremor can also occur with alcohol or sedative withdrawal.
Psychogenic tremor. Tremor can be seen as part of a somatoform disorder commonly referred to as conversion disorder or conversion reaction. Psychogenic tremor is characterized by acute onset, commonly following a psychosocial stressor; it is often atypical, variable in frequency, amplitude, and body-part involvement, and it can readily be interrupted on examination by distracting the patient.
Neurologic disorders. The postural and kinetic elements of essential tremor may also be seen in the following neurologic conditions:
- Holmes (rubral) tremor, a combination of resting, postural, kinetic, and intentional tremor of low frequency and high amplitude. It usually has a proximal component and is often unilateral. It commonly is due to a lesion that involves the brainstem, eg, red nucleus, inferior olive, cerebellum, or thalamus. Common causes include stroke, prolonged hypoxia, and head trauma (including closed-head trauma with negative imaging). This type of tremor is usually associated with ataxia.14
- Dystonic tremor is predominantly postural and is associated with abnormal dystonic posturing of the affected body part, commonly the head, hands, or feet. Unlike the rhythmic oscillations of essential tremor, dystonic tremor is often irregular in rhythm.
- Multiple sclerosis can present with a combination of postural, kinetic, and intentional tremor. Patients usually have a clear history of recurrent neurologic deficits and show a combination of pyramidal, cerebellar, and sensory signs on examination consistent with multiple sclerosis.15
- Neuropathic tremor is seen in a small proportion of patients with peripheral neuropathy, especially demyelinating neuropathy.16 The tremor is usually posturalkinetic and is associated with signs of neuropathy, such as a glove-and-stocking pattern of hypoesthesia, reduced reflexes, and sensory ataxia (including intentional tremor when the eyes are closed).
- Posttraumatic tremor can occur after severe or even mild head trauma, especially in children. It is commonly rubral, but other types have been reported, including a presentation resembling essential tremor.17
- Monosymptomatic or isolated tremor. A number of conditions related to essential tremor with location-specific or task-specific tremor have been described. These rare conditions historically have been classified as “possible essential tremor” or “essential tremor variants” but are now considered separate entities. These include task-specific tremor (eg, writing tremor), isolated head tremor, isolated voice tremor, and orthostatic tremor (tremor in the legs and trunk upon standing in place, but not when sitting or walking).18,19
DIAGNOSIS IS CLINICAL
Essential tremor is a clinical diagnosis. After a thorough review of the medical history and medication exposures, laboratory and imaging tests may be ordered to rule out a secondary cause. A complete metabolic panel, including blood glucose and thyroid-stimulating hormone levels, is usually sufficient. Brain imaging or other imaging is ordered for patients with an atypical presentation.
TREATMENT IS SYMPTOMATIC
Treatment of essential tremor is symptomatic. Several drugs of different pharmacologic classes can reduce the severity of the tremor and improve function.
Choosing the appropriate treatment depends on the type of tremor and the presence of associated conditions. The response to treatment and the development of side effects guide further adjustments. The following is a brief description of the available antitremor agents.
FIRST-LINE AGENTS
Propranolol
Propranolol (Inderal), a nonselective beta blocker, is the most widely used antitremor drug and the only agent approved by the US Food and Drug Administration for essential tremor. It should be started at a low dose and titrated upward gradually. The usual starting dose is 10 mg three times daily. The average effective dose is 120 mg daily. The dose can be increased up to 320 mg if needed and tolerated.
Sustained-release preparations are equally effective and are given as a single daily dose to improve compliance.20
Propranolol improves tremor in 50% to 70% of patients with essential tremor and achieves an average tremor reduction of 50% to 60%.1,21–25 Side effects include bronchoconstriction, bradycardia, hypotension, depression, impotence, fatigue, and gastrointestinal disturbances.
Other beta-blockers, such as nadolol (Corgard) and timolol, are also effective against tremor but are less potent than propranolol.26,27 The selective beta-1-blocker metoprolol (Lopressor) may be effective and has fewer noncardiac side effects than propranolol.28 It can be used in patients who discontinue propranolol because of adverse effects. Atenolol (Tenormin) and pindolol (Visken) have little or no effect on tremor.29
A good candidate for propranolol therapy in essential tremor is:
- A patient with no known contraindication to propranolol
- A patient with hypertension, coronary heart disease, or tachyarrhythmia
- A patient with anxiety or social phobia.
