User login
Which tube placement is best for a patient requiring enteral nutrition?
Comparative advantages of EN tubes
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
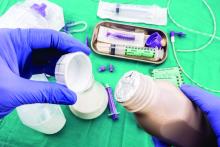
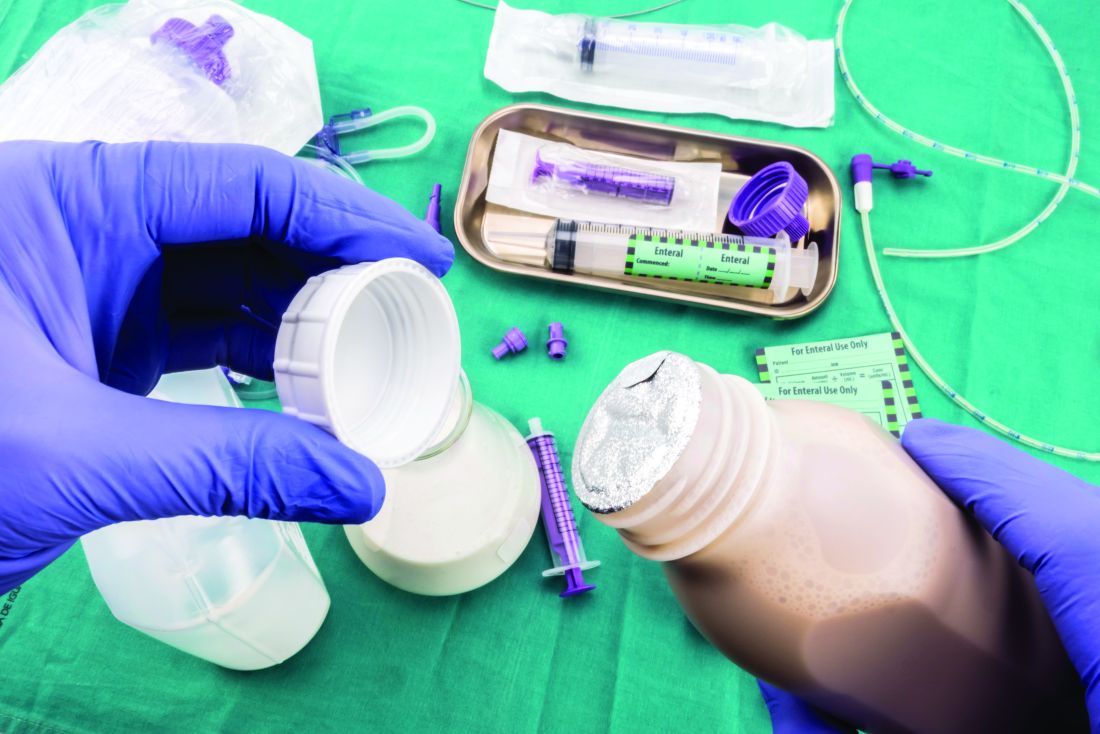
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
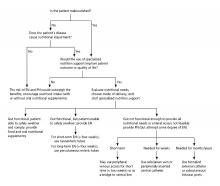
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
Comparative advantages of EN tubes
Comparative advantages of EN tubes
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
Case
A 68-year-old diabetic nonverbal female presents to the ED because of “seizure” 1 hour ago. On exam, her blood glucose is 200. She is unable to speak and has dysphagia because of a stroke she sustained last month. The patient’s husband adds that she hasn’t been eating and drinking sufficiently in the past couple of days. Imaging was negative for any acute intracranial bleeds or lesions. Labs showed a serum sodium level of 150 milliequivalents/L. D5W is started, and the following day, the patient has a sodium level of 154 milliequivalents/L.
Brief overview
Many hospitalized patients are unable to maintain hydration and/or nutritional status by mouth and will need enteral nutrition. Variables such as past medical history, swallowing ability, history of aspiration, prognosis, and functional capacity of each gastrointestinal segment will determine the best option for enteral nutrition for each patient. Each type of enteral tube feeding has advantages, disadvantages, and complications.
