User login
Cannabinoid hyperemesis syndrome: Marijuana is both antiemetic and proemetic
With the growing use of marijuana, reports have appeared of a newly recognized condition in long-term heavy users termed cannabinoid hyperemesis syndrome.1
This syndrome is interesting for at least two reasons. First, paradoxically, marijuana appears to have an emetic effect with chronic use, whereas it usually has the opposite effect and is used as an antiemetic in patients undergoing chemotherapy. Second, patients develop a compulsion to bathe or shower in extremely hot water to relieve the symptoms.
In this article, we review the pathophysiology, clinical presentation, diagnosis, and management of this emerging condition.
MARIJUANA USE ON THE RISE
Marijuana is the most widely used illicit drug worldwide. Although statistics on its use vary, a report from the Pew Research Center2 stated that 49% of Americans say they have tried it. Several states now allow the use of marijuana for medicinal purposes, and Colorado and Washington have legalized it for recreational use. This marks a major turning point and may accelerate the slow-growing acceptance of marijuana use in the United States.
Marijuana has been used to treat HIV-associated anorexia and wasting, convulsions, glaucoma, headache, and chemotherapy-induced nausea and vomiting.3–5
Cannabinoid hyperemesis syndrome was first described in 2004 in South Australia.1 Since its recognition, an increasing number of cases have been identified worldwide. However, there are still no population-based studies to estimate its exact prevalence.
THC PREVENTS VOMITING—AND CAUSES IT
Delta-9-tetrahydrocannabinol (THC) is the principal psychoactive component in marijuana.6,7 There are two types of cannabinoid receptors in humans: CB1 and CB2. Both are found in the central nervous system and autonomic nervous system. Activation of CB1 receptors is responsible for the psychoactive effects of cannabinoids such as altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time. The physiologic role of CB2 is not known.
THC as an antiemetic
The antiemetic property of THC is not well understood but has been linked to activation of CB1 receptors found on the enteric plexus, presynaptic parasympathetic system, and central nervous system, particularly the cerebellum, hypothalamus, and vomiting center in the medulla.1,8–12 Stimulation and blockade of CB1 receptors can inhibit and induce vomiting in a dose-dependent manner, implicating endogenous cannabinoids in emetic circuits.12
THC as a proemetic
The mechanism of the paradoxical hyperemetic effect of THC is unknown, but several concepts have been proposed.
Chronic cannabis use can lead to down-regulation of CB1 receptors.13 Simonetto et al10 suggested that the central effects of long-term cannabis use on the hypothalamic-pituitary-adrenal axis may play a central role in the development of hyperemesis.10
Cannabinoids have a long half-life and are lipophilic.1 When used infrequently, they prevent vomiting. But with chronic use, high concentrations of THC can accumulate in the body, including cerebral fat, and can cause severe nausea and vomiting.8,9 This paradoxic hyperemesis was observed in people using intravenous crude marijuana extract.7 The same response was also noted in ferrets injected with 2-arachidonoylglycerol, a potent cannabinoid agonist.11
Patients who experience hyperemesis from chronic cannabis use may also have a genetic variation in their hepatic drug-transforming enzymes that results in excessive levels of cannabis metabolites that promote emesis.1,14
Delayed gastric emptying has also been linked to the proemetic effect of THC. However, this association became controversial when a large case series study showed that only 30% of patients with cannabinoid hyperemesis syndrome had delayed emptying on gastric scintigraphy.10
It is also possible that excessive stimulation of cannabinoid receptors in the gut can cause diffuse splanchnic vasodilation and contribute to the abdominal pain.13
DIAGNOSING CANNABINOID HYPEREMESIS SYNDROME
Cannabinoid hyperemesis syndrome is a clinical diagnosis typically seen in young patients (under age 50) with a long history of marijuana use. They present with severe, cyclic nausea and vomiting and admit to compulsively taking extremely hot showers or baths. Most patients report using marijuana for more than a year before developing episodes of severe vomiting. However, one study found that as many as 32% of patients had used it less than 1 year before experiencing symptoms.10
Other associated nonspecific symptoms are diaphoresis, bloating, abdominal discomfort, flushing, and weight loss. Symptoms are relieved with long, hot showers or baths and cessation of marijuana use. Taking a complete history is key to making the diagnosis.
In 2004, Allen et al1 first defined cannabinoid hyperemesis as excessive marijuana use associated with cyclical vomiting and abdominal pain.1 In 2012, Simonetto et al10 proposed diagnostic criteria (Table 1). Although not yet validated, these criteria are based on the largest series of cases of cannabinoid hyperemesis syndrome to date (98 patients).10
THE THREE PHASES OF CANNABINOID HYPEREMESIS
The clinical presentation of cannabinoid hyperemesis syndrome can be divided into three phases: prodromal, vomiting, and resolution.
Prodromal phase
During this phase, patients often appear anxious and agitated and display a spectrum of autonomic symptoms such as sweating, flushing, and constantly sipping water due to thirst. They may sometimes have abdominal pain that is usually epigastric but may also be diffuse. Their symptoms are associated with severe nausea, usually early in the morning or when they see or smell food. Appetite and eating patterns remain normal. Compulsive hot bathing or showering is minimal at this phase.
Vomiting phase
In this next phase, patients experience incapacitating nausea and vomiting that may occur without warning and are resistant to conventional antiemetics such as ondansetron and promethazine.14 However, patients eventually learn that hot baths or showers relieve the symptoms, and this behavior eventually becomes a compulsion. The higher the temperature of the water, the better the effect on symptoms.1 Low-grade pyrexia, excessive thirst, orthostasis, abdominal tenderness, weight loss, and sometimes even superficial skin burns have been reported.1,9,15–18
Recovery phase
During the final phase of cannabinoid hyperemesis syndrome, most patients experience marked resolution of symptoms after 24 to 48 hours of conservative management (bowel rest until symptoms resolve, slowly advancing diet as tolerated, intravenous fluids, and electrolyte monitoring and repletion as necessary), and most importantly, cessation of cannabis use. However, the time from cessation of marijuana use to resolution of symptoms may be as long as 1 week to 1 month.1,10,14 Patients begin to resume their normal diet and daily activities. The bathing-showering compulsion subsides, and patients regain lost weight after 3 to 6 months.1
In all case series and reports, resumption of cannabis use causes the symptoms to recur. This recurrence is compelling evidence that cannabis is the cause of the hyperemesis and should be part of the essential criteria for the diagnosis of cannabinoid hyperemesis syndrome.
