User login
James J. Stevermer is in the Department of Family and Community Medicine at the University of Missouri–Columbia.
When to “Undiagnose” Asthma
Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
Should you reassess your patient’s asthma diagnosis?
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
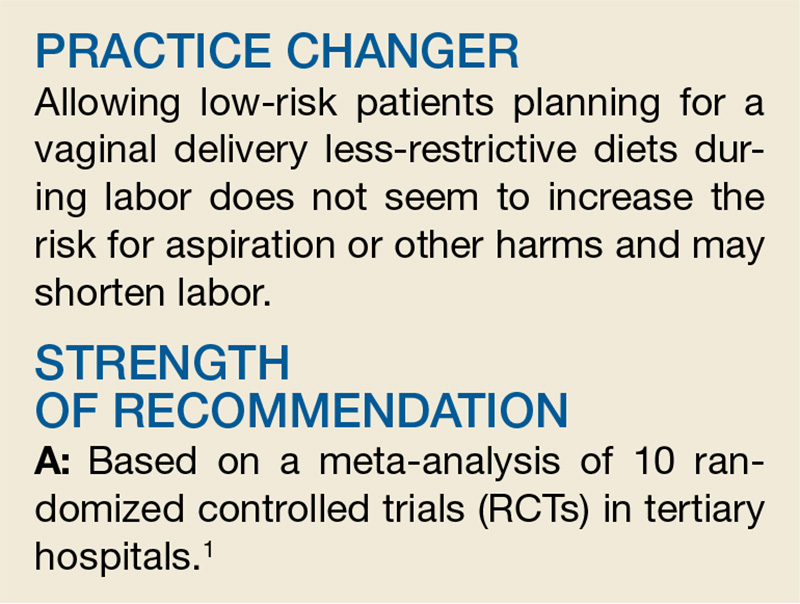
PRACTICE CHANGER
Consider tapering medications and retesting spirometry in adults with well-controlled asthma, as many may no longer have the disease.1
STRENGTH OF RECOMMENDATION
A: Based on a high-quality prospective cohort study and consistent findings in other studies.
Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
Let Low-risk Moms Eat During Labor?
A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
Let low-risk moms eat during labor?
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
PRACTICE CHANGER
Allowing low-risk patients planning for a vaginal delivery less restrictive diets during labor does not seem to increase the risk of aspiration or other harms and may shorten labor.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 10 randomized controlled trials (RCTs) in tertiary hospitals.
Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
PPIs With Warfarin Regimens: Balancing the Perks and Pitfalls
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
The benefits—and limits—of PPIs with warfarin regimens
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had gastroesophageal reflux disease (GERD) or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefits of preventing embolization with the risks of serious bleeding. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and other antiplatelet agents further increases the risk of the latter.2
Physicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most efficacious drugs for healing peptic ulcers.3,4 However, while previous case-control studies show that PPIs reduce the risk of upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 Further reflecting the confusion and uncertainty surrounding this issue is that while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk of upper GI bleeding,2 other guidelines regarding anticoagulant therapy do not address this clinical question.2,7,8
[polldaddy:9860876]
STUDY SUMMARY
Study lends support to PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding for Medicare and Medicaid patients taking warfarin with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of a severe bleed or certain illnesses that would predispose a patient to GI bleeding (such as esophageal varices). Patients with risk factors for an upper GI bleed (such as abdominal pain, peptic ulcer disease, anemia, etc.) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed over 75,000 person-years of active warfarin therapy (more than 52,000 person-years in the Medicaid cohort and more than 23,000 person-years in the Medicare cohort). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Looking at all patients taking warfarin (regardless of whether or not they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR]=0.76; 95% confidence interval [CI], 0.63 to 0.91), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk of lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk of hospitalization for upper GI bleeding by about half (HR=0.55; 95% CI, 0.39-0.77). Hospitalizations decreased by 128/10,000 person-years (95% CI, -66 to -173), yielding an NNT of 78 person-years. For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly decrease the risk of hospitalization for upper GI bleeding (HR=0.86; 95% CI, 0.70-1.06).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk of upper GI bleeding whether or not the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to patients taking warfarin alone
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk of upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Study was good, but it wasn’t a randomized controlled trial
This study is observational, and not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken over-the-counter medications that influenced or obscured results, but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk of upper GI bleeds. We need RCTs to determine whether PPIs cause a lower risk.
