User login
Early pregnancy loss: Pretreat with mifepristone?
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Jenny is a 29-year-old G2P1001 woman who presents to your clinic for a missed period. Her last menstrual period was about 10 weeks ago. She is found to have a positive pregnancy test in the office. On examination, her uterus is nontender and consistent in size with gestation of 7 weeks. She denies any bleeding or cramping. On ultrasound, you see a gestational sac measuring 28 mm and no embryo. You confirm early pregnancy loss. Jenny is sad about this diagnosis. She does not wish to proceed with expectant management and is hopeful to avoid a surgical procedure. How do you counsel her regarding medical management?
Early pregnancy loss or first trimester miscarriage is estimated to occur in about 1 million women in the United States annually and is the most common complication of early pregnancy.2,3 Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 weeks 6 days of gestation.4
Once early pregnancy loss is confirmed by ultrasound, expectant management with no intervention is an acceptable treatment option. Women generally prefer active management, either medically or with surgical evacuation.5,6 Misoprostol 800 mcg administered vaginally or orally has been the accepted medication regimen for medical management.5 However, failure rates with misoprostol have been reported to be as high as 40%, particularly among women with a closed cervical os, who then require repeat dosing of misoprostol or surgical evacuation.6
STUDY SUMMARY
Mifepristone before misoprostol improves efficacy for early pregnancy loss
The PreFaiR (Comparative Effectiveness of Pregnancy Failure Management Regimens) study was a randomized trial that took place at 3 US centers. The study was designed to assess the safety and efficacy of pretreatment with oral mifepristone prior to use of vaginal misoprostol for the medical management of early pregnancy loss.1
Three hundred women, ≥ 18 years and undergoing medical management for early pregnancy loss, were randomized to receive misoprostol 800 mcg vaginally alone or mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later.
Inclusion and exclusion criteria. Women who showed a nonviable intrauterine pregnancy at 5 to 12 weeks’ gestation by ultrasound were eligible for the study. Exclusion criteria included incomplete or inevitable abortion, contraindications to either study drug, viable or ectopic pregnancy, hemoglobin < 9.5 g/dL, current use of anticoagulants or the presence of a clotting disorder, and pregnancy with an intrauterine device in place.
Outcomes. The primary outcome was gestational sac expulsion by the first follow-up visit and no additional interventions within 30 days of treatment. Secondary outcomes included acceptability of treatment, adverse events, and clinical characteristics associated with successful expulsion.
Continue to: Demographics
Demographics. The mean age of the study participants in both groups was ~30 years, and there was a similar percentage of participants by self-reported race and ethnicity in both groups (~44% black, ~35% white, and ~25% Hispanic). The majority of participants in both groups were at 6 to 8 weeks’ gestation and had been pregnant at least 3 times.
Results. Researchers were able to evaluate 297 women at the initial follow-up. Of the women who received mifepristone and misoprostol, 83.8% (124 of 148 women; 95% confidence interval [CI], 76.8-89.3) had complete expulsion within 1 to 3 days, compared to 67.1% (100 of 149 women; 95% CI, 59-74.6) in the misoprostol alone group. The number needed to treat with mifepristone and misoprostol to achieve complete expulsion at the first follow-up visit was 6. The percentage of patients receiving uterine aspiration was lower in the mifepristone and misoprostol group (8.8%) than in the misoprostol alone group (23.5%; relative risk = 0.37; 95% CI, 0.21-0.68). There were no significant differences in adverse events including bleeding intensity, pelvic infection, or pain.
WHAT’S NEW
A high-quality RCT demonstrates improved efficacy
Prior studies that have looked at combined mifepristone and misoprostol treatment for early pregnancy loss had heterogeneity in outcome definitions and study designs leading to variable reports of effectiveness.1,5 This is the first high-quality, randomized trial to demonstrate the safety and efficacy of oral mifepristone pretreatment prior to misoprostol vaginal administration in the medical management of early pregnancy loss.
CAVEATS
Would a placebo group—or other forms of misoprostol—change the results?
The study did not include a placebo group; however, an investigator who was blinded to the treatment group allocation determined the primary outcome, and the lack of placebo did not introduce bias related to the outcomes.