WHY COMPULSIVE HOT BATHING?
The mechanism behind this unique characteristic of cannabinoid hyperemesis syndrome is not known. Several theories have been suggested, but no study has identified the exact explanation for this phenomenon.1,9,10,13–15,17–31
One suggested mechanism is a response by the thermoregulatory center of the brain to the dose-dependent hypothermic effects of THC, or even a direct effect of CB1 receptor activation in the hypothalamus.9 Cannabis toxicity could disrupt the equilibrium of satiety, thirst, digestive, and thermoregulatory systems of the hypothalamus, and this interference could resolve with hot bathing.1
The so-called “cutaneous steal” syndrome has also been proposed, in which cutaneous vasodilation caused by hot water decreases the blood volume available for the splanchnic circulation thought to be responsible for the abdominal pain and vomiting.13 The compulsive hot bathing may also be a response by the brain to the anxiety or psychological stress induced by severe nausea and vomiting.14
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of cannabinoid hyperemesis syndrome includes mainly cyclic vomiting syndrome and psychogenic vomiting. A careful history is useful, as is ruling out medication-induced reactions, toxins, pregnancy, and gastrointestinal, neurologic, metabolic, and endocrine causes. All three of these vomiting syndromes can present with a cyclic pattern of nausea and vomiting. Cannabis use is common in all three and so is not helpful in differentiating them. But the characteristic compulsive hot bathing and showering is unique and pathognomonic of cannabinoid hyperemesis syndrome.32
Endoscopic examination may reveal esophagitis and gastritis from severe bouts of retching.26
Cyclic vomiting syndrome
The Rome III criteria for the diagnosis of cyclic vomiting syndrome include three or more stereotypic episodes of acute-onset nausea and vomiting lasting less than 1 week, alternating with intervals of completely normal health. The criteria should be fulfilled for the previous 3 months with symptom onset at least 6 months before diagnosis.33
In a series of 17 patients with adult-onset cyclic vomiting syndrome,18 the average age at onset was 30, and 13 (76%) of the patients were women. Fifteen (88%) of the patients experienced a prodrome or aura of abdominal pain or headache, and in this group, a trigger such as emotional stress and infection could also be identified in 9 (60%).
Unlike in cannabinoid hyperemesis syndrome, most patients with cyclic vomiting syndrome have a family history of migraine headache, and the prevalence of psychological stressors is high.31 Also, patients with cannabinoid hyperemesis syndrome do not respond to medications that usually abort migraine episodes,15 whereas patients with cyclic vomiting syndrome, especially those who have a family history of migraines, may respond to antimigraine medications such as triptans. There is evidence of clinical psychological overlap between cyclic vomiting syndrome, abdominal migraine, and migraine headaches. Some authors recommend antimigraine therapy even in the absence of a family or personal history of migraine if, after a careful history and physical examination, the diagnosis of cyclic vomiting syndrome seems likely. Moreover, nonmedical management such as sleep, dark rooms, and quiet environment are not as effective in cannabinoid hyperemesis syndrome as they are in cyclic vomiting syndrome.18
Psychogenic vomiting
Psychogenic vomiting is classically defined as vomiting caused by psychological mechanisms without any obvious organic cause.13 It occurs most commonly in patients with major depressive disorder or conversion disorder.34 The mechanism appears to be a combination of past organic or gastrointestinal functional abnormalities and emotional problems, and multiple patterns of vomiting can occur. Most of these patients can be treated with behavioral therapy, antidepressant drug therapy, and supportive psychotherapy.34,35
ASKING A SERIES OF QUESTIONS
Most patients with cannabinoid hyperemesis syndrome have a history of frequent visits to emergency departments or clinics for persistent nausea and vomiting, and they may have undergone extensive diagnostic workups to exclude structural, inflammatory, infectious, and functional diseases of the bowel.23,24
To prevent unnecessary testing and use of healthcare resources, Wallace et al32 proposed an algorithm to help guide clinicians in diagnosing and treating patients with suspected cannabinoid hyperemesis syndrome. A patient presenting with severe nausea and vomiting should prompt a series of questions:
Do the signs and symptoms suggest a severe underlying medical cause? If so, this should be pursued.
Do symptoms improve while taking a hot shower or bath? If not, pursue an appropriate diagnostic evaluation and treatment for conditions other than cannabinoid hyperemesis syndrome.
Is the bathing compulsive? If not, consider other diagnoses, but remain suspicious about cannabinoid hyperemesis syndrome.
Does the patient currently use cannabis daily or almost daily, and has the patient done so for at least the past year? If the patient denies using cannabis, a urine drug screen for THC may be useful. If the patient admits to use, a presumptive diagnosis of cannabinoid hyperemesis syndrome can be made.
Does the patient have signs or symptoms of volume depletion, or is the patient unable to tolerate oral hydration? Encourage oral hydration or provide intravenous hydration, and provide cannabis cessation counseling.
Do the symptoms improve? If yes, great! Provide cessation counseling, resources, and follow-up. If not:
Is the patient still using cannabis? If not, it is time to rethink the diagnosis.
Treatment in the acute setting is supportive and includes intravenous hydration and correction of electrolytes. Conventional antiemetics such as ondansetron, metoclopramide, prochlorperazine, and promethazine have not been effective in relieving hyperemesis.9,12,14 This implies that the mechanism of emesis likely does not involve dopaminergic and serotonin pathways in the central and autonomic nervous systems.
Cessation of cannabis use is key for long-term resolution of symptoms. Efforts should be made to provide counseling and encourage patients to stop using the drug entirely (Figure 1).