Overview of the data
Enteral nutrition should be started within 24-48 hours in a critically ill patient who is unable to maintain intake according to the American Society of Parenteral and Enteral Nutrition.1 This can be provided through a nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) tube, PEG tube with jejunal extension (PEG-J), or a percutaneous endoscopic jejunal (PEJ) tube.1
NG tubes are often the first method deployed because of their low cost and convenience. They are also suitable for the patient who requires this type of feeding for less than 4 weeks. However, NG tubes do require some patient cooperation (to place and maintain)and are contraindicated in some patients with orofacial trauma, upper GI tumors, inadequate lower esophageal sphincter tone, and gastroparesis.2
Another option is a PEG tube, which is a good alternative for patients who are sedated; ventilated; or have neurodegenerative processes, stroke with dysphagia, or head and neck cancers. These are typically recommended when enteral nutrition will be needed for more than 4 weeks. Disadvantages of PEG tubes include tube obstruction or displacement, gastroesophageal reflux, and leakage of gastric content around the percutaneous site or into the peritoneum.
PEG-J tubes, PEJ tubes, or jejunostomy tubes are best suited for patients with GI dysmotility, patients who have unsuccessfully undergone the aforementioned methods, patients with histories of partial gastrectomies, or patients with gastric or pancreatic cancers/multiple traumas. The PEG-J tube extends into the distal duodenum; because it is longer and more narrow, it is more likely to coil and occlude the flow of nutrients during feedings.2,3 Jejunal feeding methods incorporate a continuous pump controlled infusion; if set too rapidly, this could cause dumping syndrome. A benefit of jejunal nutrition is a lower risk of aspiration, compared with other enteral tubes.4
It is best to appraise the selected method for its efficacy and patient preference. The American College of Gastroenterology recommends starting with orogastric or nasogastric feeds, and switching to postpyloric or jejunal feeds for those intolerant to or at high risk for aspiration.5 The most important aspect is early enteral nutrition in hospitalized patients unable to maintain oral nutrition.
Application of the data to the original case
This is a severely hypernatremic diabetic patient unable to swallow. On day 2 of her hospitalization, the clinical team provided the patient with an NG tube for increased free-water intake to gradually decrease her serum sodium. By hospital day 4, the patient’s sodium had normalized. Considering the patient’s long-term prognosis and dysphagia, discussions were held with the patient and husband for PEG tube placement. The patient received a PEG tube and was subsequently discharged 2 days later.
Bottom line
Enteral nutrition is a common need among hospitalized patients. Modality of enteral nutrition will depend on the patient’s past medical history, anticipated duration, and preferences.
Dr. Basnet is the hospitalist program director for Apogee Physicians Group at Eastern New Mexico Medical Center in Roswell. Ms. Tayes is a third-year medical student at Burrell College of Osteopathic Medicine in Las Cruces, N.M., with interests in surgery, internal medicine, and emergency medicine. Ms. Gallivan is a third-year medical student at Burrell College of Osteopathic Medicine, with interests in cardiothoracic surgery, general surgery, and internal medicine.
References
1. Boullata JI et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J Parenter Enteral Nutr. 2016;1-89.
2. Kirby DF et al. American Gastroenterological Association technical review on tube feeding for enteral nutrition. Gastroenterology. 1995;108:1282.
3. Lazarus BA et al. Aspiration associated with long-term gastric versus jejunal feeding: A critical analysis of the literature. Arch Phys Med Rehabil. 1990;71:46.
4. Alkhawaja S et al. Postpyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015 Aug 4;(8):CD008875.
5. McCalve SA et al. ACG Clinical Guideline: Nutrition therapy in the hospitalized patient. Am J Gastroenterol. 2016;111:315-34. doi: 10.1038/ajg.2016.28.
Additional reading
Bellini LM. Nutrition Support in Advanced Lung Disease. UptoDate. https://www-uptodate-com.ezproxy.ad.bcomnm.org/contents/nutritional-support-in-advanced-lung-disease?. Published April 20, 2018.
Commercial Formulas for the Feeding Tube. The Oral Cancer Foundation. https://oralcancerfoundation.org/nutrition/commercial-formulas-feeding-tube/. Published June 5, 2018.
Marik Z. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11). doi: 10.1007/s00134-008-1213-6.
Wischmeyer PE. Enteral nutrition can be given to patients on vasopressors. Crit Care Med. 2020;48(1):122-5. doi: 10.1097/CCM.0000000000003965.
When do I stop the code?
