Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration. It returns at 700 ng/mL. Should you order CT pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a D-dimer concentration can exclude PE without CTPA in 20% to 30% of patients.4 However, due to the complexity of the algorithm and insufficient time in busy emergency departments, adherence to recommended diagnostic strategies is variable.5
Further, it is common for a D-dimer test to be obtained before clinical assessment by a provider.6 A fixed cutoff D-dimer concentration of 500 ng/mL is commonly used, despite an absolute reduction of 11.6% in the need for CTPA using an age-adjusted D-dimer concentration threshold (age × 10 ng/mL for patients older than 50).7
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D-dimer concentration thresholds could retain sensitivity and decrease unnecessary CTPA. Decreasing CTPA would avoid contrast-induced nephropathy and decrease cancers associated with radiation exposure.9-11 Significant cost savings could also be achieved, as the estimated cost of one CTPA is $648, while a D-dimer concentration is estimated to cost $14.12
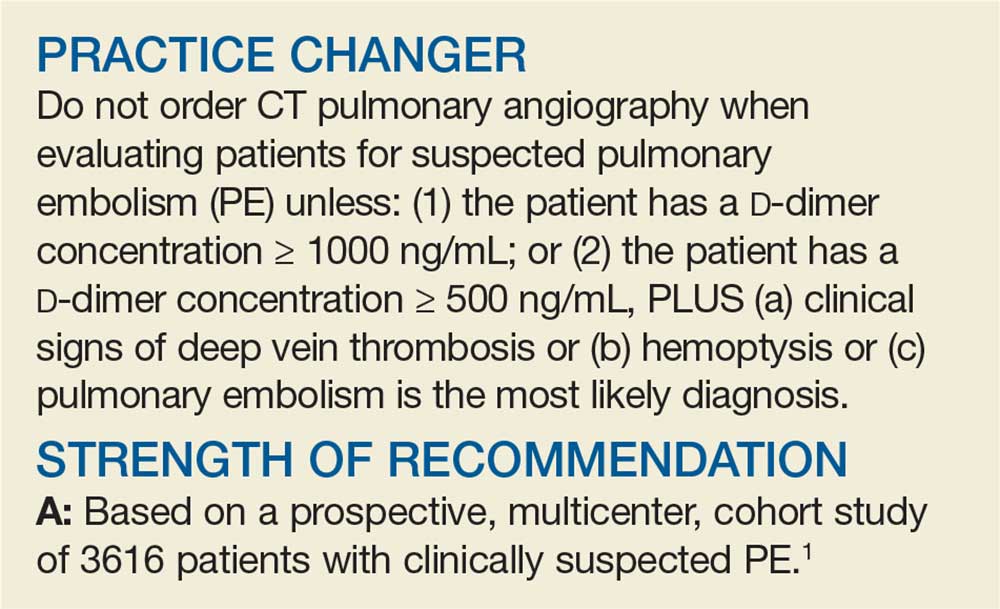
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a D-dimer concentration in all patients and assessing for the 3 items in the YEARS clinical decision rule: clinical signs of deep vein thrombosis; hemoptysis; and whether PE was the most likely diagnosis.
PE was considered excluded if a patient had a D-dimer concentration < 1000 ng/mL and no positive YEARS items or if the patient had a D-dimer concentration < 500 ng/mL and 1 or more YEARS items. The primary outcome was venous thromboembolism (VTE) events at 3 months’ follow-up once PE was excluded. The secondary outcome was the number of required CTPAs using the YEARS decision rule compared with the number that would have been required if the Wells diagnostic algorithm had been implemented.
Continue to: Of the 1743 patients...