SOMETHING TO THINK ABOUT
With the high prevalence of chronic cannabis abuse and the recent legalization of recreational marijuana use, we will all likely encounter a patient with cannabinoid hyperemesis. With adequate knowledge of this phenomenon, we can avoid unnecessary workups and inappropriate medical and surgical treatment in patients presenting with recurrent vomiting of unknown cause. The diagnosis can easily be made by simply asking for a history of chronic marijuana use and symptoms related to cannabinoid hyperemesis syndrome, such as relief of symptoms with hot baths or showers and with marijuana cessation.
Conservative management and fluid resuscitation is important in the acute setting, but cessation of marijuana use and follow-up counseling are the key components for treating patients with cannabinoid hyperemesis syndrome and for preventing recurrence.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Motel S. 6 facts about marijuana. Factank. News in the Numbers Pew Research Center. www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed June 2, 2015.
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 2003; 11:137–143.
- Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323:16–21.
- Davis M, Maida V, Daeninck P, Pergolizzi J. The emerging role of cannabinoid neuromodulators in symptom management. Support Care Cancer 2007; 15:63–71.
- National Institutes of Health (NIH). National Institute on Drug Abuse. Drug facts: marijuana. www.nida.nih.gov/infofacts/marijuana. Accessed April 29, 2015.
- Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol 1981; 18:353–366.
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–1949.
- Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc 2009; 84:76–78.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther 2002; 300:34–42.
- Darmani NA, Sim-Selley LJ, Martin BR, et al. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur J Pharmacol 2003; 459:83–95.
- Leibovich MA. Psychogenic vomiting. Psychotherapeutic considerations. Psychother Psychosom 1973; 22:263–268.
- Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci 2010; 55:3113–3119.
- Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol 2009; 15:1264–1266.
- Cox B, Chhabra A, Adler M, Simmons J, Randlett D. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med 2012; 2012:757696.
- Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc 2011; 111:166–169.
- Lee LY, Abbott L, Moodie S, Anderson S. Cyclic vomiting syndrome in 28 patients: demographics, features and outcomes. Eur J Gastroenterol Hepatol 2012; 24:939–943.
- Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry 2007; 15:156–158.
- Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth 1999; 83:637–649.
- Cota D, Steiner MA, Marsicano G, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 2007; 148:1574–1581.
- McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther 1999; 13:77–80.
- Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med 2011; 40:e63–e66.
- Singh E, Coyle W. Cannabinoid hyperemesis. Am J Gastroenterol 2008; 103:1048–1049.
- Carnett JB. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet 1926; 42:625–632.
- Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med 2010; 23:790–793.
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut 2008; 57:1140–1155.
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48:859–867.
- Choung RS, Locke GR 3rd, Lee RM, Schleck CD, Zinsmeister AR, Talley NJ. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroenterol Motil 2012; 24:20–26,e1.
- Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics 2012; 53:212–219.
- Miller JB, Walsh M, Patel PA, et al. Pediatric cannabinoid hyperemesis: two cases. Pediatr Emerg Care 2010; 26:919–920.
- Wallace EA, Andrews SE, Garmany CL, Jelley MJ. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J 2011; 104:659–664.
- Tack J, Taley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130:1466–1479.
- Muraoka M, Mine K, Matsumoto K, Nakai Y, Nakagawa T. Psychogenic vomiting: the relation between patterns of vomiting and psychiatric diagnoses. Gut 1990; 31:526–528.
- Stravynski A. Behavioral treatment of psychogenic vomiting in the context of social phobia. J Nerv Ment Dis 1983; 171:448–451.
With the growing use of marijuana, reports have appeared of a newly recognized condition in long-term heavy users termed cannabinoid hyperemesis syndrome.1
This syndrome is interesting for at least two reasons. First, paradoxically, marijuana appears to have an emetic effect with chronic use, whereas it usually has the opposite effect and is used as an antiemetic in patients undergoing chemotherapy. Second, patients develop a compulsion to bathe or shower in extremely hot water to relieve the symptoms.
In this article, we review the pathophysiology, clinical presentation, diagnosis, and management of this emerging condition.
MARIJUANA USE ON THE RISE
Marijuana is the most widely used illicit drug worldwide. Although statistics on its use vary, a report from the Pew Research Center2 stated that 49% of Americans say they have tried it. Several states now allow the use of marijuana for medicinal purposes, and Colorado and Washington have legalized it for recreational use. This marks a major turning point and may accelerate the slow-growing acceptance of marijuana use in the United States.
Marijuana has been used to treat HIV-associated anorexia and wasting, convulsions, glaucoma, headache, and chemotherapy-induced nausea and vomiting.3–5
Cannabinoid hyperemesis syndrome was first described in 2004 in South Australia.1 Since its recognition, an increasing number of cases have been identified worldwide. However, there are still no population-based studies to estimate its exact prevalence.
THC PREVENTS VOMITING—AND CAUSES IT
Delta-9-tetrahydrocannabinol (THC) is the principal psychoactive component in marijuana.6,7 There are two types of cannabinoid receptors in humans: CB1 and CB2. Both are found in the central nervous system and autonomic nervous system. Activation of CB1 receptors is responsible for the psychoactive effects of cannabinoids such as altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time. The physiologic role of CB2 is not known.
THC as an antiemetic
The antiemetic property of THC is not well understood but has been linked to activation of CB1 receptors found on the enteric plexus, presynaptic parasympathetic system, and central nervous system, particularly the cerebellum, hypothalamus, and vomiting center in the medulla.1,8–12 Stimulation and blockade of CB1 receptors can inhibit and induce vomiting in a dose-dependent manner, implicating endogenous cannabinoids in emetic circuits.12
THC as a proemetic
The mechanism of the paradoxical hyperemetic effect of THC is unknown, but several concepts have been proposed.
