User login
Asthma management: How the guidelines compare
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.
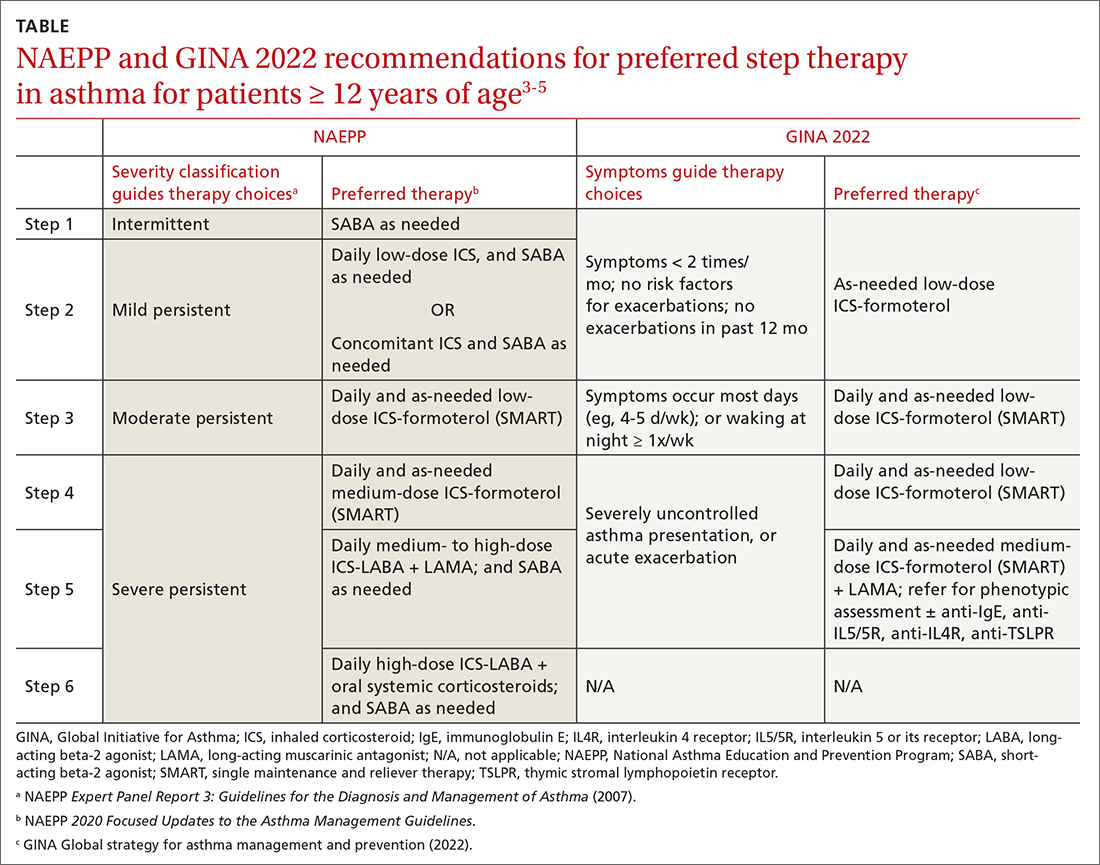
A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
PRACTICE RECOMMENDATIONS
› Consider early initiation of intermittent inhaled corticosteroid (ICS)- formoterol over a short-acting beta-2 agonist for reliever therapy. A
› Start prescribing single maintenance and reliever therapy (SMART) with ICS-formoterol to reduce exacerbation rates and simplify application. A
› Consider FeNO assessment when the diagnosis of asthma remains unclear despite history and spirometry findings. B
› Consider adding a longacting antimuscarinic agent to a medium- or high-dose ICS-LABA (long-acting beta-2 agonist) combination in uncontrolled asthma. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Depression and Heart Failure? Put Down the SSRI
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
A 60-year-old man presents for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association class 3 heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and based on his self-administered 9-item Patient Health Questionnaire (PHQ-9) score of 17, you determine that he is having a concomitant major depressive episode. Should you start him on an SSRI?
