User login
Acute gout: Oral steroids work as well as NSAIDs
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
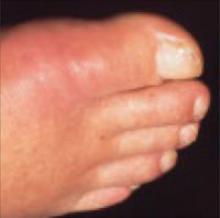
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Use a short course of oral steroids (prednisone 30-40 mg/d for 5 days) for treatment of acute gout when nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. Steroids are also a reasonable choice as first-line treatment.1,2
Strength of recommendation
B: 2 good-quality, randomized controlled trials (RCTs)
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomized equivalence trial. Lancet. 2008;371:1854-1860.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
ILLUSTRATIVE CASE
A 68-year-old man with a history of ulcer disease and mild renal insufficiency comes to your office complaining of severe pain in his right foot. You note swelling and redness around the base of the big toe and diagnose acute gout. Wishing to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine because of the patient’s medical history, you wonder what you can safely prescribe for pain relief.
NSAIDs have become the mainstay of treatment for acute gout,3,4 replacing colchicine—widely used for gout pain relief since the early 19th century.5 Colchicine fell out of favor because it routinely causes diarrhea and requires caution in patients with renal insufficiency.6 Now, however, there is growing concern about the adverse effects of NSAIDs.
Comorbidities, age, mean fewer options
NSAIDs increase the risk of gastrointestinal (GI) bleeding, especially in the first week of use.7 Cyclooxygenase-2 (COX-2) inhibitors, considered as effective as NSAIDs in treating acute gout pain,8 are also associated with GI bleeds.9 In addition, NSAIDs and COX-2 inhibitors increase cardiovascular risks, prompting the American Heart Association to recommend restricted use of both.10 NSAIDs’ effect on renal function, fluid retention, and interactions with anticoagulants are additional concerns, because gout patients are generally older and often have comorbid renal and cardiovascular diseases.3,11-13
In the United States, nearly 70% of patients who develop acute gout seek treatment from primary care physicians.12 Family physicians need a safe alternative to NSAIDs to relieve the severe pain associated with this condition. Will oral corticosteroids fit the bill?
STUDY SUMMARIES: Oral steroids: A safe and effective alternative
Janssens et al1 conducted a double-blind, randomized equivalence trial of 118 patients to compare the efficacy of prednisolone and naproxen for the treatment of monoarticular gout, confirmed by crystal analysis of synovial fluid. The study was conducted in the eastern Netherlands at a trial center patients were referred to by their family physicians. Those with major comorbidities, including a history of GI bleed or peptic ulcer, were excluded.
Participants were randomized to receive either prednisolone 35 mg* or naproxen 500 mg twice a day, with look-alike placebo tablets of the alternate drug, for 5 days. Pain, the primary outcome, was scored on a validated visual analog scale from 0 mm (no pain) to 100 mm (worst pain experienced).15 The reduction in the pain score at 90 hours was similar in both groups. Only a few minor side effects were reported in both groups, and all completely resolved in 3 weeks.
The study by Man et al2 was a randomized trial that compared indomethacin with oral prednisolone in 90 patients presenting to an emergency department in Hong Kong. Diagnosis of gout was made by clinical impression. Participants in the indomethacin group also received an intramuscular (IM) injection of diclofenac 75 mg, and those in both groups were monitored for acetaminophen use as a secondary endpoint.
Pain reduction, the primary endpoint, was assessed with a 10-point visual analog score, and was slightly better statistically in the oral steroid group. The study was not designed to evaluate for safety, but the authors noted that patients in the indomethacin group experienced more adverse effects (number needed to harm [NNH] for any adverse event: 3; NNH for serious events: 6).
Short-term steroids have few side effects
In both studies, patients receiving oral steroids experienced no significant side effects. This finding is consistent with other studies that have investigated short-term oral steroid use in the treatment of both rheumatoid arthritis and asthma.16,17
WHAT’S NEW?: Evidence supports use of steroids for acute gout
In the United States, prednisone is prescribed as treatment for acute gout only about 9% of the time.12 These 2 studies—the first randomized trials comparing oral steroids with NSAIDs, the usual gout treatment—may lead to greater use of steroids for this painful condition.
Both studies were well designed and conducted in an outpatient (or emergency) setting. Both showed that a short course of oral steroids is as effective as NSAIDs, and without significant side effects.
Previous studies have compared IM steroids with NSAIDs, and IM steroids with IM adrenocorticotropic hormone (ACTH).18,19 However, these studies were not blinded—just one of their methodological problems.4
CAVEATS: Joint aspiration is not the norm
In the Janssens study, participants were diagnosed with gout after monosodium urate crystals were found in joint aspirate.1 This may not be the usual practice in primary care settings, where a clinical diagnosis of gout is typically made. The authors indicate that the failure to perform joint aspiration will lead to occasional cases of septic arthritis being treated with oral steroids. We recommend joint aspiration or a referral for such a procedure when clinical evidence (eg, fever and leukocytosis) is suggestive of septic arthritis.
Possible impact of acetaminophen
In the study by Man et al, acetaminophen was used by both groups as an adjunct for pain relief, and the amount used was higher (mean 10.3 g vs 6.4 g over 14 days) in the oral steroid group. It is possible that some of the pain relief experienced by those in the steroid group may have been from acetaminophen; however, a difference of 4 g over a 14-day period makes that unlikely. Even if additional acetaminophen is required, the advantages of oral steroids rather than NSAIDs or colchicine for patients with contraindications remain.
Also of note: These trials examined short-term treatment of acute gout. These findings cannot be extrapolated to the treatment of intercurrent gout or chronic gouty arthritis, since long-term steroid use has severe adverse effects.
CHALLENGES TO IMPLEMENTATION: No significant barriers
We found little to prevent physicians from adopting this practice changer. Oral steroids are readily available and inexpensive, and most primary care clinicians regularly prescribe them for other conditions. This practice change recommendation should be readily implemented.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
* Prednisone is the precursor of prednisolone and is activated in the liver. The activity of both drugs is comparable, and prednisone and prednisolone can be converted milligram to milligram. However, prednisolone may be preferred for patients with severe liver disease.14 (In the United States, prednisolone is available as a liquid and prednisone as a tablet.)
PURL METHODOLOGY
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
1. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
2. Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670-677.
3. Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout—a systematic review. Rheumatology. 2006;45:1422-1431.
4. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
5. Weinberger A, Pinkhas J. The history of colchicine. Korot. 1980;7:760-763.
6. Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. ANZ J Med. 1987;17:301-304.
7. Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320-326.
8. Rubin BR, Burton R, Navarra S, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598-606.
9. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27:411-420.
10. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
11. Petersel D, Schlesinger N. Treatment of acute gout in hospitalized patients. J Rheumatol. 2007;34:1566-1568.
12. Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35:498-501.
13. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104-1110.
14. Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5:501-505.
15. Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23:285-295.
16. Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2004;(3):CD000189.-
17. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002308.-
18. Alloway JA, Moriarty MJ, Hoogland YT, Nashel DJ. Comparison of triamcinolone acetonide with indomethacin in the treatment of acute gouty arthritis. J Rheumatol. 1993;20:111-113.
19. Siegel LB, Alloway JA, Nashel DJ. Comparison of adrenocorticotropic hormone and triamcinolone acetonide in the treatment of acute gouty arthritis. J Rheumatol. 1994;21:1325-1327.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Sequential therapy boosts H pylori eradication rates
Prescribe sequential therapy rather than the standard (concurrent) therapy to improve H pylori eradication rates, particularly in treatment-naïve patients.1
Strength of recommendation
B: Based on a well-done meta-analysis with disease-oriented outcomes
Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
ILLUSTRATIVE CASE
A 40-year-old woman with a peptic ulcer has been diagnosed with Helicobacter pylori infection, and schedules a visit to discuss treatment. You’re aware of the declining eradication rates associated with standard therapy, and have heard that sequential therapy may be a more effective option. Should you offer it to this patient?
An estimated 30% to 40% of the US population is infected with H pylori,2 a bacterium that plays a crucial role in the pathogenesis of peptic ulcer disease, chronic gastritis, and gastric cancer.1 The triple-drug regimen (a proton-pump inhibitor [PPI] and clarithromycin with amoxicillin, tinidazole, or another imidazole) is commonly used to treat H pylori in Europe and in the United States.3 Yet the eradication rate associated with this standard 3-drug regimen in this country is <80%, and appears to be on the decline. The likely problem: the increase in antibiotic-resistant strains of H pylori.2
Put amoxicillin first
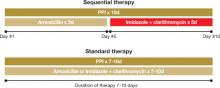
In Italy, eradication rates of >90% have been reported with a sequential therapy: a PPI and amoxicillin for 5 days, followed by the PPI, clarithromycin, and tinidazole for an additional 5 days (FIGURE).4 (Because tinidazole is a relatively new drug in the United States and physicians may use other drugs in its class instead, we refer to the drug class—imidazoles—rather than a specific medication in the FIGURE and much of the text that follows.) Using amoxicillin before the other antibiotics weakens bacterial cell walls, preventing the formation of drug efflux channels that can inhibit clarithromycin and other antibiotics, according to 1 theory.1 Thus, clarithromycin and an imidazole are more effective in the second phase of treatment.1
Should US physicians adopt the Italian protocol and prescribe tinidazole? Would metronidazole or other imidazoles be equally effective?
FIGURE
Sequential vs standard therapy: A comparison
STUDY SUMMARY: Sequential therapy is better on all counts
This meta-analysis found 9 randomized controlled trials (RCTs) that compared H pylori eradication rates with standard 7- to 10-day triple-drug therapy to 10-day sequential therapy; an additional small study used a 5-day triple-drug comparison group. The authors performed a thorough search and used standard meta-analysis methods for data synthesis and analysis. The patients were all H pylori treatment naïve and had not used PPIs, histamine-2 receptor antagonists, or antibiotics in the month preceding the study. All patients (n=2747) had documented H pylori infection based on fecal antigen test, histologic evaluation, biopsy urease test, or urea breath test.
All the trials were conducted in Italy, although 2 of the studies included patients from the United States. Nine RCTs compared a triple-therapy regimen with PPI to sequential therapy, 1 RCT compared a triple-drug regimen with ranitidine to sequential therapy, and 1 included only pediatric patients. Pooled eradication rates were 93.4% (95% confidence interval [CI,] 91.3%-95.5%) for sequential therapy and 76.9% (CI, 71.0%-82.8%) for standard therapy, with a relative risk reduction of 71% (CI, 64%-77%).1 The authors estimated that for every 6.3 patients (95% CI, 5.2-7.1) treated with sequential therapy, there would be 1 additional cure compared to standard therapy. Standard 7- to 10-day triple therapy remained inferior to sequential therapy in all subgroup analyses, including patients with risk factors for eradication failure.
Adherence rates were similar in both groups. Sequential therapy resulted in a median adherence rate of 97.4% (range, 90.0%-98.9%), with standard therapy at 96.8% (range, 93.0%-100%).1 Reported side effects were also similar in both treatment groups.1
WHAT’S NEW?: This meta-analysis reduces doubt
The latest American College of Gastroenterology guidelines on the management of H pylori infection acknowledge that sequential therapy has shown promise in Europe, but the organization has not supported a change from the standard regimen to sequential therapy as first-line treatment. The standard triple-therapy regimen is approved by the US Food and Drug Administration, and is the most commonly used H pylori treatment in the United States.1 This is the first meta-analysis based solely on RCTs, and it clearly demonstrates that sequential therapy increased eradication rates.
Helicobacter pylori (called H pylori for short) is a type of bacteria that can cause an ulcer (sore) to develop within the lining of your stomach and intestines. Ulcers can cause pain and bleeding, so it is important to get rid of this bacteria. To do this, you will need to take 4 different medicines over the next 10 days. They must be taken in a certain order, exactly as your doctor has prescribed.
One of the drugs you will be taking (for the entire 10-day period) is a proton pump inhibitor (called a PPI), a medicine that helps reduce the amount of acid in your stomach. ______________ is the name of the PPI your doctor has ordered. Take it twice a day, once in the morning and once in the evening, for 10 days.
You will also take 3 different kinds of antibiotics to eliminate the H pylori bacteria. For the first 5 days, you will take amoxicillin twice a day (2 pills in the morning and 2 in the evening), along with the PPI.
After 5 days, all the amoxicillin pills should be gone, and you will switch to 2 other antibiotics: clarithromycin AND ______________. For the next 5 days, you will take 1 PPI plus 1 clarithromycin and 1 ______________ every morning AND every night.
These medications may cause nausea, diarrhea, and a bad taste in your mouth. These side effects are usually not serious, but if they bother you so much that you cannot continue to take the medicine, it is important to call our office right away.
To remember to take each of the medicines in the morning AND evening on the correct day, use this chart to follow along. Put a check mark in the morning box or the evening box every time you take your medicine.
CAVEATS: Drug resistance, previous Tx could skew results
This meta-analysis only included studies performed in Italy, although 2 of the trials did include US recruits. Drug resistance patterns may be different in this country, which could alter sequential therapy’s eradication rates.
Because 3 antibiotics are used in sequential therapy, we may have fewer remaining options for patients who do not respond to this regimen, and we do not have information about patients with previous treatment failures. In addition, this meta-analysis only evaluated H pylori eradication rates in treatment-naïve patients, so we have no information about the effectiveness of this regimen in patients with previous treatment failures.
Would other sequential regimens—or drugs—work?
Patients who are allergic to amoxicillin would not be candidates for this sequential therapy protocol. While sequential therapy was compared with 7- to 10-day standard triple therapy, this study did not compare it with other regimens—eg, quadruple therapy or a 14-day course of standard triple-drug therapy.
Other drugs may also affect outcomes. The sequential therapy studies all used tinidazole, a relatively new agent in the United States; we don’t know whether metronidazole or other imidazoles are as effective.
The authors of this meta-analysis also noted the possibility of publication bias, but we doubt that there are enough unpublished data to invalidate the findings. Also, the selected RCTs only addressed eradication rates and not patient-oriented outcomes. However, most patient-oriented outcomes, including cancer and ulcers, can take years, even decades, to develop.
CHALLENGES TO IMPLEMENTATION: Sequential therapy may be confusing
While adherence rates were similar in the standard and sequential treatment groups in the meta-analysis, sequential therapy—which requires switching medications midway through treatment—might be more confusing for patients in actual practice. This has the potential to negatively affect adherence to the sequential regimen.
It will be important for physicians who prescribe sequential therapy to counsel patients on the importance of completing the treatment regimen exactly as prescribed and to provide clear instructions for doing so, ideally in the form of a patient handout like the one on page 653. Cost is not a problem; the price of sequential therapy is about equal to, or possibly less than, that of standard therapy.5
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
2. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825.
3. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781.
4. Zullo A, De FV, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357.
5. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563.
PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe sequential therapy rather than the standard (concurrent) therapy to improve H pylori eradication rates, particularly in treatment-naïve patients.1
Strength of recommendation
B: Based on a well-done meta-analysis with disease-oriented outcomes
Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
ILLUSTRATIVE CASE
A 40-year-old woman with a peptic ulcer has been diagnosed with Helicobacter pylori infection, and schedules a visit to discuss treatment. You’re aware of the declining eradication rates associated with standard therapy, and have heard that sequential therapy may be a more effective option. Should you offer it to this patient?
An estimated 30% to 40% of the US population is infected with H pylori,2 a bacterium that plays a crucial role in the pathogenesis of peptic ulcer disease, chronic gastritis, and gastric cancer.1 The triple-drug regimen (a proton-pump inhibitor [PPI] and clarithromycin with amoxicillin, tinidazole, or another imidazole) is commonly used to treat H pylori in Europe and in the United States.3 Yet the eradication rate associated with this standard 3-drug regimen in this country is <80%, and appears to be on the decline. The likely problem: the increase in antibiotic-resistant strains of H pylori.2
Put amoxicillin first
In Italy, eradication rates of >90% have been reported with a sequential therapy: a PPI and amoxicillin for 5 days, followed by the PPI, clarithromycin, and tinidazole for an additional 5 days (FIGURE).4 (Because tinidazole is a relatively new drug in the United States and physicians may use other drugs in its class instead, we refer to the drug class—imidazoles—rather than a specific medication in the FIGURE and much of the text that follows.) Using amoxicillin before the other antibiotics weakens bacterial cell walls, preventing the formation of drug efflux channels that can inhibit clarithromycin and other antibiotics, according to 1 theory.1 Thus, clarithromycin and an imidazole are more effective in the second phase of treatment.1
Should US physicians adopt the Italian protocol and prescribe tinidazole? Would metronidazole or other imidazoles be equally effective?
