User login
Acute paraplegia in a patient with AIDS and a normal CSF examination
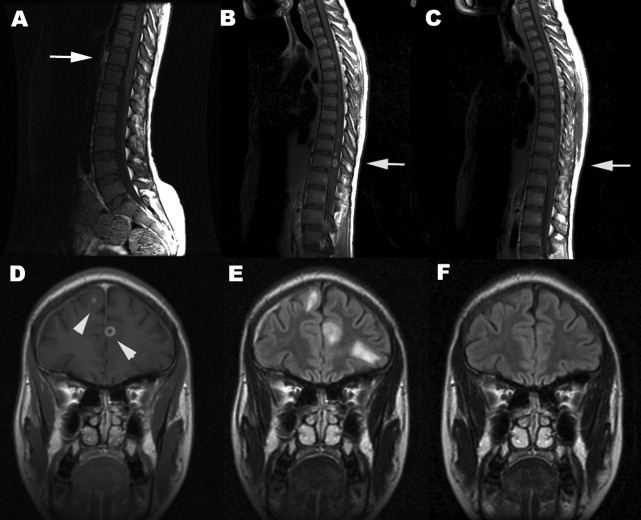
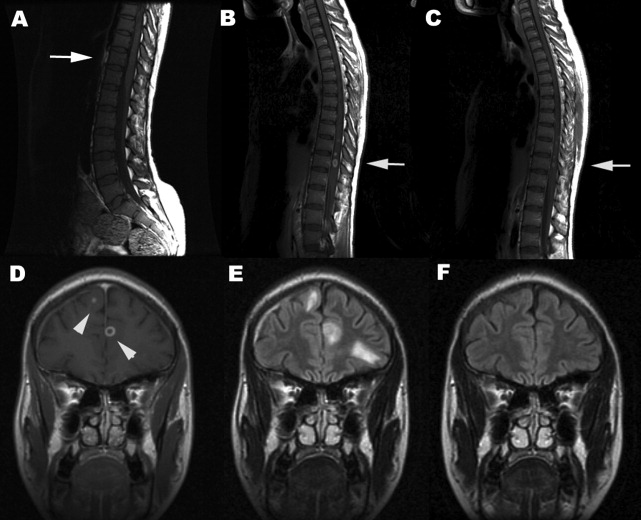
A 35‐year‐old Zimbabwean woman living in London, England, presented to the local accident and emergency department with a history of cough, shortness of breath, nausea, vomiting, diarrhea, and weight loss of 3 months' duration. Her chest X‐ray showed miliary shadowing. She was admitted to the hospital and commenced on antituberculous medications and offered an HIV test. Two weeks later, her sputum grew acid‐fast bacilli (AFB) and her HIV‐1 test came back positive. Her baseline T‐lymphocyte CD4 cell count was 6 cells/mm3 (reference range 4551320), and her HIV‐1 RNA viral load (VL) was 4,760,000 copies/mL. However, because of her multiple symptoms, her antiretroviral therapy was postponed. Four weeks later the patient's general condition improved, but she started to experience pain in her right thigh, followed by progressive right leg weakness without sensory loss. This new change affected her mobility, and she fell while walking. Within a week the patient developed progressive flaccid paraparesis with radicular sensory disturbance. This was followed by acute onset of flaccid paraplegia, extensor plantars, sensory loss to T10, and urinary incontinence. Her cerebrospinal fluid (CSF) examination revealed normal protein, glucose, and white cell count and no AFB or malignant cells, A second CSF after 3 weeks was abnormal, with protein of 0.79 g/L, normal glucose, 7 white cells (no malignant cells), and positive oligoclonal bands. The CSF screening for AFB‐PCR, treponemes, toxoplasma, cryptococcal antigen Epstein‐Barr virus, cytomegalovirus (CMV), herpes simplex virus, human T‐lymphotropic virus, and JC virus was negative. Neurophysiological studies confirmed the presence of severe multilevel radiculopathy. The MRI scan of her head and spine showed multiple intraparenchymal cerebral tuberculomata and cavitation at T 911 that enhanced postgadolinium (see Fig. 1). She was commenced on highly active antiretroviral therapy (HAART) and steroids. Ten weeks later her HIV‐1 RNA VL became undetectable in the ultrasensitive assay (cutoff 50 copies/mL), and she regained sensation in both legs and was able to stand with support. Posttreatment MRI appearances demonstrated improvement, and 14 months after treatment the MRI brain was normal (see Fig. 1).
