User login
Management of Acute and Chronic Pain Associated With Hidradenitis Suppurativa: A Comprehensive Review of Pharmacologic and Therapeutic Considerations in Clinical Practice
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
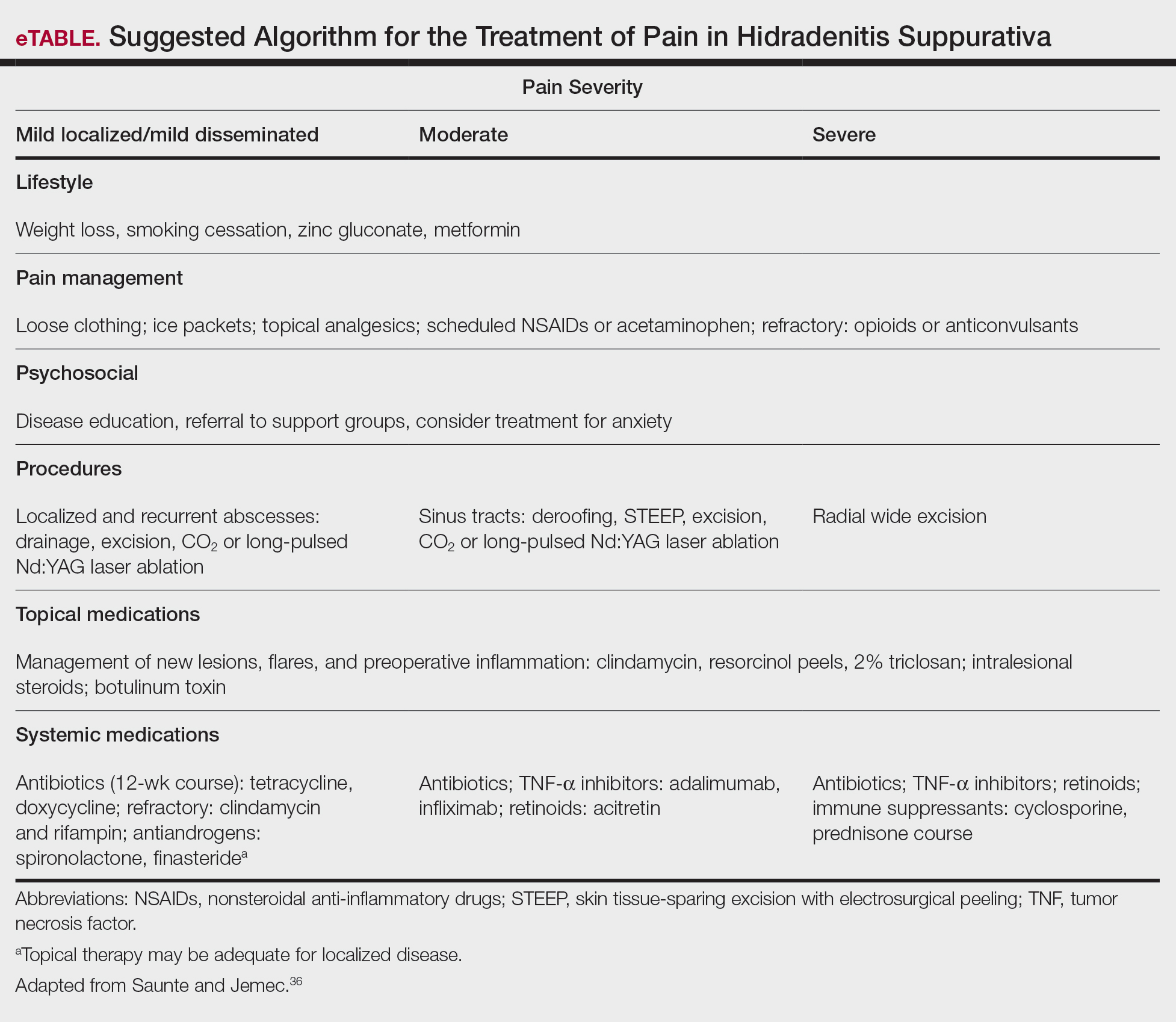
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Practice Points
- First-line therapies may not provide adequate pain control in many patients with hidradenitis suppurativa.
- Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.
- Long-term management involves lifestyle modifications and pharmacologic agents.
- The most effective pain remedies developed thus far are limited to surgery and tumor necrosis factor α inhibitors.
The Impact of Fellowship Training on Scholarly Productivity in Academic Dermatology
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).
There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.
Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.
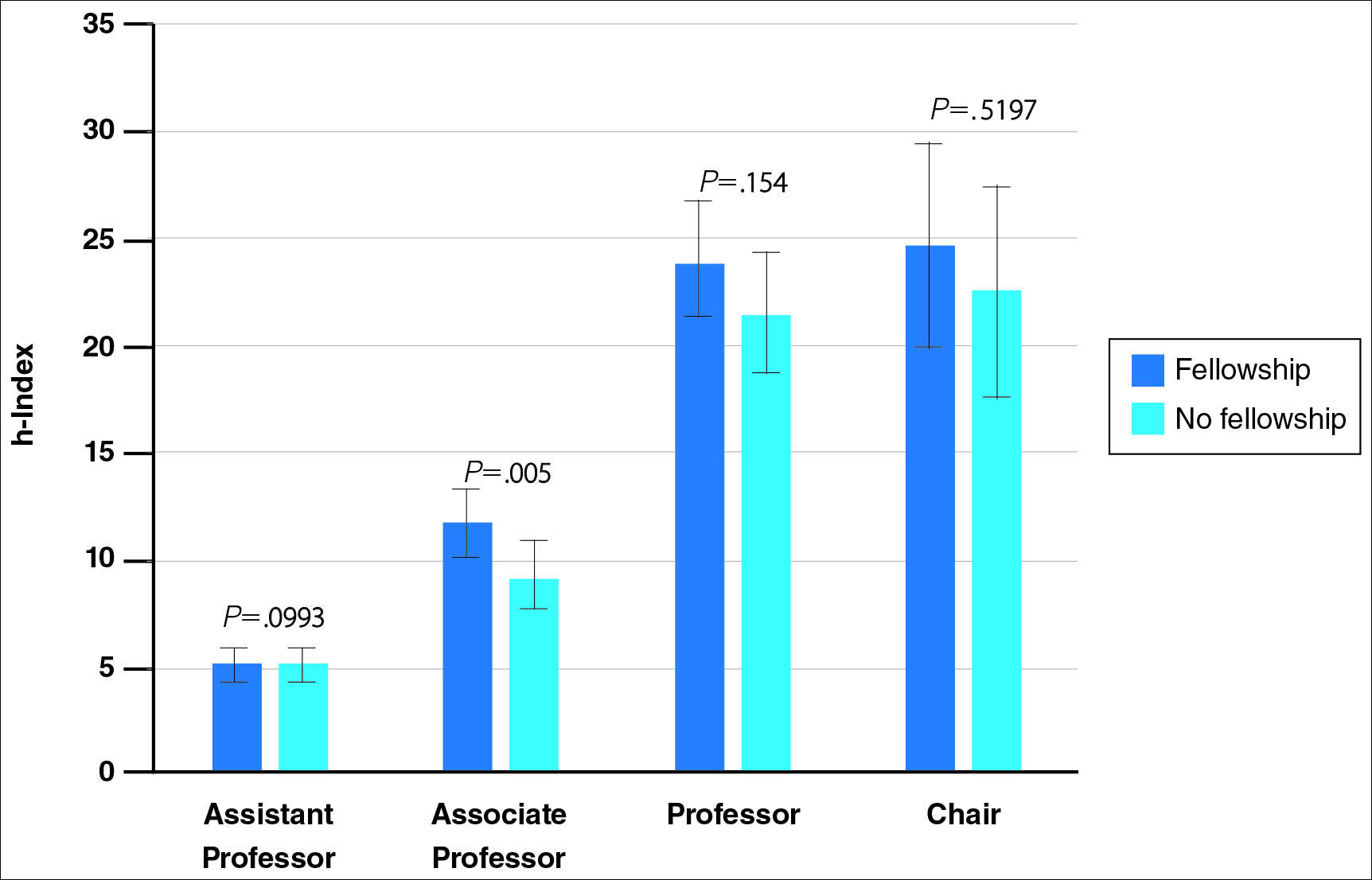
When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).