A hospitalist’s dilemma
I had just received my sign-out for the day. My pager beeped, and I heard it overhead “Code Blue Room X.” Hospitalist physicians lead the code team in our hospital; I quickly headed to the room.
A young man in his forties was found to be unconscious on the floor. One of the nurses had started cardiopulmonary resuscitation (CPR) as the patient was unconscious and had no palpable pulse. It was a long, drawn-out battle: CPR, cracking bones, shouting, lots of needles – an extreme roller-coaster-style situation. The patient had recently had a hip surgery and our suspicion was a massive pulmonary embolism. We ran the exhaustive code for more than an hour and then I started to debrief with my code team; discussed that treatment was getting futile and asked for opinions. Finally, I asked the team to stop and pronounced the patient dead. I felt terrible. Later that day I returned to my house, tossed my bag in the corner, and sympathized with myself – “Hello Dr. B, It was a tough one.”
Stopping resuscitation was one of the toughest decisions I had ever made, and I wondered if I would be able to make such a decision the next day. What if I had carried on? I had led code teams during my residency training and as an attending physician; but there was something different that day. This patient was a young man with no history of medical problems. Every physician knows how to initiate resuscitation for cardiopulmonary arrest (CPA); only a few know when to stop it. Did I miss this learning during my internal medicine training? I checked my red pocket leaflet with advanced cardiac life support (ACLS) algorithms, and it had no mention of it. I searched Google Scholar, PubMed, and UpToDate and surprisingly, I found no predetermined rule but only a few recommendations on when CPR should be stopped. The American Heart Association is clear that the decision to terminate resuscitative efforts rests with the treating physician in the hospital.
In my experience, the length of time to continue a code can vary widely and is mostly dependent on the physician running the code. I have seen it last 15 minutes (which is reasonable) and I have seen it last for 50 minutes when the initial rhythm was ventricular fibrillation. And if perhaps the patient regains a pulse temporarily, only to lose it again, we restart the clock. One needs to take into account various factors including time to CPR, time to defibrillation, comorbid disease, prearrest state, and initial arrest rhythm in making these decisions. It’s well understood that none of these factors alone or in combination is clearly predictive of outcome.1
Some selected patients potentially have good outcomes with prolonged, aggressive resuscitation. So when should we stop, and when should we continue resuscitation? This is always challenging. Physicians hate to stop CPR even when they know it’s time. We are guided by the Hippocratic Oath to save lives. Sometimes, even if we want to stop, we tend to continue to avoid being criticized for stopping; we are systematically biased against stopping CPR. We routinely run long codes, in part because we are not sure which patients we can bring back.
A 2012 Lancet study highlighted that the median duration of resuscitation was 12 minutes for patients achieving the return of spontaneous circulation and 20 minutes for nonsurvivors.2 The ethical guidelines issued by AHA in 2018 highlight that, in the absence of mitigating factors, prolonged resuscitative efforts for adults and children are unlikely to be successful and can be discontinued if there is no return of spontaneous circulation at any time during 30 minutes of cumulative ACLS. If the return of spontaneous circulation of any duration occurs at any time, however, it may be appropriate to consider extending the resuscitative effort.3
I believe a careful balance of the patient’s prognosis for both length of life and quality of life will determine whether continued CPR is appropriate. The responsible clinician should stop the resuscitative effort when he or she determines with a high degree of certainty that the arrest victim will not respond to further efforts. But what will help me guide my decisions next time if I ever come across this situation again?
I discussed my dilemma with one of our intensivist physicians; he expressed that in a similar scenario he would ask for opinions from other members of the code team. The role of good communication among code team members is necessary to exchange relevant knowledge in real time in a collaborative, nonhierarchical environment. The code team can provide the team leader with quick, accurate information about the patient’s clinical history that is critical to good decision making.
Family support is also an essential part of any resuscitation. Health care providers need to offer the opportunity to be present to family members during the resuscitation attempts whenever possible. One team member should be assigned to the family to answer questions, clarify information, and offer comfort, but physicians should not be asking family members to decide to stop the code. It is important to note that the decision should be made by the team leader and not the patient’s family members. Regardless of the age or condition of the patient, the loss of a loved one is difficult to deal with, even if expected. The issue becomes more difficult with changes in legal, cultural, or personal perspectives.