Chronic cannabis use can lead to down-regulation of CB1 receptors.13 Simonetto et al10 suggested that the central effects of long-term cannabis use on the hypothalamic-pituitary-adrenal axis may play a central role in the development of hyperemesis.10
Cannabinoids have a long half-life and are lipophilic.1 When used infrequently, they prevent vomiting. But with chronic use, high concentrations of THC can accumulate in the body, including cerebral fat, and can cause severe nausea and vomiting.8,9 This paradoxic hyperemesis was observed in people using intravenous crude marijuana extract.7 The same response was also noted in ferrets injected with 2-arachidonoylglycerol, a potent cannabinoid agonist.11
Patients who experience hyperemesis from chronic cannabis use may also have a genetic variation in their hepatic drug-transforming enzymes that results in excessive levels of cannabis metabolites that promote emesis.1,14
Delayed gastric emptying has also been linked to the proemetic effect of THC. However, this association became controversial when a large case series study showed that only 30% of patients with cannabinoid hyperemesis syndrome had delayed emptying on gastric scintigraphy.10
It is also possible that excessive stimulation of cannabinoid receptors in the gut can cause diffuse splanchnic vasodilation and contribute to the abdominal pain.13
DIAGNOSING CANNABINOID HYPEREMESIS SYNDROME
Cannabinoid hyperemesis syndrome is a clinical diagnosis typically seen in young patients (under age 50) with a long history of marijuana use. They present with severe, cyclic nausea and vomiting and admit to compulsively taking extremely hot showers or baths. Most patients report using marijuana for more than a year before developing episodes of severe vomiting. However, one study found that as many as 32% of patients had used it less than 1 year before experiencing symptoms.10
Other associated nonspecific symptoms are diaphoresis, bloating, abdominal discomfort, flushing, and weight loss. Symptoms are relieved with long, hot showers or baths and cessation of marijuana use. Taking a complete history is key to making the diagnosis.
In 2004, Allen et al1 first defined cannabinoid hyperemesis as excessive marijuana use associated with cyclical vomiting and abdominal pain.1 In 2012, Simonetto et al10 proposed diagnostic criteria (Table 1). Although not yet validated, these criteria are based on the largest series of cases of cannabinoid hyperemesis syndrome to date (98 patients).10
THE THREE PHASES OF CANNABINOID HYPEREMESIS
The clinical presentation of cannabinoid hyperemesis syndrome can be divided into three phases: prodromal, vomiting, and resolution.
Prodromal phase
During this phase, patients often appear anxious and agitated and display a spectrum of autonomic symptoms such as sweating, flushing, and constantly sipping water due to thirst. They may sometimes have abdominal pain that is usually epigastric but may also be diffuse. Their symptoms are associated with severe nausea, usually early in the morning or when they see or smell food. Appetite and eating patterns remain normal. Compulsive hot bathing or showering is minimal at this phase.
Vomiting phase
In this next phase, patients experience incapacitating nausea and vomiting that may occur without warning and are resistant to conventional antiemetics such as ondansetron and promethazine.14 However, patients eventually learn that hot baths or showers relieve the symptoms, and this behavior eventually becomes a compulsion. The higher the temperature of the water, the better the effect on symptoms.1 Low-grade pyrexia, excessive thirst, orthostasis, abdominal tenderness, weight loss, and sometimes even superficial skin burns have been reported.1,9,15–18
Recovery phase
During the final phase of cannabinoid hyperemesis syndrome, most patients experience marked resolution of symptoms after 24 to 48 hours of conservative management (bowel rest until symptoms resolve, slowly advancing diet as tolerated, intravenous fluids, and electrolyte monitoring and repletion as necessary), and most importantly, cessation of cannabis use. However, the time from cessation of marijuana use to resolution of symptoms may be as long as 1 week to 1 month.1,10,14 Patients begin to resume their normal diet and daily activities. The bathing-showering compulsion subsides, and patients regain lost weight after 3 to 6 months.1
In all case series and reports, resumption of cannabis use causes the symptoms to recur. This recurrence is compelling evidence that cannabis is the cause of the hyperemesis and should be part of the essential criteria for the diagnosis of cannabinoid hyperemesis syndrome.
WHY COMPULSIVE HOT BATHING?
The mechanism behind this unique characteristic of cannabinoid hyperemesis syndrome is not known. Several theories have been suggested, but no study has identified the exact explanation for this phenomenon.1,9,10,13–15,17–31
One suggested mechanism is a response by the thermoregulatory center of the brain to the dose-dependent hypothermic effects of THC, or even a direct effect of CB1 receptor activation in the hypothalamus.9 Cannabis toxicity could disrupt the equilibrium of satiety, thirst, digestive, and thermoregulatory systems of the hypothalamus, and this interference could resolve with hot bathing.1
The so-called “cutaneous steal” syndrome has also been proposed, in which cutaneous vasodilation caused by hot water decreases the blood volume available for the splanchnic circulation thought to be responsible for the abdominal pain and vomiting.13 The compulsive hot bathing may also be a response by the brain to the anxiety or psychological stress induced by severe nausea and vomiting.14
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of cannabinoid hyperemesis syndrome includes mainly cyclic vomiting syndrome and psychogenic vomiting. A careful history is useful, as is ruling out medication-induced reactions, toxins, pregnancy, and gastrointestinal, neurologic, metabolic, and endocrine causes. All three of these vomiting syndromes can present with a cyclic pattern of nausea and vomiting. Cannabis use is common in all three and so is not helpful in differentiating them. But the characteristic compulsive hot bathing and showering is unique and pathognomonic of cannabinoid hyperemesis syndrome.32
Endoscopic examination may reveal esophagitis and gastritis from severe bouts of retching.26
Cyclic vomiting syndrome
The Rome III criteria for the diagnosis of cyclic vomiting syndrome include three or more stereotypic episodes of acute-onset nausea and vomiting lasting less than 1 week, alternating with intervals of completely normal health. The criteria should be fulfilled for the previous 3 months with symptom onset at least 6 months before diagnosis.33
In a series of 17 patients with adult-onset cyclic vomiting syndrome,18 the average age at onset was 30, and 13 (76%) of the patients were women. Fifteen (88%) of the patients experienced a prodrome or aura of abdominal pain or headache, and in this group, a trigger such as emotional stress and infection could also be identified in 9 (60%).