Don’t overlook the risk of PPIs. This study assessed the ability of PPIs to prevent bleeds, but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include an increased risk of pneumonia, infection with Clostridium difficile, hip and spine fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another consideration is the option to prescribe a direct oral anticoagulant (DOAC), such as dabigatran, rivaroxaban, or apixaban, instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Rivaroxaban has been shown to result in fewer fatal bleeding events than warfarin due to fatal intracranial bleeds, although it is associated with more GI bleedding.13 Compared with warfarin, apixaban is associated with fewer GI bleeds and lower bleeding rates overall.13 Further research is warranted to determine if PPI therapy is beneficial to patients taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When chronic anticoagulation is necessary, physicians and patients must attempt to prevent thrombotic events while minimizing the risk of GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical rials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von HK, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe a proton pump inhibitor for patients taking dual antiplatelet/antithrombotic therapy to reduce the risk of upper gastrointestinal bleeding.
STRENGTH OF RECOMMENDATION
B: Based on a cohort study
Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.1
Oral Rehydration Therapy for KidsA More Palatable Alternative
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
A more palatable alternative to oral rehydration therapy for kids
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend that parents give half-strength apple juice to children ≥24 months of age who are minimally dehydrated following a case of simple viral gastroenteritis. The juice reduces the need for further interventions better than oral hydration therapy.
Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.1
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality randomized controlled trial.
Notice of retraction
The study1 that served as the basis for the PURL entitled, “Ramipril for claudication?” (J Fam Pract. 2013;62:579-580), has been retracted from the Journal of the American Medical Association.2 Therefore we, on behalf of all of the authors of the PURL, are retracting the PURL, as well.
According to JAMA’s retraction statement, the first author of the article admitted to data fabrication following an internal investigation.2 The source article does not provide subgroup analysis to determine how much of an effect the fabricated data may have had on the final reported outcome. However, a separately reported (and also retracted) sub-analysis of this study indicates that 165/212 (77.8%) patients were enrolled from the site of the first author.3
The question remains: Does ramipril work for symptoms of claudication? A completely separate group of researchers conducted a similar, but smaller, randomized clinical trial of ramipril in patients with intermittent claudication.4 In this study, 33 patients were randomized to ramipril or placebo for a 24-week trial. The ramipril group (n=14) improved maximum treadmill walking distance by an adjusted mean of 131 meters (m) (95% confidence interval [CI], 62-199; P=.001), improved treadmill intermittent claudication distance by 122 m (95% CI, 56-188; P=.001), and improved patient-reported walking distance by 159 m (95% CI, 66-313; P=.043).
The 2004 Heart Outcomes Prevention Evaluation (HOPE) study indicates that ramipril maintains a mortality benefit for patients with intermittent claudication.5 A subgroup of this study included 1725 patients with baseline peripheral artery disease who were randomized to ramipril at 10 mg, which yielded a relative risk (RR) of 0.75 (95% CI, 0.61-0.92) for the primary outcome (cardiovascular mortality, myocardial infarction, stroke). This alone validates the use of ramipril in patients with intermittent claudication. But with the retraction of the large randomized controlled trial, we are not sure how much it may improve walk distances. Further studies might better clarify if ramipril provides symptomatic benefit by reducing claudication symptoms, in addition to the known cardiovascular mortality benefit.
Luke Stephens, MD, MSPH
Park Ridge, IL
James J. Stevermer, MD, MSPH
Columbia, MO
1. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA. 2013;309:453-460.
2. Notice of Retraction: Ahimastos AA, et al. Effect of Ramipril on Walking Times and Quality of Life Among Patients with Peripheral Artery Disease and Intermittent Claudication: A Randomized Controlled Trial. JAMA. 2013;309(5):453-460. JAMA. 2015;314:1520-1521.
3. Notice of Retraction: Potential vascular mechanisms of ramipril induced increases in walking ability in patients with intermittent claudication. Circ Res. 2014. Circ Res. 2015;117:e64.
4. Shahin Y, Cockcroft JR, Chetter IC. Randomized clinical trial of angiotensin-converting enzyme inhibitor, ramipril, in patients with intermittent claudication. Br J Surg. 2013;100:1154-1163.
5. Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17-24.