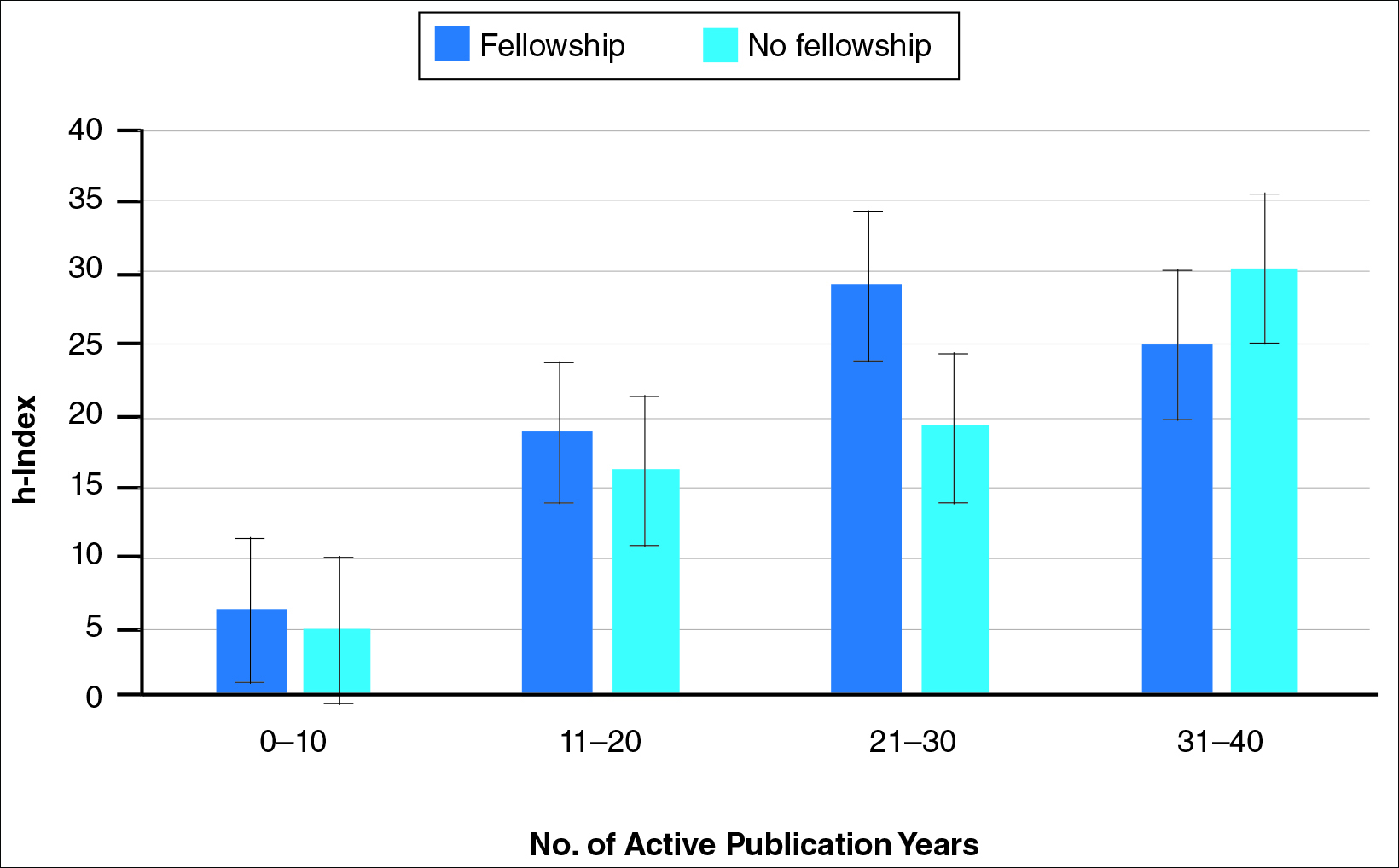
Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).


There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.

Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.

When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).

Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
The percentage of dermatology residents pursuing fellowship training is steadily increasing. A report from the American Board of Dermatology described an increase in the percentage of residents entering fellowships approved by the American Board of Dermatology and Accreditation Council for Graduate Medical Education from 10% in 2006 to 24% in 2010.1 The American Medical Association Residency & Fellowship Database FREIDA Online showed that 30% of dermatology residents or fellows pursued further fellowship training in 2013.2 The number of dermatology fellowship positions offered also is steadily increasing. Data from SF Match showed that the number of participating applicants in Mohs micrographic surgery (MMS) fellowships increased from 64 in 2002 to 86 in 2014, and the number of programs increased from 48 to 56, respectively.3 Similarly, in pediatric dermatology the SF Match reported an increase from 14 to 22 in participating applicants and an increase in available programs from 14 to 20 in 2009 and 2012, respectively.4 Reports on dermatopathology programs also have suggested either a stable or increased percentage of residents pursuing fellowships in this specialty.5,6
There are several reported factors that influence the pursuit of dermatology fellowships. Fellows often hope to gain further exposure to a dermatology subspecialty,7 which is especially applicable to procedural dermatology, as the prevailing opinion among dermatologists is that residency training should emphasize medical dermatology much more than surgery.8,9 Increased financial compensation, responsibility to provide for a family, and increased levels of educational debt do not notably influence the desire to pursue a fellowship, though these factors often play a role in the decision to pursue a career in academia.6,10-12 Additionally, it has been reported that fellowship-trained dermatologists are more likely to teach students, residents, and fellows and are up to 8 times more likely to participate in research than non–fellowship-trained dermatologists.6,8,11 Research activity also correlates with the decision to pursue an academic career. As such, fellowship training may present physicians with opportunities to improve clinical care, garner more research opportunities, and advance in academic rank.13
Scholarly productivity, measured by contribution to research, is a heavily weighted factor when hiring and promoting within academic medicine.14-17 Despite the importance of scholarly productivity, it is difficult to accurately quantify the measure. Commonly used metrics include number of publications, number of citations, amount of National Institutes of Health funding, number of research presentations, and number of lectures.18,19 However, taken individually, none of these measures entirely represents an individual’s research contribution. For example, a physician may have a large number of relatively low-quality publications. Additionally, if considering the number of citations, one of an author’s publications may have many citations, while the remaining publications do not.
The h-index, introduced in 2005 by Hirsch,20,21 is a measure of academic productivity that takes into account both the quantity and impact of research measured by recording the number of published articles and the number of citations in peer-reviewed journals. A high h-index indicates a high number of significant publications. For example, if a physician has 10 published articles cited 10 times each, his/her h-index is 10. Another physician with an h-index of 10 may have published 50 articles, which indicates that the remaining 40 articles were cited fewer than 10 times. Prior studies on the use of the h-index in fields as diverse as otolaryngology, radiology, anesthesiology, neurosurgery, ophthalmology, and urology indicate a strong association between the h-index and academic rank.22-28 Other studies indicate that fellowship-trained individuals tend to have a higher h-index than their non–fellowship-trained counterparts.29,30 One study demonstrated that fellowship-trained dermatologic surgeons had significantly increased academic productivity (P=.001), as measured by the number of publications in PubMed, compared to non–fellowship-trained dermatologic surgeons.11
The goal of this study was to determine whether dermatology fellowship training impacts scholarly productivity and academic promotion. Additionally, the scholarly productivity of procedural dermatology/MMS, dermatopathology, and pediatric dermatology fellows is compared to determine if type of subspecialty affects research productivity.
Methods
A list of academic dermatology departments was accessed using FREIDA Online. Individual departmental websites were visited to compile a list of academic faculty members. Additional recorded data included academic rank, gender, and fellowship training. Academic rank was classified as assistant professor, associate professor, professor, and chair. Physicians listed as chairs were not listed as professors to avoid duplication of these individuals. Voluntary, nonclinical, and nonacademic faculty members were excluded from the analysis. Departments that did not list the academic rank of faculty members also were excluded. Faculty members were organized by fellowship type: procedural dermatology/MMS, dermatopathology, pediatric dermatology, other fellowship, and no fellowship. Individuals with multiple fellowships were counted in multiple categories.