Absolute contraindications to propranolol are:
- Moderate to severe bronchial asthma
- Significant bradycardia or heart block
- Symptomatic hypotension
- End-stage heart failure
- Concurrent use of a calcium channel blocker.
Relative contraindications are:
- Wheezing (eg, chronic obstructive pulmonary disease)
- Depression
- Diabetes mellitus in a patient more prone to hypoglycemia (propranolol masks the warning signs of hypoglycemia)
- Reduced sexual potency in a male patient.
Primidone
Primidone (Mysoline) is an antiepileptic drug structurally similar to barbiturates. Its antitremor effect is equal to that of propranolol, though some studies suggest it is slightly more efficacious.30,31
It should be started at a low dose, ie, 25 mg once daily at bedtime. The dose should then be increased gradually until satisfactory and tolerable tremor control is achieved. Most patients respond to doses of around 250 mg per day.1,22,24–25 The dose can be increased if needed and tolerated.
Primidone reduces tremor by about 50% to 60%.1,22,24–25 Side effects include sedation, dizziness, fatigue, nausea, and depression, as well as ataxia and confusion in severe cases.
A good candidate for primidone in essential tremor is:
- A patient with no known contraindication to primidone
- A patient with contraindications to propranolol
- A younger patient
- A patient with epilepsy.
Absolute contraindications to primidone include:
- Confusion or dementia
- Oral anticoagulant therapy with difficulty controlling the International Normalized Ratio (primidone is a potent enzyme inducer).
Relative contraindications to primidone in essential tremor are:
- Depression
- Alcohol abuse
- Ongoing therapy with sedating drugs
- Ataxia or vertigo.
SECOND-LINE AGENTS
Other antiepileptics
Topiramate (Topamax) is a broad-spectrum antiepileptic shown to be significantly effective against essential tremor.32 It is usually started at a single daily dose of 25 mg and increased gradually to the most effective dose, usually around 300 mg.
Side effects include reduced appetite, weight loss, cognitive dysfunction, and paresthesia.
Favorable candidates include patients who are epileptic or overweight. Contraindications include cognitive impairment and low body weight. It is also not recommended in children so as to avoid any possible negative effect on cognitive development. In rare cases, topiramate has been reported to cause significant visual disturbances.
Gabapentin (Neurontin) is an antiepileptic that is now more often used as a symptomatic treatment for neuropathic pain. Studies have suggested a beneficial effect on essential tremor,33,34 but some investigators have questioned its efficacy.35
Like other antitremor agents, it should be started at a low dose, ie, around 300 mg, and escalated gradually until the tremor is controlled. The usual effective dose is 1,200 mg.
Gabapentin is generally well tolerated, and side effects such as dizziness, drowsiness, sedation, and unsteadiness are rare and usually mild.
The favorable candidate is a patient with associated neuropathy or multiple comorbidities. Gabapentin has also been reported to alleviate neuropathic tremor.
Contraindications are minimal and include intolerability or hypersensitivity to the drug. It also should be avoided in patients at a high risk of falling.
Levetiracetam (Keppra) is a novel antiepileptic effective against partial seizures. Studies have shown contradictory results regarding its antitremor effect. One double-blind, placebo-controlled study demonstrated significant reduction in essential tremor with 1,000 mg of levetiracetam.36 However, its effect on tremor is believed to be short-lived, and some studies argue against its efficacy.37 It has a favorable side-effect profile and is generally very well tolerated. It can be used as an adjunct to other antitremor agents and is preferred for patients with coexisting partial seizures or myoclonus.
Benzodiazepines. Minor tranquilizers are often used to control tremor, especially in coexisting anxiety or insomnia. Alprazolam (Xanax) is the one most widely used for this indication.38 It can be started in a dose of 0.25 mg once at bedtime and increased gradually up to 0.75 to 2 mg. Clonazepam (Klonopin) is particularly useful for orthostatic tremor, a variant of essential tremor characterized by tremor of the legs and trunk upon standing.39
Common side effects of benzodiazepines include sedation, cognitive dysfunction, hypotension, respiratory inhibition, and addiction after prolonged use. In the elderly, they can lead to confusion and disinhibition and can increase the risk of falling. They should be avoided in the elderly and in alcoholic patients and those with a high risk of substance abuse.
Stopping benzodiazepines should be done gradually to avoid withdrawal symptoms, including aggravation of tremor.
