User login
Progressive Axillary Hyperpigmentation
The Diagnosis: Dowling-Degos Disease
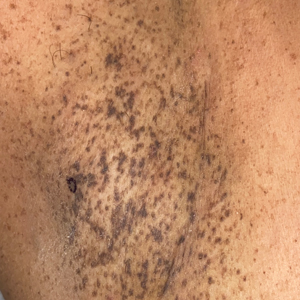
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
A 50-year-old Hispanic woman presented with asymptomatic, progressive, brown hyperpigmentation involving the axillae, neck, upper back, and inframammary areas of 5 years’ duration. She had no other notable medical history; family history was unremarkable. She had been treated with topical hydroquinone and tretinoin by an outside physician without improvement. Physical examination revealed reticulated hyperpigmented macules and patches involving the inverse regions of the neck, axillae, and inframammary regions. Additionally, acneform pitted scars involving the perioral region were seen. A 4.0-mm punch biopsy of the right axilla was performed.
Patient Questionnaire to Reduce Anxiety Prior to Full-Body Skin Examination
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
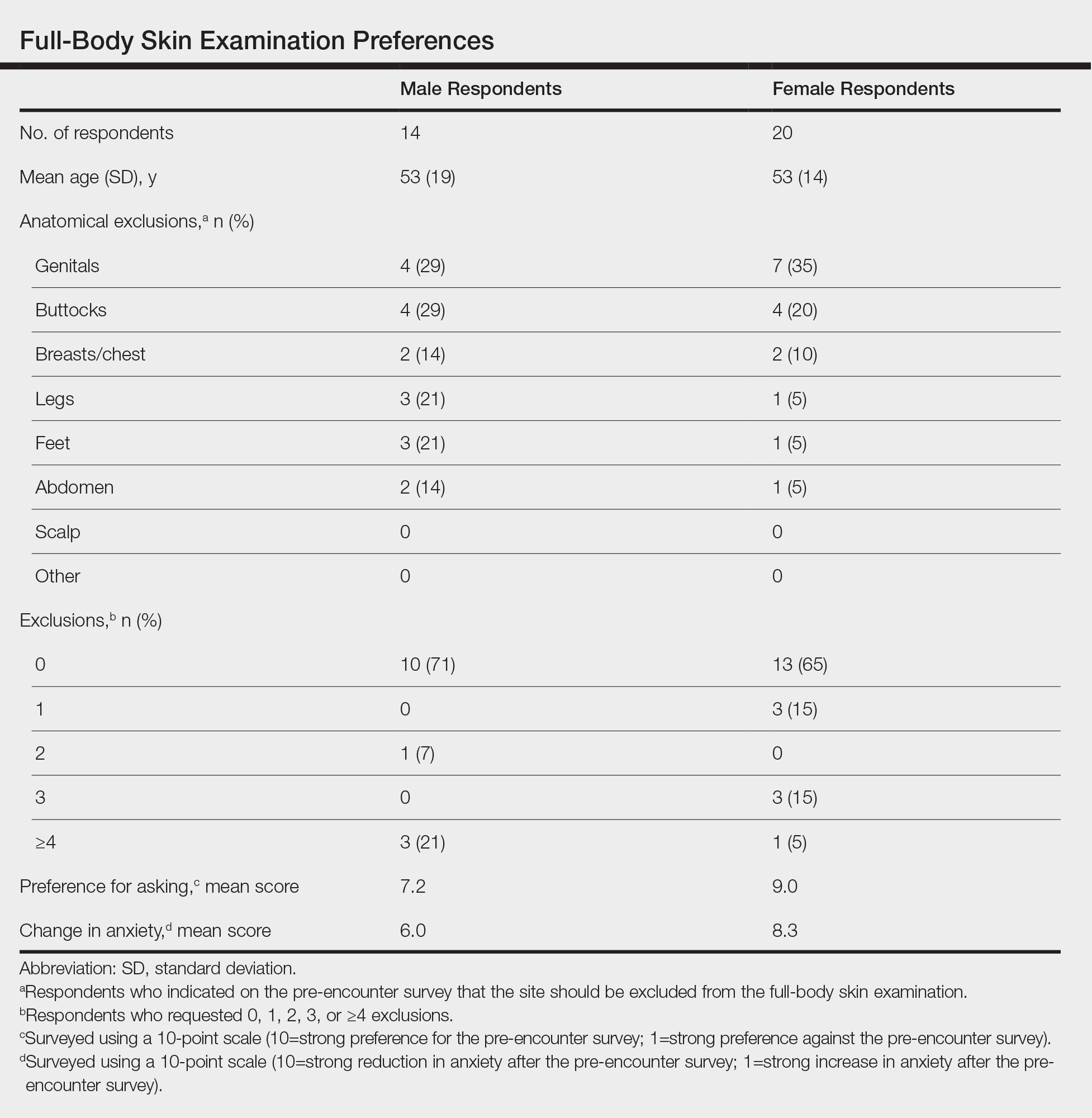
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Practice Points
- Full-body skin examination (FBSE) is an assessment that requires examination of sensitive body areas, any of which can be seen as intrusive by certain patients.