Unlike in cannabinoid hyperemesis syndrome, most patients with cyclic vomiting syndrome have a family history of migraine headache, and the prevalence of psychological stressors is high.31 Also, patients with cannabinoid hyperemesis syndrome do not respond to medications that usually abort migraine episodes,15 whereas patients with cyclic vomiting syndrome, especially those who have a family history of migraines, may respond to antimigraine medications such as triptans. There is evidence of clinical psychological overlap between cyclic vomiting syndrome, abdominal migraine, and migraine headaches. Some authors recommend antimigraine therapy even in the absence of a family or personal history of migraine if, after a careful history and physical examination, the diagnosis of cyclic vomiting syndrome seems likely. Moreover, nonmedical management such as sleep, dark rooms, and quiet environment are not as effective in cannabinoid hyperemesis syndrome as they are in cyclic vomiting syndrome.18
Psychogenic vomiting
Psychogenic vomiting is classically defined as vomiting caused by psychological mechanisms without any obvious organic cause.13 It occurs most commonly in patients with major depressive disorder or conversion disorder.34 The mechanism appears to be a combination of past organic or gastrointestinal functional abnormalities and emotional problems, and multiple patterns of vomiting can occur. Most of these patients can be treated with behavioral therapy, antidepressant drug therapy, and supportive psychotherapy.34,35
ASKING A SERIES OF QUESTIONS
Most patients with cannabinoid hyperemesis syndrome have a history of frequent visits to emergency departments or clinics for persistent nausea and vomiting, and they may have undergone extensive diagnostic workups to exclude structural, inflammatory, infectious, and functional diseases of the bowel.23,24
To prevent unnecessary testing and use of healthcare resources, Wallace et al32 proposed an algorithm to help guide clinicians in diagnosing and treating patients with suspected cannabinoid hyperemesis syndrome. A patient presenting with severe nausea and vomiting should prompt a series of questions:
Do the signs and symptoms suggest a severe underlying medical cause? If so, this should be pursued.
Do symptoms improve while taking a hot shower or bath? If not, pursue an appropriate diagnostic evaluation and treatment for conditions other than cannabinoid hyperemesis syndrome.
Is the bathing compulsive? If not, consider other diagnoses, but remain suspicious about cannabinoid hyperemesis syndrome.
Does the patient currently use cannabis daily or almost daily, and has the patient done so for at least the past year? If the patient denies using cannabis, a urine drug screen for THC may be useful. If the patient admits to use, a presumptive diagnosis of cannabinoid hyperemesis syndrome can be made.
Does the patient have signs or symptoms of volume depletion, or is the patient unable to tolerate oral hydration? Encourage oral hydration or provide intravenous hydration, and provide cannabis cessation counseling.
Do the symptoms improve? If yes, great! Provide cessation counseling, resources, and follow-up. If not:
Is the patient still using cannabis? If not, it is time to rethink the diagnosis.
Treatment in the acute setting is supportive and includes intravenous hydration and correction of electrolytes. Conventional antiemetics such as ondansetron, metoclopramide, prochlorperazine, and promethazine have not been effective in relieving hyperemesis.9,12,14 This implies that the mechanism of emesis likely does not involve dopaminergic and serotonin pathways in the central and autonomic nervous systems.
Cessation of cannabis use is key for long-term resolution of symptoms. Efforts should be made to provide counseling and encourage patients to stop using the drug entirely (Figure 1).
SOMETHING TO THINK ABOUT
With the high prevalence of chronic cannabis abuse and the recent legalization of recreational marijuana use, we will all likely encounter a patient with cannabinoid hyperemesis. With adequate knowledge of this phenomenon, we can avoid unnecessary workups and inappropriate medical and surgical treatment in patients presenting with recurrent vomiting of unknown cause. The diagnosis can easily be made by simply asking for a history of chronic marijuana use and symptoms related to cannabinoid hyperemesis syndrome, such as relief of symptoms with hot baths or showers and with marijuana cessation.
Conservative management and fluid resuscitation is important in the acute setting, but cessation of marijuana use and follow-up counseling are the key components for treating patients with cannabinoid hyperemesis syndrome and for preventing recurrence.
With the growing use of marijuana, reports have appeared of a newly recognized condition in long-term heavy users termed cannabinoid hyperemesis syndrome.1
This syndrome is interesting for at least two reasons. First, paradoxically, marijuana appears to have an emetic effect with chronic use, whereas it usually has the opposite effect and is used as an antiemetic in patients undergoing chemotherapy. Second, patients develop a compulsion to bathe or shower in extremely hot water to relieve the symptoms.
In this article, we review the pathophysiology, clinical presentation, diagnosis, and management of this emerging condition.
MARIJUANA USE ON THE RISE
Marijuana is the most widely used illicit drug worldwide. Although statistics on its use vary, a report from the Pew Research Center2 stated that 49% of Americans say they have tried it. Several states now allow the use of marijuana for medicinal purposes, and Colorado and Washington have legalized it for recreational use. This marks a major turning point and may accelerate the slow-growing acceptance of marijuana use in the United States.
Marijuana has been used to treat HIV-associated anorexia and wasting, convulsions, glaucoma, headache, and chemotherapy-induced nausea and vomiting.3–5
Cannabinoid hyperemesis syndrome was first described in 2004 in South Australia.1 Since its recognition, an increasing number of cases have been identified worldwide. However, there are still no population-based studies to estimate its exact prevalence.
THC PREVENTS VOMITING—AND CAUSES IT
Delta-9-tetrahydrocannabinol (THC) is the principal psychoactive component in marijuana.6,7 There are two types of cannabinoid receptors in humans: CB1 and CB2. Both are found in the central nervous system and autonomic nervous system. Activation of CB1 receptors is responsible for the psychoactive effects of cannabinoids such as altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time. The physiologic role of CB2 is not known.
THC as an antiemetic
The antiemetic property of THC is not well understood but has been linked to activation of CB1 receptors found on the enteric plexus, presynaptic parasympathetic system, and central nervous system, particularly the cerebellum, hypothalamus, and vomiting center in the medulla.1,8–12 Stimulation and blockade of CB1 receptors can inhibit and induce vomiting in a dose-dependent manner, implicating endogenous cannabinoids in emetic circuits.12
THC as a proemetic
The mechanism of the paradoxical hyperemetic effect of THC is unknown, but several concepts have been proposed.