The study1 that served as the basis for the PURL entitled, “Ramipril for claudication?” (J Fam Pract. 2013;62:579-580), has been retracted from the Journal of the American Medical Association.2 Therefore we, on behalf of all of the authors of the PURL, are retracting the PURL, as well.
According to JAMA’s retraction statement, the first author of the article admitted to data fabrication following an internal investigation.2 The source article does not provide subgroup analysis to determine how much of an effect the fabricated data may have had on the final reported outcome. However, a separately reported (and also retracted) sub-analysis of this study indicates that 165/212 (77.8%) patients were enrolled from the site of the first author.3
The question remains: Does ramipril work for symptoms of claudication? A completely separate group of researchers conducted a similar, but smaller, randomized clinical trial of ramipril in patients with intermittent claudication.4 In this study, 33 patients were randomized to ramipril or placebo for a 24-week trial. The ramipril group (n=14) improved maximum treadmill walking distance by an adjusted mean of 131 meters (m) (95% confidence interval [CI], 62-199; P=.001), improved treadmill intermittent claudication distance by 122 m (95% CI, 56-188; P=.001), and improved patient-reported walking distance by 159 m (95% CI, 66-313; P=.043).
The 2004 Heart Outcomes Prevention Evaluation (HOPE) study indicates that ramipril maintains a mortality benefit for patients with intermittent claudication.5 A subgroup of this study included 1725 patients with baseline peripheral artery disease who were randomized to ramipril at 10 mg, which yielded a relative risk (RR) of 0.75 (95% CI, 0.61-0.92) for the primary outcome (cardiovascular mortality, myocardial infarction, stroke). This alone validates the use of ramipril in patients with intermittent claudication. But with the retraction of the large randomized controlled trial, we are not sure how much it may improve walk distances. Further studies might better clarify if ramipril provides symptomatic benefit by reducing claudication symptoms, in addition to the known cardiovascular mortality benefit.
Luke Stephens, MD, MSPH
Park Ridge, IL
James J. Stevermer, MD, MSPH
Columbia, MO
The study1 that served as the basis for the PURL entitled, “Ramipril for claudication?” (J Fam Pract. 2013;62:579-580), has been retracted from the Journal of the American Medical Association.2 Therefore we, on behalf of all of the authors of the PURL, are retracting the PURL, as well.
According to JAMA’s retraction statement, the first author of the article admitted to data fabrication following an internal investigation.2 The source article does not provide subgroup analysis to determine how much of an effect the fabricated data may have had on the final reported outcome. However, a separately reported (and also retracted) sub-analysis of this study indicates that 165/212 (77.8%) patients were enrolled from the site of the first author.3
The question remains: Does ramipril work for symptoms of claudication? A completely separate group of researchers conducted a similar, but smaller, randomized clinical trial of ramipril in patients with intermittent claudication.4 In this study, 33 patients were randomized to ramipril or placebo for a 24-week trial. The ramipril group (n=14) improved maximum treadmill walking distance by an adjusted mean of 131 meters (m) (95% confidence interval [CI], 62-199; P=.001), improved treadmill intermittent claudication distance by 122 m (95% CI, 56-188; P=.001), and improved patient-reported walking distance by 159 m (95% CI, 66-313; P=.043).
The 2004 Heart Outcomes Prevention Evaluation (HOPE) study indicates that ramipril maintains a mortality benefit for patients with intermittent claudication.5 A subgroup of this study included 1725 patients with baseline peripheral artery disease who were randomized to ramipril at 10 mg, which yielded a relative risk (RR) of 0.75 (95% CI, 0.61-0.92) for the primary outcome (cardiovascular mortality, myocardial infarction, stroke). This alone validates the use of ramipril in patients with intermittent claudication. But with the retraction of the large randomized controlled trial, we are not sure how much it may improve walk distances. Further studies might better clarify if ramipril provides symptomatic benefit by reducing claudication symptoms, in addition to the known cardiovascular mortality benefit.
Luke Stephens, MD, MSPH
Park Ridge, IL
James J. Stevermer, MD, MSPH
Columbia, MO
1. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA. 2013;309:453-460.
2. Notice of Retraction: Ahimastos AA, et al. Effect of Ramipril on Walking Times and Quality of Life Among Patients with Peripheral Artery Disease and Intermittent Claudication: A Randomized Controlled Trial. JAMA. 2013;309(5):453-460. JAMA. 2015;314:1520-1521.