The AHA in 2018 stated that the treating physician is expected to understand the patient and the arrest features, and the system factors that have prognostic importance for resuscitation.3 For clinicians who work in critical care settings, the framework presented by AHA is intuitive. As a code leader, I can always give more epinephrine, try a clot-busting drug or deliver another shock. Situations vary greatly during a code, and the amount of time spent resuscitating a patient before terminating efforts is not set in stone. In many cases, it is a judgment call. The process of CPR is almost as disheartening as its bleak outcomes.
In-hospital CPAs are inevitably gruesome. Each day as an attending physician, we are faced with difficult decisions, but experiencing these incredibly difficult and life-changing events can make for good learning. A CPA situation in action is very difficult for all concerned, particularly when there is almost no chance of success. But an unsuccessful or aborted resuscitation is also a huge loss for both the family and the code team. One of the critical functions of the code team leader is to review the events of a code and exercise judgment while evaluating the length of a code. This can be an intense and emotional experience, but with these principles in mind, we can feel reassured that we are making the best decision possible, for the patient, the family, and our team.
Dr. Basnet is a hospitalist physician in the department of internal medicine at Eastern New Mexico Medical Center, Roswell.
References
1. Part 2: Ethical aspects of CPR and ECC. Circulation. 2000;102(8):I12.
2. Goldberger ZD et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: An observational study. The Lancet. 2012;380(9852):1473-81.
3. Sirbaugh PE et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174-84.
A hospitalist’s dilemma
A hospitalist’s dilemma
I had just received my sign-out for the day. My pager beeped, and I heard it overhead “Code Blue Room X.” Hospitalist physicians lead the code team in our hospital; I quickly headed to the room.
A young man in his forties was found to be unconscious on the floor. One of the nurses had started cardiopulmonary resuscitation (CPR) as the patient was unconscious and had no palpable pulse. It was a long, drawn-out battle: CPR, cracking bones, shouting, lots of needles – an extreme roller-coaster-style situation. The patient had recently had a hip surgery and our suspicion was a massive pulmonary embolism. We ran the exhaustive code for more than an hour and then I started to debrief with my code team; discussed that treatment was getting futile and asked for opinions. Finally, I asked the team to stop and pronounced the patient dead. I felt terrible. Later that day I returned to my house, tossed my bag in the corner, and sympathized with myself – “Hello Dr. B, It was a tough one.”
Stopping resuscitation was one of the toughest decisions I had ever made, and I wondered if I would be able to make such a decision the next day. What if I had carried on? I had led code teams during my residency training and as an attending physician; but there was something different that day. This patient was a young man with no history of medical problems. Every physician knows how to initiate resuscitation for cardiopulmonary arrest (CPA); only a few know when to stop it. Did I miss this learning during my internal medicine training? I checked my red pocket leaflet with advanced cardiac life support (ACLS) algorithms, and it had no mention of it. I searched Google Scholar, PubMed, and UpToDate and surprisingly, I found no predetermined rule but only a few recommendations on when CPR should be stopped. The American Heart Association is clear that the decision to terminate resuscitative efforts rests with the treating physician in the hospital.
In my experience, the length of time to continue a code can vary widely and is mostly dependent on the physician running the code. I have seen it last 15 minutes (which is reasonable) and I have seen it last for 50 minutes when the initial rhythm was ventricular fibrillation. And if perhaps the patient regains a pulse temporarily, only to lose it again, we restart the clock. One needs to take into account various factors including time to CPR, time to defibrillation, comorbid disease, prearrest state, and initial arrest rhythm in making these decisions. It’s well understood that none of these factors alone or in combination is clearly predictive of outcome.1
Some selected patients potentially have good outcomes with prolonged, aggressive resuscitation. So when should we stop, and when should we continue resuscitation? This is always challenging. Physicians hate to stop CPR even when they know it’s time. We are guided by the Hippocratic Oath to save lives. Sometimes, even if we want to stop, we tend to continue to avoid being criticized for stopping; we are systematically biased against stopping CPR. We routinely run long codes, in part because we are not sure which patients we can bring back.