FIGURE
Sequential vs standard therapy: A comparison
STUDY SUMMARY: Sequential therapy is better on all counts
This meta-analysis found 9 randomized controlled trials (RCTs) that compared H pylori eradication rates with standard 7- to 10-day triple-drug therapy to 10-day sequential therapy; an additional small study used a 5-day triple-drug comparison group. The authors performed a thorough search and used standard meta-analysis methods for data synthesis and analysis. The patients were all H pylori treatment naïve and had not used PPIs, histamine-2 receptor antagonists, or antibiotics in the month preceding the study. All patients (n=2747) had documented H pylori infection based on fecal antigen test, histologic evaluation, biopsy urease test, or urea breath test.
All the trials were conducted in Italy, although 2 of the studies included patients from the United States. Nine RCTs compared a triple-therapy regimen with PPI to sequential therapy, 1 RCT compared a triple-drug regimen with ranitidine to sequential therapy, and 1 included only pediatric patients. Pooled eradication rates were 93.4% (95% confidence interval [CI,] 91.3%-95.5%) for sequential therapy and 76.9% (CI, 71.0%-82.8%) for standard therapy, with a relative risk reduction of 71% (CI, 64%-77%).1 The authors estimated that for every 6.3 patients (95% CI, 5.2-7.1) treated with sequential therapy, there would be 1 additional cure compared to standard therapy. Standard 7- to 10-day triple therapy remained inferior to sequential therapy in all subgroup analyses, including patients with risk factors for eradication failure.
Adherence rates were similar in both groups. Sequential therapy resulted in a median adherence rate of 97.4% (range, 90.0%-98.9%), with standard therapy at 96.8% (range, 93.0%-100%).1 Reported side effects were also similar in both treatment groups.1
WHAT’S NEW?: This meta-analysis reduces doubt
The latest American College of Gastroenterology guidelines on the management of H pylori infection acknowledge that sequential therapy has shown promise in Europe, but the organization has not supported a change from the standard regimen to sequential therapy as first-line treatment. The standard triple-therapy regimen is approved by the US Food and Drug Administration, and is the most commonly used H pylori treatment in the United States.1 This is the first meta-analysis based solely on RCTs, and it clearly demonstrates that sequential therapy increased eradication rates.
Helicobacter pylori (called H pylori for short) is a type of bacteria that can cause an ulcer (sore) to develop within the lining of your stomach and intestines. Ulcers can cause pain and bleeding, so it is important to get rid of this bacteria. To do this, you will need to take 4 different medicines over the next 10 days. They must be taken in a certain order, exactly as your doctor has prescribed.
One of the drugs you will be taking (for the entire 10-day period) is a proton pump inhibitor (called a PPI), a medicine that helps reduce the amount of acid in your stomach. ______________ is the name of the PPI your doctor has ordered. Take it twice a day, once in the morning and once in the evening, for 10 days.
You will also take 3 different kinds of antibiotics to eliminate the H pylori bacteria. For the first 5 days, you will take amoxicillin twice a day (2 pills in the morning and 2 in the evening), along with the PPI.
After 5 days, all the amoxicillin pills should be gone, and you will switch to 2 other antibiotics: clarithromycin AND ______________. For the next 5 days, you will take 1 PPI plus 1 clarithromycin and 1 ______________ every morning AND every night.
These medications may cause nausea, diarrhea, and a bad taste in your mouth. These side effects are usually not serious, but if they bother you so much that you cannot continue to take the medicine, it is important to call our office right away.
To remember to take each of the medicines in the morning AND evening on the correct day, use this chart to follow along. Put a check mark in the morning box or the evening box every time you take your medicine.
CAVEATS: Drug resistance, previous Tx could skew results
This meta-analysis only included studies performed in Italy, although 2 of the trials did include US recruits. Drug resistance patterns may be different in this country, which could alter sequential therapy’s eradication rates.
Because 3 antibiotics are used in sequential therapy, we may have fewer remaining options for patients who do not respond to this regimen, and we do not have information about patients with previous treatment failures. In addition, this meta-analysis only evaluated H pylori eradication rates in treatment-naïve patients, so we have no information about the effectiveness of this regimen in patients with previous treatment failures.
Would other sequential regimens—or drugs—work?
Patients who are allergic to amoxicillin would not be candidates for this sequential therapy protocol. While sequential therapy was compared with 7- to 10-day standard triple therapy, this study did not compare it with other regimens—eg, quadruple therapy or a 14-day course of standard triple-drug therapy.
Other drugs may also affect outcomes. The sequential therapy studies all used tinidazole, a relatively new agent in the United States; we don’t know whether metronidazole or other imidazoles are as effective.
The authors of this meta-analysis also noted the possibility of publication bias, but we doubt that there are enough unpublished data to invalidate the findings. Also, the selected RCTs only addressed eradication rates and not patient-oriented outcomes. However, most patient-oriented outcomes, including cancer and ulcers, can take years, even decades, to develop.
CHALLENGES TO IMPLEMENTATION: Sequential therapy may be confusing
While adherence rates were similar in the standard and sequential treatment groups in the meta-analysis, sequential therapy—which requires switching medications midway through treatment—might be more confusing for patients in actual practice. This has the potential to negatively affect adherence to the sequential regimen.
It will be important for physicians who prescribe sequential therapy to counsel patients on the importance of completing the treatment regimen exactly as prescribed and to provide clear instructions for doing so, ideally in the form of a patient handout like the one on page 653. Cost is not a problem; the price of sequential therapy is about equal to, or possibly less than, that of standard therapy.5
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Prescribe sequential therapy rather than the standard (concurrent) therapy to improve H pylori eradication rates, particularly in treatment-naïve patients.1
Strength of recommendation
B: Based on a well-done meta-analysis with disease-oriented outcomes
Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
ILLUSTRATIVE CASE
A 40-year-old woman with a peptic ulcer has been diagnosed with Helicobacter pylori infection, and schedules a visit to discuss treatment. You’re aware of the declining eradication rates associated with standard therapy, and have heard that sequential therapy may be a more effective option. Should you offer it to this patient?
An estimated 30% to 40% of the US population is infected with H pylori,2 a bacterium that plays a crucial role in the pathogenesis of peptic ulcer disease, chronic gastritis, and gastric cancer.1 The triple-drug regimen (a proton-pump inhibitor [PPI] and clarithromycin with amoxicillin, tinidazole, or another imidazole) is commonly used to treat H pylori in Europe and in the United States.3 Yet the eradication rate associated with this standard 3-drug regimen in this country is <80%, and appears to be on the decline. The likely problem: the increase in antibiotic-resistant strains of H pylori.2
Put amoxicillin first
In Italy, eradication rates of >90% have been reported with a sequential therapy: a PPI and amoxicillin for 5 days, followed by the PPI, clarithromycin, and tinidazole for an additional 5 days (FIGURE).4 (Because tinidazole is a relatively new drug in the United States and physicians may use other drugs in its class instead, we refer to the drug class—imidazoles—rather than a specific medication in the FIGURE and much of the text that follows.) Using amoxicillin before the other antibiotics weakens bacterial cell walls, preventing the formation of drug efflux channels that can inhibit clarithromycin and other antibiotics, according to 1 theory.1 Thus, clarithromycin and an imidazole are more effective in the second phase of treatment.1
Should US physicians adopt the Italian protocol and prescribe tinidazole? Would metronidazole or other imidazoles be equally effective?
FIGURE
Sequential vs standard therapy: A comparison
STUDY SUMMARY: Sequential therapy is better on all counts
This meta-analysis found 9 randomized controlled trials (RCTs) that compared H pylori eradication rates with standard 7- to 10-day triple-drug therapy to 10-day sequential therapy; an additional small study used a 5-day triple-drug comparison group. The authors performed a thorough search and used standard meta-analysis methods for data synthesis and analysis. The patients were all H pylori treatment naïve and had not used PPIs, histamine-2 receptor antagonists, or antibiotics in the month preceding the study. All patients (n=2747) had documented H pylori infection based on fecal antigen test, histologic evaluation, biopsy urease test, or urea breath test.
All the trials were conducted in Italy, although 2 of the studies included patients from the United States. Nine RCTs compared a triple-therapy regimen with PPI to sequential therapy, 1 RCT compared a triple-drug regimen with ranitidine to sequential therapy, and 1 included only pediatric patients. Pooled eradication rates were 93.4% (95% confidence interval [CI,] 91.3%-95.5%) for sequential therapy and 76.9% (CI, 71.0%-82.8%) for standard therapy, with a relative risk reduction of 71% (CI, 64%-77%).1 The authors estimated that for every 6.3 patients (95% CI, 5.2-7.1) treated with sequential therapy, there would be 1 additional cure compared to standard therapy. Standard 7- to 10-day triple therapy remained inferior to sequential therapy in all subgroup analyses, including patients with risk factors for eradication failure.
Adherence rates were similar in both groups. Sequential therapy resulted in a median adherence rate of 97.4% (range, 90.0%-98.9%), with standard therapy at 96.8% (range, 93.0%-100%).1 Reported side effects were also similar in both treatment groups.1
WHAT’S NEW?: This meta-analysis reduces doubt
The latest American College of Gastroenterology guidelines on the management of H pylori infection acknowledge that sequential therapy has shown promise in Europe, but the organization has not supported a change from the standard regimen to sequential therapy as first-line treatment. The standard triple-therapy regimen is approved by the US Food and Drug Administration, and is the most commonly used H pylori treatment in the United States.1 This is the first meta-analysis based solely on RCTs, and it clearly demonstrates that sequential therapy increased eradication rates.
Helicobacter pylori (called H pylori for short) is a type of bacteria that can cause an ulcer (sore) to develop within the lining of your stomach and intestines. Ulcers can cause pain and bleeding, so it is important to get rid of this bacteria. To do this, you will need to take 4 different medicines over the next 10 days. They must be taken in a certain order, exactly as your doctor has prescribed.
One of the drugs you will be taking (for the entire 10-day period) is a proton pump inhibitor (called a PPI), a medicine that helps reduce the amount of acid in your stomach. ______________ is the name of the PPI your doctor has ordered. Take it twice a day, once in the morning and once in the evening, for 10 days.
You will also take 3 different kinds of antibiotics to eliminate the H pylori bacteria. For the first 5 days, you will take amoxicillin twice a day (2 pills in the morning and 2 in the evening), along with the PPI.
After 5 days, all the amoxicillin pills should be gone, and you will switch to 2 other antibiotics: clarithromycin AND ______________. For the next 5 days, you will take 1 PPI plus 1 clarithromycin and 1 ______________ every morning AND every night.
These medications may cause nausea, diarrhea, and a bad taste in your mouth. These side effects are usually not serious, but if they bother you so much that you cannot continue to take the medicine, it is important to call our office right away.
To remember to take each of the medicines in the morning AND evening on the correct day, use this chart to follow along. Put a check mark in the morning box or the evening box every time you take your medicine.
CAVEATS: Drug resistance, previous Tx could skew results
This meta-analysis only included studies performed in Italy, although 2 of the trials did include US recruits. Drug resistance patterns may be different in this country, which could alter sequential therapy’s eradication rates.
Because 3 antibiotics are used in sequential therapy, we may have fewer remaining options for patients who do not respond to this regimen, and we do not have information about patients with previous treatment failures. In addition, this meta-analysis only evaluated H pylori eradication rates in treatment-naïve patients, so we have no information about the effectiveness of this regimen in patients with previous treatment failures.
Would other sequential regimens—or drugs—work?
Patients who are allergic to amoxicillin would not be candidates for this sequential therapy protocol. While sequential therapy was compared with 7- to 10-day standard triple therapy, this study did not compare it with other regimens—eg, quadruple therapy or a 14-day course of standard triple-drug therapy.
Other drugs may also affect outcomes. The sequential therapy studies all used tinidazole, a relatively new agent in the United States; we don’t know whether metronidazole or other imidazoles are as effective.
The authors of this meta-analysis also noted the possibility of publication bias, but we doubt that there are enough unpublished data to invalidate the findings. Also, the selected RCTs only addressed eradication rates and not patient-oriented outcomes. However, most patient-oriented outcomes, including cancer and ulcers, can take years, even decades, to develop.
CHALLENGES TO IMPLEMENTATION: Sequential therapy may be confusing
While adherence rates were similar in the standard and sequential treatment groups in the meta-analysis, sequential therapy—which requires switching medications midway through treatment—might be more confusing for patients in actual practice. This has the potential to negatively affect adherence to the sequential regimen.
It will be important for physicians who prescribe sequential therapy to counsel patients on the importance of completing the treatment regimen exactly as prescribed and to provide clear instructions for doing so, ideally in the form of a patient handout like the one on page 653. Cost is not a problem; the price of sequential therapy is about equal to, or possibly less than, that of standard therapy.5
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
2. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825.
3. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781.
4. Zullo A, De FV, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357.
5. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563.
PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931.
2. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825.
3. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781.
4. Zullo A, De FV, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357.
5. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563.
PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Can metformin undo weight gain induced by antipsychotics?
Lack of evidence for weight loss drugs
The most recent guideline on this topic does not recommend any medication, citing a lack of evidence. In its 2003 consensus statement, a panel representing the American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity3 recommends:
- That patients taking second-generation antipsychotics have the following assessments at baseline and regular intervals: weight, height, waist circumference, blood pressure, fasting plasma glucose, and fasting lipids.
- Providing nutrition and exercise counseling to all patients who are over-weight or obese at baseline.
- Initiating treatment with one of the second-generation antipsychotics with a lower risk of weight gain for patients at high risk of diabetes (ie, family history) and for patients who gain 5% or more of their initial weight or develop worsening hyperglycemia or dyslipidemia during treatment.
This guideline does not recommend metformin to reduce weight gain.
A 2007 Cochrane review of interventions to reduce weight gain in patients with schizophrenia included 23 randomized controlled trials of a variety of weight loss interventions, including cognitive/behavioral interventions and a variety of medications, including sibutramine, orlistat, fluoxetine, topiramate, and metformin. The authors highlighted the limited number of studies of short duration and with small sample sizes and concluded that the evidence was insufficient for the use of pharmacologic interventions to prevent or treat weight gain.5
STUDY SUMMARY: Lifestyle changes and metformin compared
This randomized controlled trial was conducted in China and included 128 adults aged 18 to 45 with a first psychotic episode of schizophrenia. All patients had to have gained more than 10% of their pretreatment body weight during the first year of treatment with an antipsychotic medication (clozapine, olanzapine, risperidone, or sulpiride [not approved for use in the United States]). All study participants had to be under the care of an adult caregiver who monitored and recorded food intake, exercise, and medication intake. Patients with diabetes, cardiovascular disease, liver or renal dysfunction, substance abuse, or psychiatric diagnoses other than schizophrenia were excluded.
Patients were randomized to 1 of 4 groups for the 12 weeks of the study:
- Metformin alone, 250 mg 3 times daily
- Placebo alone
- Lifestyle intervention plus metformin
- Lifestyle intervention plus placebo
The lifestyle intervention included 3 components: (1) education: monthly programs on nutrition and physical activity; (2) diet: the American Heart Association step 2 diet (<30% calories from fat, 55% carbohydrates, >15% protein, with at least 15 g fiber per 1000 kcal); and (3) exercise: 1 week of sessions with an exercise physiologist followed by an individualized home-based exercise program.