Faculty members were subsequently searched on the Scopus database to determine the h-index and publication range in years. Correct author identity was ensured by confirming correct departmental affiliations and publications related to dermatology. (Results collected from the Scopus database have been shown to correlate well with those ofISI Web of Knowledge.23)
Kruskal-Wallis tests were used to compare continuous variables, and the Pearson χ2 test was used to compare categorical variables. Statistical significance was set at P<.05. All statistical analyses were performed using SAS software. This study qualified as nonhuman subject research per the institutional review board of Rutgers New Jersey Medical School (Newark, New Jersey).
Results
The analysis included 1043 faculty members from 103 academic departments. There were 144 dermatologists (13.8%) with procedural dermatology/MMS fellowships, 162 (15.5%) with dermatopathology fellowships, 71 (6.8%) with pediatric dermatology fellowships, 124 (11.9%) with other fellowships, and 542 (52.0%) with no fellowships (Figure 1). Fellowships classified as other included immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology. Fellowship-trained dermatologists had a higher mean h-index than dermatologists without fellowships (13.2 vs 11.7; P<.001)(Figure 2).


There were significant statistical differences among the fellowships examined (Kruskal-Wallis analysis of variance, P<.05). Academic dermatologists who completed dermatopathology or other fellowships had higher scholarly productivity than those who completed pediatric dermatology and procedural dermatology/MMS fellowships (P<.05)(Figure 3). Those who did not complete a fellowship had a higher mean h-index than those who completed pediatric dermatology and procedural dermatology/MMS fellowships; however, the difference was not statistically significant.

Regarding academic rank, there was a significant increase in scholarly productivity (as measured by the h-index) from assistant professor to professor (P<.05). There was no statistical difference in scholarly productivity between professors and chairs. When controlling for academic rank, there were no statistically significant differences in h-index between fellowship-trained versus non–fellowship-trained dermatologists, except at the level of associate professor. However, fellowship-trained dermatologists consistently had a higher mean h-index compared to non–fellowship-trained dermatologists in each rank (Figure 4). Fellowship-trained dermatologists made up 48.2% (222/461) of assistant professors, 45.2% (103/228) of associate professors, 47.3% (125/264) of professors, and 56.7% (51/90) of chairs.

When controlling for the number of active publication years, no statistically significant differences were found between scholarly productivity in fellowship-trained versus non–fellowship-trained dermatologists. However, fellowship-trained academic dermatologists consistently had a higher mean h-index than non–fellowship-trained dermatologists within each 10-year range, except for the 31- to 40-year range (Figure 5).

Comment
The proportion of dermatology residents who pursue fellowship training has been steadily increasing, according to data from the American Medical Association and American Board of Dermatology.1,2 Fellowship training allows graduating residents to have greater exposure to a dermatology subspecialty and often provides a narrower focus for future clinical activities. In our study, we found that fellowship-trained dermatologists had significantly higher research productivity, as measured by the h-index, than academic dermatologists without fellowship, which is likely because fellowship training offers an opportunity to hone teaching skills and pursue more research activity.13 For instance, several fellowship programs allow focused research time during training.11 Additionally, residents pursuing fellowships may be more likely to engage in research activities.
Greater scholarly productivity is especially important for academic physicians, as it plays an important role in hiring and promoting.14,15,19,31 Additionally, increased research productivity has been found to be associated with improved teaching and clinical activity.19 Research productivity of faculty members also influences the reputation and prestige of the department and the institution’s subsequent ability to attract higher-quality residents and faculty members.28
There were significant differences in mean h-index between dermatology subspecialties. Academic dermatologists who completed procedural dermatology/MMS fellowships had the lowest mean h-index, while those who completed dermatopathology or other fellowships had the highest mean h-index. These findings suggest that an emphasis on research productivity may be greater in dermatopathology. Additionally, dermatologists who completed other fellowships, such as immunodermatology or dermatopharmacology, may have received such fellowships prior to dermatology training. It would be interesting to determine the amount of time allocated for research within each subspecialty fellowship training.
