User login
Perianal Ulceration and Verrucous Papules
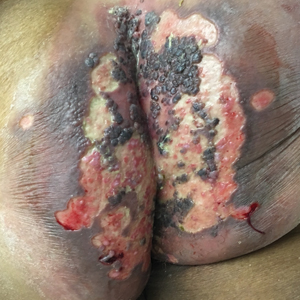
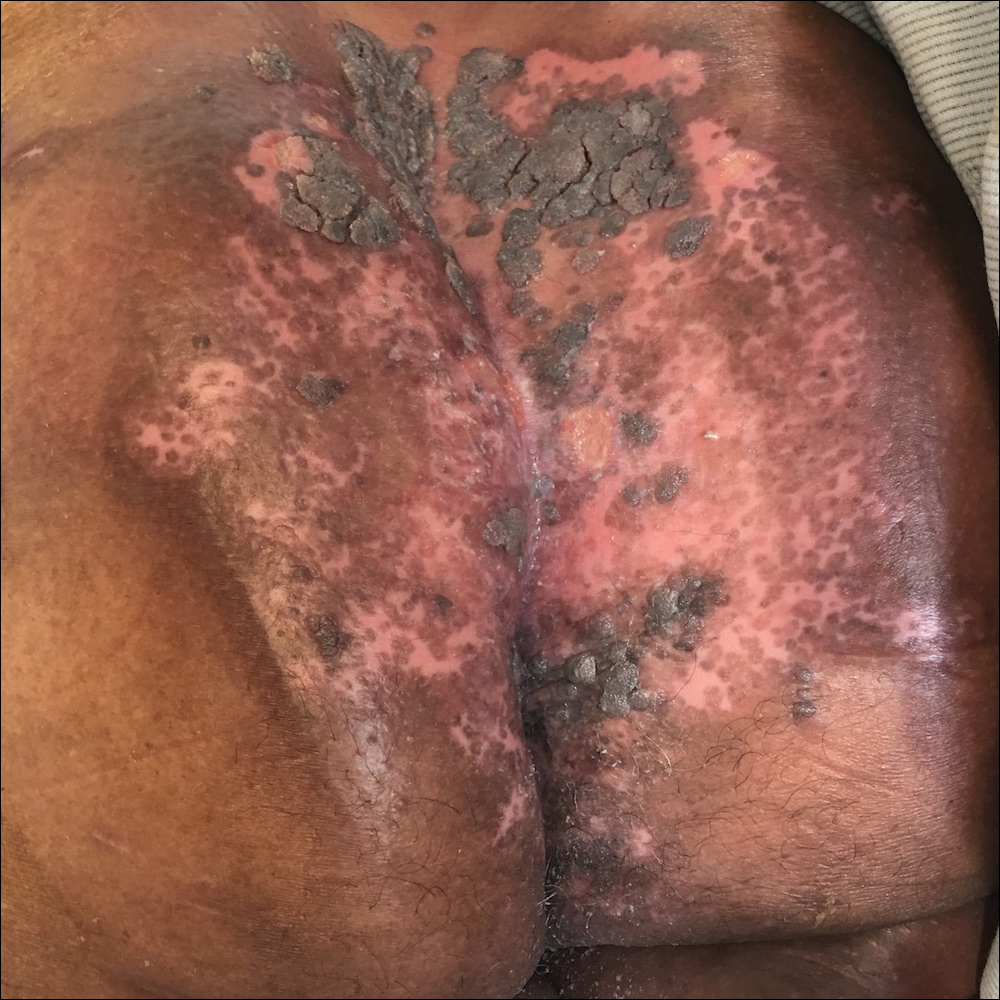
The Diagnosis: Herpes Simplex Virus Infection
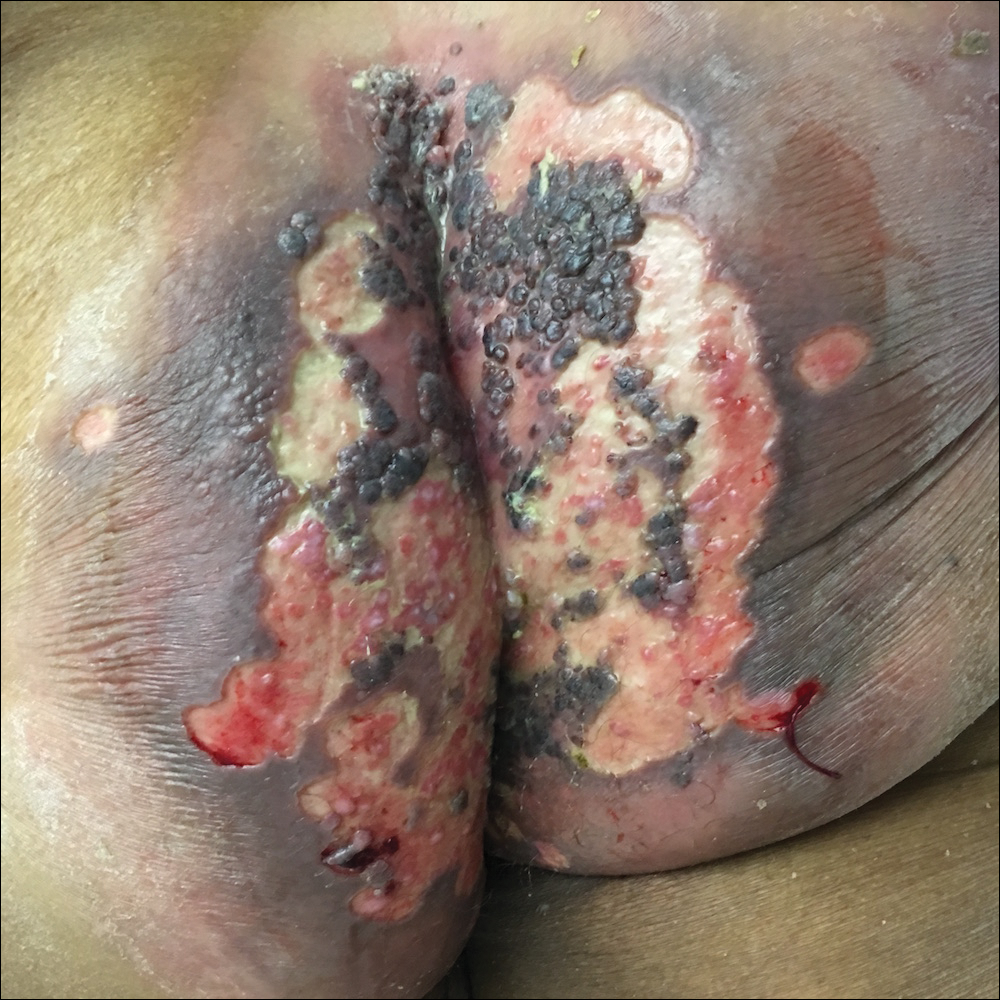
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.
Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
The Diagnosis: Herpes Simplex Virus Infection
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.

Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
The Diagnosis: Herpes Simplex Virus Infection
Viral culture of the ulcer was positive for herpes simplex virus type 2 (HHV-2). Bacterial culture grew enteric flora. The patient was started on intravenous acyclovir 5 mg/kg every 8 hours for 7 days and then transitioned to oral acyclovir for chronic suppressive therapy. One month later, there was near-complete reepithelialization with 2 remaining 1-cm shallow ulcers. The verrucous lesions had dried up and were flaking off (Figure). At 6-month follow-up, the ulcers and verrucous lesions had completely resolved.