A 2012 Lancet study highlighted that the median duration of resuscitation was 12 minutes for patients achieving the return of spontaneous circulation and 20 minutes for nonsurvivors.2 The ethical guidelines issued by AHA in 2018 highlight that, in the absence of mitigating factors, prolonged resuscitative efforts for adults and children are unlikely to be successful and can be discontinued if there is no return of spontaneous circulation at any time during 30 minutes of cumulative ACLS. If the return of spontaneous circulation of any duration occurs at any time, however, it may be appropriate to consider extending the resuscitative effort.3
I believe a careful balance of the patient’s prognosis for both length of life and quality of life will determine whether continued CPR is appropriate. The responsible clinician should stop the resuscitative effort when he or she determines with a high degree of certainty that the arrest victim will not respond to further efforts. But what will help me guide my decisions next time if I ever come across this situation again?
I discussed my dilemma with one of our intensivist physicians; he expressed that in a similar scenario he would ask for opinions from other members of the code team. The role of good communication among code team members is necessary to exchange relevant knowledge in real time in a collaborative, nonhierarchical environment. The code team can provide the team leader with quick, accurate information about the patient’s clinical history that is critical to good decision making.
Family support is also an essential part of any resuscitation. Health care providers need to offer the opportunity to be present to family members during the resuscitation attempts whenever possible. One team member should be assigned to the family to answer questions, clarify information, and offer comfort, but physicians should not be asking family members to decide to stop the code. It is important to note that the decision should be made by the team leader and not the patient’s family members. Regardless of the age or condition of the patient, the loss of a loved one is difficult to deal with, even if expected. The issue becomes more difficult with changes in legal, cultural, or personal perspectives.
The AHA in 2018 stated that the treating physician is expected to understand the patient and the arrest features, and the system factors that have prognostic importance for resuscitation.3 For clinicians who work in critical care settings, the framework presented by AHA is intuitive. As a code leader, I can always give more epinephrine, try a clot-busting drug or deliver another shock. Situations vary greatly during a code, and the amount of time spent resuscitating a patient before terminating efforts is not set in stone. In many cases, it is a judgment call. The process of CPR is almost as disheartening as its bleak outcomes.
In-hospital CPAs are inevitably gruesome. Each day as an attending physician, we are faced with difficult decisions, but experiencing these incredibly difficult and life-changing events can make for good learning. A CPA situation in action is very difficult for all concerned, particularly when there is almost no chance of success. But an unsuccessful or aborted resuscitation is also a huge loss for both the family and the code team. One of the critical functions of the code team leader is to review the events of a code and exercise judgment while evaluating the length of a code. This can be an intense and emotional experience, but with these principles in mind, we can feel reassured that we are making the best decision possible, for the patient, the family, and our team.
Dr. Basnet is a hospitalist physician in the department of internal medicine at Eastern New Mexico Medical Center, Roswell.
References
1. Part 2: Ethical aspects of CPR and ECC. Circulation. 2000;102(8):I12.
2. Goldberger ZD et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: An observational study. The Lancet. 2012;380(9852):1473-81.
3. Sirbaugh PE et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174-84.
I had just received my sign-out for the day. My pager beeped, and I heard it overhead “Code Blue Room X.” Hospitalist physicians lead the code team in our hospital; I quickly headed to the room.
A young man in his forties was found to be unconscious on the floor. One of the nurses had started cardiopulmonary resuscitation (CPR) as the patient was unconscious and had no palpable pulse. It was a long, drawn-out battle: CPR, cracking bones, shouting, lots of needles – an extreme roller-coaster-style situation. The patient had recently had a hip surgery and our suspicion was a massive pulmonary embolism. We ran the exhaustive code for more than an hour and then I started to debrief with my code team; discussed that treatment was getting futile and asked for opinions. Finally, I asked the team to stop and pronounced the patient dead. I felt terrible. Later that day I returned to my house, tossed my bag in the corner, and sympathized with myself – “Hello Dr. B, It was a tough one.”
Stopping resuscitation was one of the toughest decisions I had ever made, and I wondered if I would be able to make such a decision the next day. What if I had carried on? I had led code teams during my residency training and as an attending physician; but there was something different that day. This patient was a young man with no history of medical problems. Every physician knows how to initiate resuscitation for cardiopulmonary arrest (CPA); only a few know when to stop it. Did I miss this learning during my internal medicine training? I checked my red pocket leaflet with advanced cardiac life support (ACLS) algorithms, and it had no mention of it. I searched Google Scholar, PubMed, and UpToDate and surprisingly, I found no predetermined rule but only a few recommendations on when CPR should be stopped. The American Heart Association is clear that the decision to terminate resuscitative efforts rests with the treating physician in the hospital.