Primary outcomes included changes in weight, body mass index (BMI), waist circumference, and fasting glucose ( TABLE 2 ). Ten of the 128 randomized patients either discontinued the study or were lost to follow up, but all 128 patients were included in the analysis.
TABLE 2
Mean difference between baseline and endpoint (week 12) of treatment outcomes (95% confidence intervals)1
| LIFESTYLE + METFORMIN | METFORMIN | LIFESTYLE | PLACEBO | |
|---|---|---|---|---|
| Weight, kg | -4.7 (-5.7 to -3.4) | -3.2 (-3.9 to -2.5) | -1.4 (-2.0 to -0.7) | 3.1 (2.4 to 3.8) |
| BMI, kg/m2 | -1.8 (-2.3 to -1.3) | -1.2 (-1.5 to -0.9) | -0.5 (-0.8 to -0.3) | 1.2 (0.9 to 1.5) |
| Waist circumference, cm | -2.0 (-2.4 to -1.5) | -1.3 (-1.5 to -1.1) | 0.1 (-0.5 to 0.7) | 2.2 (1.7 to 2.8) |
| Fasting glucose, mg/dL | -7.2 (-10.8 to -5.4) | -10.8 (-16.2 to -7.2) | -7.2 (-9.0 to -3.6) | 1.8 (-1.8 to 3.6) |
Best result: Lifestyle changes plus metformin
Compared with baseline, weight decreased by 7.3% in the lifestyle plus metformin group, by 4.9% in the metformin-only group, and by 2.2% in the lifestyle-only group; in the placebo group, weight increased by 4.8%.
Participants in all 3 intervention groups also showed significant decreases in the mean fasting glucose, insulin levels, and insulin resistance index (IRI). The insulin levels and the IRI increased in the placebo group.
No significant differences in adverse effects were noted among the 4 treatment groups.1
WHAT’S NEW: Convincing evidence
This is the first randomized controlled trial to show convincingly that metformin alone or in combination with lifestyle changes is superior to lifestyle changes alone or placebo for reducing weight gain and other adverse metabolic outcomes induced by second-generation antipsychotics.
Intensive lifestyle interventions
Prior studies found that intensive lifestyle interventions can help reduce antipsychotic-related weight gain. A 3-month randomized controlled trial compared an early behavioral intervention (dietary counseling, an exercise program, and behavior therapy) with routine care in 61 patients with first-episode psychosis who were taking risperidone, olanzapine, or haloperidol;6 significantly fewer patients assigned to behavioral intervention had an increased initial body weight of more than 7%: 39% in the behavioral intervention group vs 79% in the routine care group (P<.002).
Small samples, small effect sizes
Past studies of metformin for antipsychotic-associated weight gain have generally shown a small benefit, though small sample sizes and small effect sizes prohibited definitive conclusions. Unlike the study by Wu and colleagues,1 none of these past studies were designed to compare the combination of metformin and lifestyle intervention with metformin alone, lifestyle intervention alone, or placebo alone.
Klein et al conducted a randomized placebo-controlled trial of metformin in 39 children ages 10 to 17 whose weight had increased more than 10% on atypical antipsychotic therapy.7 The children treated with placebo gained a mean of 4 kg and increased their mean BMI by 1.12 kg/m2 during 16 weeks of treatment, while those in the metformin group did not gain weight and decreased their mean BMI by 0.43 kg/m2.
Baptista et al randomized 40 in-patients with schizophrenia, who were being switched from conventional antipsychotics to olanzapine, to either metformin (850-1750 mg/d) or placebo. Both groups gained a similar amount of weight after the 14-week study (5.5 vs 6.3 kg, metformin vs placebo). Three patients who started with high fasting glucose had decreases while taking metformin, and 3 patients given placebo developed elevated fasting glucose during the study.8
In another randomized controlled trial of metformin vs placebo in 80 patients who had been taking olanzapine for at least 4 months, Baptista et al found only a small, insignificant difference in weight loss after 12 weeks of treatment (metformin group lost 1.4 kg, placebo group lost 0.18 kg, P=.09). They reported that both groups were highly motivated to lose weight and were compliant with the healthy lifestyle recommendations.9
An adequately powered study
The trial1 highlighted in this PURL had an adequate sample size to compare metformin plus a lifestyle intervention with either treatment alone or placebo. It showed a clinically important effect of metformin both by itself and in conjunction with the lifestyle intervention.
CAVEATS: Consider switching drugs
Before adding metformin to help with weight loss, primary care clinicians should contact the patient’s psychiatrist to discuss the option of switching antipsychotic medications. Switching from a medication with a higher risk for weight gain, such as olanzapine, to one with a lower risk, such as aripiprazole or ziprasidone, can lead to significant weight loss.10
Not an option for some
However, some patients, especially those taking clozapine, may have already tried multiple antipsychotic agents without success, and switching is not an option for them.
Prescribing metformin
CHALLENGES TO IMPLEMENTATION: Adherence
These study participants were under the care of an adult caregiver who monitored and recorded food and medication intake and exercise level. The lifestyle intervention was thorough and structured and this kind of program is often not available to us for our patients. As a consequence, we may not obtain the same results as in this study. However, even the metformin-alone group showed improvements, and if our patients can reliably take their second-generation antipsychotic, they should also be able to take metformin reliably.
Patient resistance
Some patients may resist taking an additional medication to treat the side effects of their antipsychotic medication. Taking the time to educate them about the increased risk of diabetes and cardiovascular disease related to weight gain may help convince them to do so. Warn them about possible gastrointestinal adverse effects of metformin, which tend to lessen or disappear with time.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Wu R-R, Zhao J-P, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185-193.
2. Rowland K, Schumann SA. Have pedometer, will travel. J Fam Pract. 2008;57:90-93.
3. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
4. Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007;68(suppl 1):20-27.
5. Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. 2007;(1):CD005148.-
6. Alvarez-Jiménez M, González-Blanch C, Vázquez-Barquero JL, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naïve first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry. 2006;67:1253-1260.
7. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072-2079.
8. Baptista T, Martínez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192-196.
9. Baptista T, Rangel N, Fernández V, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Res. 2007;93:99-108.
10. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
Lack of evidence for weight loss drugs
The most recent guideline on this topic does not recommend any medication, citing a lack of evidence. In its 2003 consensus statement, a panel representing the American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity3 recommends:
- That patients taking second-generation antipsychotics have the following assessments at baseline and regular intervals: weight, height, waist circumference, blood pressure, fasting plasma glucose, and fasting lipids.
- Providing nutrition and exercise counseling to all patients who are over-weight or obese at baseline.
- Initiating treatment with one of the second-generation antipsychotics with a lower risk of weight gain for patients at high risk of diabetes (ie, family history) and for patients who gain 5% or more of their initial weight or develop worsening hyperglycemia or dyslipidemia during treatment.
This guideline does not recommend metformin to reduce weight gain.
A 2007 Cochrane review of interventions to reduce weight gain in patients with schizophrenia included 23 randomized controlled trials of a variety of weight loss interventions, including cognitive/behavioral interventions and a variety of medications, including sibutramine, orlistat, fluoxetine, topiramate, and metformin. The authors highlighted the limited number of studies of short duration and with small sample sizes and concluded that the evidence was insufficient for the use of pharmacologic interventions to prevent or treat weight gain.5
STUDY SUMMARY: Lifestyle changes and metformin compared
This randomized controlled trial was conducted in China and included 128 adults aged 18 to 45 with a first psychotic episode of schizophrenia. All patients had to have gained more than 10% of their pretreatment body weight during the first year of treatment with an antipsychotic medication (clozapine, olanzapine, risperidone, or sulpiride [not approved for use in the United States]). All study participants had to be under the care of an adult caregiver who monitored and recorded food intake, exercise, and medication intake. Patients with diabetes, cardiovascular disease, liver or renal dysfunction, substance abuse, or psychiatric diagnoses other than schizophrenia were excluded.
Patients were randomized to 1 of 4 groups for the 12 weeks of the study:
- Metformin alone, 250 mg 3 times daily
- Placebo alone
- Lifestyle intervention plus metformin
- Lifestyle intervention plus placebo
The lifestyle intervention included 3 components: (1) education: monthly programs on nutrition and physical activity; (2) diet: the American Heart Association step 2 diet (<30% calories from fat, 55% carbohydrates, >15% protein, with at least 15 g fiber per 1000 kcal); and (3) exercise: 1 week of sessions with an exercise physiologist followed by an individualized home-based exercise program.
Primary outcomes included changes in weight, body mass index (BMI), waist circumference, and fasting glucose ( TABLE 2 ). Ten of the 128 randomized patients either discontinued the study or were lost to follow up, but all 128 patients were included in the analysis.
TABLE 2
Mean difference between baseline and endpoint (week 12) of treatment outcomes (95% confidence intervals)1
| LIFESTYLE + METFORMIN | METFORMIN | LIFESTYLE | PLACEBO | |
|---|---|---|---|---|
| Weight, kg | -4.7 (-5.7 to -3.4) | -3.2 (-3.9 to -2.5) | -1.4 (-2.0 to -0.7) | 3.1 (2.4 to 3.8) |
| BMI, kg/m2 | -1.8 (-2.3 to -1.3) | -1.2 (-1.5 to -0.9) | -0.5 (-0.8 to -0.3) | 1.2 (0.9 to 1.5) |
| Waist circumference, cm | -2.0 (-2.4 to -1.5) | -1.3 (-1.5 to -1.1) | 0.1 (-0.5 to 0.7) | 2.2 (1.7 to 2.8) |
| Fasting glucose, mg/dL | -7.2 (-10.8 to -5.4) | -10.8 (-16.2 to -7.2) | -7.2 (-9.0 to -3.6) | 1.8 (-1.8 to 3.6) |
Best result: Lifestyle changes plus metformin
Compared with baseline, weight decreased by 7.3% in the lifestyle plus metformin group, by 4.9% in the metformin-only group, and by 2.2% in the lifestyle-only group; in the placebo group, weight increased by 4.8%.
Participants in all 3 intervention groups also showed significant decreases in the mean fasting glucose, insulin levels, and insulin resistance index (IRI). The insulin levels and the IRI increased in the placebo group.
No significant differences in adverse effects were noted among the 4 treatment groups.1
WHAT’S NEW: Convincing evidence
This is the first randomized controlled trial to show convincingly that metformin alone or in combination with lifestyle changes is superior to lifestyle changes alone or placebo for reducing weight gain and other adverse metabolic outcomes induced by second-generation antipsychotics.
Intensive lifestyle interventions
Prior studies found that intensive lifestyle interventions can help reduce antipsychotic-related weight gain. A 3-month randomized controlled trial compared an early behavioral intervention (dietary counseling, an exercise program, and behavior therapy) with routine care in 61 patients with first-episode psychosis who were taking risperidone, olanzapine, or haloperidol;6 significantly fewer patients assigned to behavioral intervention had an increased initial body weight of more than 7%: 39% in the behavioral intervention group vs 79% in the routine care group (P<.002).
Small samples, small effect sizes
Past studies of metformin for antipsychotic-associated weight gain have generally shown a small benefit, though small sample sizes and small effect sizes prohibited definitive conclusions. Unlike the study by Wu and colleagues,1 none of these past studies were designed to compare the combination of metformin and lifestyle intervention with metformin alone, lifestyle intervention alone, or placebo alone.
Klein et al conducted a randomized placebo-controlled trial of metformin in 39 children ages 10 to 17 whose weight had increased more than 10% on atypical antipsychotic therapy.7 The children treated with placebo gained a mean of 4 kg and increased their mean BMI by 1.12 kg/m2 during 16 weeks of treatment, while those in the metformin group did not gain weight and decreased their mean BMI by 0.43 kg/m2.
Baptista et al randomized 40 in-patients with schizophrenia, who were being switched from conventional antipsychotics to olanzapine, to either metformin (850-1750 mg/d) or placebo. Both groups gained a similar amount of weight after the 14-week study (5.5 vs 6.3 kg, metformin vs placebo). Three patients who started with high fasting glucose had decreases while taking metformin, and 3 patients given placebo developed elevated fasting glucose during the study.8
In another randomized controlled trial of metformin vs placebo in 80 patients who had been taking olanzapine for at least 4 months, Baptista et al found only a small, insignificant difference in weight loss after 12 weeks of treatment (metformin group lost 1.4 kg, placebo group lost 0.18 kg, P=.09). They reported that both groups were highly motivated to lose weight and were compliant with the healthy lifestyle recommendations.9
An adequately powered study
The trial1 highlighted in this PURL had an adequate sample size to compare metformin plus a lifestyle intervention with either treatment alone or placebo. It showed a clinically important effect of metformin both by itself and in conjunction with the lifestyle intervention.
CAVEATS: Consider switching drugs
Before adding metformin to help with weight loss, primary care clinicians should contact the patient’s psychiatrist to discuss the option of switching antipsychotic medications. Switching from a medication with a higher risk for weight gain, such as olanzapine, to one with a lower risk, such as aripiprazole or ziprasidone, can lead to significant weight loss.10
Not an option for some
However, some patients, especially those taking clozapine, may have already tried multiple antipsychotic agents without success, and switching is not an option for them.
Prescribing metformin
CHALLENGES TO IMPLEMENTATION: Adherence
These study participants were under the care of an adult caregiver who monitored and recorded food and medication intake and exercise level. The lifestyle intervention was thorough and structured and this kind of program is often not available to us for our patients. As a consequence, we may not obtain the same results as in this study. However, even the metformin-alone group showed improvements, and if our patients can reliably take their second-generation antipsychotic, they should also be able to take metformin reliably.
Patient resistance
Some patients may resist taking an additional medication to treat the side effects of their antipsychotic medication. Taking the time to educate them about the increased risk of diabetes and cardiovascular disease related to weight gain may help convince them to do so. Warn them about possible gastrointestinal adverse effects of metformin, which tend to lessen or disappear with time.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Lack of evidence for weight loss drugs
The most recent guideline on this topic does not recommend any medication, citing a lack of evidence. In its 2003 consensus statement, a panel representing the American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity3 recommends:
- That patients taking second-generation antipsychotics have the following assessments at baseline and regular intervals: weight, height, waist circumference, blood pressure, fasting plasma glucose, and fasting lipids.
- Providing nutrition and exercise counseling to all patients who are over-weight or obese at baseline.
- Initiating treatment with one of the second-generation antipsychotics with a lower risk of weight gain for patients at high risk of diabetes (ie, family history) and for patients who gain 5% or more of their initial weight or develop worsening hyperglycemia or dyslipidemia during treatment.
This guideline does not recommend metformin to reduce weight gain.
A 2007 Cochrane review of interventions to reduce weight gain in patients with schizophrenia included 23 randomized controlled trials of a variety of weight loss interventions, including cognitive/behavioral interventions and a variety of medications, including sibutramine, orlistat, fluoxetine, topiramate, and metformin. The authors highlighted the limited number of studies of short duration and with small sample sizes and concluded that the evidence was insufficient for the use of pharmacologic interventions to prevent or treat weight gain.5
STUDY SUMMARY: Lifestyle changes and metformin compared
This randomized controlled trial was conducted in China and included 128 adults aged 18 to 45 with a first psychotic episode of schizophrenia. All patients had to have gained more than 10% of their pretreatment body weight during the first year of treatment with an antipsychotic medication (clozapine, olanzapine, risperidone, or sulpiride [not approved for use in the United States]). All study participants had to be under the care of an adult caregiver who monitored and recorded food intake, exercise, and medication intake. Patients with diabetes, cardiovascular disease, liver or renal dysfunction, substance abuse, or psychiatric diagnoses other than schizophrenia were excluded.
Patients were randomized to 1 of 4 groups for the 12 weeks of the study:
- Metformin alone, 250 mg 3 times daily
- Placebo alone
- Lifestyle intervention plus metformin
- Lifestyle intervention plus placebo
The lifestyle intervention included 3 components: (1) education: monthly programs on nutrition and physical activity; (2) diet: the American Heart Association step 2 diet (<30% calories from fat, 55% carbohydrates, >15% protein, with at least 15 g fiber per 1000 kcal); and (3) exercise: 1 week of sessions with an exercise physiologist followed by an individualized home-based exercise program.