THIRD-LINE AGENTS
Clozapine
Clozapine (Clozaril) is a novel antipsychotic drug with no extrapyramidal side effects. It has been reported effective in essential tremor and drug-induced tremor,40,41 but the results of these early studies have not been confirmed.
Clozapine is started as a single daily dose of 12.5 mg and is increased up to 75 mg or 100 mg. It is an attractive option for patients with coexisting psychosis, bipolar disorder, or chorea. Its main side effects are sedation, salivation, weight gain, hypertension, diabetes, and seizures.
One especially serious side effect is agranulocytosis. This potentially fatal effect is rare, occurring in about 1.3% of patients receiving this drug. Weekly monitoring of the white blood cell count is mandated during treatment with clozapine, and this has made clozapine a less attractive option for the routine treatment of essential tremor.
Mirtazapine
Mirtazapine (Remeron) is a novel antidepressant widely used in Parkinson disease as both an antidepressant and a sleeping aid. Case studies have reported efficacy in both essential tremor and parkinsonian tremor,42 but controlled studies have not confirmed this.43 Mirtazapine is a reasonable option in patients with coexisting depression or insomnia. It is usually given as a single bedtime dose of 15 to 30 mg.
Other drugs
Studies of other agents for the treatment of essential tremor—eg, carbonic anhydrase enzyme inhibitors, calcium channel blockers, isoniazid (Tubizid), clonidine (Catapres), phenobarbital, and theophylline—have yielded highly contradictory results. Thus, they are not recommended as first- or second-line agents for essential tremor.
SPECIALTY-LEVEL CARE
When essential tremor does not respond to drug therapy or the patient cannot tolerate drug therapy, the patient should be referred to a center specializing in movement disorders for more advanced treatment options, ie, botulinum toxin injection and deep brain stimulation surgery.
Botulinum toxin
Botulinum toxin type A has been studied for the treatment of essential tremor with variable degrees of success. It has been effective in reducing hand tremor in essential tremor, but without a concomitant improvement in functional disability.44 This limited functional improvement has been attributed to the development of muscle weakness after injection of the neurotoxin. This has also raised questions about unintentional unblinding when interpreting study results. Therefore, most clinicians restrict its use to focal forms of tremor such as voice tremor,45 head tremor, and task-specific tremor.
Side effects are limited and temporary and include muscle weakness, pain at the injection site, dysphagia (when injected for head or voice tremor), and a breathy vocal quality (when injected for voice tremor). Botulinum toxin injection is the treatment of choice for focal dystonia, and therefore would be a good option for dystonic tremor.
Thalamic deep brain stimulation
This technique involves stereotactic implantation of a stimulation lead in the ventral intermediate nucleus of the thalamus. The lead connects via a subcutaneous wire to an intermittent pulse generator, implanted subcutaneously in the infraclavicular region. The stimulation lead produces continuous stimulation of the ventralis intermedius nucleus that is functionally equal to lesional surgery, thus antagonizing the relay of tremor signals at the thalamus.
The battery of the pulse generator must be replaced every 4 to 7 years depending on usage and stimulation parameters. Battery replacement can be performed with minor surgery at the infraclavicular region.
Thalamic deep brain stimulation is indicated for patients with severe, disabling essential tremor who have tremor resistant to drug therapy or who cannot tolerate drug therapy.
The procedure has been shown to provide benefit in 90% of patients, with more than an 80% improvement in tremor severity and functional impact.46–49 Deep brain stimulation is effective against tremor affecting parts of the body other than the limbs, including the head; an exception to this is voice tremor, which usually does not improve dramatically. The procedure can be done unilaterally or bilaterally, depending on symptoms. Patients with asymmetrical tremor and those at risk of side effects can undergo unilateral surgery. Bilateral treatment is recommended for patients with symmetric tremor or significant head tremor, or who are young and healthy.
Surgical risks include brain hemorrhage and infection. Side effects of the stimulation include paresthesias, paresis, imbalance, dysarthria, and, in rare cases, dysphagia.
CHOOSING THE BEST MANAGEMENT PLAN FOR YOUR PATIENT
The choice of treatment may be challenging, given the multiple treatment options and the variability of tremor severity from one patient to another. The following guidelines can be used to help make this decision.
All patients should be advised to reduce caffeine intake, to have sufficient hours of sleep, and to avoid stressful situations.
Patients with minor, nondisabling tremor can be left untreated if the tremors are not bothersome or if the patient prefers not to pursue active treatment.