Depression is widely recognized as an independent risk factor for cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying link between depression and CVD risk.2,6 Conversely, a recent systematic review found that positive constructs, mediated primarily through lifestyle behaviors, may have a protective effect on outcomes.7
Recently, researchers have focused on treating depression to simultaneously improve CVD outcomes. While some studies have shown SSRIs to be a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD trial did suggest that SSRI treatment may improve mortality and morbidity post-MI.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with lower quality of life (QoL), poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure; it is an independent predictor of mortality in this patient population.1 Until recently, only one RCT (the SADHART-CHF study) looked at SSRI treatment in patients with heart failure and depression.12 In that 12-week trial, sertraline did not improve depression or CVD outcomes when compared with placebo—but the study period may have been too short to capture long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 mo).1 Specifically, this RCT assessed whether treatment with escitalopram could reduce morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients with New York Heart Association class 2 to 4 heart failure and left ventricular ejection fractions < 45% were screened for depression using the PHQ-9. Patients with PHQ-9 scores ≥ 12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist, and those diagnosed with major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months, in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every six months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The study used a composite endpoint of all-cause death or hospitalization; the primary outcome was time to first event of this composite. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale, and health-related QoL as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 escitalopram, 187 placebo). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR], 0.99).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean MADRS score changed from 20.2 at baseline to 11.2 at 12 weeks with escitalopram, and from 21.4 to 12.5 in the placebo group (between-group difference, –0.9).10 Overall, the two treatment groups had comparable daily medication doses and mean treatment duration (18 mo), and both groups demonstrated partial remission of depression symptoms, improved health status, and improved QoL over the study period.
Interestingly, the placebo group experienced significantly improved QoL at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by conducting the study over a much longer duration (up to 24 mo vs 12 wk). Also, in contrast to SADHART-CHF, this trial studied escitalopram rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: SSRI treatment for patients with heart failure and depression did not reduce the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram versus placebo. Given that this high-quality trial replicated the findings of SADHART-CHF with a longer treatment period and a potentially more effective SSRI, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at initiating SSRIs for depression in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to mitigate the elevated risk for adverse clinical outcomes remains nuanced and elusive. The same can be said of non-CVD chronic conditions (eg, diabetes) based on recent systematic reviews.13 In sum, these studies suggest that a traditional screen-and-treat approach using SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
A recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought.14 Nevertheless, this area of research should continue to be explored, given the worsened chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change, we expect that it will be a challenge to convince providers to stop initiating SSRI treatment to affect cardiovascular outcomes in patients with depression and heart failure—especially given the body of evidence denoting depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquires Network and The Journal of Family Practice (2017;66[9]:564-567).
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
1. Angermann CE, Gelbrich G, Störk S, et al; MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315(24):2683-2693.
2. Sin NL, Kumar AD, Gehi AK, Whooley MA. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50(4):523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Investigators. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(8):1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1773-1782.
SSRIs for depression/heart failure patients? Not so fast
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Do not prescribe selective serotonin reuptake inhibitors to improve depression and reduce cardiovascular risk in patients with congestive heart failure.
STRENGTH OF RECOMMENDATION
B: Based on one large randomized controlled trial.
Angermann CE, Gelbrick G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.1
The shrinking case for saw palmetto
Advise men with benign prostatic hyperplasia (BPH) not to take saw palmetto for urinary symptoms. Explain that it has not been found to alleviate symptoms, even at triple the standard dose.1
A: Based on evidence from a high-quality randomized controlled trial (RCT)1 and a 2009 meta-analysis.2
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
ILLUSTRATIVE CASE
A 66-year-old man comes to your office complaining of urinary frequency and straining to begin urination. He was recently diagnosed with BPh by a urologist, but is hesitant to begin taking a prescription drug. The patient, who is on a fixed income, asks you if saw palmetto extract might relieve his urinary symptoms. What should you tell him?