Primary outcomes included changes in weight, body mass index (BMI), waist circumference, and fasting glucose ( TABLE 2 ). Ten of the 128 randomized patients either discontinued the study or were lost to follow up, but all 128 patients were included in the analysis.
TABLE 2
Mean difference between baseline and endpoint (week 12) of treatment outcomes (95% confidence intervals)1
| LIFESTYLE + METFORMIN | METFORMIN | LIFESTYLE | PLACEBO | |
|---|---|---|---|---|
| Weight, kg | -4.7 (-5.7 to -3.4) | -3.2 (-3.9 to -2.5) | -1.4 (-2.0 to -0.7) | 3.1 (2.4 to 3.8) |
| BMI, kg/m2 | -1.8 (-2.3 to -1.3) | -1.2 (-1.5 to -0.9) | -0.5 (-0.8 to -0.3) | 1.2 (0.9 to 1.5) |
| Waist circumference, cm | -2.0 (-2.4 to -1.5) | -1.3 (-1.5 to -1.1) | 0.1 (-0.5 to 0.7) | 2.2 (1.7 to 2.8) |
| Fasting glucose, mg/dL | -7.2 (-10.8 to -5.4) | -10.8 (-16.2 to -7.2) | -7.2 (-9.0 to -3.6) | 1.8 (-1.8 to 3.6) |
Best result: Lifestyle changes plus metformin
Compared with baseline, weight decreased by 7.3% in the lifestyle plus metformin group, by 4.9% in the metformin-only group, and by 2.2% in the lifestyle-only group; in the placebo group, weight increased by 4.8%.
Participants in all 3 intervention groups also showed significant decreases in the mean fasting glucose, insulin levels, and insulin resistance index (IRI). The insulin levels and the IRI increased in the placebo group.
No significant differences in adverse effects were noted among the 4 treatment groups.1
WHAT’S NEW: Convincing evidence
This is the first randomized controlled trial to show convincingly that metformin alone or in combination with lifestyle changes is superior to lifestyle changes alone or placebo for reducing weight gain and other adverse metabolic outcomes induced by second-generation antipsychotics.
Intensive lifestyle interventions
Prior studies found that intensive lifestyle interventions can help reduce antipsychotic-related weight gain. A 3-month randomized controlled trial compared an early behavioral intervention (dietary counseling, an exercise program, and behavior therapy) with routine care in 61 patients with first-episode psychosis who were taking risperidone, olanzapine, or haloperidol;6 significantly fewer patients assigned to behavioral intervention had an increased initial body weight of more than 7%: 39% in the behavioral intervention group vs 79% in the routine care group (P<.002).
Small samples, small effect sizes
Past studies of metformin for antipsychotic-associated weight gain have generally shown a small benefit, though small sample sizes and small effect sizes prohibited definitive conclusions. Unlike the study by Wu and colleagues,1 none of these past studies were designed to compare the combination of metformin and lifestyle intervention with metformin alone, lifestyle intervention alone, or placebo alone.
Klein et al conducted a randomized placebo-controlled trial of metformin in 39 children ages 10 to 17 whose weight had increased more than 10% on atypical antipsychotic therapy.7 The children treated with placebo gained a mean of 4 kg and increased their mean BMI by 1.12 kg/m2 during 16 weeks of treatment, while those in the metformin group did not gain weight and decreased their mean BMI by 0.43 kg/m2.
Baptista et al randomized 40 in-patients with schizophrenia, who were being switched from conventional antipsychotics to olanzapine, to either metformin (850-1750 mg/d) or placebo. Both groups gained a similar amount of weight after the 14-week study (5.5 vs 6.3 kg, metformin vs placebo). Three patients who started with high fasting glucose had decreases while taking metformin, and 3 patients given placebo developed elevated fasting glucose during the study.8
In another randomized controlled trial of metformin vs placebo in 80 patients who had been taking olanzapine for at least 4 months, Baptista et al found only a small, insignificant difference in weight loss after 12 weeks of treatment (metformin group lost 1.4 kg, placebo group lost 0.18 kg, P=.09). They reported that both groups were highly motivated to lose weight and were compliant with the healthy lifestyle recommendations.9
An adequately powered study
The trial1 highlighted in this PURL had an adequate sample size to compare metformin plus a lifestyle intervention with either treatment alone or placebo. It showed a clinically important effect of metformin both by itself and in conjunction with the lifestyle intervention.
CAVEATS: Consider switching drugs
Before adding metformin to help with weight loss, primary care clinicians should contact the patient’s psychiatrist to discuss the option of switching antipsychotic medications. Switching from a medication with a higher risk for weight gain, such as olanzapine, to one with a lower risk, such as aripiprazole or ziprasidone, can lead to significant weight loss.10
Not an option for some
However, some patients, especially those taking clozapine, may have already tried multiple antipsychotic agents without success, and switching is not an option for them.
Prescribing metformin
CHALLENGES TO IMPLEMENTATION: Adherence
These study participants were under the care of an adult caregiver who monitored and recorded food and medication intake and exercise level. The lifestyle intervention was thorough and structured and this kind of program is often not available to us for our patients. As a consequence, we may not obtain the same results as in this study. However, even the metformin-alone group showed improvements, and if our patients can reliably take their second-generation antipsychotic, they should also be able to take metformin reliably.
Patient resistance
Some patients may resist taking an additional medication to treat the side effects of their antipsychotic medication. Taking the time to educate them about the increased risk of diabetes and cardiovascular disease related to weight gain may help convince them to do so. Warn them about possible gastrointestinal adverse effects of metformin, which tend to lessen or disappear with time.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Wu R-R, Zhao J-P, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185-193.
2. Rowland K, Schumann SA. Have pedometer, will travel. J Fam Pract. 2008;57:90-93.
3. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
4. Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007;68(suppl 1):20-27.
5. Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. 2007;(1):CD005148.-
6. Alvarez-Jiménez M, González-Blanch C, Vázquez-Barquero JL, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naïve first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry. 2006;67:1253-1260.
7. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072-2079.
8. Baptista T, Martínez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192-196.
9. Baptista T, Rangel N, Fernández V, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Res. 2007;93:99-108.
10. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
1. Wu R-R, Zhao J-P, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185-193.
2. Rowland K, Schumann SA. Have pedometer, will travel. J Fam Pract. 2008;57:90-93.
3. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
4. Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007;68(suppl 1):20-27.
5. Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. 2007;(1):CD005148.-
6. Alvarez-Jiménez M, González-Blanch C, Vázquez-Barquero JL, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naïve first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry. 2006;67:1253-1260.
7. Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072-2079.
8. Baptista T, Martínez J, Lacruz A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192-196.
9. Baptista T, Rangel N, Fernández V, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Res. 2007;93:99-108.
10. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Patients insist on antibiotics for sinusitis? Here is a good reason to say “no”
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
ILLUSTRATIVE CASE
A 23-year-old woman presents to your office with a 1-week history of cough, purulent nasal discharge, and unilateral facial pain. You diagnose acute sinusitis.
Should you prescribe an antibiotic?
No. Yet it’s no wonder that most adults treated for acute sinusitis leave the doctor’s office with a prescription for antibiotics. Until the publication of the meta-analysis by Young and colleagues1 featured in this PURL, we have lacked A-level evidence from studies conducted in realistic settings—like your practice and ours.
Review of serial data from the National Ambulatory Medical Care Surveys (NAMCS) from 1999 through 2005 does show a slight downward trend in antibiotic prescribing for acute sinusitis: 1999-2002 data showed that 83% of cases of acute sinusitis were treated with an antibiotic.2 Data from the 2004 and 2005 NAMCS reveal that family physicians prescribed antibiotics for 80% of patients with acute sinusitis in 2004 and 76% of patients in 2005 (S. Medvedev, unpublished data, NAMCS database, March 2008).
Is this continued high rate of antibiotic prescribing justified?
Do antibiotics improve symptoms and shorten the duration of illness or not?
These questions are important, obviously, not only because of the high rate of prescribing but also because sinusitis is one of the most common diagnoses: approximately 20 million cases annually in the United States, or about 21% of all outpatient antibiotic prescriptions for adults.2
Which patients might benefit from antibiotics?
Common clinical signs and symptoms cannot identify patients with rhinosinusitis for whom treatment is clearly justified, given the cost, adverse events, and bacterial resistance associated with antibiotic use
- Severity of symptoms is important only in that signs suggestive of a serious complication are the sole reason for immediate antibiotic treatment
- Purulent discharge noted in the pharynx on exam was associated with a higher likelihood of benefit from antibiotics, but NNT was 8
- Antibiotics are not justified even if a patient reports having symptoms for longer than 7-10 days
Source: Young et al.1
A diagnostic dilemma
Before we discuss the evidence that is summarized in the excellent meta-analysis by Young and colleagues,1 let’s acknowledge that acute sinusitis is undeniably a diagnostic dilemma. Distinguishing bacterial from viral infection is nearly impossible on clinical grounds because the symptoms are so similar. A litany of identical upper respiratory symptoms accompanies both viral and bacterial sinus infections. Purulent nasal secretions, maxillary facial pain (especially with unilateral predominance), maxillary tooth pain (which is uncommon with sinus infection), altered sense of smell, and worsening illness after improvement constitute the short list of signs and symptoms that has some predictive value, but even the presence of all of these is not a terrific predictor of bacterial sinus infection. Plain x-rays have low accuracy in distinguishing viral from bacterial infection. Computed tomography (CT) sinus scans are better but far from perfect, are not readily available in practice, and are expensive.
Sinusitis in the real world
How effective are antibiotics for patients diagnosed not by sinus x-rays or CTs, but by signs and symptoms—as we typically do in daily practice?
A meta-analysis3 of 13 randomized controlled trials (RCTs) found that sinusitis improved without antibiotics, but it included trials in which patients were recruited based on results of imaging studies and cultures, which are not normally used in primary care clinical practice. That study compared antibiotic treatment to placebo for acute uncomplicated sinusitis; 35% of placebo-treated patients were clinically cured by 7 to 12 days and 73% were improved after 7 days. Antibiotic therapy increased cure rates by 15% and improvement rates by 14%, yielding a number needed to treat of 7 to achieve 1 additional positive outcome at 7 days.
STUDY SUMMARY: Meta-analysis of primary care trials
Young and colleagues1 aggregated and analyzed individual patient-level data from all known placebo-controlled, randomized, antibiotic treatment trials of adults with clinical symptoms of acute sinusitis that were conducted in primary care settings. They excluded trials that used imaging or bacterial culture as part of patient recruitment.
Studies were included that allowed the use of concomitant medication such as nonsteroidal anti-inflammatory drugs, decongestants, or nasal steroids, as long as patients in both groups had access to the same medications. All trials excluded patients with severe symptoms such as high fever, periorbital swelling or erythema, or intense facial pain, important exclusions that we will discuss below.
They identified 10 such studies and completed an intent-to-treat analysis of the 9 double-blind trials for which patient level data were available. Using individual data from 2547 patients, the odds ratio for an overall antibiotic treatment effect was 1.37 (95% confidence interval, 1.13-1.66), with a number needed to treat (NNT) of 15.
This finding means that 15 patients needed to be given an antibiotic for 1 additional patient to be cured at 8 to 15 days after treatment commenced. Using statistical modeling, they determined that 64% of patients treated with placebo were cured at 14 days compared with 70% given an antibiotic. One patient out of 1381 treated with placebo experienced a serious complication, a brain abscess.
Do antibiotics benefit any subgroups?
The investigators also analyzed the prognostic value of specific signs and symptoms to answer the question: Is there any subgroup of patients who might benefit more from antibiotic treatment?
Duration. Patients with a longer duration of symptoms, more severe symptoms, or increased age took longer to cure, but were no more likely to benefit from antibiotic treatment than other patients.
Symptoms, such as a previous common cold, pain on bending, unilateral facial pain, tooth pain, and purulent nasal discharge did not have any prognostic value.
Only one sign—purulent discharge noted in the pharynx on examination—was associated with a higher likelihood of benefit from treatment with antibiotics, but the NNT was still 8 in this group. Patients with symptoms for 7 days or longer were no more likely to respond to antibiotics than those with symptoms for fewer than 7 days.1
WHAT’S NEW: Realistic evidence from realistic settings
We believe this meta-analysis provides a high level of evidence against routine treatment of sinusitis with antibiotics in primary care practice. Treating 15 patients with an antibiotic to possibly benefit 1 patient 2 weeks after treatment commences does not seem like a good idea when one considers the cost and complications of antibiotic use. Diarrhea and other adverse outcomes are 80% more common among patients with sinusitis who are treated with an antibiotic compared with placebo.3 As noted above, prior meta-analyses of antibiotic treatment for acute sinusitis have been more encouraging than this meta-analysis, with a number needed to treat of 7, but those meta-analyses are clearly overly optimistic for the results one will achieve in primary care practice using clinical signs and symptoms to diagnose acute sinusitis.3,4 Unlike the Young study, they included trials in specialty clinics with CT scans and sinus puncture and culture used for the diagnostic standard.
Symptoms >1 week are not a reason to prescribe
One very important new finding in this meta-analysis that should change practice is that the duration of illness did not predict a positive response to antibiotics.
Current national recommendations are to use an antibiotic for patients with a duration of illness longer than 1 week, as these patients are presumably more likely to have a bacterial infection.5-7 However, that recommendation had been based on expert opinion, not on data from clinical trials. A longer duration of symptoms should not be a reason to prescribe an antibiotic for sinusitis symptoms.
How can you help your patient?
What to do, then, for patients with acute sinusitis? Treat the symptoms, which means recommending pain medication for facial pain or headache and saline nasal spray for the nasal discharge, not antibiotics or nasal corticosteroids. Side effects will be fewer and costs will be lower.
- Saline irrigation. A 2007 Cochrane review of 8 chronic and recurrent sinusitis trials showed that nasal saline irrigation is effective for reducing symptoms of chronic and recurrent sinusitis.8 Although we do not have high-quality RCT data on saline nasal irrigation for treatment of acute sinusitis, nasal saline irrigation is harmless and inexpensive.
- What about nasal steroids? The evidence is equivocal, and the most recent high-quality RCT of nasal steroids showed no effect.9
CAVEATS: Refer seriously ill patients and complicated cases
A very important caveat to our recommendation is that seriously ill patients must be managed differently. Very infrequently a patient develops a serious complication of acute sinusitis such as brain abscess, periorbital cellulitis, or meningitis. Therefore, seriously ill patients with signs and symptoms of acute bacterial sinusitis, such as high fever, periorbital erythema or edema, severe headache, or intense facial pain must be carefully evaluated and treated with great caution and close follow-up. These patients should be referred immediately for consultation with an otolaryngologist.
Of course, mildly ill patients today may become quite ill tomorrow, so always provide advice to patients to return if they are getting worse, a good clinical practice for any condition that is usually benign but occasionally serious.
Patients who have prolonged or recurrent sinusitis symptoms need further evaluation for other diagnoses such as allergies, cystic fibrosis, fungal sinus infection, and other illnesses associated with immune compromise. These complicated patients benefit from consultation with an otolaryngologist who has a specific interest in chronic and recurrent sinusitis, and perhaps consultation from an infectious disease specialist as well.
CHALLENGES TO IMPLEMENTATION: The patient who wants a pill
Some patients may be accustomed to receiving an antibiotic prescription for their “sinus infections” and may resist conservative management. It may be difficult to convince them that antibiotics won’t make a difference when they attribute past resolution of symptoms to antibiotics.
Take enough time to educate your patients on the natural course of illness, the positive benefits of nasal saline, and the reasons not to use unnecessary antibiotics (eg, they are not effective, have potential adverse effects, and can contribute to future antibiotic resistance); this effort will save you time in future visits.10 A “just in case you don’t get better” prescription to be filled only if the patient is not improving in the next few days is about 50% effective in reducing antibiotic usage for upper respiratory infections.11
Acknowledgement
We acknowledge Sofia Medvedev of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the National Ambulatory Medical Care Survey data.