3. Notice of Retraction: Potential vascular mechanisms of ramipril induced increases in walking ability in patients with intermittent claudication. Circ Res. 2014. Circ Res. 2015;117:e64.
4. Shahin Y, Cockcroft JR, Chetter IC. Randomized clinical trial of angiotensin-converting enzyme inhibitor, ramipril, in patients with intermittent claudication. Br J Surg. 2013;100:1154-1163.
5. Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17-24.
1. Ahimastos AA, Walker PJ, Askew C, et al. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA. 2013;309:453-460.
2. Notice of Retraction: Ahimastos AA, et al. Effect of Ramipril on Walking Times and Quality of Life Among Patients with Peripheral Artery Disease and Intermittent Claudication: A Randomized Controlled Trial. JAMA. 2013;309(5):453-460. JAMA. 2015;314:1520-1521.
3. Notice of Retraction: Potential vascular mechanisms of ramipril induced increases in walking ability in patients with intermittent claudication. Circ Res. 2014. Circ Res. 2015;117:e64.
4. Shahin Y, Cockcroft JR, Chetter IC. Randomized clinical trial of angiotensin-converting enzyme inhibitor, ramipril, in patients with intermittent claudication. Br J Surg. 2013;100:1154-1163.
5. Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17-24.
“Go Low” or “Say No” to Aggressive Systolic BP Goals?
PRACTICE CHANGER
Consider treating nondiabetic patients ages 50 and older to a systolic blood pressure (SBP) target < 120 mm Hg (as compared to < 140 mm Hg) when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of Recommendation
B: Based on a single, good-quality randomized controlled trial (RCT). 1
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) presents for routine care. His blood pressure is 135/85 mm Hg, and he is currently taking lisinopril 40 mg/d. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events in patients with diabetes, but the study may have been underpowered.5 The members of the Eighth Joint National Committee (JNC 8) recommended treating patients older than 60 to BP goals < 150/90 mm Hg.6 This was based on evidence from six RCTs, but there remains debate—even among the JNC 8 committee members—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg. 7-13
Continue for the study summary >>
STUDY SUMMARY
Treating to SBP < 120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in nondiabetic patients at high risk for CV events improves outcomes, compared with standard care. Patients were at least 50, had an SBP of 130 to 180 mm Hg, and were at increased CV risk; the last was defined as clinical or subclinical CV disease other than stroke; CKD with a glomerular filtration rate (GFR) of 20 to 60 mL/min/1.73 m2; 10-year risk for CV disease > 15% on Framingham risk score; or age 75 or older. Patients with diabetes, prior stroke, polycystic kidney disease, significant proteinuria or symptomatic heart failure within the past six months, or left ventricular ejection fraction < 35% were excluded.1
Patients (N = 9,361) were randomly assigned to an SBP target < 120 mm Hg in the intensive group or < 140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), ACE inhibitors or angiotensin receptor blockers, calcium channel blockers, and ß-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
Medication dosing adjustments were based on the average of three BP measurements taken with an automated measurement system with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was < 130 mm Hg at a single visit or < 135 mm Hg at two consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome; death from any cause; and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group versus the standard therapy group (1.65% vs 2.19% per year, respectively; hazard ratio [HR], 0.75 with intensive treatment). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk for the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR, 0.73).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR, 1.04), with a number needed to harm (NNH) of 46 over the study period.1
Rates of serious adverse events that were identified as likely associated with the intervention were 4.7% vs 2.5%, respectively. Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls, although higher in the intensive treatment group, did not reach statistical significance. In the subgroup of patients 75 or older, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. Patients in the intensive therapy group required, on average, one additional BP medication, compared to those in the standard treatment group (2.8 vs 1.8, respectively).1
Continue for what's new >>
WHAT’S NEW
Lower SBP produces mortality benefits in those younger, and older, than 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to < 150 mm Hg7,11,12 by demonstrating benefits, including reduced all-cause mortality, for lower SBP targets in nondiabetic patients at high risk for CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients 75 or older.
The incidence of the primary outcome in the cohort 75 or older receiving intensive therapy was 7.7%, compared with 10.9% for those receiving standard therapy (HR, 0.67; NNT, 31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients 75 or older: 5.5% vs 8.04% (HR, 0.68; NNT, 38).1
CAVEATS
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1,000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of three readings after the patient had rested for 5 minutes) and what occurs typically in clinical practice could potentially lead to overtreatment in a “real world” setting.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups; this is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied nondiabetic patients at high risk for CV disease who were 50 or older, limiting generalizability to other populations.