Roughly 40% of American men older than 60 years and nearly 90% of men older than 80 suffer from BPH and the troublesome lower urinary tract symptoms (LUTS) that it causes.3 Established medical and surgical options, as well as over-the-counter (OTC) plant-based products, are used for symptom relief. The OTC remedy most commonly used for BPH is Serenoa repens, derived from the saw palmetto dwarf palm tree. In a 2007 survey, 1.6 million US adults reported using saw palmetto extract, often as a treatment for BPH, in the 30 days prior to the survey.4
Until now, more questions than answers
As a family physician, you undoubtedly have many patients who are taking or considering taking saw palmetto for relief of BPH symptoms. The significant adverse effects of alpha-blockers and 5-alpha-reductase inhibitors, which are typically prescribed for LUTS—including decreased libido and dizziness—may help account for their interest in this alternative treatment.5,6
Until recently, evidence of saw palmetto’s efficacy has been limited and conflicting, despite widespread use of the extract. That has left many of us wondering whether we should recommend that men with BPH try saw palmetto despite the limited evidence; whether it is effective for some, but not all, BPH symptoms; and whether an increase in dose would increase its efficacy.
A 2002 Cochrane meta-analysis of 21 trials of saw palmetto extract for LUTS reported reduced nocturia, improved self-reported symptoms, and increased peak uroflow compared with placebo, without significant adverse effects.7 An updated Cochrane review published in 2009 included several more rigorous trials—and had very different results: This meta-analysis, which was based on 30 trials, found a reduction in nocturia, but failed to show improvement in other self-reported symptoms or peak uroflow.2
The largest trial included in the 2009 review was the Saw Palmetto Treatment for Enlarged Prostates (STEP) study,8 a one-year study with 225 participants. Its findings: no improvement in the treatment group compared with the placebo group in symptom scores or any secondary endpoints, and no important toxicity.8 Of note, the STEP study and most trials included in the 2009 Cochrane review used the standard saw palmetto extract dose of 160 mg twice daily.1,2
STUDY SUMMARY: Saw palmetto is ineffective, even at triple the dose
Barry et al conducted a 72-week double-blind, multicenter placebo-controlled trial to assess the effect of double (640 mg/d) and triple (960 mg/d) the standard dose of saw palmetto extract on BPH symptoms.1 The study included 369 men with moderate LUTS who had not recently received treatment for BPH. Exclusion criteria included a history of invasive BPH treatment, recent treatment with either an alpha-blocker or a 5-alpha-reductase inhibitor; recent phytotherapy, including saw palmetto; and a history of prostate or bladder cancer. Participants were randomized to receive either saw palmetto extract or an identical-looking placebo gel cap. Doses started at 320 mg/d and were increased to 640 mg/d at 24 weeks and 960 mg/d at 48 weeks.
The primary outcome was the change in the American Urological Association Symptom Index (AUASI) score from baseline to 72 weeks. AUASI, a scale of 0 to 35 in which higher numbers represent increased symptoms, is the same scoring tool used in both the Cochrane review and the STEP trial. Secondary measures included other symptom scales, peak uroflow, and poststudy satisfaction. The treatment and placebo groups had statistically identical baseline characteristics, and the sample size was large enough to detect clinically significant differences.
The AUASI score decreased by a mean of 2.20 points (95% confidence interval [CI], -3.04 to -0.36) in the group that received saw palmetto and by 2.99 points (95% CI, -3.81 to -2.17) in the placebo group—a mean difference of 0.79 in favor of the placebo group (P=.91). The proportion of participants achieving a 3-point reduction in AUASI score was statistically similar between the 2 groups (P=0.66). There was no significant dose response difference between the 2 groups, and saw palmetto proved to be no better than placebo for any of the secondary outcomes.