- A pre-encounter survey on the FBSE can offer an efficient means by which to determine patient preference and reduce visit-associated anxiety.
Tense Bullae on the Hands
The Diagnosis: Epidermolysis Bullosa Acquisita
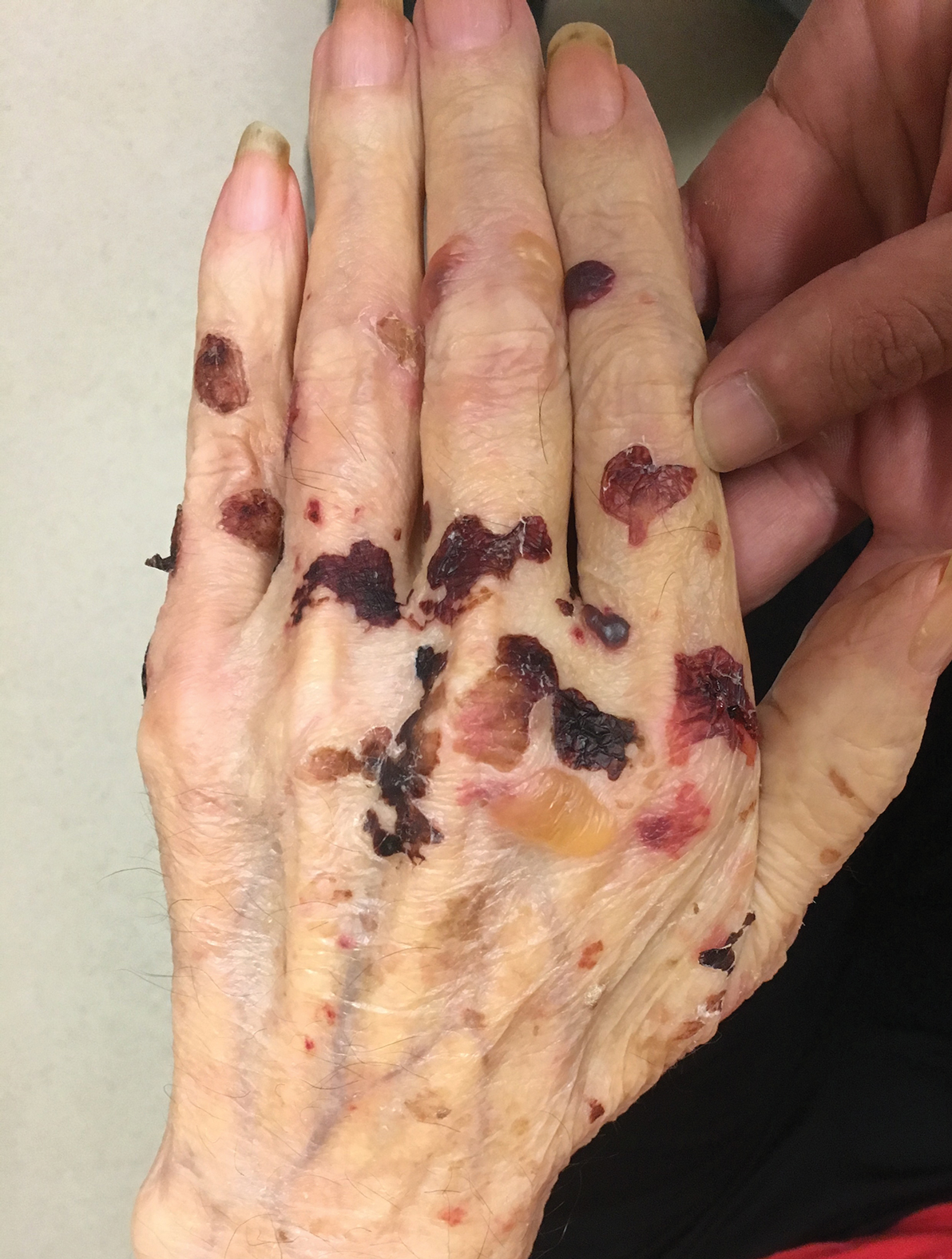
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
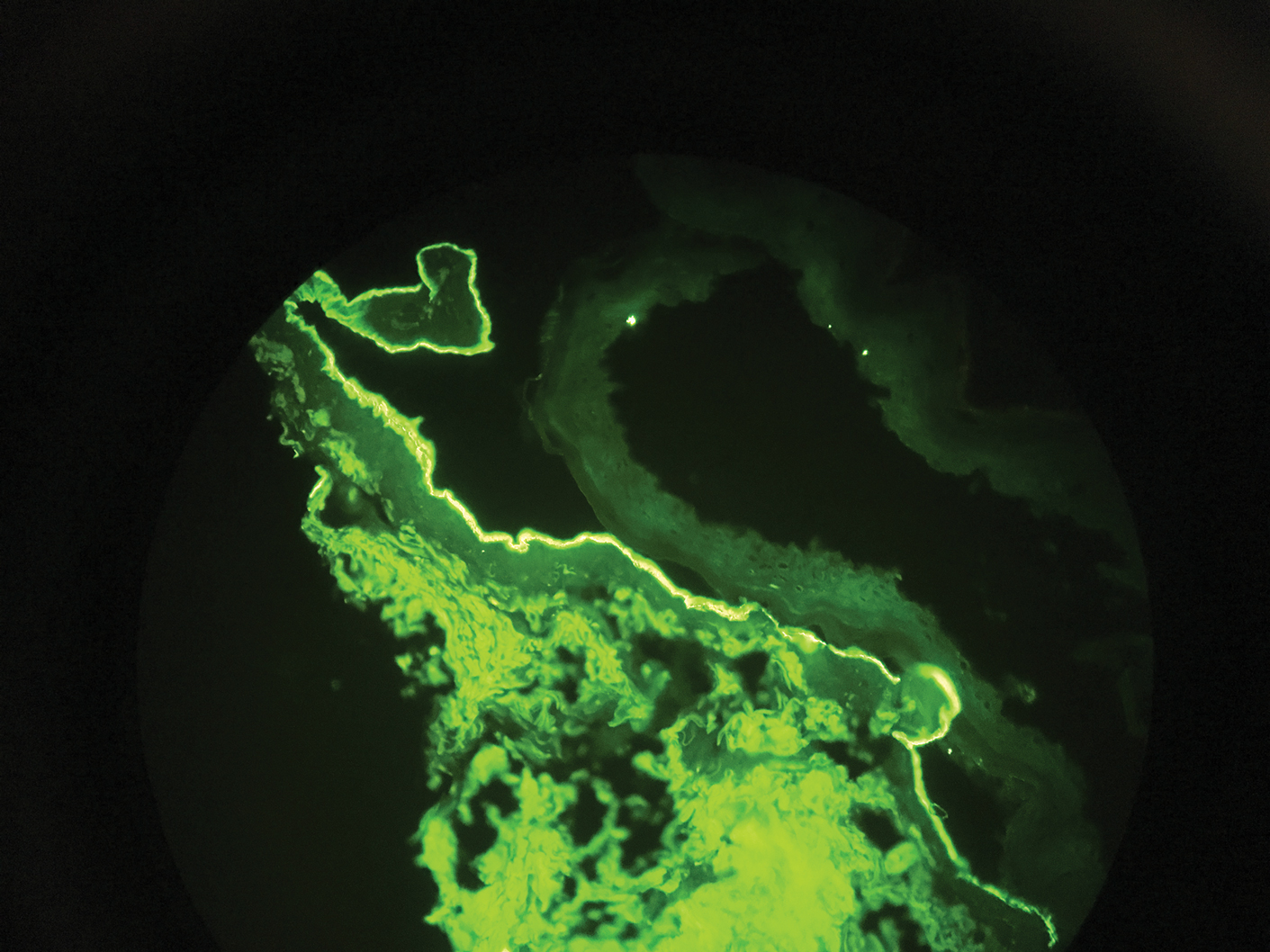
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
A 75-year-old man presented to our clinic with nonpainful, nonpruritic, tense bullae and erosions on the dorsal aspects of the hands and extensor surfaces of the elbows of 1 month's duration. The patient also had erythematous erosions and crusted papules on the left cheek and surrounding the left eye. He denied any new medications, history of liver or kidney disease, or history of hepatitis or human immunodeficiency virus. There were no obvious exacerbating factors, including exposure to sunlight. Direct immunofluorescence using a salt-split preparation was performed on a biopsy of the perilesional skin.
Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
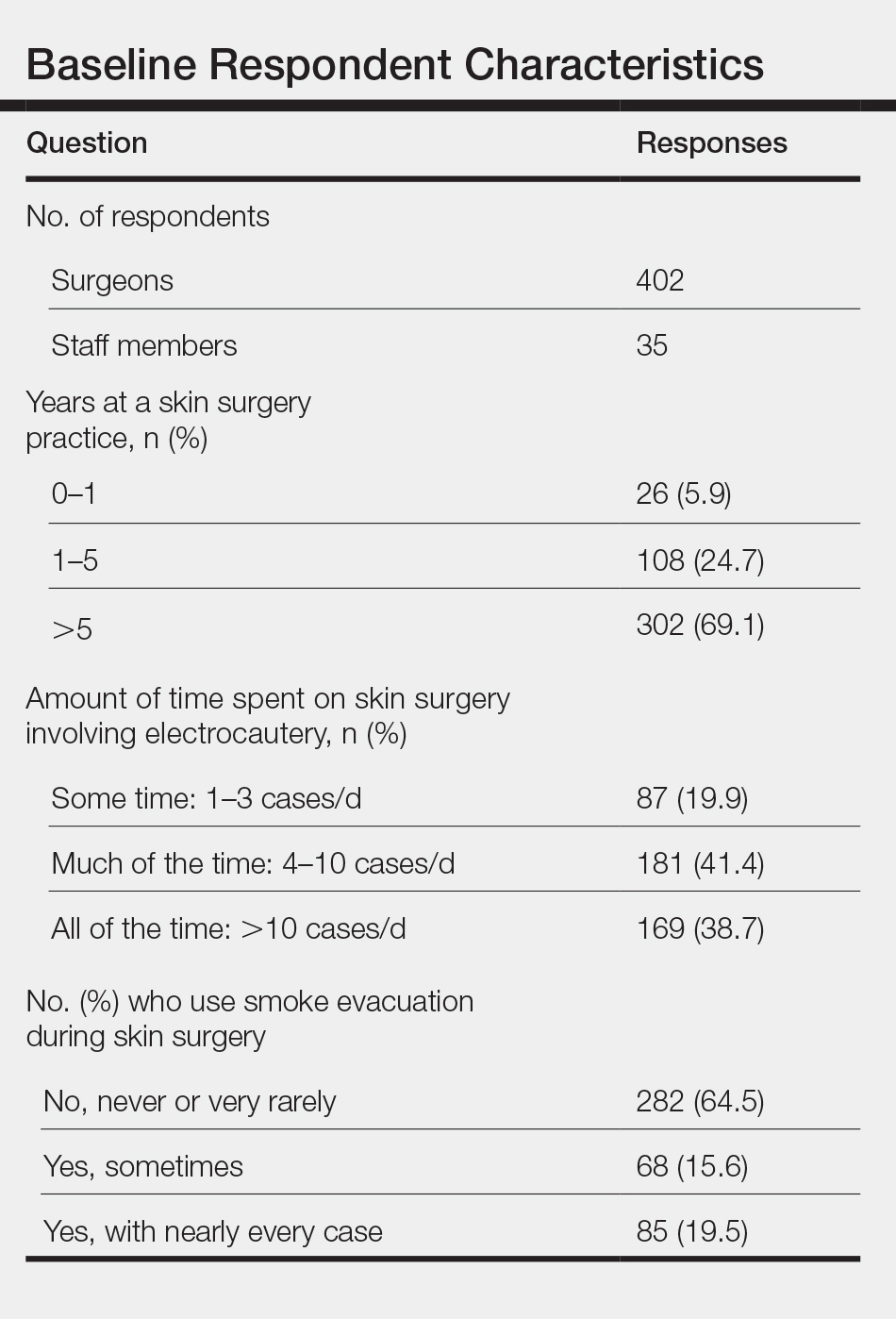
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