In my experience, the length of time to continue a code can vary widely and is mostly dependent on the physician running the code. I have seen it last 15 minutes (which is reasonable) and I have seen it last for 50 minutes when the initial rhythm was ventricular fibrillation. And if perhaps the patient regains a pulse temporarily, only to lose it again, we restart the clock. One needs to take into account various factors including time to CPR, time to defibrillation, comorbid disease, prearrest state, and initial arrest rhythm in making these decisions. It’s well understood that none of these factors alone or in combination is clearly predictive of outcome.1
Some selected patients potentially have good outcomes with prolonged, aggressive resuscitation. So when should we stop, and when should we continue resuscitation? This is always challenging. Physicians hate to stop CPR even when they know it’s time. We are guided by the Hippocratic Oath to save lives. Sometimes, even if we want to stop, we tend to continue to avoid being criticized for stopping; we are systematically biased against stopping CPR. We routinely run long codes, in part because we are not sure which patients we can bring back.
A 2012 Lancet study highlighted that the median duration of resuscitation was 12 minutes for patients achieving the return of spontaneous circulation and 20 minutes for nonsurvivors.2 The ethical guidelines issued by AHA in 2018 highlight that, in the absence of mitigating factors, prolonged resuscitative efforts for adults and children are unlikely to be successful and can be discontinued if there is no return of spontaneous circulation at any time during 30 minutes of cumulative ACLS. If the return of spontaneous circulation of any duration occurs at any time, however, it may be appropriate to consider extending the resuscitative effort.3
I believe a careful balance of the patient’s prognosis for both length of life and quality of life will determine whether continued CPR is appropriate. The responsible clinician should stop the resuscitative effort when he or she determines with a high degree of certainty that the arrest victim will not respond to further efforts. But what will help me guide my decisions next time if I ever come across this situation again?
I discussed my dilemma with one of our intensivist physicians; he expressed that in a similar scenario he would ask for opinions from other members of the code team. The role of good communication among code team members is necessary to exchange relevant knowledge in real time in a collaborative, nonhierarchical environment. The code team can provide the team leader with quick, accurate information about the patient’s clinical history that is critical to good decision making.
Family support is also an essential part of any resuscitation. Health care providers need to offer the opportunity to be present to family members during the resuscitation attempts whenever possible. One team member should be assigned to the family to answer questions, clarify information, and offer comfort, but physicians should not be asking family members to decide to stop the code. It is important to note that the decision should be made by the team leader and not the patient’s family members. Regardless of the age or condition of the patient, the loss of a loved one is difficult to deal with, even if expected. The issue becomes more difficult with changes in legal, cultural, or personal perspectives.
The AHA in 2018 stated that the treating physician is expected to understand the patient and the arrest features, and the system factors that have prognostic importance for resuscitation.3 For clinicians who work in critical care settings, the framework presented by AHA is intuitive. As a code leader, I can always give more epinephrine, try a clot-busting drug or deliver another shock. Situations vary greatly during a code, and the amount of time spent resuscitating a patient before terminating efforts is not set in stone. In many cases, it is a judgment call. The process of CPR is almost as disheartening as its bleak outcomes.
In-hospital CPAs are inevitably gruesome. Each day as an attending physician, we are faced with difficult decisions, but experiencing these incredibly difficult and life-changing events can make for good learning. A CPA situation in action is very difficult for all concerned, particularly when there is almost no chance of success. But an unsuccessful or aborted resuscitation is also a huge loss for both the family and the code team. One of the critical functions of the code team leader is to review the events of a code and exercise judgment while evaluating the length of a code. This can be an intense and emotional experience, but with these principles in mind, we can feel reassured that we are making the best decision possible, for the patient, the family, and our team.
Dr. Basnet is a hospitalist physician in the department of internal medicine at Eastern New Mexico Medical Center, Roswell.
References
1. Part 2: Ethical aspects of CPR and ECC. Circulation. 2000;102(8):I12.
2. Goldberger ZD et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: An observational study. The Lancet. 2012;380(9852):1473-81.
3. Sirbaugh PE et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174-84.