Subgroup analysis did not reveal any results that differed from the main outcomes. The only adverse events that were significantly different between the 2 groups related to physical injury or trauma, which were unlikely to be due to the intervention.1
By using the same symptom scoring system (AUASI) as many studies in the previous Cochrane reviews, Barry et al were able to compare their findings with those of other high-quality studies with similar methodologies and outcome measures. Despite using an even higher dose of the extract, the results of this trial are remarkably consistent with previous conclusions: Saw palmetto is not an effective treatment for symptoms associated with BPH. Moreover, this trial had a broad base of participants similar to the population in a primary care practice, including patients who would typically choose a natural remedy for LUTS.1
WHAT’S NEW: We now have answers to queries about saw palmetto
This trial is the first to compare higher doses of saw palmetto with placebo to assess a dosage threshold for effectiveness. While the study found no evidence of saw palmetto toxicity even at these higher doses, the extract did not outperform placebo for any measured outcome.1
This high-quality study confirmed the recent series of rigorous studies with negative outcomes by showing that the use of a standard dosage was not a study limitation and that saw palmetto extract is not effective for treating LUTS at any dosage. This trial should substantially affect future guideline recommendations that were limited by methodological concerns in the past.9-11
CAVEATS: In theory, individual preparations could work differently
It is not possible to be absolutely certain that these findings apply to all saw palmetto extract preparations, given the unknown active ingredients and unknown mechanism of action. However, the researchers used a high-quality preparation (a proprietary lipidic ethanolic extract) of saw palmetto at higher doses than the STEP trial and came to a similar conclusion, making it highly unlikely that another preparation would perform differently.
CHALLENGES TO IMPLEMENTATION: There are none
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
2. Tacklind J, MacDonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423.-
3. Roehrborn CG, McConnell JD. Etiology, Pathophysiology, Epidemiology, and Natural History of Benign Prostatic Hyperplasia. 8th ed. Philadelphia, Pa: Campbell’s Urology; 2002.
4. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
5. Traish AM, Hassani J, Guay AT, et al. Adverse side effects of 5-alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872-884.
6. Clifford GM, Farmer RD. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
7. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(3):CD001423.-
8. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
9. Benign prostatic hyperplasia: treatment {saw palmetto}. In: DynaMed. Available at: http://www.DynamicMedical.com. Accessed April 16, 2012.
10. Saper R. Clinical use of saw palmetto {saw palmetto}. In: Basow DS, ed. UpToDate [database online]. Waltham, Mass: UpToDate 2012. Available at: http://www.uptodate.com. Accessed April 16, 2012.
11. Agency for Healthcare Research and Quality. Guidelines on the treatment of non-neurogenic male LUTS. Available at: http://www.guideline.gov/content.aspx?id=34066. Accessed June 17, 2012.
Advise men with benign prostatic hyperplasia (BPH) not to take saw palmetto for urinary symptoms. Explain that it has not been found to alleviate symptoms, even at triple the standard dose.1
A: Based on evidence from a high-quality randomized controlled trial (RCT)1 and a 2009 meta-analysis.2
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
ILLUSTRATIVE CASE
A 66-year-old man comes to your office complaining of urinary frequency and straining to begin urination. He was recently diagnosed with BPh by a urologist, but is hesitant to begin taking a prescription drug. The patient, who is on a fixed income, asks you if saw palmetto extract might relieve his urinary symptoms. What should you tell him?
Roughly 40% of American men older than 60 years and nearly 90% of men older than 80 suffer from BPH and the troublesome lower urinary tract symptoms (LUTS) that it causes.3 Established medical and surgical options, as well as over-the-counter (OTC) plant-based products, are used for symptom relief. The OTC remedy most commonly used for BPH is Serenoa repens, derived from the saw palmetto dwarf palm tree. In a 2007 survey, 1.6 million US adults reported using saw palmetto extract, often as a treatment for BPH, in the 30 days prior to the survey.4
Until now, more questions than answers
As a family physician, you undoubtedly have many patients who are taking or considering taking saw palmetto for relief of BPH symptoms. The significant adverse effects of alpha-blockers and 5-alpha-reductase inhibitors, which are typically prescribed for LUTS—including decreased libido and dizziness—may help account for their interest in this alternative treatment.5,6
Until recently, evidence of saw palmetto’s efficacy has been limited and conflicting, despite widespread use of the extract. That has left many of us wondering whether we should recommend that men with BPH try saw palmetto despite the limited evidence; whether it is effective for some, but not all, BPH symptoms; and whether an increase in dose would increase its efficacy.