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
2. Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999-2002. Arch Otolaryngol Head Neck Surg. 2007;133:260-265.
3. Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S32-S45.
4. Williams JW, Jr, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2003;(2):CD000243.-
5. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. For the American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control; Infectious Diseases Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498-505.
6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
7. Anon JB, Jacobs MR, Poole MD, et al. For the Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 suppl):S1-S45.
8. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394.-
9. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
10. Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ. 1997;315:350-352.
11. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract. 2003;53:871-877.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Use anesthetic drops to relieve acute otitis media pain
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
ILLUSTRATIVE CASE
A mother brings her 3-year-old son to your office first thing in the morning. The boy has fever and right ear pain. You see that the tympanic membrane is dull, red, and bulging. The mother has been up most of the night with her child. She implores you to “do something.” She is exhausted and her son is crying and holding his right ear. You know that antibiotics will not provide immediate pain relief and oral analgesics will take a while to help. What can you offer that will help right away?
Until this study, we’ve had only 1 placebo-controlled trial to guide how we manage a big problem. Big in terms of distress to parents and children, and in sheer numbers—acute otitis media (AOM) is extremely common in children.2,3
Routine antibiotics: Woeful lack of evidence
Even when antibiotics are indicated, pain relief is minimal and takes several days.4-8 Despite “a woeful lack of substantial evidence on the question of antibiotic therapy” for AOM, it is the most common reason for the prescription of antibiotics in children.4,6 A Cochrane review showed that antibiotics have no effect on recurrence of AOM or complications, including hearing impairment.5 The same review showed no pain reduction in 24 hours and only 30% pain reduction in 2 to 7 days with antibiotic use.5 Antibiotics clearly have a minimal role in providing pain relief for AOM.
Oral analgesics too slow
Oral analgesic use in AOM has been studied and has shown good results. We calculated the number needed to treat (1.0 divided by the absolute risk reduction) for both ibuprofen (number needed to treat [NNT]=5) and acetaminophen (NNT=6) from data in the 1996 trial by Bertin et al.9
It is a common practice in the United States to treat AOM with oral analgesics. However, the onset of pain relief with oral medications can be slow and the relief is generally not complete, so oral medications are not immediately helpful to meet the needs of our crying 3-year-old patient and his exhausted mother.
Topical anesthetics
To our knowledge, prior evidence on the efficacy of topical anesthetics is limited to 1 placebo-controlled trial. A randomized trial by Hoberman et al,10 with 54 subjects, showed a statistically significant 25% reduction in pain with the analgesic drops Auralgan (containing antipyrine, benzocaine, and glycerine) at 30 minutes when compared with olive oil. A 2006 Cochrane review11 did not include the Bolt et al trial1 described in this PURL, but did include the Hoberman trial10 and 3 trials that compared a topical anesthetic with naturopathic herbal ear drops for AOM pain, and the review concluded that evidence was insufficient.
Topical anesthetic plus oraI analgesia for earache relief
Bolt P et al.1
STUDY SUMMARY: Pain measured by visual analogue, Bieri faces scales
This double-blind, randomized, placebo-controlled trial1 compared aqueous lidocaine 2% drops with saline drops in the ear, for reducing pain due to AOM in patients 3 to 17 years of age. The trial was conducted at an Australian children’s hospital emergency room. The study evaluated “lignocaine,” the name for lidocaine in Australia.
Emergency physicians instilled 3 drops of either lidocaine or saline into the affected ear in the 2 groups (n=31 in the study group and n=32 in the placebo group). Patients, parents, treating physicians, and staff administering ear drops and assessing pain were blinded to group assignment. Doctors measured pain at baseline and after 30 minutes and patients measured pain at baseline, 10, 20, and 30 minutes after the drops were instilled, using the Bieri faces pain scale and a visual analogue scale.12
Lidocaine reduced pain scores by 50% from baseline at 10 and 30 minutes compared with saline. No serious side effects were noted at 30 minutes, although 3 patients in the lidocaine group complained of mild dizziness the next day. The treating physician prescribed paracetamol (equivalent to acetaminophen) for participants in both the lidocaine and the placebo group at their discretion. The proportion given paracetamol was similar in both groups.
WHAT’S NEW: Pain relief is immediate
Family physicians have used topical anethestics for otitis externa for decades. This RCT adds evidence that topical anesthetics are useful for providing immediate pain relief from AOM, as well. The 2004 guidelines from the American Academy of Pediatrics and the American Academy of Family Physicians indicate that management of AOM should include the assessment and treatment of pain.
We think that topical agents such as lidocaine and benzocaine are useful adjuncts to oral analgesics in providing immediate pain relief, especially in the face of evidence showing that antibiotics do not offer significant pain relief.
Previous studies have shown that aqueous lidocaine is ineffective on uninflamed tympanic membrane.13 The results of this study are consistent with increased uptake of the drug through the inflamed tympanic membrane.
CAVEATS: Children >3 years studied
This trial included only children older than 3 years, so the results may not apply to younger children and infants.
This essentially was a study of ear pain treatment, which, in our view, does not detract from the clinical usefulness of the findings. Topical anesthetics seem to be useful for ear pain in general.
Concurrent analgesics
Some variability existed in the oral analgesics the children received, as these agents were given at the discretion of the parents and treating physicians. We think that this does not detract from the study findings, as it represents a practical, real-world setting, which is desirable in an effectiveness RCT. Moreover, the extent of pain reduction was over and above that conferred by analgesic administration.
CHALLENGES TO IMPLEMENTATION: Lidocaine is not sold in a dropper
Aqueous lidocaine is not sold in a bottle with a dropper: our search on www.drugstore.com and www.drugs.com did not yield an otic preparation with only aqueous lidocaine. However, 2% injectable lidocaine, which is readily available, can be used with a dropper. Aqueous lidocaine can be compounded at a compounding pharmacy and placed in a bottle with a dropper.
Benzocaine might be a suitable substitute
In preparing this PURL, we learned from several colleagues that they always keep a bottle of benzocaine in their pocket and they apply the drops to the ears of their pediatric patients with AOM when they see them in their offices or in the emergency department.
Benzocaine is available in various brand names (eg, Auralgan, Americaine, A/B otic drops, among others). These are oil suspensions and not an aqueous solution as used in this study, so it would be important not to use these preparations in the presence of a ruptured tympanic membrane because the oil suspension may not be absorbed. The aqueous solution will be absorbed so it can be used even if a ruptured tympanic membrane is suspected.
In most situations rupture of the tympanic membrane gives immediate pain relief. However, in some, the pain persists and it may be difficult to tell if the tympanic membrane has ruptured or the debris and pus is from otitis externa. In those situations, the aqueous lidocaine solution might be preferred.
Should parents give drops at home?
This study does not address home use of these drops. However, benzocaine preparations have been used safely for many years for otitis externa at home, and we cannot think of any reason why drops could not be prescribed for home use in acute otitis media.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
1. Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child. 2008;93:40-44.
2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
3. Arnold J. Otitis media and its complications. In: Nelson W, Behrman R, Kliegman R, Arvin A, eds. Nelson Textbook of Pediatrics. 15th ed. Philadelphia: Saunders; 1996:1814-1824.
4. Chan LS, Takata GS, Shekelle P, Morton SC, Mason W, Marcy SM. Evidence assessment of management of acute otitis media: II. Research gaps and priorities for future research. Pediatrics. 2001;108:248-254.
5. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2004;(1):CD000219.
6. Hendley JO. Clinical practice. Otitis media. N Engl J Med. 2002;347:1169-1174.
7. Rovers MM, Glasziou P, Appelman CL, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet. 2006;368:1429-1435.
8. Gasper K, St Anna L, Montgomery L. Clinical inquiries. Are antibiotics effective for otitis media with effusion? J Fam Pract. 2003;52:321-323.
9. Bertin L, Pons G, d’Athis P, et al. A randomized, double-blind, multicentre controlled trial of ibuprofen versus acetaminophen and placebo for symptoms of acute otitis media in children. Fundam Clin Pharmacol. 1996;10:387-392.
10. Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med. 1997;151:675-678.
11. Cochrane Database Syst Rev 2006 Jul 19;3: CD005657.
12. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173-183.
13. Moller A, Grontved A. Topical anaesthesia of the normal tympanic membrane: a controlled clinical trial of different suspensions of lidocaine. ORL J Otorhinolaryngol Relat Spec. 1990;52:168-173.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
New PURLs® INSTANT POLL: Tell us what works in daily practice
Do you use anesthetic drops for immediate relief of pain in children with acute otitis media, as we advise in the Priority Update from the Research Literature (PURL), page 370? Do colleagues like you around the country do so?
You can find out in a few seconds, at www.jfponline.com. Starting this month, our PURLs Instant Poll—a Reality Check on the Research Literature, lets you join an online dialogue about practice changes recommended in the PURLs series in JFP.
We have surveyed thousands of research studies in the past 8 months, with input from our “Reality Checkers” listserv and colleagues around the country, to help us figure out which studies are relevant, valid, practice changing, and usable. Our small group at the University of Chicago culled a dozen studies that met those criteria. Some of the questions that arose from these studies were: Do you use tamsulosin to help pass ureteral stones? …vitamin C to prevent pain syndrome after fracture?… more thiazides and fewer beta-blockers for hypertension in the elderly—even those with diabetes and metabolic syndrome? … zoledronic acid to prevent osteoporotic fracture? If so, why? If not, why not?
There is more to adopting a new practice than reading a study. Clear reasons (cost, insurance, patient factors) sometimes support a decision to maintain the status quo. But more often, we’ve discovered in our PURL-diving thus far, the reason is that we are unaware of the new evidence or unconvinced of its usefulness.
“Show me”
Diffusion theory suggests that we doctors will not adopt a practice change until we are convinced that our patients will benefit. Like those of us from Missouri, most doctors prudently think, “show me.” We have to be convinced that the science is good and that it is relevant to our patients, but we also have to be convinced that in the reality where practicing doctors and patients live, it works, it is acceptable, and it can actually be implemented.
We want to facilitate practice changes that will benefit patients. But one must know what the current practice is to determine if a study will change practice. We hope that we can all learn from each other about what works and makes sense for our practices and patients, and what does not.
By participating in the online PURLs Instant Poll, you can take part in selecting, adopting, and disseminating worthwhile new practice recommendations.
Please join us in defining what makes sense in the reality of family practice by logging in at www.jfponline.com, and responding to PURLs Instant Polls.
Do you use anesthetic drops for immediate relief of pain in children with acute otitis media, as we advise in the Priority Update from the Research Literature (PURL), page 370? Do colleagues like you around the country do so?
You can find out in a few seconds, at www.jfponline.com. Starting this month, our PURLs Instant Poll—a Reality Check on the Research Literature, lets you join an online dialogue about practice changes recommended in the PURLs series in JFP.
We have surveyed thousands of research studies in the past 8 months, with input from our “Reality Checkers” listserv and colleagues around the country, to help us figure out which studies are relevant, valid, practice changing, and usable. Our small group at the University of Chicago culled a dozen studies that met those criteria. Some of the questions that arose from these studies were: Do you use tamsulosin to help pass ureteral stones? …vitamin C to prevent pain syndrome after fracture?… more thiazides and fewer beta-blockers for hypertension in the elderly—even those with diabetes and metabolic syndrome? … zoledronic acid to prevent osteoporotic fracture? If so, why? If not, why not?
There is more to adopting a new practice than reading a study. Clear reasons (cost, insurance, patient factors) sometimes support a decision to maintain the status quo. But more often, we’ve discovered in our PURL-diving thus far, the reason is that we are unaware of the new evidence or unconvinced of its usefulness.
“Show me”
Diffusion theory suggests that we doctors will not adopt a practice change until we are convinced that our patients will benefit. Like those of us from Missouri, most doctors prudently think, “show me.” We have to be convinced that the science is good and that it is relevant to our patients, but we also have to be convinced that in the reality where practicing doctors and patients live, it works, it is acceptable, and it can actually be implemented.
We want to facilitate practice changes that will benefit patients. But one must know what the current practice is to determine if a study will change practice. We hope that we can all learn from each other about what works and makes sense for our practices and patients, and what does not.
By participating in the online PURLs Instant Poll, you can take part in selecting, adopting, and disseminating worthwhile new practice recommendations.
Please join us in defining what makes sense in the reality of family practice by logging in at www.jfponline.com, and responding to PURLs Instant Polls.
Do you use anesthetic drops for immediate relief of pain in children with acute otitis media, as we advise in the Priority Update from the Research Literature (PURL), page 370? Do colleagues like you around the country do so?
You can find out in a few seconds, at www.jfponline.com. Starting this month, our PURLs Instant Poll—a Reality Check on the Research Literature, lets you join an online dialogue about practice changes recommended in the PURLs series in JFP.
We have surveyed thousands of research studies in the past 8 months, with input from our “Reality Checkers” listserv and colleagues around the country, to help us figure out which studies are relevant, valid, practice changing, and usable. Our small group at the University of Chicago culled a dozen studies that met those criteria. Some of the questions that arose from these studies were: Do you use tamsulosin to help pass ureteral stones? …vitamin C to prevent pain syndrome after fracture?… more thiazides and fewer beta-blockers for hypertension in the elderly—even those with diabetes and metabolic syndrome? … zoledronic acid to prevent osteoporotic fracture? If so, why? If not, why not?
There is more to adopting a new practice than reading a study. Clear reasons (cost, insurance, patient factors) sometimes support a decision to maintain the status quo. But more often, we’ve discovered in our PURL-diving thus far, the reason is that we are unaware of the new evidence or unconvinced of its usefulness.
“Show me”
Diffusion theory suggests that we doctors will not adopt a practice change until we are convinced that our patients will benefit. Like those of us from Missouri, most doctors prudently think, “show me.” We have to be convinced that the science is good and that it is relevant to our patients, but we also have to be convinced that in the reality where practicing doctors and patients live, it works, it is acceptable, and it can actually be implemented.
We want to facilitate practice changes that will benefit patients. But one must know what the current practice is to determine if a study will change practice. We hope that we can all learn from each other about what works and makes sense for our practices and patients, and what does not.
By participating in the online PURLs Instant Poll, you can take part in selecting, adopting, and disseminating worthwhile new practice recommendations.
Please join us in defining what makes sense in the reality of family practice by logging in at www.jfponline.com, and responding to PURLs Instant Polls.
Drugs help pass more ureteral stones
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
Give vitamin C to avert lingering pain after fracture
Vitamin C 500 mg daily for 50 days reduced the risk of complex regional pain syndrome for patients with a wrist fracture.
We think vitamin C 500 mg a day for 7 weeks is well worth recommending.1
Strength of recommendation
A: Based on 2 consistent, well-designed randomized controlled trials (RCTs)
Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007; 89:1424–1431.1
Illustrative case
Your patient is an 83-year-old woman who fell at home. radiographs show a distal radial fracture, which is treated with casting. You know that a quarter of patients with wrist fractures will suffer from complex regional pain syndrome, and there is no well-established treatment for it. Is there any way to reduce this woman’s chance of getting this painful syndrome?
BACKGROUND: Often misdiagnosed
We were surprised to learn how commonly patients suffer from complex regional pain syndrome (CRPS) after a fracture.
We think this diagnosis is frequently missed or misdiagnosed. Even with resolution, however, symptoms can be bothersome—and in 1% to 2% of cases, quite severe.
CRPS, a syndrome of pain and autonomic dysfunction after trauma, is divided into 2 types:
- type I has no obvious damage to nerves
- type II is due to definite damage to nerves (causalgia).