Chronic cannabis use can lead to down-regulation of CB1 receptors.13 Simonetto et al10 suggested that the central effects of long-term cannabis use on the hypothalamic-pituitary-adrenal axis may play a central role in the development of hyperemesis.10
Cannabinoids have a long half-life and are lipophilic.1 When used infrequently, they prevent vomiting. But with chronic use, high concentrations of THC can accumulate in the body, including cerebral fat, and can cause severe nausea and vomiting.8,9 This paradoxic hyperemesis was observed in people using intravenous crude marijuana extract.7 The same response was also noted in ferrets injected with 2-arachidonoylglycerol, a potent cannabinoid agonist.11
Patients who experience hyperemesis from chronic cannabis use may also have a genetic variation in their hepatic drug-transforming enzymes that results in excessive levels of cannabis metabolites that promote emesis.1,14
Delayed gastric emptying has also been linked to the proemetic effect of THC. However, this association became controversial when a large case series study showed that only 30% of patients with cannabinoid hyperemesis syndrome had delayed emptying on gastric scintigraphy.10
It is also possible that excessive stimulation of cannabinoid receptors in the gut can cause diffuse splanchnic vasodilation and contribute to the abdominal pain.13
DIAGNOSING CANNABINOID HYPEREMESIS SYNDROME
Cannabinoid hyperemesis syndrome is a clinical diagnosis typically seen in young patients (under age 50) with a long history of marijuana use. They present with severe, cyclic nausea and vomiting and admit to compulsively taking extremely hot showers or baths. Most patients report using marijuana for more than a year before developing episodes of severe vomiting. However, one study found that as many as 32% of patients had used it less than 1 year before experiencing symptoms.10
Other associated nonspecific symptoms are diaphoresis, bloating, abdominal discomfort, flushing, and weight loss. Symptoms are relieved with long, hot showers or baths and cessation of marijuana use. Taking a complete history is key to making the diagnosis.
In 2004, Allen et al1 first defined cannabinoid hyperemesis as excessive marijuana use associated with cyclical vomiting and abdominal pain.1 In 2012, Simonetto et al10 proposed diagnostic criteria (Table 1). Although not yet validated, these criteria are based on the largest series of cases of cannabinoid hyperemesis syndrome to date (98 patients).10
THE THREE PHASES OF CANNABINOID HYPEREMESIS
The clinical presentation of cannabinoid hyperemesis syndrome can be divided into three phases: prodromal, vomiting, and resolution.
Prodromal phase
During this phase, patients often appear anxious and agitated and display a spectrum of autonomic symptoms such as sweating, flushing, and constantly sipping water due to thirst. They may sometimes have abdominal pain that is usually epigastric but may also be diffuse. Their symptoms are associated with severe nausea, usually early in the morning or when they see or smell food. Appetite and eating patterns remain normal. Compulsive hot bathing or showering is minimal at this phase.
Vomiting phase
In this next phase, patients experience incapacitating nausea and vomiting that may occur without warning and are resistant to conventional antiemetics such as ondansetron and promethazine.14 However, patients eventually learn that hot baths or showers relieve the symptoms, and this behavior eventually becomes a compulsion. The higher the temperature of the water, the better the effect on symptoms.1 Low-grade pyrexia, excessive thirst, orthostasis, abdominal tenderness, weight loss, and sometimes even superficial skin burns have been reported.1,9,15–18
Recovery phase
During the final phase of cannabinoid hyperemesis syndrome, most patients experience marked resolution of symptoms after 24 to 48 hours of conservative management (bowel rest until symptoms resolve, slowly advancing diet as tolerated, intravenous fluids, and electrolyte monitoring and repletion as necessary), and most importantly, cessation of cannabis use. However, the time from cessation of marijuana use to resolution of symptoms may be as long as 1 week to 1 month.1,10,14 Patients begin to resume their normal diet and daily activities. The bathing-showering compulsion subsides, and patients regain lost weight after 3 to 6 months.1
In all case series and reports, resumption of cannabis use causes the symptoms to recur. This recurrence is compelling evidence that cannabis is the cause of the hyperemesis and should be part of the essential criteria for the diagnosis of cannabinoid hyperemesis syndrome.
WHY COMPULSIVE HOT BATHING?
The mechanism behind this unique characteristic of cannabinoid hyperemesis syndrome is not known. Several theories have been suggested, but no study has identified the exact explanation for this phenomenon.1,9,10,13–15,17–31
One suggested mechanism is a response by the thermoregulatory center of the brain to the dose-dependent hypothermic effects of THC, or even a direct effect of CB1 receptor activation in the hypothalamus.9 Cannabis toxicity could disrupt the equilibrium of satiety, thirst, digestive, and thermoregulatory systems of the hypothalamus, and this interference could resolve with hot bathing.1
The so-called “cutaneous steal” syndrome has also been proposed, in which cutaneous vasodilation caused by hot water decreases the blood volume available for the splanchnic circulation thought to be responsible for the abdominal pain and vomiting.13 The compulsive hot bathing may also be a response by the brain to the anxiety or psychological stress induced by severe nausea and vomiting.14
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of cannabinoid hyperemesis syndrome includes mainly cyclic vomiting syndrome and psychogenic vomiting. A careful history is useful, as is ruling out medication-induced reactions, toxins, pregnancy, and gastrointestinal, neurologic, metabolic, and endocrine causes. All three of these vomiting syndromes can present with a cyclic pattern of nausea and vomiting. Cannabis use is common in all three and so is not helpful in differentiating them. But the characteristic compulsive hot bathing and showering is unique and pathognomonic of cannabinoid hyperemesis syndrome.32
Endoscopic examination may reveal esophagitis and gastritis from severe bouts of retching.26
Cyclic vomiting syndrome
The Rome III criteria for the diagnosis of cyclic vomiting syndrome include three or more stereotypic episodes of acute-onset nausea and vomiting lasting less than 1 week, alternating with intervals of completely normal health. The criteria should be fulfilled for the previous 3 months with symptom onset at least 6 months before diagnosis.33
In a series of 17 patients with adult-onset cyclic vomiting syndrome,18 the average age at onset was 30, and 13 (76%) of the patients were women. Fifteen (88%) of the patients experienced a prodrome or aura of abdominal pain or headache, and in this group, a trigger such as emotional stress and infection could also be identified in 9 (60%).