A 2002 Cochrane meta-analysis of 21 trials of saw palmetto extract for LUTS reported reduced nocturia, improved self-reported symptoms, and increased peak uroflow compared with placebo, without significant adverse effects.7 An updated Cochrane review published in 2009 included several more rigorous trials—and had very different results: This meta-analysis, which was based on 30 trials, found a reduction in nocturia, but failed to show improvement in other self-reported symptoms or peak uroflow.2
The largest trial included in the 2009 review was the Saw Palmetto Treatment for Enlarged Prostates (STEP) study,8 a one-year study with 225 participants. Its findings: no improvement in the treatment group compared with the placebo group in symptom scores or any secondary endpoints, and no important toxicity.8 Of note, the STEP study and most trials included in the 2009 Cochrane review used the standard saw palmetto extract dose of 160 mg twice daily.1,2
STUDY SUMMARY: Saw palmetto is ineffective, even at triple the dose
Barry et al conducted a 72-week double-blind, multicenter placebo-controlled trial to assess the effect of double (640 mg/d) and triple (960 mg/d) the standard dose of saw palmetto extract on BPH symptoms.1 The study included 369 men with moderate LUTS who had not recently received treatment for BPH. Exclusion criteria included a history of invasive BPH treatment, recent treatment with either an alpha-blocker or a 5-alpha-reductase inhibitor; recent phytotherapy, including saw palmetto; and a history of prostate or bladder cancer. Participants were randomized to receive either saw palmetto extract or an identical-looking placebo gel cap. Doses started at 320 mg/d and were increased to 640 mg/d at 24 weeks and 960 mg/d at 48 weeks.
The primary outcome was the change in the American Urological Association Symptom Index (AUASI) score from baseline to 72 weeks. AUASI, a scale of 0 to 35 in which higher numbers represent increased symptoms, is the same scoring tool used in both the Cochrane review and the STEP trial. Secondary measures included other symptom scales, peak uroflow, and poststudy satisfaction. The treatment and placebo groups had statistically identical baseline characteristics, and the sample size was large enough to detect clinically significant differences.
The AUASI score decreased by a mean of 2.20 points (95% confidence interval [CI], -3.04 to -0.36) in the group that received saw palmetto and by 2.99 points (95% CI, -3.81 to -2.17) in the placebo group—a mean difference of 0.79 in favor of the placebo group (P=.91). The proportion of participants achieving a 3-point reduction in AUASI score was statistically similar between the 2 groups (P=0.66). There was no significant dose response difference between the 2 groups, and saw palmetto proved to be no better than placebo for any of the secondary outcomes.
Subgroup analysis did not reveal any results that differed from the main outcomes. The only adverse events that were significantly different between the 2 groups related to physical injury or trauma, which were unlikely to be due to the intervention.1
By using the same symptom scoring system (AUASI) as many studies in the previous Cochrane reviews, Barry et al were able to compare their findings with those of other high-quality studies with similar methodologies and outcome measures. Despite using an even higher dose of the extract, the results of this trial are remarkably consistent with previous conclusions: Saw palmetto is not an effective treatment for symptoms associated with BPH. Moreover, this trial had a broad base of participants similar to the population in a primary care practice, including patients who would typically choose a natural remedy for LUTS.1
WHAT’S NEW: We now have answers to queries about saw palmetto
This trial is the first to compare higher doses of saw palmetto with placebo to assess a dosage threshold for effectiveness. While the study found no evidence of saw palmetto toxicity even at these higher doses, the extract did not outperform placebo for any measured outcome.1
This high-quality study confirmed the recent series of rigorous studies with negative outcomes by showing that the use of a standard dosage was not a study limitation and that saw palmetto extract is not effective for treating LUTS at any dosage. This trial should substantially affect future guideline recommendations that were limited by methodological concerns in the past.9-11
CAVEATS: In theory, individual preparations could work differently
It is not possible to be absolutely certain that these findings apply to all saw palmetto extract preparations, given the unknown active ingredients and unknown mechanism of action. However, the researchers used a high-quality preparation (a proprietary lipidic ethanolic extract) of saw palmetto at higher doses than the STEP trial and came to a similar conclusion, making it highly unlikely that another preparation would perform differently.