CHALLENGES TO IMPLEMENTATION
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 Caution should be exercised particularly in the subgroup of patients 75 or older. Despite a lower NNT than the rest of the study population, this group experienced serious adverse events more frequently. Also, this particular cohort of volunteers may not be representative of those 75 or older in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP < 120 mm Hg.1 And in a 2011-2012 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target < 140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals in the management of hypertension, requiring clinicians to carefully assess an individual patient’s likelihood of benefit versus harm.
REFERENCES
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials.Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents: a systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8).JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older.N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al; the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension.Lancet. 1997;350:757-764.
12. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014;312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016 Feb 23. [Epub ahead of print]
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(5):342-344.
PRACTICE CHANGER
Consider treating nondiabetic patients ages 50 and older to a systolic blood pressure (SBP) target < 120 mm Hg (as compared to < 140 mm Hg) when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of Recommendation
B: Based on a single, good-quality randomized controlled trial (RCT). 1
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) presents for routine care. His blood pressure is 135/85 mm Hg, and he is currently taking lisinopril 40 mg/d. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events in patients with diabetes, but the study may have been underpowered.5 The members of the Eighth Joint National Committee (JNC 8) recommended treating patients older than 60 to BP goals < 150/90 mm Hg.6 This was based on evidence from six RCTs, but there remains debate—even among the JNC 8 committee members—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg. 7-13
Continue for the study summary >>
STUDY SUMMARY
Treating to SBP < 120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in nondiabetic patients at high risk for CV events improves outcomes, compared with standard care. Patients were at least 50, had an SBP of 130 to 180 mm Hg, and were at increased CV risk; the last was defined as clinical or subclinical CV disease other than stroke; CKD with a glomerular filtration rate (GFR) of 20 to 60 mL/min/1.73 m2; 10-year risk for CV disease > 15% on Framingham risk score; or age 75 or older. Patients with diabetes, prior stroke, polycystic kidney disease, significant proteinuria or symptomatic heart failure within the past six months, or left ventricular ejection fraction < 35% were excluded.1
Patients (N = 9,361) were randomly assigned to an SBP target < 120 mm Hg in the intensive group or < 140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), ACE inhibitors or angiotensin receptor blockers, calcium channel blockers, and ß-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
Medication dosing adjustments were based on the average of three BP measurements taken with an automated measurement system with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was < 130 mm Hg at a single visit or < 135 mm Hg at two consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome; death from any cause; and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group versus the standard therapy group (1.65% vs 2.19% per year, respectively; hazard ratio [HR], 0.75 with intensive treatment). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk for the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR, 0.73).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR, 1.04), with a number needed to harm (NNH) of 46 over the study period.1
Rates of serious adverse events that were identified as likely associated with the intervention were 4.7% vs 2.5%, respectively. Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls, although higher in the intensive treatment group, did not reach statistical significance. In the subgroup of patients 75 or older, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. Patients in the intensive therapy group required, on average, one additional BP medication, compared to those in the standard treatment group (2.8 vs 1.8, respectively).1
Continue for what's new >>
WHAT’S NEW
Lower SBP produces mortality benefits in those younger, and older, than 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to < 150 mm Hg7,11,12 by demonstrating benefits, including reduced all-cause mortality, for lower SBP targets in nondiabetic patients at high risk for CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients 75 or older.
The incidence of the primary outcome in the cohort 75 or older receiving intensive therapy was 7.7%, compared with 10.9% for those receiving standard therapy (HR, 0.67; NNT, 31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients 75 or older: 5.5% vs 8.04% (HR, 0.68; NNT, 38).1
CAVEATS
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1,000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of three readings after the patient had rested for 5 minutes) and what occurs typically in clinical practice could potentially lead to overtreatment in a “real world” setting.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups; this is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied nondiabetic patients at high risk for CV disease who were 50 or older, limiting generalizability to other populations.