Unlike in cannabinoid hyperemesis syndrome, most patients with cyclic vomiting syndrome have a family history of migraine headache, and the prevalence of psychological stressors is high.31 Also, patients with cannabinoid hyperemesis syndrome do not respond to medications that usually abort migraine episodes,15 whereas patients with cyclic vomiting syndrome, especially those who have a family history of migraines, may respond to antimigraine medications such as triptans. There is evidence of clinical psychological overlap between cyclic vomiting syndrome, abdominal migraine, and migraine headaches. Some authors recommend antimigraine therapy even in the absence of a family or personal history of migraine if, after a careful history and physical examination, the diagnosis of cyclic vomiting syndrome seems likely. Moreover, nonmedical management such as sleep, dark rooms, and quiet environment are not as effective in cannabinoid hyperemesis syndrome as they are in cyclic vomiting syndrome.18
Psychogenic vomiting
Psychogenic vomiting is classically defined as vomiting caused by psychological mechanisms without any obvious organic cause.13 It occurs most commonly in patients with major depressive disorder or conversion disorder.34 The mechanism appears to be a combination of past organic or gastrointestinal functional abnormalities and emotional problems, and multiple patterns of vomiting can occur. Most of these patients can be treated with behavioral therapy, antidepressant drug therapy, and supportive psychotherapy.34,35
ASKING A SERIES OF QUESTIONS
Most patients with cannabinoid hyperemesis syndrome have a history of frequent visits to emergency departments or clinics for persistent nausea and vomiting, and they may have undergone extensive diagnostic workups to exclude structural, inflammatory, infectious, and functional diseases of the bowel.23,24
To prevent unnecessary testing and use of healthcare resources, Wallace et al32 proposed an algorithm to help guide clinicians in diagnosing and treating patients with suspected cannabinoid hyperemesis syndrome. A patient presenting with severe nausea and vomiting should prompt a series of questions:
Do the signs and symptoms suggest a severe underlying medical cause? If so, this should be pursued.
Do symptoms improve while taking a hot shower or bath? If not, pursue an appropriate diagnostic evaluation and treatment for conditions other than cannabinoid hyperemesis syndrome.
Is the bathing compulsive? If not, consider other diagnoses, but remain suspicious about cannabinoid hyperemesis syndrome.
Does the patient currently use cannabis daily or almost daily, and has the patient done so for at least the past year? If the patient denies using cannabis, a urine drug screen for THC may be useful. If the patient admits to use, a presumptive diagnosis of cannabinoid hyperemesis syndrome can be made.
Does the patient have signs or symptoms of volume depletion, or is the patient unable to tolerate oral hydration? Encourage oral hydration or provide intravenous hydration, and provide cannabis cessation counseling.
Do the symptoms improve? If yes, great! Provide cessation counseling, resources, and follow-up. If not:
Is the patient still using cannabis? If not, it is time to rethink the diagnosis.
Treatment in the acute setting is supportive and includes intravenous hydration and correction of electrolytes. Conventional antiemetics such as ondansetron, metoclopramide, prochlorperazine, and promethazine have not been effective in relieving hyperemesis.9,12,14 This implies that the mechanism of emesis likely does not involve dopaminergic and serotonin pathways in the central and autonomic nervous systems.
Cessation of cannabis use is key for long-term resolution of symptoms. Efforts should be made to provide counseling and encourage patients to stop using the drug entirely (Figure 1).
SOMETHING TO THINK ABOUT
With the high prevalence of chronic cannabis abuse and the recent legalization of recreational marijuana use, we will all likely encounter a patient with cannabinoid hyperemesis. With adequate knowledge of this phenomenon, we can avoid unnecessary workups and inappropriate medical and surgical treatment in patients presenting with recurrent vomiting of unknown cause. The diagnosis can easily be made by simply asking for a history of chronic marijuana use and symptoms related to cannabinoid hyperemesis syndrome, such as relief of symptoms with hot baths or showers and with marijuana cessation.
Conservative management and fluid resuscitation is important in the acute setting, but cessation of marijuana use and follow-up counseling are the key components for treating patients with cannabinoid hyperemesis syndrome and for preventing recurrence.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Motel S. 6 facts about marijuana. Factank. News in the Numbers Pew Research Center. www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed June 2, 2015.
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 2003; 11:137–143.
- Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323:16–21.
- Davis M, Maida V, Daeninck P, Pergolizzi J. The emerging role of cannabinoid neuromodulators in symptom management. Support Care Cancer 2007; 15:63–71.
- National Institutes of Health (NIH). National Institute on Drug Abuse. Drug facts: marijuana. www.nida.nih.gov/infofacts/marijuana. Accessed April 29, 2015.
- Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol 1981; 18:353–366.
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–1949.
- Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc 2009; 84:76–78.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther 2002; 300:34–42.
- Darmani NA, Sim-Selley LJ, Martin BR, et al. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur J Pharmacol 2003; 459:83–95.
- Leibovich MA. Psychogenic vomiting. Psychotherapeutic considerations. Psychother Psychosom 1973; 22:263–268.
- Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci 2010; 55:3113–3119.
- Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol 2009; 15:1264–1266.
- Cox B, Chhabra A, Adler M, Simmons J, Randlett D. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med 2012; 2012:757696.
- Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc 2011; 111:166–169.
- Lee LY, Abbott L, Moodie S, Anderson S. Cyclic vomiting syndrome in 28 patients: demographics, features and outcomes. Eur J Gastroenterol Hepatol 2012; 24:939–943.
- Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry 2007; 15:156–158.
- Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth 1999; 83:637–649.
- Cota D, Steiner MA, Marsicano G, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 2007; 148:1574–1581.
- McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther 1999; 13:77–80.
- Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med 2011; 40:e63–e66.
- Singh E, Coyle W. Cannabinoid hyperemesis. Am J Gastroenterol 2008; 103:1048–1049.