DISCUSSION
Tuberculous myeloradiculitis is a rarely reported manifestation of tuberculosis, and there are no accurate figures for its incidence.13 It may arise as a primary manifestation of the infection, by downward extension of tuberculous meningitis, or by spread from a vertebral osteomyelitis.3 It is not uncommon for it to develop during treatment for a primary infection elsewhere.4 The clinical features of this patient were those of radiculitis followed by rapid flaccid paraparesis. The acute onset of the paraplegia with a normal CSF could be mistakenly interpreted as purely peripheral HIV‐related conditions such as CMV or PML. The extensor plantars and acute development of a paraplegia and sensory level (a combination of radiculopathy, myelopathy, and spinal shock) betrayed the central involvement of the thoracolumbar region.2 In many cases there is a copious leptomeningeal exudate, which helps to explain the fairly typical clinical presentation. This case was unusual in that there was no visible imaging evidence of exudate. The presentation should be differentiated from the vacuolar myelopathy seen with HIV infection alone.1, 5 A further unusual and unique feature was the normality of the CSF examination. Although in cases of tuberculous abscess, the CSF may be normal until rupture occurs into the subarachnoid space, a lymhocytosis, raised protein and possibly hypoglycorrachia would have been expected even in a treated and sterile CSF.3 A normal CSF examination was reported in a previous study.6 This case strongly emphasizes that the diagnosis of opportunistic tuberculosis in the setting of HIV infection can be elusive. Immune reconstruction inflammatory syndrome is an unlikely cause of her neurological symptoms, as she developed these symptoms before HAART. Antituberculous medications and antimycobacterial treatment must be modified to take into account the altered pharmacokinetics as a result of HAART and steroids. There are no published guidelines on how long steroids should be maintained despite their efficacy as an adjunct in TB meningitis.7 There is no evidence to guide their use in patients with coexistent HIV. Treatment until significant improvement or a 6‐month tailed trial of steroids (in the absence of other contraindications) is probably acceptable. Even though the consequences of a well‐placed mycobacterial lesion may be devastating, appropriate treatment may lead to partial and clinically important reversal of disability.
- ,,, et al.Spectrum of myelopathies in HIV seropositive South African patients.Neurology.2001;57:348–351.
- ,.Diagnosis and management of tuberculous paraplegia with special reference to tuberculous radiculomyelitis.J Neurol Neurosurg Psychiatry.1979;42:12–18.
- ,,, et al.Tuberculous radiculomyelitis complicating tuberculous meningitis: case report and review.Clin Infect Dis.2000;30:915–921.
- ,,.Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: a report of 10 patients and review of the literature.Q J Med.1987;63:449–460.
- ,.Infectious myelopathies.Semin Neurol.2002;22:133–141.
- ,,,,,, et al.Tuberculous meningitis in patients infected with the human immunodeficiency virus.New Engl J Med.1992;326:668–672.
- ,,, et al.Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults.N Engl J Med.2004;351:1741–1751.
A 35‐year‐old Zimbabwean woman living in London, England, presented to the local accident and emergency department with a history of cough, shortness of breath, nausea, vomiting, diarrhea, and weight loss of 3 months' duration. Her chest X‐ray showed miliary shadowing. She was admitted to the hospital and commenced on antituberculous medications and offered an HIV test. Two weeks later, her sputum grew acid‐fast bacilli (AFB) and her HIV‐1 test came back positive. Her baseline T‐lymphocyte CD4 cell count was 6 cells/mm3 (reference range 4551320), and her HIV‐1 RNA viral load (VL) was 4,760,000 copies/mL. However, because of her multiple symptoms, her antiretroviral therapy was postponed. Four weeks later the patient's general condition improved, but she started to experience pain in her right thigh, followed by progressive right leg weakness without sensory loss. This new change affected her mobility, and she fell while walking. Within a week the patient developed progressive flaccid paraparesis with radicular sensory disturbance. This was followed by acute onset of flaccid paraplegia, extensor plantars, sensory loss to T10, and urinary incontinence. Her cerebrospinal fluid (CSF) examination revealed normal protein, glucose, and white cell count and no AFB or malignant cells, A second CSF after 3 weeks was abnormal, with protein of 0.79 g/L, normal glucose, 7 white cells (no malignant cells), and positive oligoclonal bands. The CSF screening for AFB‐PCR, treponemes, toxoplasma, cryptococcal antigen Epstein‐Barr virus, cytomegalovirus (CMV), herpes simplex virus, human T‐lymphotropic virus, and JC virus was negative. Neurophysiological studies confirmed the presence of severe multilevel radiculopathy. The MRI scan of her head and spine showed multiple intraparenchymal cerebral tuberculomata and cavitation at T 911 that enhanced postgadolinium (see Fig. 1). She was commenced on highly active antiretroviral therapy (HAART) and steroids. Ten weeks later her HIV‐1 RNA VL became undetectable in the ultrasensitive assay (cutoff 50 copies/mL), and she regained sensation in both legs and was able to stand with support. Posttreatment MRI appearances demonstrated improvement, and 14 months after treatment the MRI brain was normal (see Fig. 1).