In patients who have bothersome tremor only when anxious or in certain social situations, give propranolol or alprazolam (or both) to be taken as needed. Relaxation techniques and meditation are also useful for these patients.
Patients with constant bothersome tremor should be started on either propranolol or primidone based on the patient’s profile and propensity to develop side effects from each of these drugs. The dosing should be optimized gradually according to the patient’s response and the drug’s tolerability.
If essential tremor is not sufficiently controlled with one first-line agent (propranolol or primidone), try combining the two first-line agents if the patient finds it tolerable.
A second-line agent can be added to either of the first-line agents or to the combination of both if tremor control is not yet sufficient. A second-line or third-line agent can also be used as the primary treatment if both first-line agents are contraindicated or intolerable. Combining two or more second- and third-line agents is another option. The choice of second- or third-line agent should be guided by the patient’s characteristics and comorbidities in relation to the agent’s side effects and contraindications as detailed in the above section.
Patients should be referred to a movement disorders specialist in cases of resistant tremor, intolerance to oral medications, severe disability, and atypical presentation. Types of tremor known to be poorly responsive to oral medications (eg, head tremor, voice tremor) deserve a specialist evaluation if they contribute significantly to the patient’s morbidity.
The usual specialist treatment of severe voice tremor and head tremor is botulinum toxin injection. Patients with resistant and disabling hand tremor are evaluated for thalamic deep brain stimulation.
Patients with residual disability despite medical and surgical treatment should be referred for occupational therapy. Occupational therapy can improve quality of life through the use of special utensils, pens, computer gadgets, and arm weights, among other devices.
- Zesiewicz TA, Chari A, Jahan I, et al. Overview of essential tremor. Neuropsychiatr Dis Treat 2010; 6:401–408.
- Elble RJ. Essential tremor frequency decreases with time. Neurology 2000; 55:1547–1551.
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998; 13:5–10.
- Louis ED, Dure LS, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord 2001; 16:921–923.
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord 1994; 9:193–196.
- Deuschl G, Wenzelburger R, Löffler K, et al. Essential tremor and cerebellar dysfunction. Clinical and kinematic analysis of intention tremor. Brain 2000; 123:1568–1580.
- Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord 2010; 25:481–485.
- Ondo WG, Sutton L, Dat Vuong K, et al. Hearing impairment in essential tremor. Neurology 2003; 61:1093–1097.
- Schneier FR, Barnes LF, Albert SM, et al. Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001; 62:367–372.
- Whaley NR, Putzke JD, Baba Y, et al. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord 2007; 13:333–339.
- Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain 2007; 130:1456–1464.
- Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol 2003; 60:405–410.
- Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord 2007; 13:67–76.
- Yang YW, Chang FC, Tsai CH, et al. Clinical and magnetic resonance imaging manifestations of Holmes tremor. Acta Neurol Taiwan 2005; 14:9–15.
- Alusi SH, Worthington J, Glickman S, et al. A study of tremor in multiple sclerosis. Brain 2001; 124:720–730.
- Breit S, Wächter T, Schöls L, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. J Neurol Neurosurg Psychiatry 2009; 80:235–236.
- Koller WC, Wong GF, Lang A. Posttraumatic movement disorders: a review. Mov Disord 1989; 4:20–36.
- Jankovic J. Essential tremor: a heterogenous disorder. Mov Disord 2002; 17:638–644.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13(suppl 3):2–23.
- Calzetti S, Findley LJ, Gresty MA, et al. Effect of a single oral dose of propranolol on essential tremor: a double-blind controlled study. Ann Neurol 1983; 13:165–171.
- Larsen TA, Teräväinen H, Calne DB. Atenolol vs propranolol in essential tremor. A controlled, quantitative study. Acta Neurol Scand 1982; 66:547–554.
- Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2005; 64:2008–2020.
- Lyons KE, Pahwa R, Comella CL, et al.Benefits and risks of pharmacological treatments for essential tremor. Drug Saf 2003; 26:461–481.
- Pahwa R, Lyons KE. Essential tremor: differential diagnosis and current therapy. Am J Med 2003; 115:134–142.
- Louis ED. Clinical practice. Essential tremor. N Engl J Med 2001; 345:887–891.
- Koller WC. Nadolol in essential tremor. Neurology 1983; 33:1076–1077.