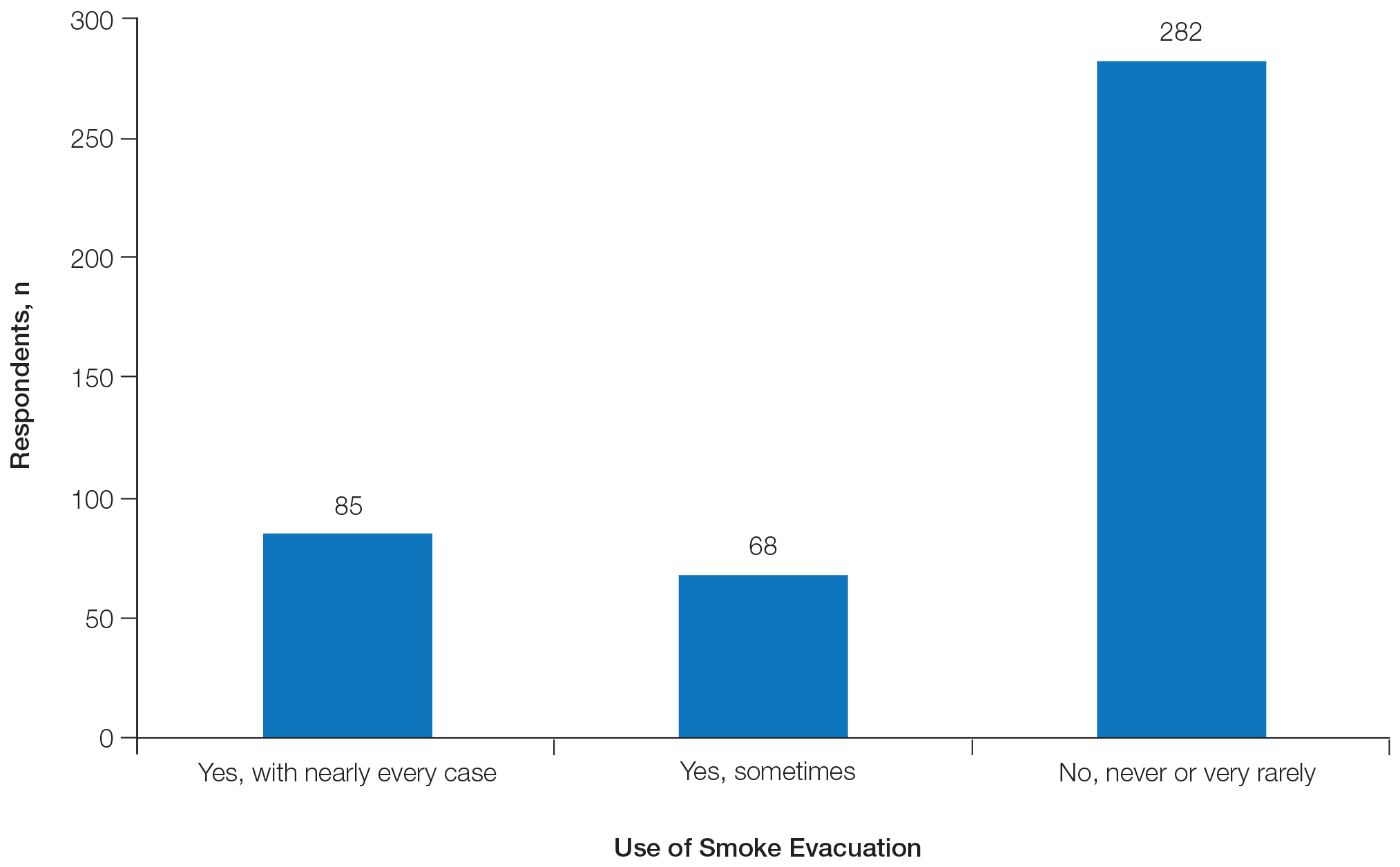
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
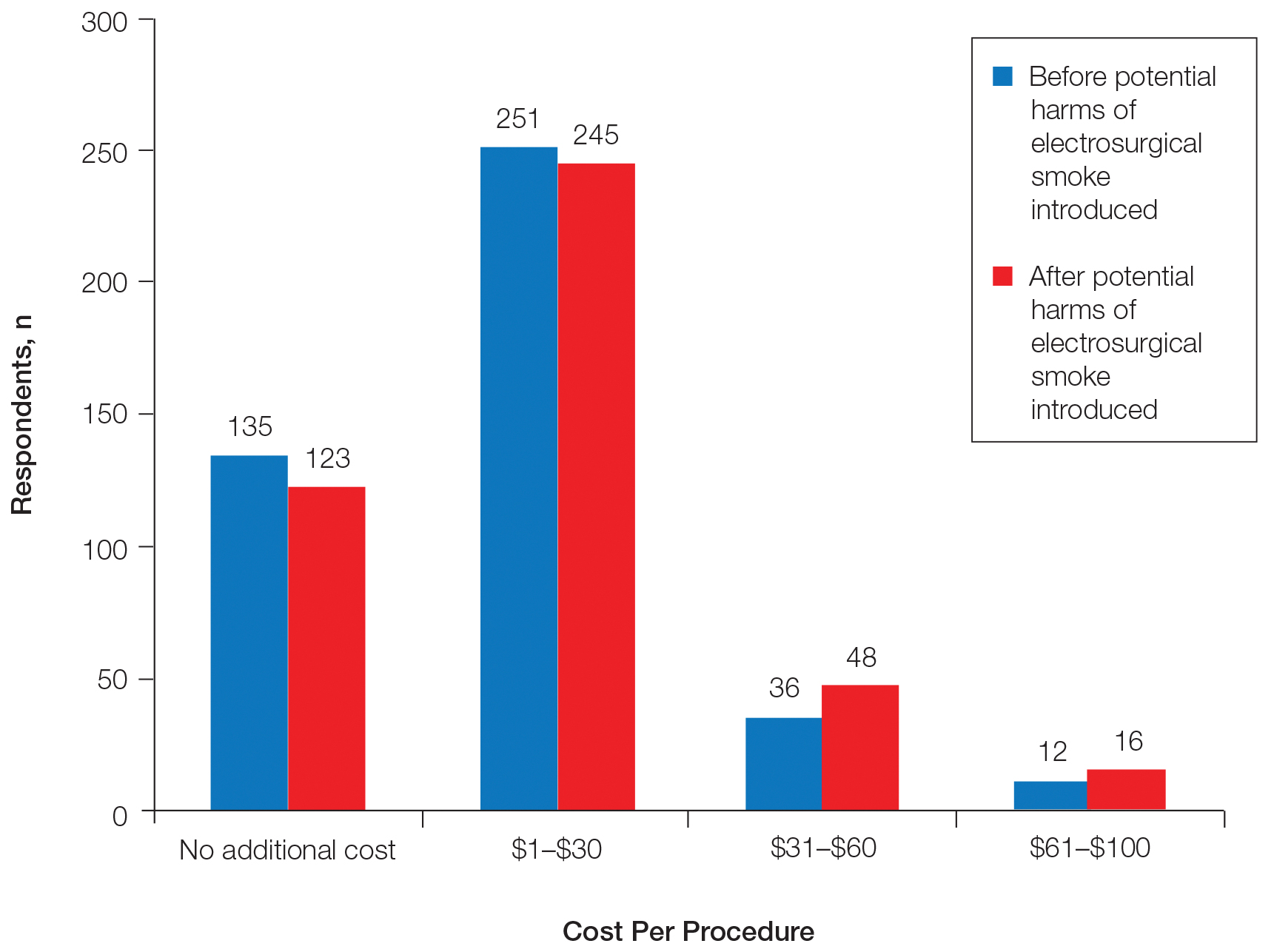
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
