Article
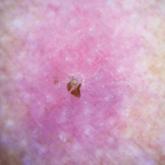
Punked By the Punctum: Domestically Acquired Cutaneous Myiasis
- Author:
- Jeffrey Globerson, DO
- Danielle Yee, MD
- Stephen Olsen, MD
- Brett Bender, DO
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on...
Article
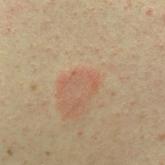
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
- Author:
- Brittany Valk, DO
- Brett Bender, DO
We report a clinically typical case of pityriasis rosea that developed following COVID-19 vaccination.
Article
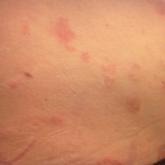
Autoimmune Progesterone Dermatitis
- Author:
- Ivy DeRosa, DO
- Brett Bender, DO
- Michael Centilli, DO