Intravaginal misoprostol was used in this study, rather than oral, rectal, buccal, or sublingual misoprostol.7 It is not clear from this study if the results of pretreatment with mifepristone would be different if misoprostol was administered via one of these other routes.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
FDA restrictions limit availability of mifepristone
The main challenge to implementation is the availability of mifepristone. Mifepristone was approved by the US Food and Drug Administration in 2000. The approval included Risk Evaluation and Mitigation Strategy (REMS) restrictions, stipulating that a health provider be specially certified for prescribing; dispensing must occur in clinics, medical offices, or hospitals; and patients must sign a patient agreement form prior to obtaining the agent.8
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
1. Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
2. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
3. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 200. Early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
4. National Institute for Health and Clinical Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. Clinical guideline 154. www.nice.org.uk/guidance/cg154/resources/guidance-ectopic-pregnancy-and-miscarriage-pdf. Published December 2012. Accessed December 5, 2019.
5. Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006;CD002253.
6. Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
7. Ngoc NT, Blum J, Westheimer E, et al. Medical treatment of missed abortion using misoprostol. Int J Gynaecol Obstet. 2004;87:138-142.
8. US Food and Drug Administration. Mifeprex (mifepristone) information. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information. Updated February 5, 2018. Accessed December 5, 2019.
PRACTICE CHANGER
Pretreat patients with oral mifepristone prior to using vaginal misoprostol to increase the efficacy of medical management of early pregnancy loss over that with misoprostol alone.
STRENGTH OF RECOMMENDATION
B: Based on a single, well-executed, randomized controlled trial.1
Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
A better approach to the diagnosis of PE
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
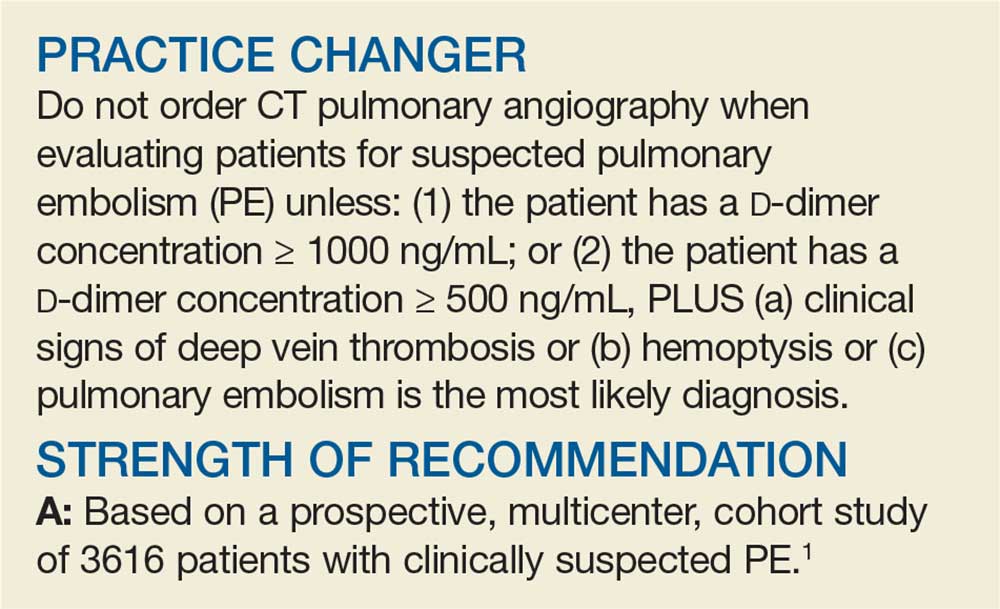
PRACTICE CHANGER
Do not order computed tomography pulmonary angiography when evaluating patients for suspected pulmonary embolism unless: (1) the patient has a
STRENGTH OF RECOMMENDATION
A: Based on a prospective, multicenter, cohort study of 3616 patients with clinically suspected pulmonary embolism.1
van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
First-time, Mild Diverticulitis: Antibiotics or Watchful Waiting?
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
First-time, mild diverticulitis: Antibiotics or watchful waiting?
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
PRACTICE CHANGER
For mild, computed tomography-proven acute diverticulitis, consider observation only instead of antibiotic therapy.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.
Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.1
Depression and Heart Failure? Put Down the SSRI
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
Direct Oral Anticoagulants or Warfarin for A-fib?
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
Direct oral anticoagulants or warfarin for A fib?
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Use direct oral anticoagulants instead of warfarin in patients with atrial fibrillation because they are just as effective at preventing ischemic stroke and systemic emboli as warfarin, and because apixaban and dabigatran have lower bleeding rates.
STRENGTH OF RECOMMENDATION
B: Based on a single, prospective, cohort study.
Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.1
Deliver or Wait with Late Preterm Membrane Rupture?
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
Deliver or wait with late preterm membrane rupture?
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.