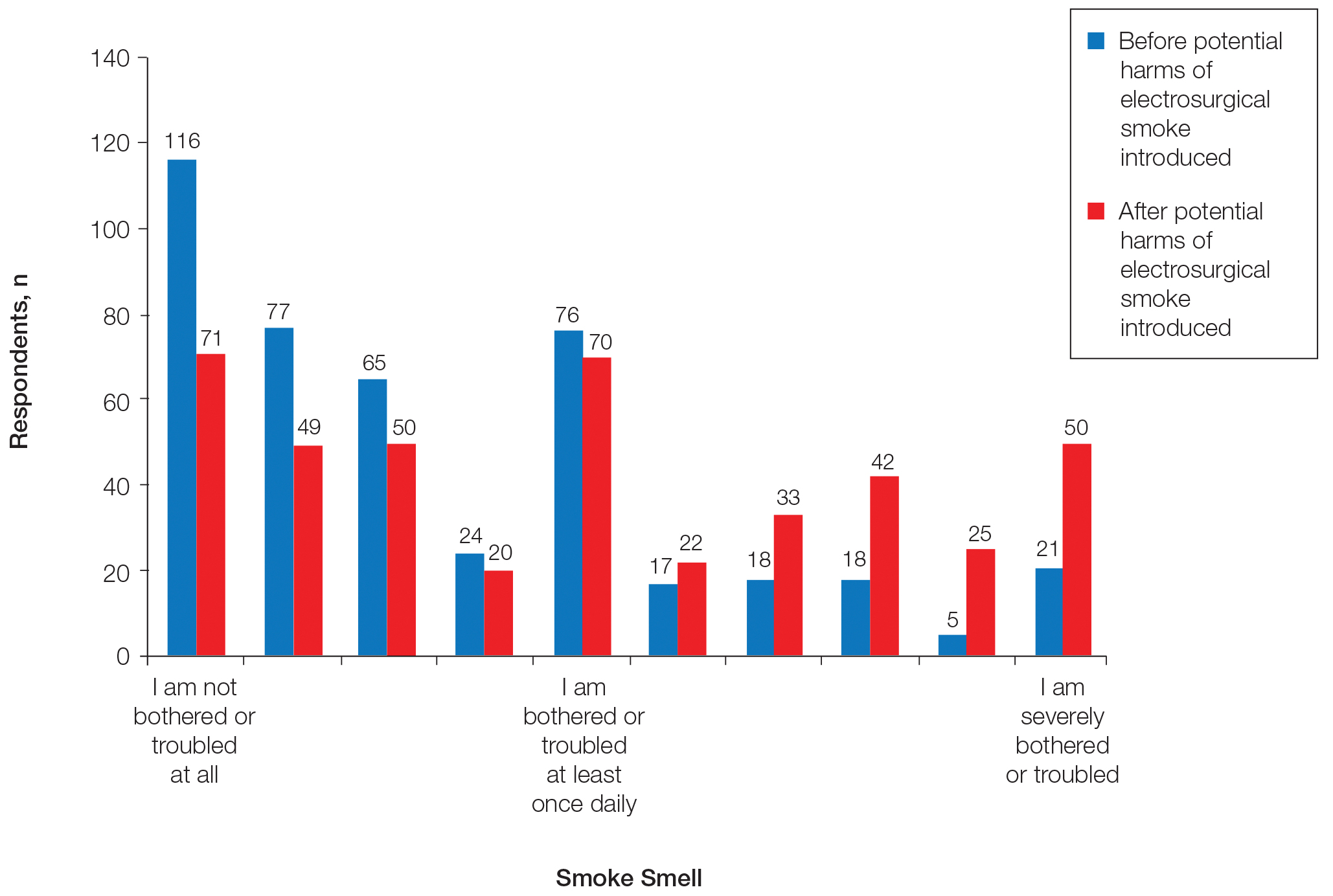
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
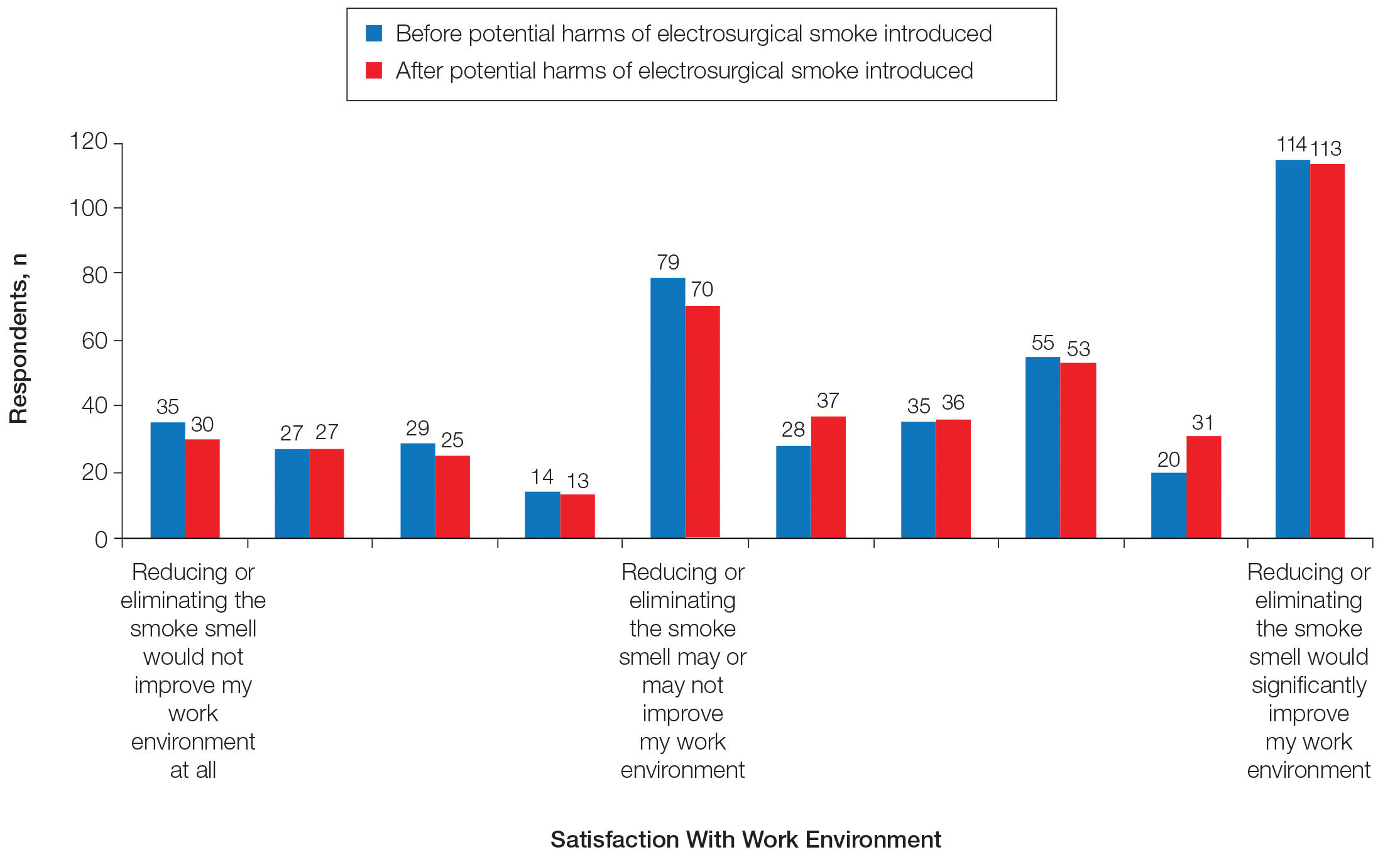
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
Practice Points
- Growing evidence suggests that the surgical smoke plume generated during electrosurgery may be harmful if inhaled.
- Our survey indicates that this information may affect clinician and staff perceptions about exposure to electrosurgical smoke and its remediation.