- Dietrichson P, Espen E. Effects of timolol and atenolol on benign essential tremor: placebo-controlled studies based on quantitative tremor recording. J Neurol Neurosurg Psychiatry 1981; 44:677–683.
- Calzetti S, Findley LJ, Gresty MA, et al. Metoprolol and propranolol in essential tremor: a double-blind, controlled study. J Neurol Neurosurg Psychiatry 1981; 44:814–819.
- Teravainen H, Larsen A, Fogelholm R. Comparison between the effects of pindolol and propranolol on essential tremor. Neurology 1977; 27:439–442.
- Gorman WP, Cooper R, Pocock P, et al. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry 1986; 49:64–68.
- Koller WC, Royse VL. Efficacy of primidone in essential tremor. Neurology 1986; 36:121–124.
- Connor GS. A double-blind placebo-controlled trial of topiramate treatment for essential tremor. Neurology 2002; 59:132–134.
- Gironell A, Kulisevsky J, Barbanoj M, et al. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol 1999; 56:475–480.
- Ondo W, Hunter C, Vuong KD, et al. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov Disord 2000; 15:678–682.
- Pahwa R, Lyons K, Hubble JP, et al. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord 1998; 13:465–467.
- Bushara KO, Malik T, Exconde RE. The effect of levetiracetam on essential tremor. Neurology 2005; 64:1078–1080.
- Sullivan KL, Hauser RA, Zesiewicz TA. Levetiracetam for the treatment of essential tremor. Mov Disord 2005; 20:640.
- Huber SJ, Paulson GW. Efficacy of alprazolam for essential tremor. Neurology 1988; 38:241–243.
- McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve 1993; 16:1254–1260.
- Ceravolo R, Salvetti S, Piccini P, et al. Acute and chronic effects of clozapine in essential tremor. Mov Disord 1999; 14:468–472.
- Pakkenberg H, Pakkenberg B. Clozapine in the treatment of tremor. Acta Neurol Scand 1986; 73:295–297.
- Pact V, Giduz T. Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 1999; 53:1154.
- Lyons KE, Pahwa R. A double-blind, placebo-controlled, pilot study of mirtazapine in essential tremor. Presented at the 54th Annual Meeting of the American Academy of Neurology, Denver, Colorado. Neurology 2002; 58(suppl 3):A254.
- Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001; 56:1523–1528.
- Blitzer A, Brin MF, Stewart C, et al. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope 1992; 102:163–167.
- Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000; 342:461–468.
- Flora ED, Perera CL, Cameron AL, et al. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 2010; 25:1550–1559.
- Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg 1986; 65:296–302.
- Zirh A, Reich SG, Dougherty PM, et al. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry 1999; 66:772–775.
- Zesiewicz TA, Chari A, Jahan I, et al. Overview of essential tremor. Neuropsychiatr Dis Treat 2010; 6:401–408.
- Elble RJ. Essential tremor frequency decreases with time. Neurology 2000; 55:1547–1551.
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998; 13:5–10.
- Louis ED, Dure LS, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord 2001; 16:921–923.
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord 1994; 9:193–196.
- Deuschl G, Wenzelburger R, Löffler K, et al. Essential tremor and cerebellar dysfunction. Clinical and kinematic analysis of intention tremor. Brain 2000; 123:1568–1580.
- Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord 2010; 25:481–485.
- Ondo WG, Sutton L, Dat Vuong K, et al. Hearing impairment in essential tremor. Neurology 2003; 61:1093–1097.
- Schneier FR, Barnes LF, Albert SM, et al. Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001; 62:367–372.
- Whaley NR, Putzke JD, Baba Y, et al. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord 2007; 13:333–339.
- Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain 2007; 130:1456–1464.
- Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol 2003; 60:405–410.
- Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord 2007; 13:67–76.
- Yang YW, Chang FC, Tsai CH, et al. Clinical and magnetic resonance imaging manifestations of Holmes tremor. Acta Neurol Taiwan 2005; 14:9–15.
- Alusi SH, Worthington J, Glickman S, et al. A study of tremor in multiple sclerosis. Brain 2001; 124:720–730.
- Breit S, Wächter T, Schöls L, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. J Neurol Neurosurg Psychiatry 2009; 80:235–236.
- Koller WC, Wong GF, Lang A. Posttraumatic movement disorders: a review. Mov Disord 1989; 4:20–36.
