User login
Fecal microbiota transplantation for recurrent C difficile infection: Ready for prime time?
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
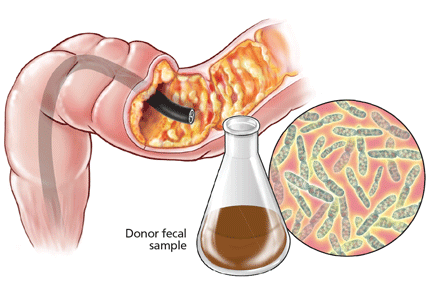
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
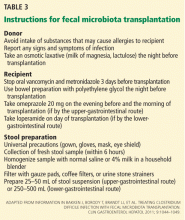
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
KEY POINTS
- Fecal microbiota transplantation involves instilling gut microbiota from a healthy donor into the diseased gut of a patient who has recurrent or recalcitrant episodes of diarrhea despite antibiotic treatment for C difficile infection. The instillation can be done via nasogastric tube, endoscope, or enema.
- Donor screening is necessary to prevent transmission of communicable diseases to the recipient.
- Recently published studies indicate that this procedure is effective for treating recurrent C difficile infection. Randomized clinical trials to assess its efficacy and safety are underway.
- The field of microbiota therapy is rapidly progressing. More physicians are learning to embrace the concept of fecal microbiota transplantation, and patients are beginning to overcome the “yuck factor” and accept its benefits.
Making the most of currently available bowel preparations for colonoscopy
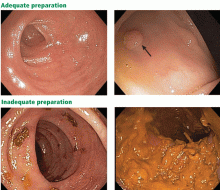
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
KEY POINTS
- Polyethylene glycol solutions are fast, effective, and preferred for cleansing the colon.
- Use of split dosing, a low-volume solution, or both can increase patient acceptability without compromising efficacy.
- Sodium phosphate can be prescribed for patients who cannot tolerate polyethylene glycol solutions, provided they are not at risk of electrolyte or fluid imbalances.
- Enemas, bisacodyl, magnesium citrate, and metoclopramide (Reglan) can be useful as adjuncts to polyethylene glycol but by themselves are inadequate for cleansing the entire colon.
- Educating patients about bowel preparation instructions, including correct dosing and adequate hydration, helps reduce the risk of adverse events and serious adverse events.