DISCUSSION
Tuberculous myeloradiculitis is a rarely reported manifestation of tuberculosis, and there are no accurate figures for its incidence.13 It may arise as a primary manifestation of the infection, by downward extension of tuberculous meningitis, or by spread from a vertebral osteomyelitis.3 It is not uncommon for it to develop during treatment for a primary infection elsewhere.4 The clinical features of this patient were those of radiculitis followed by rapid flaccid paraparesis. The acute onset of the paraplegia with a normal CSF could be mistakenly interpreted as purely peripheral HIV‐related conditions such as CMV or PML. The extensor plantars and acute development of a paraplegia and sensory level (a combination of radiculopathy, myelopathy, and spinal shock) betrayed the central involvement of the thoracolumbar region.2 In many cases there is a copious leptomeningeal exudate, which helps to explain the fairly typical clinical presentation. This case was unusual in that there was no visible imaging evidence of exudate. The presentation should be differentiated from the vacuolar myelopathy seen with HIV infection alone.1, 5 A further unusual and unique feature was the normality of the CSF examination. Although in cases of tuberculous abscess, the CSF may be normal until rupture occurs into the subarachnoid space, a lymhocytosis, raised protein and possibly hypoglycorrachia would have been expected even in a treated and sterile CSF.3 A normal CSF examination was reported in a previous study.6 This case strongly emphasizes that the diagnosis of opportunistic tuberculosis in the setting of HIV infection can be elusive. Immune reconstruction inflammatory syndrome is an unlikely cause of her neurological symptoms, as she developed these symptoms before HAART. Antituberculous medications and antimycobacterial treatment must be modified to take into account the altered pharmacokinetics as a result of HAART and steroids. There are no published guidelines on how long steroids should be maintained despite their efficacy as an adjunct in TB meningitis.7 There is no evidence to guide their use in patients with coexistent HIV. Treatment until significant improvement or a 6‐month tailed trial of steroids (in the absence of other contraindications) is probably acceptable. Even though the consequences of a well‐placed mycobacterial lesion may be devastating, appropriate treatment may lead to partial and clinically important reversal of disability.
A 35‐year‐old Zimbabwean woman living in London, England, presented to the local accident and emergency department with a history of cough, shortness of breath, nausea, vomiting, diarrhea, and weight loss of 3 months' duration. Her chest X‐ray showed miliary shadowing. She was admitted to the hospital and commenced on antituberculous medications and offered an HIV test. Two weeks later, her sputum grew acid‐fast bacilli (AFB) and her HIV‐1 test came back positive. Her baseline T‐lymphocyte CD4 cell count was 6 cells/mm3 (reference range 4551320), and her HIV‐1 RNA viral load (VL) was 4,760,000 copies/mL. However, because of her multiple symptoms, her antiretroviral therapy was postponed. Four weeks later the patient's general condition improved, but she started to experience pain in her right thigh, followed by progressive right leg weakness without sensory loss. This new change affected her mobility, and she fell while walking. Within a week the patient developed progressive flaccid paraparesis with radicular sensory disturbance. This was followed by acute onset of flaccid paraplegia, extensor plantars, sensory loss to T10, and urinary incontinence. Her cerebrospinal fluid (CSF) examination revealed normal protein, glucose, and white cell count and no AFB or malignant cells, A second CSF after 3 weeks was abnormal, with protein of 0.79 g/L, normal glucose, 7 white cells (no malignant cells), and positive oligoclonal bands. The CSF screening for AFB‐PCR, treponemes, toxoplasma, cryptococcal antigen Epstein‐Barr virus, cytomegalovirus (CMV), herpes simplex virus, human T‐lymphotropic virus, and JC virus was negative. Neurophysiological studies confirmed the presence of severe multilevel radiculopathy. The MRI scan of her head and spine showed multiple intraparenchymal cerebral tuberculomata and cavitation at T 911 that enhanced postgadolinium (see Fig. 1). She was commenced on highly active antiretroviral therapy (HAART) and steroids. Ten weeks later her HIV‐1 RNA VL became undetectable in the ultrasensitive assay (cutoff 50 copies/mL), and she regained sensation in both legs and was able to stand with support. Posttreatment MRI appearances demonstrated improvement, and 14 months after treatment the MRI brain was normal (see Fig. 1).