- Jankovic J. Essential tremor: a heterogenous disorder. Mov Disord 2002; 17:638–644.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13(suppl 3):2–23.
- Calzetti S, Findley LJ, Gresty MA, et al. Effect of a single oral dose of propranolol on essential tremor: a double-blind controlled study. Ann Neurol 1983; 13:165–171.
- Larsen TA, Teräväinen H, Calne DB. Atenolol vs propranolol in essential tremor. A controlled, quantitative study. Acta Neurol Scand 1982; 66:547–554.
- Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2005; 64:2008–2020.
- Lyons KE, Pahwa R, Comella CL, et al.Benefits and risks of pharmacological treatments for essential tremor. Drug Saf 2003; 26:461–481.
- Pahwa R, Lyons KE. Essential tremor: differential diagnosis and current therapy. Am J Med 2003; 115:134–142.
- Louis ED. Clinical practice. Essential tremor. N Engl J Med 2001; 345:887–891.
- Koller WC. Nadolol in essential tremor. Neurology 1983; 33:1076–1077.
- Dietrichson P, Espen E. Effects of timolol and atenolol on benign essential tremor: placebo-controlled studies based on quantitative tremor recording. J Neurol Neurosurg Psychiatry 1981; 44:677–683.
- Calzetti S, Findley LJ, Gresty MA, et al. Metoprolol and propranolol in essential tremor: a double-blind, controlled study. J Neurol Neurosurg Psychiatry 1981; 44:814–819.
- Teravainen H, Larsen A, Fogelholm R. Comparison between the effects of pindolol and propranolol on essential tremor. Neurology 1977; 27:439–442.
- Gorman WP, Cooper R, Pocock P, et al. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry 1986; 49:64–68.
- Koller WC, Royse VL. Efficacy of primidone in essential tremor. Neurology 1986; 36:121–124.
- Connor GS. A double-blind placebo-controlled trial of topiramate treatment for essential tremor. Neurology 2002; 59:132–134.
- Gironell A, Kulisevsky J, Barbanoj M, et al. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol 1999; 56:475–480.
- Ondo W, Hunter C, Vuong KD, et al. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov Disord 2000; 15:678–682.
- Pahwa R, Lyons K, Hubble JP, et al. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord 1998; 13:465–467.
- Bushara KO, Malik T, Exconde RE. The effect of levetiracetam on essential tremor. Neurology 2005; 64:1078–1080.
- Sullivan KL, Hauser RA, Zesiewicz TA. Levetiracetam for the treatment of essential tremor. Mov Disord 2005; 20:640.
- Huber SJ, Paulson GW. Efficacy of alprazolam for essential tremor. Neurology 1988; 38:241–243.
- McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve 1993; 16:1254–1260.
- Ceravolo R, Salvetti S, Piccini P, et al. Acute and chronic effects of clozapine in essential tremor. Mov Disord 1999; 14:468–472.
- Pakkenberg H, Pakkenberg B. Clozapine in the treatment of tremor. Acta Neurol Scand 1986; 73:295–297.
- Pact V, Giduz T. Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 1999; 53:1154.
- Lyons KE, Pahwa R. A double-blind, placebo-controlled, pilot study of mirtazapine in essential tremor. Presented at the 54th Annual Meeting of the American Academy of Neurology, Denver, Colorado. Neurology 2002; 58(suppl 3):A254.
- Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001; 56:1523–1528.
- Blitzer A, Brin MF, Stewart C, et al. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope 1992; 102:163–167.
- Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000; 342:461–468.
- Flora ED, Perera CL, Cameron AL, et al. Deep brain stimulation for essential tremor: a systematic review. Mov Disord 2010; 25:1550–1559.
- Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg 1986; 65:296–302.
- Zirh A, Reich SG, Dougherty PM, et al. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry 1999; 66:772–775.
KEY POINTS
- In addition to motor dysfunction, the tremor can also have a significant psychological impact on the patient, especially since it usually gets worse in social situations.
- Essential tremor is a clinical diagnosis. After a thorough review of the medical history and medication exposures, laboratory and imaging tests may be ordered to rule out a secondary cause.
- The two first-line agents in drug therapy for essential tremor are the nonselective beta-blocker propranolol (Inderal) and the antiepileptic primidone (Mysoline). They can be used alone or in combination.
- Botulinum toxin injection and deep brain stimulation are reserved for resistant tremor or for patients who do not tolerate drug therapy.