The etiology of CRPS (all further references here will be to type I) is still unclear, but not for lack of proposed theories. Sigmund Freud suggested that it has an origin in personality; there is little evidence to support his theory.2
Other theories include microtrauma to nerves, sympathetic nervous system abnormalities (hence the former name, reflex sympathetic dystrophy), abnormalities of the inflammatory response, and physiologic responses to immobilization. It is often described as a biphasic syndrome, with early edema followed by contracture and stiffness. It typically affects the extremities.2
CRPS more likely in women. A population-based study in Olmsted County, MN found an incidence of 5.5 per 100,000 person-years, with a prevalence of 20.6 per 100,000. Women were affected 4 times more than men.3 Fracture (46%) and sprain (12%) were the leading triggers, followed by other injuries, including crush, stroke, and contusion.
Bernard Ewigman, MD, MSPH
Department of Family Medicine
The University of Chicago
Apparently quite a few patients with complex regional pain syndrome (CRPS) saw me during my 20 years of practice, but I did not see them, or at least I did not recognize their symptoms.
As many as 50% of cast complaints—or post-cast complaints—probably represented CRPS. I wrote them off in all but the most severe cases, which were so dramatic they couldn’t be written off.
I vividly remember the suffering those patients experienced, as well as my feelings of helplessness to offer any relief. CRPS is still not treatable, but now we know that vitamin C can help prevent it, or at least shorten its severity and duration. Granted, the symptoms can be mild, and most resolve spontaneously, but it can go on for months and it can be truly horrific in a small percent (1%–2%).
Here is an instance in which an ounce of prevention is worth more than a pound of cure.
Over half of patients may suffer
Despite typically reported rates of 1% to 2% for severe, chronic CRPS following these injuries, it appears that milder cases are substantially more common.
In a cohort of 274 patients with Colles’s fractures, at 2 weeks, 24% met all 4 of the criteria used in the study to define CRPS, specifically:
- tenderness
- vascular instability
- stiffness
- objective swelling of areas distal to and distinctive from the fracture.
Although 48% met none of the criteria, the remaining 28% had at least some of the criteria.4
Most patients recover. Patients with partial CRPS improved more rapidly than those with CRPS; by 5 months, most had recovered completely. Patients with definitive CRPS also improved, although about 65% still reported stiffness in hands and wrists at 5 months. Another cohort study of 100 patients, also with Colles’s fractures, found similar rates of CRPS 9 weeks.5
CLINICAL CONTEXT: Treatments are not very effective
A systematic review of therapeutic options found 18 randomized controlled studies evaluating possible therapies for CRPS.6 The overall quality of data was low. The authors concluded there was little to no evidence for sympathetic blockade (either via stellate ganglion block or RIS block), radical scavenging with DMSO, prednisolone, acupuncture, or manual lymph drainage. Bisphosphonates and calcitonin, as well as qigong exercises, did show some potential, but data were too limited to draw a firm conclusion.
Guidelines recommend early intervention with physical and psychological therapy, as well as adequate pain control.7
The systematic review also identified 2 studies of preventive interventions. A pilot study of vitamin C (by the authors of the trial that is the subject of this PURL) showed some efficacy.8 An RCT of IV guanethidine found no benefit compared with saline.6
STUDY SUMMARY: Vitamin C reduced the rate of CRPS
This randomized, multicenter, dose-ranging, placebo-controlled trial was performed at 3 hospitals in the Netherlands.1 Any adult (>18 years of age) with a fracture of one or both wrists treated in the emergency departments of these hospitals was invited to participate. During 2001–2004, there were 2137 patients with wrist fractures. Of these, 416 (19.5%) patients (with 427 fractures) were enrolled in the study. The study was double-blinded, and allocation was adequately concealed. There was 100% follow-up of all patients. Of enrolled patients, 82.4% were women, and the average age of all subjects was 62.4 years.
Patients were randomized into 1 of 4 groups: placebo, vitamin C 200 mg daily, vitamin C 500 mg daily, or vitamin C 1500 mg daily for 50 days. Other fracture therapy was undertaken at the discretion of the treating physician. Patients were evaluated at 1 week, 4 or 5 weeks (or cast removal), 6 or 7 weeks, 12 weeks, and 26 weeks. After 1 year, patients were contacted by phone or mail to confirm their status using Veldman’s criteria (TABLE 1).8
TABLE 1
Veldman’s criteria for diagnosis of complex regional pain syndrome, type 1
| Must have 4 of the 5 symptoms below, at the affected hand or wrist, or during activity with that wrist: |
|---|
|
10% of the placebo group had CRPS after 1 year
One year after the fracture, 10% of placebo patients had a diagnosis of CRPS. Rates of CRPS were 4%, 2%, and 2% in the 200 mg, 500 mg, 1500 mg daily dosing of vitamin C, respectively (TABLE 2).
There was no association between the development of CRPS and site of fracture, whether the fracture was displaced or intraarticular, or whether surgical therapy was chosen (although 90% of the fractures were treated with casting). Of note: all of the patients who developed CRPS were female; however, only 75 men were enrolled (18%).
Older patients were at increased risk, and patients with complaints about their cast were substantially more likely to have CRPS (number needed to harm [NNH]=2.6; odds ratio [OR]=5.73; 95% confidence interval [CI], 2.11–15.57).
TABLE 2
Rates of complex regional pain syndrome at 1 year: 10% placebo, 2.4% vitamin C
| TREATMENT GROUP | PERCENT WITH CRPS | RR (95% CI ) | ARR | NNT |
|---|---|---|---|---|
| Placebo | 10% | |||
| Vitamin C | ||||
| All doses combined | 2.4% | 0.24 (0.10–0.60) | 0.076 | 13 |
| 200 mg | 4% | 0.41 (0.13–1.27) | 0.06 | 17 |
| 500 mg | 2% | 0.17 (0.04–0.77) | 0.08 | 13 |
| 1500 mg | 2% | 0.17 (0.04–0.75) | 0.08 | 13 |
| CRPS, complex regional pain syndrome; RR, relative risk; CI, confidence interval; ARR, absolute risk reduction; NNT, number needed to treat. | ||||
| Source: Zollinger et al, 2007.1 | ||||
WHAT’S NEW?: Effective dose: 500 mg/d vitamin C for 50 days
Vitamin C at a dose of at least 500 mg/day for 50 days reduced the rate of CRPS from 10% to 2% (number needed to treat [NNT]=13). This is the second study undertaken by the same investigators to demonstrate risk reduction.8 The previous study enrolled only 129 patients, but found an absolute risk reduction of 15% (NNT=7; P<.05) for patients taking 500 mg of vitamin C.
Patients who complained about their casts were at substantially higher risk of being diagnosed with CRPS (OR=10.0; 95% CI, 2.9–33), suggesting that cast complaints may be a harbinger.
The more recent study was also designed to determine the effective dose for vitamin C. Doses of 200 mg daily reduced the risk, but the effect was not statistically significant. The effect size for the 500 mg and 1500 mg doses, on the other hand, were essentially identical, and both statistically and clinically significant.1
CAVEATS: Selection bias?
The study enrolled less than 20% of potentially eligible patients, raising the possibility that only patients who might benefit from vitamin C prophylaxis were enrolled. However, almost two thirds of those eligible were either never approached due to the emergency department being busy (43%), or refused randomization (14%) after they were informed in the consent process that there was evidence of benefit of vitamin C, based on the prior trial.8 Therefore, selection bias seems an unlikely explanation for the positive results. This is the second trial to show the same finding, which is reassuring.
No men had CRPS in either group in this study, so there is no evidence to show whether or not this intervention works in men. We are unaware of any physiologic reason to suggest that vitamin C would have a differential effect in men. In the earlier study, only 1 man (in the placebo group) got CRPS, for an absolute risk difference of 0.08 (95% CI, –0.07 to 0.27).8
What are the diagnostic criteria?
There is no universal agreement on the diagnostic criteria for CRPS. This study used Veldman’s criteria (TABLE 1), which is the standard criteria used in The Netherlands, and has the best inter-rater reliability of the current criteria.9 Criteria from the International Association of Studies in Pain are the most widely cited in the literature, but are not particularly specific or reproducible.9
CHALLENGES TO IMPLEMENTATION: Getting vitamin C started
Recommending 500 mg of vitamin C daily for 7 weeks is a simple and low-cost intervention. Many of our patients will be treated acutely in emergency rooms or by orthopedic surgeons. If these clinicians do not initiate the vitamin C, it may be some time before the primary physician can begin this therapy. We don’t have any information on whether a delay in initiation affects the efficacy of vitamin C.
The evidence presented here is for Colles’s fractures, but CRPS definitely follows other fractures. We are unaware of any physiologic reason why vitamin C therapy would behave any differently for other fracture locations.
It’s a mistake to think CRPS is rare
Perhaps the greatest challenge to implementation is the perception that CRPS is a rare phenomenon or at least generally resolves spontaneously.
The rate in the placebo group (1 in 10 diagnosed at 1 year) and the rates reported through systematic surveillance (1 in 4 diagnosed at some point following Colles’s fracture) struck both us and the clinicians reviewing this study as high.
We suspect symptoms are often missed or misdiagnosed. Even with resolution, the symptoms can be bothersome, and quite severe in a few cases (1% to 2%). We think vitamin C 500 mg/day for 7 weeks is well worth recommending.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007;89:1424-1431
2. Atkins RM. Complex regional pain syndrome. J Bone Joint Surg Br 2003;85:1100-1106
3. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103:199-207
4. Bickerstaff DR, Kanis JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol 1994;33:240-248
5. Field J, Atkins RM. Algodystrophy is an early complication of Colles’ fracture. What are the implications? J Hand Surg [Br] 1997;22:178-182
6. Forouzanfar T, Koke AJ, van Kleef M, Weber WE. Treatment of complex regional pain syndrome type I. Eur J Pain 2002;6:105-122
7. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome: which treatments show promise? J Fam Pract 2005;54:599-603
8. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet 1999;354:2025-2028
9. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome underdiagnosed. J Fam Pract 2005;54:524-532
Vitamin C 500 mg daily for 50 days reduced the risk of complex regional pain syndrome for patients with a wrist fracture.
We think vitamin C 500 mg a day for 7 weeks is well worth recommending.1
Strength of recommendation
A: Based on 2 consistent, well-designed randomized controlled trials (RCTs)
Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007; 89:1424–1431.1
Illustrative case
Your patient is an 83-year-old woman who fell at home. radiographs show a distal radial fracture, which is treated with casting. You know that a quarter of patients with wrist fractures will suffer from complex regional pain syndrome, and there is no well-established treatment for it. Is there any way to reduce this woman’s chance of getting this painful syndrome?
BACKGROUND: Often misdiagnosed
We were surprised to learn how commonly patients suffer from complex regional pain syndrome (CRPS) after a fracture.
We think this diagnosis is frequently missed or misdiagnosed. Even with resolution, however, symptoms can be bothersome—and in 1% to 2% of cases, quite severe.
CRPS, a syndrome of pain and autonomic dysfunction after trauma, is divided into 2 types:
- type I has no obvious damage to nerves
- type II is due to definite damage to nerves (causalgia).
The etiology of CRPS (all further references here will be to type I) is still unclear, but not for lack of proposed theories. Sigmund Freud suggested that it has an origin in personality; there is little evidence to support his theory.2
Other theories include microtrauma to nerves, sympathetic nervous system abnormalities (hence the former name, reflex sympathetic dystrophy), abnormalities of the inflammatory response, and physiologic responses to immobilization. It is often described as a biphasic syndrome, with early edema followed by contracture and stiffness. It typically affects the extremities.2
CRPS more likely in women. A population-based study in Olmsted County, MN found an incidence of 5.5 per 100,000 person-years, with a prevalence of 20.6 per 100,000. Women were affected 4 times more than men.3 Fracture (46%) and sprain (12%) were the leading triggers, followed by other injuries, including crush, stroke, and contusion.
Bernard Ewigman, MD, MSPH
Department of Family Medicine
The University of Chicago
Apparently quite a few patients with complex regional pain syndrome (CRPS) saw me during my 20 years of practice, but I did not see them, or at least I did not recognize their symptoms.
As many as 50% of cast complaints—or post-cast complaints—probably represented CRPS. I wrote them off in all but the most severe cases, which were so dramatic they couldn’t be written off.
I vividly remember the suffering those patients experienced, as well as my feelings of helplessness to offer any relief. CRPS is still not treatable, but now we know that vitamin C can help prevent it, or at least shorten its severity and duration. Granted, the symptoms can be mild, and most resolve spontaneously, but it can go on for months and it can be truly horrific in a small percent (1%–2%).
Here is an instance in which an ounce of prevention is worth more than a pound of cure.
Over half of patients may suffer
Despite typically reported rates of 1% to 2% for severe, chronic CRPS following these injuries, it appears that milder cases are substantially more common.
In a cohort of 274 patients with Colles’s fractures, at 2 weeks, 24% met all 4 of the criteria used in the study to define CRPS, specifically:
- tenderness
- vascular instability
- stiffness
- objective swelling of areas distal to and distinctive from the fracture.
Although 48% met none of the criteria, the remaining 28% had at least some of the criteria.4
Most patients recover. Patients with partial CRPS improved more rapidly than those with CRPS; by 5 months, most had recovered completely. Patients with definitive CRPS also improved, although about 65% still reported stiffness in hands and wrists at 5 months. Another cohort study of 100 patients, also with Colles’s fractures, found similar rates of CRPS 9 weeks.5
CLINICAL CONTEXT: Treatments are not very effective
A systematic review of therapeutic options found 18 randomized controlled studies evaluating possible therapies for CRPS.6 The overall quality of data was low. The authors concluded there was little to no evidence for sympathetic blockade (either via stellate ganglion block or RIS block), radical scavenging with DMSO, prednisolone, acupuncture, or manual lymph drainage. Bisphosphonates and calcitonin, as well as qigong exercises, did show some potential, but data were too limited to draw a firm conclusion.
Guidelines recommend early intervention with physical and psychological therapy, as well as adequate pain control.7
The systematic review also identified 2 studies of preventive interventions. A pilot study of vitamin C (by the authors of the trial that is the subject of this PURL) showed some efficacy.8 An RCT of IV guanethidine found no benefit compared with saline.6
STUDY SUMMARY: Vitamin C reduced the rate of CRPS
This randomized, multicenter, dose-ranging, placebo-controlled trial was performed at 3 hospitals in the Netherlands.1 Any adult (>18 years of age) with a fracture of one or both wrists treated in the emergency departments of these hospitals was invited to participate. During 2001–2004, there were 2137 patients with wrist fractures. Of these, 416 (19.5%) patients (with 427 fractures) were enrolled in the study. The study was double-blinded, and allocation was adequately concealed. There was 100% follow-up of all patients. Of enrolled patients, 82.4% were women, and the average age of all subjects was 62.4 years.
Patients were randomized into 1 of 4 groups: placebo, vitamin C 200 mg daily, vitamin C 500 mg daily, or vitamin C 1500 mg daily for 50 days. Other fracture therapy was undertaken at the discretion of the treating physician. Patients were evaluated at 1 week, 4 or 5 weeks (or cast removal), 6 or 7 weeks, 12 weeks, and 26 weeks. After 1 year, patients were contacted by phone or mail to confirm their status using Veldman’s criteria (TABLE 1).8
TABLE 1
Veldman’s criteria for diagnosis of complex regional pain syndrome, type 1
| Must have 4 of the 5 symptoms below, at the affected hand or wrist, or during activity with that wrist: |
|---|
|
10% of the placebo group had CRPS after 1 year
One year after the fracture, 10% of placebo patients had a diagnosis of CRPS. Rates of CRPS were 4%, 2%, and 2% in the 200 mg, 500 mg, 1500 mg daily dosing of vitamin C, respectively (TABLE 2).
There was no association between the development of CRPS and site of fracture, whether the fracture was displaced or intraarticular, or whether surgical therapy was chosen (although 90% of the fractures were treated with casting). Of note: all of the patients who developed CRPS were female; however, only 75 men were enrolled (18%).
Older patients were at increased risk, and patients with complaints about their cast were substantially more likely to have CRPS (number needed to harm [NNH]=2.6; odds ratio [OR]=5.73; 95% confidence interval [CI], 2.11–15.57).