- Carnett JB. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet 1926; 42:625–632.
- Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med 2010; 23:790–793.
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut 2008; 57:1140–1155.
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48:859–867.
- Choung RS, Locke GR 3rd, Lee RM, Schleck CD, Zinsmeister AR, Talley NJ. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroenterol Motil 2012; 24:20–26,e1.
- Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics 2012; 53:212–219.
- Miller JB, Walsh M, Patel PA, et al. Pediatric cannabinoid hyperemesis: two cases. Pediatr Emerg Care 2010; 26:919–920.
- Wallace EA, Andrews SE, Garmany CL, Jelley MJ. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J 2011; 104:659–664.
- Tack J, Taley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130:1466–1479.
- Muraoka M, Mine K, Matsumoto K, Nakai Y, Nakagawa T. Psychogenic vomiting: the relation between patterns of vomiting and psychiatric diagnoses. Gut 1990; 31:526–528.
- Stravynski A. Behavioral treatment of psychogenic vomiting in the context of social phobia. J Nerv Ment Dis 1983; 171:448–451.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Motel S. 6 facts about marijuana. Factank. News in the Numbers Pew Research Center. www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed June 2, 2015.
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 2003; 11:137–143.
- Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323:16–21.
- Davis M, Maida V, Daeninck P, Pergolizzi J. The emerging role of cannabinoid neuromodulators in symptom management. Support Care Cancer 2007; 15:63–71.
- National Institutes of Health (NIH). National Institute on Drug Abuse. Drug facts: marijuana. www.nida.nih.gov/infofacts/marijuana. Accessed April 29, 2015.
- Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol 1981; 18:353–366.
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–1949.
- Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc 2009; 84:76–78.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther 2002; 300:34–42.
- Darmani NA, Sim-Selley LJ, Martin BR, et al. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur J Pharmacol 2003; 459:83–95.
- Leibovich MA. Psychogenic vomiting. Psychotherapeutic considerations. Psychother Psychosom 1973; 22:263–268.
- Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci 2010; 55:3113–3119.
- Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol 2009; 15:1264–1266.
- Cox B, Chhabra A, Adler M, Simmons J, Randlett D. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med 2012; 2012:757696.
- Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc 2011; 111:166–169.
- Lee LY, Abbott L, Moodie S, Anderson S. Cyclic vomiting syndrome in 28 patients: demographics, features and outcomes. Eur J Gastroenterol Hepatol 2012; 24:939–943.
- Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry 2007; 15:156–158.
- Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth 1999; 83:637–649.
- Cota D, Steiner MA, Marsicano G, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 2007; 148:1574–1581.
- McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther 1999; 13:77–80.
- Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med 2011; 40:e63–e66.
- Singh E, Coyle W. Cannabinoid hyperemesis. Am J Gastroenterol 2008; 103:1048–1049.
- Carnett JB. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet 1926; 42:625–632.
- Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med 2010; 23:790–793.
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut 2008; 57:1140–1155.
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48:859–867.
- Choung RS, Locke GR 3rd, Lee RM, Schleck CD, Zinsmeister AR, Talley NJ. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroenterol Motil 2012; 24:20–26,e1.
- Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics 2012; 53:212–219.
- Miller JB, Walsh M, Patel PA, et al. Pediatric cannabinoid hyperemesis: two cases. Pediatr Emerg Care 2010; 26:919–920.
- Wallace EA, Andrews SE, Garmany CL, Jelley MJ. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J 2011; 104:659–664.
- Tack J, Taley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130:1466–1479.
- Muraoka M, Mine K, Matsumoto K, Nakai Y, Nakagawa T. Psychogenic vomiting: the relation between patterns of vomiting and psychiatric diagnoses. Gut 1990; 31:526–528.
- Stravynski A. Behavioral treatment of psychogenic vomiting in the context of social phobia. J Nerv Ment Dis 1983; 171:448–451.
KEY POINTS
- The prodromal phase is characterized by severe anxiety and agitation. Patients display a spectrum of autonomic symptoms such as sweating, flushing, constantly sipping water due to thirst, and colicky abdominal pain.
- In the second phase, patients develop incapacitating nausea and vomiting that may occur without warning and is usually resistant to conventional antiemetics such as ondansetron and promethazine. During this phase, patients learn the immediate relieving effects of taking hot baths.
- After 24 to 48 hours of conservative management, intravenous fluid replacement, and, most importantly, cessation of cannabis use, patients experience marked resolution of symptoms. The compulsive hot-bathing behavior subsides. However, eventually, patients go back to using marijuana, and the cycle of symptoms recurs.
Fecal microbiota transplantation for recurrent C difficile infection: Ready for prime time?
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
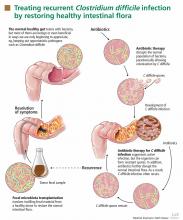
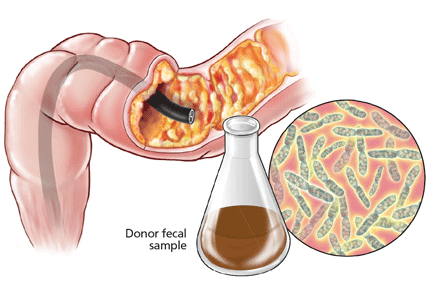
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
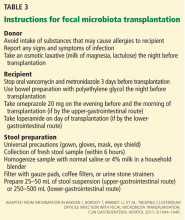
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
KEY POINTS
- Fecal microbiota transplantation involves instilling gut microbiota from a healthy donor into the diseased gut of a patient who has recurrent or recalcitrant episodes of diarrhea despite antibiotic treatment for C difficile infection. The instillation can be done via nasogastric tube, endoscope, or enema.
- Donor screening is necessary to prevent transmission of communicable diseases to the recipient.
- Recently published studies indicate that this procedure is effective for treating recurrent C difficile infection. Randomized clinical trials to assess its efficacy and safety are underway.
- The field of microbiota therapy is rapidly progressing. More physicians are learning to embrace the concept of fecal microbiota transplantation, and patients are beginning to overcome the “yuck factor” and accept its benefits.