CHALLENGES TO IMPLEMENTATION: There are none
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Advise men with benign prostatic hyperplasia (BPH) not to take saw palmetto for urinary symptoms. Explain that it has not been found to alleviate symptoms, even at triple the standard dose.1
A: Based on evidence from a high-quality randomized controlled trial (RCT)1 and a 2009 meta-analysis.2
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
ILLUSTRATIVE CASE
A 66-year-old man comes to your office complaining of urinary frequency and straining to begin urination. He was recently diagnosed with BPh by a urologist, but is hesitant to begin taking a prescription drug. The patient, who is on a fixed income, asks you if saw palmetto extract might relieve his urinary symptoms. What should you tell him?
Roughly 40% of American men older than 60 years and nearly 90% of men older than 80 suffer from BPH and the troublesome lower urinary tract symptoms (LUTS) that it causes.3 Established medical and surgical options, as well as over-the-counter (OTC) plant-based products, are used for symptom relief. The OTC remedy most commonly used for BPH is Serenoa repens, derived from the saw palmetto dwarf palm tree. In a 2007 survey, 1.6 million US adults reported using saw palmetto extract, often as a treatment for BPH, in the 30 days prior to the survey.4
Until now, more questions than answers
As a family physician, you undoubtedly have many patients who are taking or considering taking saw palmetto for relief of BPH symptoms. The significant adverse effects of alpha-blockers and 5-alpha-reductase inhibitors, which are typically prescribed for LUTS—including decreased libido and dizziness—may help account for their interest in this alternative treatment.5,6
Until recently, evidence of saw palmetto’s efficacy has been limited and conflicting, despite widespread use of the extract. That has left many of us wondering whether we should recommend that men with BPH try saw palmetto despite the limited evidence; whether it is effective for some, but not all, BPH symptoms; and whether an increase in dose would increase its efficacy.
A 2002 Cochrane meta-analysis of 21 trials of saw palmetto extract for LUTS reported reduced nocturia, improved self-reported symptoms, and increased peak uroflow compared with placebo, without significant adverse effects.7 An updated Cochrane review published in 2009 included several more rigorous trials—and had very different results: This meta-analysis, which was based on 30 trials, found a reduction in nocturia, but failed to show improvement in other self-reported symptoms or peak uroflow.2
The largest trial included in the 2009 review was the Saw Palmetto Treatment for Enlarged Prostates (STEP) study,8 a one-year study with 225 participants. Its findings: no improvement in the treatment group compared with the placebo group in symptom scores or any secondary endpoints, and no important toxicity.8 Of note, the STEP study and most trials included in the 2009 Cochrane review used the standard saw palmetto extract dose of 160 mg twice daily.1,2
STUDY SUMMARY: Saw palmetto is ineffective, even at triple the dose
Barry et al conducted a 72-week double-blind, multicenter placebo-controlled trial to assess the effect of double (640 mg/d) and triple (960 mg/d) the standard dose of saw palmetto extract on BPH symptoms.1 The study included 369 men with moderate LUTS who had not recently received treatment for BPH. Exclusion criteria included a history of invasive BPH treatment, recent treatment with either an alpha-blocker or a 5-alpha-reductase inhibitor; recent phytotherapy, including saw palmetto; and a history of prostate or bladder cancer. Participants were randomized to receive either saw palmetto extract or an identical-looking placebo gel cap. Doses started at 320 mg/d and were increased to 640 mg/d at 24 weeks and 960 mg/d at 48 weeks.
The primary outcome was the change in the American Urological Association Symptom Index (AUASI) score from baseline to 72 weeks. AUASI, a scale of 0 to 35 in which higher numbers represent increased symptoms, is the same scoring tool used in both the Cochrane review and the STEP trial. Secondary measures included other symptom scales, peak uroflow, and poststudy satisfaction. The treatment and placebo groups had statistically identical baseline characteristics, and the sample size was large enough to detect clinically significant differences.
The AUASI score decreased by a mean of 2.20 points (95% confidence interval [CI], -3.04 to -0.36) in the group that received saw palmetto and by 2.99 points (95% CI, -3.81 to -2.17) in the placebo group—a mean difference of 0.79 in favor of the placebo group (P=.91). The proportion of participants achieving a 3-point reduction in AUASI score was statistically similar between the 2 groups (P=0.66). There was no significant dose response difference between the 2 groups, and saw palmetto proved to be no better than placebo for any of the secondary outcomes.