TABLE 2
Rates of complex regional pain syndrome at 1 year: 10% placebo, 2.4% vitamin C
| TREATMENT GROUP | PERCENT WITH CRPS | RR (95% CI ) | ARR | NNT |
|---|---|---|---|---|
| Placebo | 10% | |||
| Vitamin C | ||||
| All doses combined | 2.4% | 0.24 (0.10–0.60) | 0.076 | 13 |
| 200 mg | 4% | 0.41 (0.13–1.27) | 0.06 | 17 |
| 500 mg | 2% | 0.17 (0.04–0.77) | 0.08 | 13 |
| 1500 mg | 2% | 0.17 (0.04–0.75) | 0.08 | 13 |
| CRPS, complex regional pain syndrome; RR, relative risk; CI, confidence interval; ARR, absolute risk reduction; NNT, number needed to treat. | ||||
| Source: Zollinger et al, 2007.1 | ||||
WHAT’S NEW?: Effective dose: 500 mg/d vitamin C for 50 days
Vitamin C at a dose of at least 500 mg/day for 50 days reduced the rate of CRPS from 10% to 2% (number needed to treat [NNT]=13). This is the second study undertaken by the same investigators to demonstrate risk reduction.8 The previous study enrolled only 129 patients, but found an absolute risk reduction of 15% (NNT=7; P<.05) for patients taking 500 mg of vitamin C.
Patients who complained about their casts were at substantially higher risk of being diagnosed with CRPS (OR=10.0; 95% CI, 2.9–33), suggesting that cast complaints may be a harbinger.
The more recent study was also designed to determine the effective dose for vitamin C. Doses of 200 mg daily reduced the risk, but the effect was not statistically significant. The effect size for the 500 mg and 1500 mg doses, on the other hand, were essentially identical, and both statistically and clinically significant.1
CAVEATS: Selection bias?
The study enrolled less than 20% of potentially eligible patients, raising the possibility that only patients who might benefit from vitamin C prophylaxis were enrolled. However, almost two thirds of those eligible were either never approached due to the emergency department being busy (43%), or refused randomization (14%) after they were informed in the consent process that there was evidence of benefit of vitamin C, based on the prior trial.8 Therefore, selection bias seems an unlikely explanation for the positive results. This is the second trial to show the same finding, which is reassuring.
No men had CRPS in either group in this study, so there is no evidence to show whether or not this intervention works in men. We are unaware of any physiologic reason to suggest that vitamin C would have a differential effect in men. In the earlier study, only 1 man (in the placebo group) got CRPS, for an absolute risk difference of 0.08 (95% CI, –0.07 to 0.27).8
What are the diagnostic criteria?
There is no universal agreement on the diagnostic criteria for CRPS. This study used Veldman’s criteria (TABLE 1), which is the standard criteria used in The Netherlands, and has the best inter-rater reliability of the current criteria.9 Criteria from the International Association of Studies in Pain are the most widely cited in the literature, but are not particularly specific or reproducible.9
CHALLENGES TO IMPLEMENTATION: Getting vitamin C started
Recommending 500 mg of vitamin C daily for 7 weeks is a simple and low-cost intervention. Many of our patients will be treated acutely in emergency rooms or by orthopedic surgeons. If these clinicians do not initiate the vitamin C, it may be some time before the primary physician can begin this therapy. We don’t have any information on whether a delay in initiation affects the efficacy of vitamin C.
The evidence presented here is for Colles’s fractures, but CRPS definitely follows other fractures. We are unaware of any physiologic reason why vitamin C therapy would behave any differently for other fracture locations.
It’s a mistake to think CRPS is rare
Perhaps the greatest challenge to implementation is the perception that CRPS is a rare phenomenon or at least generally resolves spontaneously.
The rate in the placebo group (1 in 10 diagnosed at 1 year) and the rates reported through systematic surveillance (1 in 4 diagnosed at some point following Colles’s fracture) struck both us and the clinicians reviewing this study as high.
We suspect symptoms are often missed or misdiagnosed. Even with resolution, the symptoms can be bothersome, and quite severe in a few cases (1% to 2%). We think vitamin C 500 mg/day for 7 weeks is well worth recommending.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Vitamin C 500 mg daily for 50 days reduced the risk of complex regional pain syndrome for patients with a wrist fracture.
We think vitamin C 500 mg a day for 7 weeks is well worth recommending.1
Strength of recommendation
A: Based on 2 consistent, well-designed randomized controlled trials (RCTs)
Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007; 89:1424–1431.1
Illustrative case
Your patient is an 83-year-old woman who fell at home. radiographs show a distal radial fracture, which is treated with casting. You know that a quarter of patients with wrist fractures will suffer from complex regional pain syndrome, and there is no well-established treatment for it. Is there any way to reduce this woman’s chance of getting this painful syndrome?
BACKGROUND: Often misdiagnosed
We were surprised to learn how commonly patients suffer from complex regional pain syndrome (CRPS) after a fracture.
We think this diagnosis is frequently missed or misdiagnosed. Even with resolution, however, symptoms can be bothersome—and in 1% to 2% of cases, quite severe.
CRPS, a syndrome of pain and autonomic dysfunction after trauma, is divided into 2 types:
- type I has no obvious damage to nerves
- type II is due to definite damage to nerves (causalgia).
The etiology of CRPS (all further references here will be to type I) is still unclear, but not for lack of proposed theories. Sigmund Freud suggested that it has an origin in personality; there is little evidence to support his theory.2
Other theories include microtrauma to nerves, sympathetic nervous system abnormalities (hence the former name, reflex sympathetic dystrophy), abnormalities of the inflammatory response, and physiologic responses to immobilization. It is often described as a biphasic syndrome, with early edema followed by contracture and stiffness. It typically affects the extremities.2
CRPS more likely in women. A population-based study in Olmsted County, MN found an incidence of 5.5 per 100,000 person-years, with a prevalence of 20.6 per 100,000. Women were affected 4 times more than men.3 Fracture (46%) and sprain (12%) were the leading triggers, followed by other injuries, including crush, stroke, and contusion.
Bernard Ewigman, MD, MSPH
Department of Family Medicine
The University of Chicago
Apparently quite a few patients with complex regional pain syndrome (CRPS) saw me during my 20 years of practice, but I did not see them, or at least I did not recognize their symptoms.
As many as 50% of cast complaints—or post-cast complaints—probably represented CRPS. I wrote them off in all but the most severe cases, which were so dramatic they couldn’t be written off.
I vividly remember the suffering those patients experienced, as well as my feelings of helplessness to offer any relief. CRPS is still not treatable, but now we know that vitamin C can help prevent it, or at least shorten its severity and duration. Granted, the symptoms can be mild, and most resolve spontaneously, but it can go on for months and it can be truly horrific in a small percent (1%–2%).
Here is an instance in which an ounce of prevention is worth more than a pound of cure.
Over half of patients may suffer
Despite typically reported rates of 1% to 2% for severe, chronic CRPS following these injuries, it appears that milder cases are substantially more common.
In a cohort of 274 patients with Colles’s fractures, at 2 weeks, 24% met all 4 of the criteria used in the study to define CRPS, specifically:
- tenderness
- vascular instability
- stiffness
- objective swelling of areas distal to and distinctive from the fracture.
Although 48% met none of the criteria, the remaining 28% had at least some of the criteria.4
Most patients recover. Patients with partial CRPS improved more rapidly than those with CRPS; by 5 months, most had recovered completely. Patients with definitive CRPS also improved, although about 65% still reported stiffness in hands and wrists at 5 months. Another cohort study of 100 patients, also with Colles’s fractures, found similar rates of CRPS 9 weeks.5
CLINICAL CONTEXT: Treatments are not very effective
A systematic review of therapeutic options found 18 randomized controlled studies evaluating possible therapies for CRPS.6 The overall quality of data was low. The authors concluded there was little to no evidence for sympathetic blockade (either via stellate ganglion block or RIS block), radical scavenging with DMSO, prednisolone, acupuncture, or manual lymph drainage. Bisphosphonates and calcitonin, as well as qigong exercises, did show some potential, but data were too limited to draw a firm conclusion.
Guidelines recommend early intervention with physical and psychological therapy, as well as adequate pain control.7
The systematic review also identified 2 studies of preventive interventions. A pilot study of vitamin C (by the authors of the trial that is the subject of this PURL) showed some efficacy.8 An RCT of IV guanethidine found no benefit compared with saline.6
STUDY SUMMARY: Vitamin C reduced the rate of CRPS
This randomized, multicenter, dose-ranging, placebo-controlled trial was performed at 3 hospitals in the Netherlands.1 Any adult (>18 years of age) with a fracture of one or both wrists treated in the emergency departments of these hospitals was invited to participate. During 2001–2004, there were 2137 patients with wrist fractures. Of these, 416 (19.5%) patients (with 427 fractures) were enrolled in the study. The study was double-blinded, and allocation was adequately concealed. There was 100% follow-up of all patients. Of enrolled patients, 82.4% were women, and the average age of all subjects was 62.4 years.
Patients were randomized into 1 of 4 groups: placebo, vitamin C 200 mg daily, vitamin C 500 mg daily, or vitamin C 1500 mg daily for 50 days. Other fracture therapy was undertaken at the discretion of the treating physician. Patients were evaluated at 1 week, 4 or 5 weeks (or cast removal), 6 or 7 weeks, 12 weeks, and 26 weeks. After 1 year, patients were contacted by phone or mail to confirm their status using Veldman’s criteria (TABLE 1).8
TABLE 1
Veldman’s criteria for diagnosis of complex regional pain syndrome, type 1
| Must have 4 of the 5 symptoms below, at the affected hand or wrist, or during activity with that wrist: |
|---|
|
10% of the placebo group had CRPS after 1 year
One year after the fracture, 10% of placebo patients had a diagnosis of CRPS. Rates of CRPS were 4%, 2%, and 2% in the 200 mg, 500 mg, 1500 mg daily dosing of vitamin C, respectively (TABLE 2).
There was no association between the development of CRPS and site of fracture, whether the fracture was displaced or intraarticular, or whether surgical therapy was chosen (although 90% of the fractures were treated with casting). Of note: all of the patients who developed CRPS were female; however, only 75 men were enrolled (18%).
Older patients were at increased risk, and patients with complaints about their cast were substantially more likely to have CRPS (number needed to harm [NNH]=2.6; odds ratio [OR]=5.73; 95% confidence interval [CI], 2.11–15.57).
TABLE 2
Rates of complex regional pain syndrome at 1 year: 10% placebo, 2.4% vitamin C
| TREATMENT GROUP | PERCENT WITH CRPS | RR (95% CI ) | ARR | NNT |
|---|---|---|---|---|
| Placebo | 10% | |||
| Vitamin C | ||||
| All doses combined | 2.4% | 0.24 (0.10–0.60) | 0.076 | 13 |
| 200 mg | 4% | 0.41 (0.13–1.27) | 0.06 | 17 |
| 500 mg | 2% | 0.17 (0.04–0.77) | 0.08 | 13 |
| 1500 mg | 2% | 0.17 (0.04–0.75) | 0.08 | 13 |
| CRPS, complex regional pain syndrome; RR, relative risk; CI, confidence interval; ARR, absolute risk reduction; NNT, number needed to treat. | ||||
| Source: Zollinger et al, 2007.1 | ||||
WHAT’S NEW?: Effective dose: 500 mg/d vitamin C for 50 days
Vitamin C at a dose of at least 500 mg/day for 50 days reduced the rate of CRPS from 10% to 2% (number needed to treat [NNT]=13). This is the second study undertaken by the same investigators to demonstrate risk reduction.8 The previous study enrolled only 129 patients, but found an absolute risk reduction of 15% (NNT=7; P<.05) for patients taking 500 mg of vitamin C.
Patients who complained about their casts were at substantially higher risk of being diagnosed with CRPS (OR=10.0; 95% CI, 2.9–33), suggesting that cast complaints may be a harbinger.
The more recent study was also designed to determine the effective dose for vitamin C. Doses of 200 mg daily reduced the risk, but the effect was not statistically significant. The effect size for the 500 mg and 1500 mg doses, on the other hand, were essentially identical, and both statistically and clinically significant.1
CAVEATS: Selection bias?
The study enrolled less than 20% of potentially eligible patients, raising the possibility that only patients who might benefit from vitamin C prophylaxis were enrolled. However, almost two thirds of those eligible were either never approached due to the emergency department being busy (43%), or refused randomization (14%) after they were informed in the consent process that there was evidence of benefit of vitamin C, based on the prior trial.8 Therefore, selection bias seems an unlikely explanation for the positive results. This is the second trial to show the same finding, which is reassuring.
No men had CRPS in either group in this study, so there is no evidence to show whether or not this intervention works in men. We are unaware of any physiologic reason to suggest that vitamin C would have a differential effect in men. In the earlier study, only 1 man (in the placebo group) got CRPS, for an absolute risk difference of 0.08 (95% CI, –0.07 to 0.27).8
What are the diagnostic criteria?
There is no universal agreement on the diagnostic criteria for CRPS. This study used Veldman’s criteria (TABLE 1), which is the standard criteria used in The Netherlands, and has the best inter-rater reliability of the current criteria.9 Criteria from the International Association of Studies in Pain are the most widely cited in the literature, but are not particularly specific or reproducible.9
CHALLENGES TO IMPLEMENTATION: Getting vitamin C started
Recommending 500 mg of vitamin C daily for 7 weeks is a simple and low-cost intervention. Many of our patients will be treated acutely in emergency rooms or by orthopedic surgeons. If these clinicians do not initiate the vitamin C, it may be some time before the primary physician can begin this therapy. We don’t have any information on whether a delay in initiation affects the efficacy of vitamin C.
The evidence presented here is for Colles’s fractures, but CRPS definitely follows other fractures. We are unaware of any physiologic reason why vitamin C therapy would behave any differently for other fracture locations.
It’s a mistake to think CRPS is rare
Perhaps the greatest challenge to implementation is the perception that CRPS is a rare phenomenon or at least generally resolves spontaneously.
The rate in the placebo group (1 in 10 diagnosed at 1 year) and the rates reported through systematic surveillance (1 in 4 diagnosed at some point following Colles’s fracture) struck both us and the clinicians reviewing this study as high.
We suspect symptoms are often missed or misdiagnosed. Even with resolution, the symptoms can be bothersome, and quite severe in a few cases (1% to 2%). We think vitamin C 500 mg/day for 7 weeks is well worth recommending.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007;89:1424-1431
2. Atkins RM. Complex regional pain syndrome. J Bone Joint Surg Br 2003;85:1100-1106
3. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103:199-207
4. Bickerstaff DR, Kanis JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol 1994;33:240-248
5. Field J, Atkins RM. Algodystrophy is an early complication of Colles’ fracture. What are the implications? J Hand Surg [Br] 1997;22:178-182
6. Forouzanfar T, Koke AJ, van Kleef M, Weber WE. Treatment of complex regional pain syndrome type I. Eur J Pain 2002;6:105-122
7. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome: which treatments show promise? J Fam Pract 2005;54:599-603
8. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet 1999;354:2025-2028
9. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome underdiagnosed. J Fam Pract 2005;54:524-532
1. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am 2007;89:1424-1431
2. Atkins RM. Complex regional pain syndrome. J Bone Joint Surg Br 2003;85:1100-1106
3. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103:199-207
4. Bickerstaff DR, Kanis JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol 1994;33:240-248
5. Field J, Atkins RM. Algodystrophy is an early complication of Colles’ fracture. What are the implications? J Hand Surg [Br] 1997;22:178-182
6. Forouzanfar T, Koke AJ, van Kleef M, Weber WE. Treatment of complex regional pain syndrome type I. Eur J Pain 2002;6:105-122
7. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome: which treatments show promise? J Fam Pract 2005;54:599-603
8. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet 1999;354:2025-2028
9. Quisel A, Gill JM, Witherell P. Complex regional pain syndrome underdiagnosed. J Fam Pract 2005;54:524-532
PURLs—Translating research into reality
You likely do not read many (or any) of the thousands of medical research studies published each month. That’s understandable, given the demands of patient care and the joys of our personal lives. You have probably tried many approaches to keeping up, but have yet to discover a method that systematically distills the literature to those few articles that are relevant, practice-changing, and able to be implemented immediately.
