Article
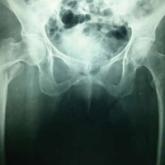
Treatment of Unstable Trochanteric Femur Fractures: Proximal Femur Nail Versus Proximal Femur Locking Compression Plate
- Author:
- Ashutosh Kumar Singh, MBBS, MS
- Nidhi Narsaria, MBBS, MD
- Arun G. R., MBBS, MS
- Vivek Srivastava, MBBS, MS
Unstable trochanteric femur fractures are common fractures that are difficult to manage. We conducted a prospective study to compare functional...