Subgroup analysis did not reveal any results that differed from the main outcomes. The only adverse events that were significantly different between the 2 groups related to physical injury or trauma, which were unlikely to be due to the intervention.1
By using the same symptom scoring system (AUASI) as many studies in the previous Cochrane reviews, Barry et al were able to compare their findings with those of other high-quality studies with similar methodologies and outcome measures. Despite using an even higher dose of the extract, the results of this trial are remarkably consistent with previous conclusions: Saw palmetto is not an effective treatment for symptoms associated with BPH. Moreover, this trial had a broad base of participants similar to the population in a primary care practice, including patients who would typically choose a natural remedy for LUTS.1
WHAT’S NEW: We now have answers to queries about saw palmetto
This trial is the first to compare higher doses of saw palmetto with placebo to assess a dosage threshold for effectiveness. While the study found no evidence of saw palmetto toxicity even at these higher doses, the extract did not outperform placebo for any measured outcome.1
This high-quality study confirmed the recent series of rigorous studies with negative outcomes by showing that the use of a standard dosage was not a study limitation and that saw palmetto extract is not effective for treating LUTS at any dosage. This trial should substantially affect future guideline recommendations that were limited by methodological concerns in the past.9-11
CAVEATS: In theory, individual preparations could work differently
It is not possible to be absolutely certain that these findings apply to all saw palmetto extract preparations, given the unknown active ingredients and unknown mechanism of action. However, the researchers used a high-quality preparation (a proprietary lipidic ethanolic extract) of saw palmetto at higher doses than the STEP trial and came to a similar conclusion, making it highly unlikely that another preparation would perform differently.
CHALLENGES TO IMPLEMENTATION: There are none
We see no challenges to implementation of this recommendation.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
2. Tacklind J, MacDonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423.-
3. Roehrborn CG, McConnell JD. Etiology, Pathophysiology, Epidemiology, and Natural History of Benign Prostatic Hyperplasia. 8th ed. Philadelphia, Pa: Campbell’s Urology; 2002.
4. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
5. Traish AM, Hassani J, Guay AT, et al. Adverse side effects of 5-alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872-884.
6. Clifford GM, Farmer RD. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
7. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(3):CD001423.-
8. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
9. Benign prostatic hyperplasia: treatment {saw palmetto}. In: DynaMed. Available at: http://www.DynamicMedical.com. Accessed April 16, 2012.
10. Saper R. Clinical use of saw palmetto {saw palmetto}. In: Basow DS, ed. UpToDate [database online]. Waltham, Mass: UpToDate 2012. Available at: http://www.uptodate.com. Accessed April 16, 2012.
11. Agency for Healthcare Research and Quality. Guidelines on the treatment of non-neurogenic male LUTS. Available at: http://www.guideline.gov/content.aspx?id=34066. Accessed June 17, 2012.
1. Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344-1351.
2. Tacklind J, MacDonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423.-
3. Roehrborn CG, McConnell JD. Etiology, Pathophysiology, Epidemiology, and Natural History of Benign Prostatic Hyperplasia. 8th ed. Philadelphia, Pa: Campbell’s Urology; 2002.
4. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
5. Traish AM, Hassani J, Guay AT, et al. Adverse side effects of 5-alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872-884.
6. Clifford GM, Farmer RD. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2-19.
7. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(3):CD001423.-
8. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
9. Benign prostatic hyperplasia: treatment {saw palmetto}. In: DynaMed. Available at: http://www.DynamicMedical.com. Accessed April 16, 2012.
10. Saper R. Clinical use of saw palmetto {saw palmetto}. In: Basow DS, ed. UpToDate [database online]. Waltham, Mass: UpToDate 2012. Available at: http://www.uptodate.com. Accessed April 16, 2012.
11. Agency for Healthcare Research and Quality. Guidelines on the treatment of non-neurogenic male LUTS. Available at: http://www.guideline.gov/content.aspx?id=34066. Accessed June 17, 2012.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.