A greater amount of clinical responsibility also may influence the difference in measures of scholarly productivity within each subspecialty. For instance, there is a known shortage of pediatric dermatologists,32 which may translate as a decreased amount of time that can be dedicated to research activity because of higher clinical volume per physician. Dermatologists with no fellowship had a higher mean h-index than those with pediatric and procedural dermatology/MMS fellowships, which may reflect the smaller number of subspecialists compared to non–fellowship-trained dermatologists (13.8% procedural dermatology/MMS; 6.8% pediatric dermatology; 52.0% no fellowship). As such, the research of subspecialists is targeted to a narrower audience and will garner fewer citations than non–fellowship-trained dermatologists. However, the lower number of subspecialists is not the only factor impacting scholarly productivity, as dermato-pathologists had higher scholarly impact than non–fellowship-trained individuals despite comprising only 15.5% of the cohort.
In corroboration with prior studies of academic medicine, the h-index increased with increasing rank from assistant professor to professor and chair.29,30,33 This increase confirms that research productivity is associated with academic rank. When stratifying the 2 cohorts of fellowship-trained and non–fellowship-trained academic dermatologists by academic rank, there was no significant difference in the h-index for both groups at each rank, except for associate professor. In addition, there was a relatively equal distribution within each rank of fellowship-trained and non–fellowship-trained individuals. This lack of statistical difference also was demonstrated when stratifying for years of active publication experience. Academic dermatologists have been shown to be more interested in pursuing research activity, and research is pivotal to pursuing a dermatology residency.11 Future studies may extend the comparison of scholarly productivity to nonacademic dermatologists.
It is important to acknowledge certain limitations in the data collection process and use of the h-index. Many of the dermatology department websites do not provide information about whether individual faculty members are pursuing a tenure track or nontenure track. This distinction may have bearing on the h-index, as research is more heavily emphasized in the tenure track. Moreover, the h-index does not take into account the type of research (ie, clinical vs basic science research). Therefore, while basic science research often is more time intensive than clinical research, a publication is weighed solely by its number of citations. As such, the h-index may not capture the true amount of time dedicated to research activities. In addition, the h-index cannot account for self-citation, which may increase this measure.34 However, to greatly influence the h-index, many self-citations of each work would be necessary, making it less concerning. Another limitation of this study is that it does not take into account time dedicated to the education of residents and medical students, an act that is necessary for preservation of the field. Although education portfolios that detail an individual’s contribution to teaching are starting to become more popular, there currently is no measure for educational activities.18,35 Finally, dermatology department websites are not frequently updated; as such, data gathered from websites regarding academic rank may not always be recent.
Conclusion
Scholarly productivity, as measured by the h-index, is a major contributory factor to hiring, promoting, and developing reputations in academic medicine. Our findings demonstrate that there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists. However, when controlling for academic rank and publication range, this difference is minimized. As such, fellowships may provide more opportunity for structured research experiences but may not be necessary for successful academic careers. In addition, individuals who wish to dedicate a substantial portion of time to research may find that fellowships in dermatopathology, immunodermatology, dermatology-rheumatology, clinical education, dermatoepidemiology, cutaneous oncology, dermatopharmacology, and photobiology are more conducive to performing research. We also recommend that other activities, including clinical and teaching activities, serve as supplemental measures to scholarly productivity when evaluating a physician’s contribution.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
- Trends in postgraduate fellowships. American Board of Dermatology website. https://www.abderm.org/media/42577/prog-dir-ite_newsletter_july_2011.pdf. Accessed February 3, 2016.
- American Medical Association. FREIDA Online. https://freida.ama-assn.org/Freida/user/specStatistics Search.do?method=viewGraduates&pageNumber=3&spcCd=080. Accessed February 3, 2016.
- Micrographic surgery and dermatologic oncology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=10&typ=1&name=Micrographic%20Surgery%20and%20Dermatologic%20Oncology#. Accessed February 3, 2016.
- Pediatric dermatology fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=16&typ=1&name=Pediatric%20Dermatology#. Accessed February 3, 2016.
- Javorsky E, Kostecki J, Kimball AB. The relative popularity of nonprocedural dermatology fellowships. J Am Acad Dermatol. 2012;66:693-694.
- Suwattee P, Cham PM, Abdollahi M, et al. Dermatopathology workforce in the United States: a survey. J Am Acad Dermatol. 2011;65:1180-1185.
- Park KK. Fellowships after dermatology residency: the traditional and beyond. Cutis. 2015;95:E31-E34.