DISCUSSION
Tuberculous myeloradiculitis is a rarely reported manifestation of tuberculosis, and there are no accurate figures for its incidence.13 It may arise as a primary manifestation of the infection, by downward extension of tuberculous meningitis, or by spread from a vertebral osteomyelitis.3 It is not uncommon for it to develop during treatment for a primary infection elsewhere.4 The clinical features of this patient were those of radiculitis followed by rapid flaccid paraparesis. The acute onset of the paraplegia with a normal CSF could be mistakenly interpreted as purely peripheral HIV‐related conditions such as CMV or PML. The extensor plantars and acute development of a paraplegia and sensory level (a combination of radiculopathy, myelopathy, and spinal shock) betrayed the central involvement of the thoracolumbar region.2 In many cases there is a copious leptomeningeal exudate, which helps to explain the fairly typical clinical presentation. This case was unusual in that there was no visible imaging evidence of exudate. The presentation should be differentiated from the vacuolar myelopathy seen with HIV infection alone.1, 5 A further unusual and unique feature was the normality of the CSF examination. Although in cases of tuberculous abscess, the CSF may be normal until rupture occurs into the subarachnoid space, a lymhocytosis, raised protein and possibly hypoglycorrachia would have been expected even in a treated and sterile CSF.3 A normal CSF examination was reported in a previous study.6 This case strongly emphasizes that the diagnosis of opportunistic tuberculosis in the setting of HIV infection can be elusive. Immune reconstruction inflammatory syndrome is an unlikely cause of her neurological symptoms, as she developed these symptoms before HAART. Antituberculous medications and antimycobacterial treatment must be modified to take into account the altered pharmacokinetics as a result of HAART and steroids. There are no published guidelines on how long steroids should be maintained despite their efficacy as an adjunct in TB meningitis.7 There is no evidence to guide their use in patients with coexistent HIV. Treatment until significant improvement or a 6‐month tailed trial of steroids (in the absence of other contraindications) is probably acceptable. Even though the consequences of a well‐placed mycobacterial lesion may be devastating, appropriate treatment may lead to partial and clinically important reversal of disability.
- ,,, et al.Spectrum of myelopathies in HIV seropositive South African patients.Neurology.2001;57:348–351.
- ,.Diagnosis and management of tuberculous paraplegia with special reference to tuberculous radiculomyelitis.J Neurol Neurosurg Psychiatry.1979;42:12–18.
- ,,, et al.Tuberculous radiculomyelitis complicating tuberculous meningitis: case report and review.Clin Infect Dis.2000;30:915–921.
- ,,.Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: a report of 10 patients and review of the literature.Q J Med.1987;63:449–460.
- ,.Infectious myelopathies.Semin Neurol.2002;22:133–141.
- ,,,,,, et al.Tuberculous meningitis in patients infected with the human immunodeficiency virus.New Engl J Med.1992;326:668–672.
- ,,, et al.Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults.N Engl J Med.2004;351:1741–1751.
- ,,, et al.Spectrum of myelopathies in HIV seropositive South African patients.Neurology.2001;57:348–351.
- ,.Diagnosis and management of tuberculous paraplegia with special reference to tuberculous radiculomyelitis.J Neurol Neurosurg Psychiatry.1979;42:12–18.
- ,,, et al.Tuberculous radiculomyelitis complicating tuberculous meningitis: case report and review.Clin Infect Dis.2000;30:915–921.
- ,,.Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: a report of 10 patients and review of the literature.Q J Med.1987;63:449–460.
- ,.Infectious myelopathies.Semin Neurol.2002;22:133–141.
- ,,,,,, et al.Tuberculous meningitis in patients infected with the human immunodeficiency virus.New Engl J Med.1992;326:668–672.
- ,,, et al.Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults.N Engl J Med.2004;351:1741–1751.