Herpes simplex virus type 2 is the most common cause of genital and perianal ulcers in immunocompromised individuals.1 Patients classically present with painful grouped vesicles followed by painful superficial ulcers that may rapidly progress to extensive confluent ulceration. A hypertrophic variant of genital herpes characterized by anogenital verrucous lesions, similar to condyloma acuminata, also can be seen in immunocompromised individuals.2 This form has almost exclusively been observed in patients with human immunodeficiency virus and may occur in isolation or together with the ulcerative form.1-5 A case of vegetative HHV infection of the genital area in a patient with common variable immunodeficiency has been reported.6 Verrucous lesions of the mouth secondary to HHV have been observed in Hodgkin lymphoma, acute myelogenous leukemia, and individuals on immunosuppressive medications.7-10
Perianal involvement of Crohn disease typically presents with fistulas, ulcers, abscesses, strictures, and skin tags in some cases. Invasive squamous cell carcinoma may arise within a chronic ulcer of the anogenital area or may itself manifest as an ulcer or anal fissure. Perianal ulcerative skin tuberculosis has been reported in the literature as a rare manifestation of extrapulmonary tuberculosis and should be considered in a patient with an appropriate clinical history. Pyoderma gangrenosum classically pre-sents as a large ulcer with irregular rolled borders, though a rare variant of vegetative pyoderma gangrenosum may manifest as a nodular or verrucous plaque.
Studies to diagnose HHV include viral cell culture, HHV polymerase chain reaction testing, HHV serology, and direct fluorescent antibody testing. Skin biopsy may be necessary to rule out underlying malignancy. Treatment of perianal HHV infection includes acyclovir, valacyclovir, or famciclovir.1,5,6 Hypertrophic lesions often are refractory to first-line antiviral therapy and may require surgical resection or treatment with alternative medications such as imiquimod, a topical immunomodulator.3,5,6,11
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
- Ranu H, Lee J, Chio M, et al. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22:181-186.
- Tong P, Mutasim DF. Herpes simplex virus infection masquerading as condylomata accuminata in a patient with HIV disease. Br J Dermatol. 1996;134:797-800.
- Mosunjac M, Park J, Wang W, et al. Genital and perianal herpes simplex simulating neoplasia in patients with AIDS. AIDS Patient Care STDS. 2009;23:153-158.
- Gubinelli E, Cocuroccia B, Lazzarotto T, et al. Nodular perianal herpes simplex with prominent plasma cell infiltration. Sexually Transm Dis. 2003;30:157-159.
- Nadal SR, Calore EE, Manzione CR, et al. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48:2289-2293.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Burke EM, Karp DL, Wu TC, et al. Atypical oral presentation of herpes simplex virus infection in a patient after orthotopic liver transplantation. Eur Arch Otorhinolaryngol. 1994;251:301-303.
- Tabaee A, Saltman B, Shutter J, et al. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:376-380.
- Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54:3043-3047.
- Burgoyne M, Burke W. Atypical herpes simplex infection in patients with acute myelogenous leukemia recovering from chemotherapy. J Am Acad Dermatol. 1989;20:1125-1126.
- Deza G, Martin-Ezquerra G, Curto-Barredo L, et al. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42:1176-1178.
A 75-year-old woman with chronic lymphocytic leukemia undergoing ibrutinib targeted therapy presented to the emergency department with fever and perianal pain of 4 months' duration. The patient denied history of genital or perianal ulcers, warts, masses, bedsores, prolonged immobilization, anal surgeries, or recent travel. She had not been previously treated for the perianal pain. On physical examination there was an 18×15-cm shallow ulceration with rolled borders involving the intergluteal cleft and perianal area. There were numerous hyperpigmented verrucous papules clustered in the center of the ulceration. No vesicles or bullae were present. Laboratory results were pertinent for a white blood cell count of 3600/µL (reference range, 4500-11,000/µL) and absolute neutrophil count of 1300/µL (reference range, 1900-8000/µL). Human immunodeficiency virus testing was negative.
Skin Cancer Mortality in Patients With Skin of Color
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.