- Tierney EP, Hanke CW, Kimball AB. Recent changes in the workforce and practice of dermatologic surgery. Dermatol Surg. 2009;35:413-419.
- Wu JJ, Markus RF, Orengo IF. The increased competitiveness of Mohs micrographic surgery training. Dermatol Online J. 2002;8:24.
- Salter SA, Kimball AB. Rising educational debt levels in recent dermatology trainees and effects on career choices. J Am Acad Dermatol. 2006;54:329-331.
- Tierney EP, Hanke CW, Kimball AB. Academic productivity and affiliation of dermatologic surgeons. Dermatol Surg. 2009;35:1886-1892.
- Nguyen JC, Jacobson CC, Rehmus W, et al. Workforce characteristics of Mohs surgery fellows. Dermatol Surg. 2004;30(2, pt 1):136-138.
- Goldenberg G, Patel MJ, Sangueza OP, et al. US dermatopathology fellows career survey: 2004-2005. J Cutan Pathol. 2007;34:487-489.
- Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18:711-716.
- Beasley BW, Wright SM, Cofrancesco J Jr, et al. Promotion criteria for clinician-educators in the United States and Canada. a survey of promotion committee chairpersons. JAMA. 1997;278:723-728.
- Dixon AK. Publishing and academic promotion. Singapore Med J. 2009;50:847-850.
- Todisco A, Souza RF, Gores GJ. Trains, tracks, and promotion in an academic medical center. Gastroenterology. 2011;141:1545-1548.
- Baldwin C, Chandran L, Gusic M. Guidelines for evaluating the educational performance of medical school faculty: priming a national conversation. Teach Learn Med. 2011;23:285-297.
- Akl EA, Meerpohl JJ, Raad D, et al. Effects of assessing the productivity of faculty in academic medical centres: a systematic review. CMAJ. 2012;184:E602-E612.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the h-index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- Lee J, Kraus KL, Couldwell WT. Use of the h-index in neurosurgery. clinical article. J Neurosurg. 2009;111:387-392.
- Kasabwala K, Morton CM, Svider PF, et al. Factors influencing scholarly impact: does urology fellowship training affect research output? J Surg Educ. 2014;71:345-352.
- Pagel PS, Hudetz JA. H-index is a sensitive indicator of academic activity in highly productive anaesthesiologists: results of a bibliometric analysis. Acta Anaesthesiol Scand. 2011;55:1085-1089.
- Rad AE, Brinjikji W, Cloft HJ, et al. The h-index in academic radiology. Acad Radiol. 2010;17:817-821.
- Svider PF, Choudhry ZA, Choudhry OJ, et al. The use of the h-index in academic otolaryngology. Laryngoscope. 2013;123:103-106.
- Svider PF, Lopez SA, Husain Q, et al. The association between scholarly impact and National Institutes of Health funding in ophthalmology. Ophthalmology. 2014;121:423-428.
- Eloy JA, Svider PF, Mauro KM, et al. Impact of fellowship training on research productivity in academic otolaryngology. Laryngoscope. 2012;122:2690-2694.
- Huang G, Fang CH, Lopez SA, et al. Impact of fellowship training on research productivity in academic ophthalmology. J Surg Educ. 2015;72:410-417.
- Ball P. Achievement index climbs the ranks. Nature. 2007;448:737.
- Dinulos JG. Pediatric dermatology: past, present and future. Curr Opin Pediatr. 2007;19:417-419.
- Agarwal N, Clark S, Svider PF, et al. Impact of fellowship training on research productivity in academic neurological surgery. World Neurosurg. 2013;80:738-744.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Lamki N, Marchand M. The medical educator teaching portfolio: its compilation and potential utility. Sultan Qaboos Univ Med J. 2006;6:7-12.
Practice Points
- As residents decide whether to pursue fellowship training, it is important to consider the importance of fellowship completion for academic promotion and productivity.
- Although there is greater scholarly productivity among fellowship-trained dermatologists compared to non–fellowship-trained dermatologists, this difference is minimized when controlling for academic rank and publication range.
- Fellowships may provide more opportunity for structured research experiences but may not be necessary for successful careers in academic dermatology.