Numerous efforts to disseminate research findings to practicing physicians share 2 major weaknesses.Azithromycin for PID beats doxycycline on all counts. This was an easy choice, a well-done randomized trial on a common family medicine problem for which 1 commonly available drug was found significantly superior to another commonly available drug. Azithromycin was not recommended in any of the frequently updated and well-referenced electronic knowledge resources that we regularly consult (UpToDate,Page 1010)
- Consider an annual infusion of zoledronic acid to prevent fractures in your patients with osteoporosis.
1. InfoPOEMS. Available at: www.infopoems.com. Accessed on November 12, 2007.
2. Dynamed. Available at: www.ebscohost.com/dynamed. Accessed on November 12, 2007.
3. Global Family Doctor: WONCA Online. Available at: www.globalfamilydoctor.com. Accessed on November 12, 2007.
4. McMaster Online Rating of Evidence (MORE). Available at: hiru.mcmaster.ca/MORE/AboutMORE.htm. Accessed on November 12, 2007.
5. QuickScan Reviews—Family Practice with KeyINFO Manager, Available at: Findarticles.com/p/articles/mi_m0689/is_n5_v45/ai_20116292. Accessed on November 12, 2007.
6. Medscape. Available at: www.medscape.com/home. Accessed on November 12, 2007.
7. BMJ Evidence Updates. Available at: bmjupdates.com. Accessed on November 12, 2007.
8. NIH Roadmap for Medical Research. Available at: nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Accessed on November 12, 2007.
9. International Development Research Center. Knowledge Translation: Basic Theories, Approaches and Applications. Available at: www.idrc.ca/en/ev-90105-201-1-DO_TOPIC.html. Accessed on November 12, 2007.
10. Family Physicians Inquiries Network. Available at: www.fpin.org. Accessed on November 12, 2007.
11. Susman J. Diving for PURLs: Introducing Priority Updates from the Research Literature. J Fam Pract 2007;56:878.-
12. National Center for Research Resources. Clinical and Translational Science Awards. Available at: www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards. Accessed on November 12, 2007.
13. UpToDate. Available at: www.uptodate.com. Accessed on November 12, 2007.
14. PEPID Online. Available at: www.pepidonline.com. Accessed on November 12, 2007.
15. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines 2002. Available at: www.cdc.gov/std/treatment/5-2002TG.htm#Treatment. Accessed on November 12, 2007.
16. Accreditation Council for Graduate Medical Education (ACGME). ACGME Program Requirements for Graduate Medical Education in Family Medicine. Available at: www.acgme.org/acWebsite/downloads/RRC_progReq/120pr07012007.pdf. Accessed on November 12, 2007.
You likely do not read many (or any) of the thousands of medical research studies published each month. That’s understandable, given the demands of patient care and the joys of our personal lives. You have probably tried many approaches to keeping up, but have yet to discover a method that systematically distills the literature to those few articles that are relevant, practice-changing, and able to be implemented immediately.
Numerous efforts to disseminate research findings to practicing physicians share 2 major weaknesses.Azithromycin for PID beats doxycycline on all counts. This was an easy choice, a well-done randomized trial on a common family medicine problem for which 1 commonly available drug was found significantly superior to another commonly available drug. Azithromycin was not recommended in any of the frequently updated and well-referenced electronic knowledge resources that we regularly consult (UpToDate,Page 1010)
- Consider an annual infusion of zoledronic acid to prevent fractures in your patients with osteoporosis.
You likely do not read many (or any) of the thousands of medical research studies published each month. That’s understandable, given the demands of patient care and the joys of our personal lives. You have probably tried many approaches to keeping up, but have yet to discover a method that systematically distills the literature to those few articles that are relevant, practice-changing, and able to be implemented immediately.
Numerous efforts to disseminate research findings to practicing physicians share 2 major weaknesses.Azithromycin for PID beats doxycycline on all counts. This was an easy choice, a well-done randomized trial on a common family medicine problem for which 1 commonly available drug was found significantly superior to another commonly available drug. Azithromycin was not recommended in any of the frequently updated and well-referenced electronic knowledge resources that we regularly consult (UpToDate,Page 1010)
- Consider an annual infusion of zoledronic acid to prevent fractures in your patients with osteoporosis.
1. InfoPOEMS. Available at: www.infopoems.com. Accessed on November 12, 2007.
2. Dynamed. Available at: www.ebscohost.com/dynamed. Accessed on November 12, 2007.
3. Global Family Doctor: WONCA Online. Available at: www.globalfamilydoctor.com. Accessed on November 12, 2007.
4. McMaster Online Rating of Evidence (MORE). Available at: hiru.mcmaster.ca/MORE/AboutMORE.htm. Accessed on November 12, 2007.
5. QuickScan Reviews—Family Practice with KeyINFO Manager, Available at: Findarticles.com/p/articles/mi_m0689/is_n5_v45/ai_20116292. Accessed on November 12, 2007.
6. Medscape. Available at: www.medscape.com/home. Accessed on November 12, 2007.
7. BMJ Evidence Updates. Available at: bmjupdates.com. Accessed on November 12, 2007.
8. NIH Roadmap for Medical Research. Available at: nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Accessed on November 12, 2007.
9. International Development Research Center. Knowledge Translation: Basic Theories, Approaches and Applications. Available at: www.idrc.ca/en/ev-90105-201-1-DO_TOPIC.html. Accessed on November 12, 2007.
10. Family Physicians Inquiries Network. Available at: www.fpin.org. Accessed on November 12, 2007.
11. Susman J. Diving for PURLs: Introducing Priority Updates from the Research Literature. J Fam Pract 2007;56:878.-
12. National Center for Research Resources. Clinical and Translational Science Awards. Available at: www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards. Accessed on November 12, 2007.
13. UpToDate. Available at: www.uptodate.com. Accessed on November 12, 2007.
14. PEPID Online. Available at: www.pepidonline.com. Accessed on November 12, 2007.
15. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines 2002. Available at: www.cdc.gov/std/treatment/5-2002TG.htm#Treatment. Accessed on November 12, 2007.
16. Accreditation Council for Graduate Medical Education (ACGME). ACGME Program Requirements for Graduate Medical Education in Family Medicine. Available at: www.acgme.org/acWebsite/downloads/RRC_progReq/120pr07012007.pdf. Accessed on November 12, 2007.
1. InfoPOEMS. Available at: www.infopoems.com. Accessed on November 12, 2007.
2. Dynamed. Available at: www.ebscohost.com/dynamed. Accessed on November 12, 2007.
3. Global Family Doctor: WONCA Online. Available at: www.globalfamilydoctor.com. Accessed on November 12, 2007.
4. McMaster Online Rating of Evidence (MORE). Available at: hiru.mcmaster.ca/MORE/AboutMORE.htm. Accessed on November 12, 2007.
5. QuickScan Reviews—Family Practice with KeyINFO Manager, Available at: Findarticles.com/p/articles/mi_m0689/is_n5_v45/ai_20116292. Accessed on November 12, 2007.
6. Medscape. Available at: www.medscape.com/home. Accessed on November 12, 2007.
7. BMJ Evidence Updates. Available at: bmjupdates.com. Accessed on November 12, 2007.
8. NIH Roadmap for Medical Research. Available at: nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Accessed on November 12, 2007.
9. International Development Research Center. Knowledge Translation: Basic Theories, Approaches and Applications. Available at: www.idrc.ca/en/ev-90105-201-1-DO_TOPIC.html. Accessed on November 12, 2007.
10. Family Physicians Inquiries Network. Available at: www.fpin.org. Accessed on November 12, 2007.
11. Susman J. Diving for PURLs: Introducing Priority Updates from the Research Literature. J Fam Pract 2007;56:878.-
12. National Center for Research Resources. Clinical and Translational Science Awards. Available at: www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards. Accessed on November 12, 2007.
13. UpToDate. Available at: www.uptodate.com. Accessed on November 12, 2007.
14. PEPID Online. Available at: www.pepidonline.com. Accessed on November 12, 2007.
15. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines 2002. Available at: www.cdc.gov/std/treatment/5-2002TG.htm#Treatment. Accessed on November 12, 2007.
16. Accreditation Council for Graduate Medical Education (ACGME). ACGME Program Requirements for Graduate Medical Education in Family Medicine. Available at: www.acgme.org/acWebsite/downloads/RRC_progReq/120pr07012007.pdf. Accessed on November 12, 2007.
What is the risk of bowel strangulation in an adult with an untreated inguinal hernia?
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
The risk of bowel strangulation is estimated to be small—less than 1% per year (strength of recommendation [SOR]: B, based on small cohort studies with short follow-up). Experts recommend repair for patients with risk factors for poor outcomes after potential strangulation. These risk factors include advanced age, limited access to emergency care, significant concomitant illness, inability to recognize symptoms of bowel incarceration, and poor operative risk (American society of Anesthesiologists class III and IV) (SOR: C, based on expert opinion and case series). It is reasonable to offer elective surgery or watchful waiting to low-risk patients who understand the risks of strangulation (SOR: C, based on expert opinion and case series).
Watchful waiting, yes, but not for high-risk seniors
Michael K. Park, MD
University of Colorado Health Sciences Center, Rose Family Medicine Residency, Denver
The evidence reinforces “watchful waiting” as a reasonable management approach. However, certain patients—say, a 66-year-old diabetic farmer—should probably undergo elective herniorrhaphy to preempt the increased risk of complications with emergent repair.
shared decision-making is an essential process in accounting for individual preferences. In addition to knowing the risks of strangulation, patients opting for surgery also need to be aware of the differences between open and laparoscopic techniques. The former may be done under local anesthesia; the latter decreases postoperative pain and recovery time, but requires general anesthesia and increases the rates of serious complications.
Evidence summary
In 2 randomized controlled trials (RCTs) comparing elective repair of inguinal hernias with watchful waiting, the cohorts who made up the control groups experienced strangulation rates of 1.8 per thousand (0.18%) and 7.9 per thousand (0.79%) occurrences per patient-year.1,2 In the first of these 2 trials,1 with 364 control group patients, median follow-up was only 3.2 years (maximum 4.5 years), and by 4 years 31% of patients had crossed over to the treatment group for elective repair. The mean follow-up time in the second trial,2 which had 80 control group participants, was 1.6 years; 29% of patients eventually crossed over for repair.
Spanish study may have overestimated the risk. A retrospective study3 of 70 patients with incarcerated inguinal hernias presenting for emergency surgery in Northern Spain reported a cumulative 2.8% probability of strangulation at 3 months, rising to 4.5% after 2 years. This study did not include patients presenting for elective repair of hernias, and therefore it likely overestimated the rate of strangulation among patients in a primary care setting.
When to repair inguinal hernia
Experts recommend repair of an inguinal hernia in patients with risk factors for poor outcomes after potential strangulation. Risk factors include advanced age and significant concomitant illness.
In 2001, a prospective study4 of 669 patients presenting for elective hernia repair in London found that only 0.3% of patients required resection of bowel or omentum.
Risk appears to be <1% a year. Collectively, these studies suggest that the risk of strangulation is less than 1% per year (0.18% to 0.79%) among all patients with inguinal hernias, at least in the first few years of the onset of the hernia. As you’d expect, the risk of strangulation is higher (2.8% to 4.5%) among patients presenting for emergency repair of incarcerated hernias. We found no prospective studies that followed patients for more than 4.5 years.
Age factors into poor outcomes
A number of studies5,6 have examined risk factors for increased rates of strangulation and poor outcomes. Older age increases the risk of a poor outcome, peaking in the seventh decade. Patient comorbidity and late hospitalization also make emergent repair more risky.3,5
Retrospective studies3,5,7 of the temporal duration and the natural history of inguinal hernias, as well as operative complication rates, have shown conflicting results.
70- and 80-year olds have greater risk. A Turkish study5 of patients needing emergent surgical repair found morbidity to be significantly related to American Society of Anesthesiologists (ASA) class, with mortality rates of 3% and 14% for ASA class III and IV patients, respectively. This was a retrospective chart review that analyzed factors responsible for unfavorable outcomes; it found increased complications in hernia patients who had coexisting disease, hernias of longer duration, as well as higher ASA class. This study5 and another retrospective study6 found the need for emergent repair peaked for patients 70 to 80 years of age.
longer history of herniation may more postop complications. The Spanish retrospective review3 of emergent surgical repair of incarcerated hernias (noted earlier) reported a 3.4% postoperative mortality rate. All deaths were among patients over 65 years of age and ASA class III or IV. This review also found more postoperative complications and a higher mortality for hernias present for more than 10 years.
Another study raises questions. A retrospective study from Israel8 also showed that patients who underwent emergency repair were older, had a longer history of herniation than those undergoing elective repair, and had higher ASA scores. However, a case-control study7 and a chart review9 found that the risk of strangulation was higher for hernias of shorter duration.
We found no studies addressing potential exacerbating conditions of inguinal hernia, such as chronic cough, bladder outlet obstruction with straining, constipation, obesity, or bilateral hernias.
Recommendations from others
All the textbooks and guidelines we identified acknowledge that many patients forego operation and remain minimally symptomatic for long periods of time, and that operations themselves have risks and complications.10–12 The avoidable risks of strangulation and emergent operation lead most experts to favor operative treatment.
In ACS Surgery: Principles & Practice 2007,10 the authors lament the difficulty of obtaining accurate studies of the natural history of inguinal hernia because surgeons have been taught that it is best to operate at diagnosis, making it hard to find an adequate population to study. The authors acknowledge that while many primary care physicians advise their patients to delay operations if the hernia is minimally asymptomatic, they do not share this belief.
The American College of Physicians’ PIER: The Physicians’ Information and Education Resource11 recommends assessing the hernia and the patient on a case-by-case basis. They recommend deferring an operation for poor-risk patients with minimal symptoms if the hernia is easily reducible and is unquestionably an inguinal hernia, if there are no past episodes of obstruction, and if the risks of untreated hernia are fully understood by the patient. Sabiston Textbook of Surgery makes virtually the same points and recommendations.12
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
1. Fitzgibbons RJ, Giobbie-Hurder A, Gibbs JO, et al. watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295:285-292.
2. O’Dwyer PJ, Norrie J, Alani A, walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: A randomised clinical trial. Ann Surg 2006;244:167-173.
3. Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez A, Alvarez JI. Incarcerated groin hernias in adults: Presentation and outcome. Hernia 2004;8:121-126.
4. Hair A, Paterson C, Wright D, Baxter JN, O’Dwyer PJ. What effect does the duration of an inguinal hernia have on patient symptoms? J Am Coll Surg 2001;193:125-129.
5. Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg 2001;182:455-459.
6. McEntee G, O’carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg 1989;76:725-726.
7. Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust N Z J Surg 1998;68:650-654.
8. Ohana manevwitch I, Weil R, et al. Inguinal hernia: challenging the traditional indication for surgery in asymptomatic patients. Hernia 2004;8:117-120.
9. Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-1173.
10. Fitzgibbons RJ, Richards AT, Quinn TH. Open hernia repair. In: Souba WW, Wilmore DW, Fink MP, et al, eds.ACS Surgery: Principles and Practice 2007.New York, NY: webMD Professional Publishing; 2007. Available at: www.acssurgery.com. Accessed on June 22, 2007.
11. Kingsnorth AN, Khan JA. Hernia. In: PIER—The Physicians’ Information and Education Resource [database online]. Philadelphia, Pa: American College of Physicians; 2007.
12. Malangoni MA, Gagliardi RJ. Hernias. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders; 2004:1199–1217. Available at: www.mdconsult.com/das/book/0/view/1235/394.html. Accessed on November 8, 2007.
Evidence-based answers from the Family Physicians Inquiries Network