CHALLENGES TO IMPLEMENTATION
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 Caution should be exercised particularly in the subgroup of patients 75 or older. Despite a lower NNT than the rest of the study population, this group experienced serious adverse events more frequently. Also, this particular cohort of volunteers may not be representative of those 75 or older in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP < 120 mm Hg.1 And in a 2011-2012 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target < 140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals in the management of hypertension, requiring clinicians to carefully assess an individual patient’s likelihood of benefit versus harm.
REFERENCES
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials.Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents: a systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8).JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older.N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al; the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension.Lancet. 1997;350:757-764.
12. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014;312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016 Feb 23. [Epub ahead of print]
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(5):342-344.
PRACTICE CHANGER
Consider treating nondiabetic patients ages 50 and older to a systolic blood pressure (SBP) target < 120 mm Hg (as compared to < 140 mm Hg) when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of Recommendation
B: Based on a single, good-quality randomized controlled trial (RCT). 1
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) presents for routine care. His blood pressure is 135/85 mm Hg, and he is currently taking lisinopril 40 mg/d. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events in patients with diabetes, but the study may have been underpowered.5 The members of the Eighth Joint National Committee (JNC 8) recommended treating patients older than 60 to BP goals < 150/90 mm Hg.6 This was based on evidence from six RCTs, but there remains debate—even among the JNC 8 committee members—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg. 7-13
Continue for the study summary >>
STUDY SUMMARY
Treating to SBP < 120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in nondiabetic patients at high risk for CV events improves outcomes, compared with standard care. Patients were at least 50, had an SBP of 130 to 180 mm Hg, and were at increased CV risk; the last was defined as clinical or subclinical CV disease other than stroke; CKD with a glomerular filtration rate (GFR) of 20 to 60 mL/min/1.73 m2; 10-year risk for CV disease > 15% on Framingham risk score; or age 75 or older. Patients with diabetes, prior stroke, polycystic kidney disease, significant proteinuria or symptomatic heart failure within the past six months, or left ventricular ejection fraction < 35% were excluded.1
Patients (N = 9,361) were randomly assigned to an SBP target < 120 mm Hg in the intensive group or < 140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), ACE inhibitors or angiotensin receptor blockers, calcium channel blockers, and ß-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
Medication dosing adjustments were based on the average of three BP measurements taken with an automated measurement system with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was < 130 mm Hg at a single visit or < 135 mm Hg at two consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome; death from any cause; and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group versus the standard therapy group (1.65% vs 2.19% per year, respectively; hazard ratio [HR], 0.75 with intensive treatment). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk for the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR, 0.73).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR, 1.04), with a number needed to harm (NNH) of 46 over the study period.1
Rates of serious adverse events that were identified as likely associated with the intervention were 4.7% vs 2.5%, respectively. Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls, although higher in the intensive treatment group, did not reach statistical significance. In the subgroup of patients 75 or older, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. Patients in the intensive therapy group required, on average, one additional BP medication, compared to those in the standard treatment group (2.8 vs 1.8, respectively).1
Continue for what's new >>
WHAT’S NEW
Lower SBP produces mortality benefits in those younger, and older, than 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to < 150 mm Hg7,11,12 by demonstrating benefits, including reduced all-cause mortality, for lower SBP targets in nondiabetic patients at high risk for CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients 75 or older.
The incidence of the primary outcome in the cohort 75 or older receiving intensive therapy was 7.7%, compared with 10.9% for those receiving standard therapy (HR, 0.67; NNT, 31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients 75 or older: 5.5% vs 8.04% (HR, 0.68; NNT, 38).1
CAVEATS
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1,000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of three readings after the patient had rested for 5 minutes) and what occurs typically in clinical practice could potentially lead to overtreatment in a “real world” setting.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups; this is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied nondiabetic patients at high risk for CV disease who were 50 or older, limiting generalizability to other populations.
CHALLENGES TO IMPLEMENTATION
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 Caution should be exercised particularly in the subgroup of patients 75 or older. Despite a lower NNT than the rest of the study population, this group experienced serious adverse events more frequently. Also, this particular cohort of volunteers may not be representative of those 75 or older in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP < 120 mm Hg.1 And in a 2011-2012 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target < 140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals in the management of hypertension, requiring clinicians to carefully assess an individual patient’s likelihood of benefit versus harm.
REFERENCES
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials.Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents: a systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8).JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older.N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al; the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension.Lancet. 1997;350:757-764.
12. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014;312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016 Feb 23. [Epub ahead of print]
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(5):342-344.